Dear Editor,
Oculopharyngodistal myopathy (OPDM) is a slowly progressive adult-onset muscle disease characterized by ptosis, ophthalmoparesis, dysphagia, and weakness of the facial, masseter, bulbar, and distal limbs muscles [1]. Ishiura et al. revealed that OPDM is caused by noncoding CGG repeat expansions in the low-density lipoprotein receptor-related protein 12 gene (LRP12) [2]. Ishiura et al. also found similar noncoding CGG repeat expansions in two clinically similar conditions: neuronal intranuclear inclusion disease (NIID) and oculopharyngeal myopathy with leukoencephalopathy (OPML), in the NBPF19 (NOTCH2NLC) and LOC642361/NUTM2B-AS1 genes, respectively [2]. As is the case between NIID and OPML as well as between OPML and OPDM, clinical or pathological similarities between OPDM and NIID are also expected. In fact, the presence of neuronal intranuclear inclusions in OPDM patients has been demonstrated [3,4]. However, the findings in neuromuscular tissues, such as the sural nerve, where biopsies are often performed, are not yet established. Here, we report a genetically confirmed case of OPDM that shared clinicopathological neuromuscular characteristics with NIID (Fig. 1A).
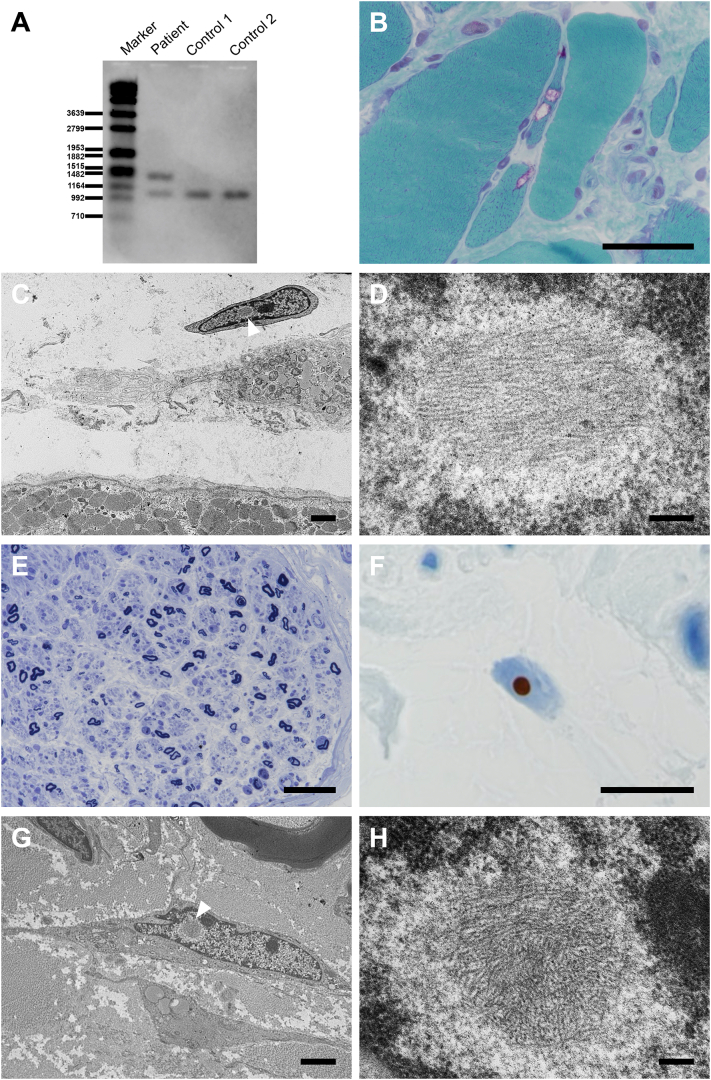
Fig. 1.
Southern blot analysis demonstrates CGG repeat expansion in the LRP12 gene (A). Histopathological findings at age 80 years. In the biceps brachii muscle, modified Gomori trichrome staining demonstrates atrophic fibers with rimmed vacuoles (B). Electron microscopy reveals that a fibroblast adjacent to the degenerated muscles contains intranuclear inclusion (arrowhead) surrounded by amorphous rough halos without limiting membrane (C). High magnification of the area indicated by arrowhead in panel C demonstrates intermingled granulofilamentous profiles (D)]. Toluidine blue staining of the sural nerve indicates severe axonal loss (E) and p62-immunoreactive intranuclear inclusions (F). Electron microscopy shows identical intranuclear inclusions in the fibroblast (arrowhead) of the sural nerve as those in the biceps brachii muscle [low (G) and high (H) magnification]. Scale bars: 50 μm (B, E); 10 μm (F); 2 μm (C, G); 0.2 μm (D, H). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
A 70-year-old man without a family history of neuromuscular disease presented with an eight-year history of hoarseness and dysphagia, followed by bilateral ptosis and weakness and atrophy of the muscle of the distal upper limb, as well as paresthesia in the toes of both feet. His past medical history included juvenile baldness, sick sinus syndrome with atrial fibrillation, and bilateral cataracts. Neurological examination revealed limited horizontal eye movements. Further examination revealed a myopathic face with facial muscle atrophy and bilateral ptosis, bilateral muscle weakness in the distal upper extremities, atrophic thenar and hypothenar muscles, and absent deep tendon reflexes in all four extremities. The other examinations revealed normal findings. Laboratory study results were normal, except for an elevated serum creatine kinase level of 339 IU/L. Brain magnetic resonance imaging did not reveal leukoencephalopathy. Computed tomography revealed bilateral partial adipose tissue replacement of the paraspinal and gastrocnemius muscles. The patient underwent a biopsy of the biceps brachii muscle, which revealed variation in the fiber size, increased number of internalized nuclei, scattered pyknotic nuclear clumps, and occasional atrophic fibers with rimmed vacuoles. No necrotic or inflammatory changes or ragged red fibers were observed.
At age 80 years, he was admitted for heart failure, which improved after diuretic therapy. At this time, he exhibited evident limited eye movements in all directions, bulbar weakness, and distal-predominant muscle atrophy as well as distal paresthesia. The patient had daytime hypercapnia, with a pCO2 of 52 mmHg on arterial blood gas analysis. His vital capacity by spirometry was 40.1% of the predicted value. Nerve conduction studies disclosed mixed axonal and demyelinating features characterized by reduction of amplitude of motor and sensory action potentials and conduction slowing, which were more prominent in his lower extremities. Needle electromyography disclosed fibrillation potentials and positive sharp waves in the biceps brachii and tibialis anterior muscles. Blood tests did not identify an underlying cause for the neuropathy. To examine the cause of the unexpected symptoms and findings suggesting neuropathy, biopsies of the biceps brachii muscle and sural nerve were performed. The histopathological findings of the muscle were similar to those of his first biopsy (Fig. 1B). Immunohistochemistry revealed a few p62- and ubiquitin- immunoreactive intramyonuclear inclusions. The sural nerve showed severe axonal loss (Fig. 1C). Even here, p62-immunoreactive intranuclear inclusions in fibroblasts were observed (Fig. 1D). Further, electron microscopy demonstrated intranuclear aggregations consisting of haphazardly arranged filaments of 10- to 15-nm diameter, admixed with granular substances, in the fibroblasts of both biceps brachii muscle and sural nerve. (Fig. 1E–F). Subsequently, genetic analysis using repeat-primed polymerase chain reaction and Southern blot demonstrated CGG repeat expansion (106 CGG repeats) in the LRP12 gene (Fig. 1A). CTG repeat expansion in the DMPK gene was 12 and 13, thus, within the normal range.
The patient followed a clinical course typical of OPDM, and developed respiratory complications, which are less frequent but known to occur in OPDM [5]. Moreover, he also developed neuropathy. Histopathological examination revealed findings consistent with OPDM in addition to severe axonal neuropathy and intranuclear inclusions comprising filaments as well as anti p62- and ubiquitin-immunoreactivity.
Neuropathy and intranuclear inclusions are representative features of NIID [6,7]. Considering that OPDM and NIID share similar genetic abnormalities (noncoding CGG repeat expansions), we believe that OPDM can present with neuropathy and intranuclear inclusions. In fact, a previous report of OPDM with genetic validation described OPDM with neuropathy [4]. Going a step further, in the current case, we clinicopathologically confirmed the presence of neuropathy and demonstrated intranuclear inclusion bodies in the fibroblasts of the sural nerve. The current case also showed intranuclear inclusions in the fibroblasts of the biceps brachii muscle. The presence of such inclusions should also be noted even in the muscle biopsies for OPDM.
In conclusion, our report of genetically confirmed OPDM showed that OPDM could share both genetic and clinicopathological characteristics of neuropathy with NIID. We suggest that neuropathy and intranuclear inclusions should be checked with more focus when examining OPDM cases.
Contributors
TM analyzed and interpreted the data and drafted and revised the manuscript. YS and SM designed and conceptualized the study and analyzed and interpreted the data. TK and MH contributed clinical data equitation and analyzed and interpreted the data. MO, AI, and IN performed genetic analyses and interpreted the data. FH, TA, and AK analyzed and interpreted the data. All authors read and approved the final manuscript.
Declarations of Competing Interest
The authors report no disclosures.
Study funding
This work was supported by Grants-in-Aid from the Research Committee of CNS Degenerative Diseases, Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health, Labour and Welfare Sciences Research Grants, the Ministry of Health, Labour and Welfare, Japan, MEXT/JSPS KAKENHI Grant Number JP16H06277 (SM), AMED under Grant Number JP18dm0107103 (YS, SM) and JP20ek0109490h0001 (IN), Intramural Research Grant (2–5 and 29–4 to IN; 2–5 and 30–9 to AI) for Neurological and Psychiatric Disorders of NCNP, and Joint Usage and Joint Research Programs, the Institute of Advanced Medical Sciences, Tokushima University (2020, 2A19 to AI). The funding bodies did not play any role in the design of the study, the collection, analysis, and interpretation of data, or the writing of the manuscript.
Acknowledgements
The authors would like to thank Dr. Narihiro Minami for the genetic analysis of DMPK, Mr. Yutaka Koga, Ms. Mieko Harada, Ms. Kyoko Okamoto, Ms. Nobuko Naoi, and Ms. Sachiko Imai for providing technical assistance.
Contributor Information
Tomoyasu Matsubara, Email: tomoyasu_matsubara@tmghig.jp.
Yuko Saito, Email: yukosm@bbarjp.net.
Takashi Kurashige, Email: takashi-kurashige@hiroshima-u.ac.jp.
Mana Higashihara, Email: mana_higashihara@tmghig.jp.
Fumio Hasegawa, Email: f_hasega@tmig.or.jp.
Masashi Ogasawara, Email: masashi.o0611@ncnp.go.jp.
Aritoshi Iida, Email: iidaa@ncnp.go.jp.
Ichizo Nishino, Email: nishino@ncnp.go.jp.
Tadashi Adachi, Email: adachi8@med.tottori-u.ac.jp.
Akatsuki Kubota, Email: akatsuki-tky@umin.net.
Shigeo Murayama, Email: smurayam@bbarjp.net.
References
- 1.Satoyoshi E., Kinoshita M. Oculopharyngodistal myopathy. Arch Neurol. 1977;34:89–92. doi: 10.1001/archneur.1977.00500140043007. [DOI] [PubMed] [Google Scholar]
- 2.Ishiura H., Shibata S., Yoshimura J., Suzuki Y., Qu W., Doi K., Almansour M.A., Kikuchi J.K., Taira M., Mitsui J., Takahashi Y., Ichikawa Y., Mano T., Iwata A., Harigaya Y., Matsukawa M.K., Matsukawa T., Tanaka M., Shirota Y., Ohtomo R., Kowa H., Date H., Mitsue A., Hatsuta H., Morimoto S., Murayama S., Shiio Y., Saito Y., Mitsutake A., Kawai M., Sasaki T., Sugiyama Y., Hamada M., Ohtomo G., Terao Y., Nakazato Y., Takeda A., Sakiyama Y., Umeda-Kameyama Y., Shinmi J., Ogata K., Kohno Y., Lim S.Y., Tan A.H., Shimizu J., Goto J., Nishino I., Toda T., Morishita S., Tsuji S. Noncoding CGG repeat expansions in neuronal intranuclear inclusion disease, oculopharyngodistal myopathy and an overlapping disease. Nat. Genet. 2019;51:1222–1232. doi: 10.1038/s41588-019-0458-z. [DOI] [PubMed] [Google Scholar]
- 3.Saito R., Shimizu H., Miura T., Hara N., Mezaki N., Higuchi Y., Miyashita A., Kawachi I., Sanpei K., Honma Y., Onodera O., Ikeuchi T., Kakita A. Oculopharyngodistal myopathy with coexisting histology of systemic neuronal intranuclear inclusion disease: Clinicopathologic features of an autopsied patient harboring CGG repeat expansions in LRP12. Acta Neuropathol Commun. 2020;8:75. doi: 10.1186/s40478-020-00945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogasawara M., Iida A., Kumutpongpanich T., Ozaki A., Oya Y., Konishi H., Nakamura A., Abe R., Takai H., Hanajima R., Doi H., Tanaka F., Nakamura H., Nonaka I., Wang Z., Hayashi S., Noguchi S., Nishino I. CGG expansion in NOTCH2NLC is associated with oculopharyngodistal myopathy with neurological manifestations. Acta Neuropathol Commun. 2020;8:204. doi: 10.1186/s40478-020-01084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durmus H., Laval S.H., Deymeer F., Parman Y., Kiyan E., Gokyigiti M., Ertekin C., Ercan I., Solakoglu S., Karcagi V., Straub V., Bushby K., Lochmuller H., Serdaroglu-Oflazer P. Oculopharyngodistal myopathy is a distinct entity: clinical and genetic features of 47 patients. Neurology. 2011;76:227–235. doi: 10.1212/WNL.0b013e318207b043. [DOI] [PubMed] [Google Scholar]
- 6.Sone J., Mori K., Inagaki T., Katsumata R., Takagi S., Yokoi S., Araki K., Kato T., Nakamura T., Koike H., Takashima H., Hashiguchi A., Kohno Y., Kurashige T., Kuriyama M., Takiyama Y., Tsuchiya M., Kitagawa N., Kawamoto M., Yoshimura H., Suto Y., Nakayasu H., Uehara N., Sugiyama H., Takahashi M., Kokubun N., Konno T., Katsuno M., Tanaka F., Iwasaki Y., Yoshida M., Sobue G. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain. 2016;139:3170–3186. doi: 10.1093/brain/aww249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morimoto S., Hatsuta H., Komiya T., Kanemaru K., Tokumaru A.M., Murayama S. Simultaneous skin-nerve-muscle biopsy and abnormal mitochondrial inclusions in intranuclear hyaline inclusion body disease. J Neurol Sci. 2017;372:447–449. doi: 10.1016/j.jns.2016.10.042. [DOI] [PubMed] [Google Scholar]