Abstract
Pseudorabies virus (PRV), the causative agent of Aujeszky's disease, is one of the most harmful pathogens to the pig industry. PRV can infect and kill a variety of mammals. Nevertheless, the underlying pathogenesis related to PRV is still unclear. This study aims to investigate the pathogenesis induced by PRV in a mouse model. The mice infected with the PRV-HLJ strain developed severe clinical manifestations at 36 h post-infection (hpi), and mortality occurred within 48–72 hpi. Hematoxylin-eosin staining and qRT-PCR methods were used to detect the pathological damage and expression of cytokines related to an immune reaction in brain tissue, respectively. The cytokine storms caused by IFN-α, IFN-β, TNF-α, IL-1β, IL-6 and IL-18 were related to the histopathological changes induced by PRV. This pattern of cytokine secretion depicts an image of typical cytokine storms, characterized by dysregulated secretion of pro-inflammatory cytokines and imbalanced pro-inflammatory and anti-inflammatory responses. In addition, the pyroptosis pathway was also activated by PRV by elevating the expression levels of nod-like receptor protein 3, Caspase-1, Gasdermin-D and interleukin-1β/18. These findings provide a way for further understanding the molecular basis in PRV pathogenesis.
Keywords: cytokine storms, pyroptosis, pseudorabies virus
1. Introduction
Pseudorabies virus (PRV) or suid α herpesvirus 1 is the pathogen of porcine Aujeszky's disease (AD) which affects the respiratory system, nervous system and reproductive systems [1]. Many mammals other than pigs, are susceptible to PRV infection, such as cattle, sheep, rabbits, cats, dogs, guinea pigs, rats and mice [2]. However, pigs are the only susceptible animals that can survive, although the prognosis of the disease largely depends on factors including inoculation site, virus strain and titer as well as the age of pigs [3,4]. AD is a highly infectious disease with high mortality in piglets. Transmission mainly occurs through direct contact with oral and nasal secretions but can also occur through aerosol and the placenta or sexual intercourse [1]. Therefore, the prevalence of PRV has led to a wide range of economic losses in the pork production industry. Inactivated and attenuated vaccines have been developed to delay or reduce swine death. However, they cannot eradicate the disease because none can prevent virus potential infection and reactivation and shedding of the virulent fields [5]. Due to the impact of AD on the pig industry, some countries are trying to eradicate AD based on the differentiating infected from vaccinated animals program. However, since 2011, the outbreak of AD occurred in pigs vaccinated with PR vaccine in China, which indicates that the AD vaccine cannot provide effective treatment to prevent wild PR infection [6].
Mice and rabbits are usually used to study PRV in the laboratory. After infection, animals showed abnormal excitement and nasal itching, accompanied by convulsions and rapid death. In mice, PRV almost manifested as a neurogenic infection of the central nervous system (CNS), accompanied by fulminant central nervous symptoms and high mortality [7]. PRV is known to cause severe encephalitis in piglets and various non-native hosts, even in humans [8]. Few studies have focused on the pathogenesis of encephalitis. It is known that pyroptosis is involved in the immune response in various types of cells, which can be triggered by various pathological stimuli, leading to the secretion of pro-inflammatory cytokines and intracellular contents [9]. Inflammation is a double-edged sword, which has a crucial role in metabolism. A mild inflammatory response could protect the body to a certain degree, help to repair damaged tissue and be beneficial to steady-state reconstruction. However, excessive inflammation may form ‘cytokine storms’, leading to tissue damage. In the present work, we describe the influence of PRV on the immune factor and pyroptosis-related factors in mice brains.
2. Material and methods
2.1. Reagents and animals
An Annexin V-FITC/PI Apoptosis kit was obtained from BD Company (Franklin Lakes, USA). Modified Bradford Protein Assay Kit, Antibody against NLRP3, Animal Total RNA Isolation Kit and Tissue Total Protein Extraction Kit were supplied by Sagon Biotech Company (Shanghai, China). Another antibody against Gasdermin D (GSDMD) was bought from Thermo Fisher Company (USA). IL-1β, IL-18 and β-actin antibodies were obtained from Bioss Company (Beijing, China). Caspase-1 antibody was obtained from Boster Biological Technology com.Ltd (Wuhan, Hubei, China). A PrimeScript RT reagent kit was bought from TAKARA company (Daliang, China). PRV-HLJ strain (MK080279.1) is a strain isolated from Heilong Jiang prpvince and provided by Professor Jingfei Wang from Harbin Veterinary Research Institute, CAAS. Eighty-six-week-old female Balb/C mice were obtained from Dossy Experimental Animal Corporation (Chengdu, China).
2.2. Experimental
One week later, the mice were divided randomly into five groups with 16 per group, including one control group and four experimental groups, in which mice were infected at 36 hpi, 48 hpi, 60 hpi and 72 hpi. The mice in the control group were injected with 0.2 ml of normal saline by subcutaneous inoculation on the back. The mice in another group received 0.2 ml PRV-HLJ strain (104 TCID50/100 µl) at the same inoculation site. The animals were fed in the room illuminated with a 12 h light-dark cycle. The ambient temperature and relative humidity were maintained at 22–24°C and 40–60%, respectively. Ten mice were fed in each cage and given the above dosage. Water and diet were provided ad libitum. Mice brain tissues in each group were collected aseptically. Subsection specimens were snap-frozen and stored at −80°C for RNA extraction. In addition, portions of the brain were fixed in 4% paraformaldehyde solution for histopathological examination.
2.3. Histopathological analysis
The histopathological observation was operated by using a standard laboratory procedure. The brain was removed from experimental animals and washed thoroughly in phosphate-buffered saline (PBS, pH 7.4). Then, the tissue was fixed in 4% paraformaldehyde for 2 d and transferred to 95% or absolute alcohol for dehydration. After that, it was processed to a paraffin embedding routine. The paraffin-embedded tissue was sliced into a 5 µm section, dewaxed in xylene and then rehydrated in graded alcohols. The section was stained with hematoxylin-eosin (H&E) and then examined under a light microscope for histopathological examination.
2.4. Detection of pyroptosis by flow cytometry in brain
The rate of pyroptosis cells in the brain was measured using an Annexin V-FITC/PI Apoptosis kit according to the instructions provided. Brains were taken from mice, which were humanely killed at the time mentioned above, ground to form a suspension and filtered with a 300-mesh nylon screen. The cells were washed three times with pre-cold PBS and adjusted at a concentration of 1 × 106 cells ml−1. Furthermore, 100 µl cells were incubated with Annexin V-FITC/PI staining at room temperature for 15 min in a culture tube in a dark atmosphere. To each tube was added 300 µl of binding buffer and then detected with an FCM (Becton Dickinson, USA). CellQuest Pro software (Becton Dickinson, USA) was used to visualize the results.
2.5. RNA extraction and qRT-PCR analysis
Total RNA from brains was isolated by an ‘Animal Total RNA Isolation Kit’ according to the kit instructions. RNA integrity was detected by 2% agarose gel electrophoresis. A spectrophotometer was used to detect the RNA quantity and quality (NanoDrop-2000, Thermofisher Company, USA). Total RNA was converted into DNA for qRT-PCR by using the PrimeScript RT reagent kit. The first strand of cDNA was amplified through SYBR staining on a LightCycler 96 apparatus (Roche, Germany). All primers used in this research were designed by Oligo.7 software and synthesized by Sagon Biotech Ltd. (Shanghai, China). Detailed information about primers was available in the electronic supplementary material, table S1. The 2−ΔΔCt method was used to analyse the mRNA expression. β-actin, a housekeeping gene, was used as an internal control for values correction.
2.6. Western blotting
A ‘Tissue Total Protein Extraction Kit’ was used for protein extracted from the brain. The protein concentration of each specimen was measured by Bradford assay. The proteins were first separated on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a polyvinylidene difluoride (PVDF) membrane. Next, the PVDF membrane was blocked with TBST solution containing 5% non-fat milk at room condition for 2 h and then incubated at 4°C condition for 12 h with the corresponding primary antibodies diluted with a solution: NLRP3 (1 : 1000), Caspase-1 (1 : 800), GSDMD (1 : 900), IL-1β (1 : 500), IL-1β (1 : 500) and β-action (1 : 2000). Then, the PVDF membrane was incubated at room temperature with HRP-labelled secondary antibody for 1 h, and blots were measured by an ECL reagent. The β-actin was used as a protein loading control.
2.7. Statistical analysis
SPSS22.0 software was used for data analysis. The results were presented as means ± standards deviations (mean ± s.d.). One-way analysis of variance package in SPSS 22.0 was used to evaluate the statistical significance between PRV and control group. GraphPad Prism6.0 software was used to perform statistical artwork. In all statistical comparisons, the p-value was introduced as a judgement of the statistical difference.
3. Results
3.1. Clinical symptoms and histopathological analysis
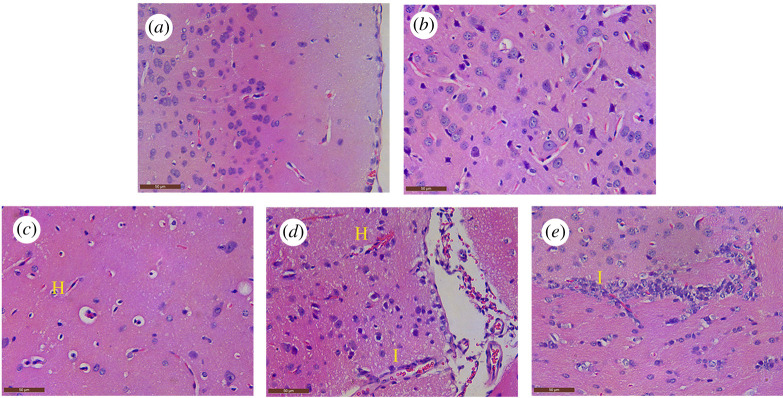
At 36 hpi, the mice infected with PRV generally appeared with typical clinical signs, including depression, anorexia and neuropathic itch. Mortality occurred within the period of 48–72 hpi. However, none of the mice in the control group exhibited clinical symptoms or died. Compared with the control group, there was a significant difference in the microscopic lesions in the PRV-inoculated group (figure 1). Normal brain structure was found in the control and PRV-inoculated mice before 36 hpi (figure 1a,b). At 48 hpi, hyperemia appeared in brain tissue, followed by perivascular space widening; perivascular lymphocytes increased, and degeneration and necrosis occurred in some neurons (figure 1c). Focal inflammatory cellular infiltration was found in the brain in 60–72 hpi (figure 1d,e).
Figure 1.
Histopathological observations of the brain of PRV-inoculated examinated by H&E staining. (a) Microscopic lesion in the control group; (b) 36 hpi group; (c) hyperemia (H, yellow letter) appeared in the brain tissue, followed by perivascular space widened; perivascular lymphocytes increased, and degeneration and necrosis occurred in some neurons in the 48 hpi group; (d,e) focal inflammatory cellular infiltration (I, yellow letter) was found in the brain of the 60 hpi and 72 hpi groups.
3.2. The mRNA expression levels about innate immune-related genes
Innate immunity recognizes invading pathogens by binding to pattern recognition receptors, leading to the expression of antiviral molecules. Interferons (IFN) are antiviral molecules, which have a pivotal role in the clearance of invading pathogenic microorganisms [10]. In this work, the transcriptional levels of IFN-α, IFN-β and IFN-γ were measured by qRT-PCR.
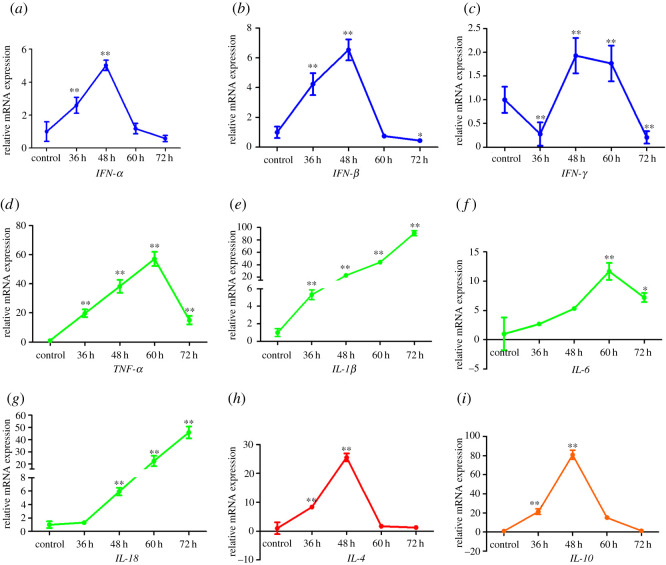
As shown in figure 2, the expression levels of IFN-α and IFN-β in the brain of PRV infected mice were upregulated from 36 hpi (p < 0.01) and peaked at 48 hpi (p < 0.01) and then downregulated in 60–72 hpi (p < 0.05, figure 2a,b) . Furthermore, IFN-γ expression in the brain was downregulated before 36 hpi and then upregulated until 60 hpi (p < 0.01, figure 2c). In addition, pro-inflammatory cytokines (TNF-α, IL-1β, IL-6 and IL-18) and anti-inflammatory cytokines (IL-4 and IL-10) were also measured in the brain, respectively. The relative mRNA levels of TNF-α and IL-6 in the brain were remarkedly upregulated, caused by PRV infection, and peaked at 60 hpi (p < 0.01), and were then downregulated at 72 hpi (figure 2d,f). After PRV infection, the IL-1β expression in the brain was upregulated from 36 hpi and lasted for the whole experiment period (p < 0.01, figure 2e). In addition, IL-18 expression was upregulated from 36 hpi and had the same tendency as IL-1β (p < 0.01, figure 2g). The cytokine storms caused by IFN-α, IFN-β, TNF-α, IL-1β, IL-6 and IL-18 were related to the histopathological changes induced by PRV (figure 1). Remarkably, the mRNA expression levels of IL-4 and IL-10 were upregulated before 48 hpi (p < 0.01) and downregulated and maintained a low level until 72 hpi (figure 2h,i). This indicated that PRV inhibited the expression of IL-4 and IL-10 since 48 hpi.
Figure 2.
The dynamic changes of mRNA expression related to immune system induced by PRV (a) IFN-α, (b) IFN-β, (c) IFN-γ, (d) TNF-α, (e) IL-1β, (f) IL-6, (g) IL-18, (h) IL-4 and (i) IL-10. **p < 0.01 and *p < 0.05 compared with the control group.
3.3. Detection of pyroptosis cells in the brain
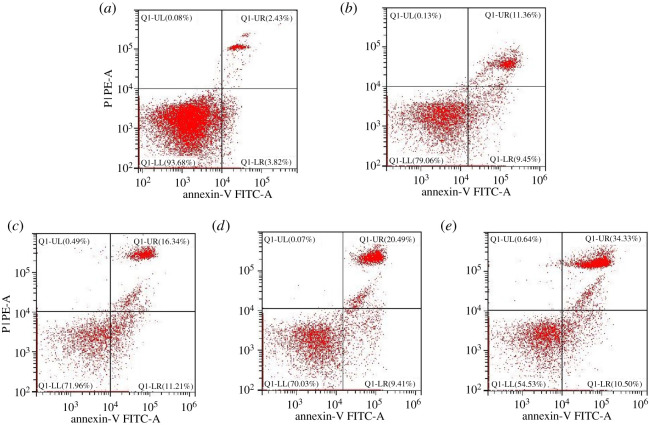
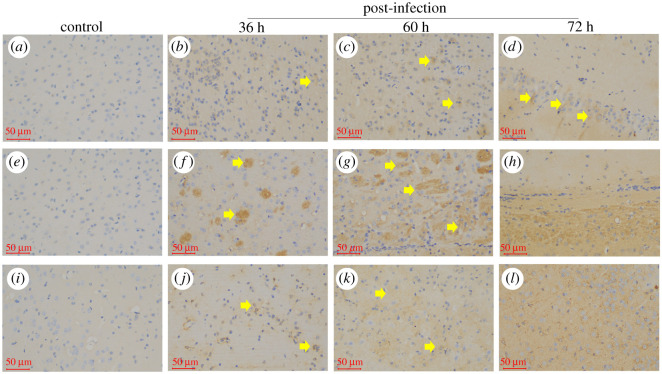
Pyroptosis in the brain was measured by Annexin V/PI double staining through flow cytometry. As shown in figure 3, a large number of PI + pyroptosis cells in the brain was observed at 36, 48, 60 and 72 hpi, which increased in a time-dependent way (p < 0.01) compared with that in the control group (electronic supplementary material, table S2). In addition, PRV significantly upregulated in situ the protein expression of Caspase-1 (figure 4b–d), IL-1β (figure 4f–h) and IL-18 (figure 4j–l) in the brain tissue in a time-dependent manner.
Figure 3.
PRV caused pyroptosis in mice brains measured by the flow cytometry method through Annexin V-FITC/PI staining (a) control group, (b) the per cent of pyroptosis cells in 36 hpi group, (c) 48 hpi group, (d) 60 hpi group and (e) 72 hpi group.
Figure 4.
The in situ expression of Caspase-1, IL-1β and IL-18 in the mice brains by HI. (a) Caspase-1 expression in the control group; (b–d) Caspase-1 expression in the brain at 36, 60 and 72 hpi; (e) IL-1β expression in the control group; (f–h) IL-1β expression in the brain at 36, 60 and 72 hpi; (i) IL-18 expression in the control group; (j–l) IL-18 expression in the brain at 36, 60 and 72 hpi; obvious positive regions could be seen in yellow in (h) and (j); positive regions are shown in yellow arrowheads in (b–d,f,g,j and k), scale bar = 50 µm.
3.4. Changes in protein expression levels related to pyroptosis in the brain
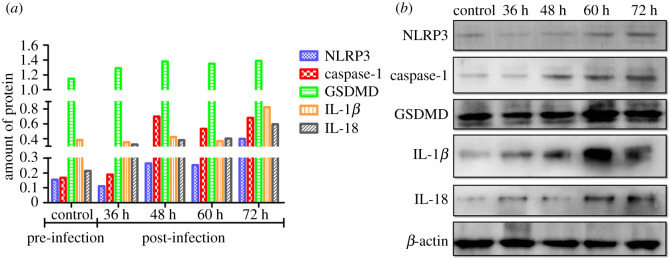
To further investigate the effect of PRV on the pyroptosis in vivo, the proteins involved in the pyroptosis signal pathway were detected by blotting, including NLRP3, Caspase-1, GSDMD, IL-1β and IL-18. From the result in figure 5, NRRP3 decreased at 36 hpi followed by an increasing tendency in a time-dependent manner. In addition, the Caspase-1 level was significantly upregulated by PRV from 36 hpi and maintained at a high level until the end of this experiment by comparing it with that in the control group. Furthermore, GSDMD, a pyroptosis marker protein, was also measured in all groups. As shown in figure 5a, the amount of GSDMD was higher in all PRV groups than in the control. In addition, the expression levels of IL-1β and IL-18, two cytokines related to pyroptosis, were determined by blotting. The result demonstrated that PRV could elevate the protein levels of IL-1β and IL-18.
Figure 5.
The protein expression levels were related to pyroptosis induced by PRV. (a) The relative expression amount protein of NRLR3, Caspase-1, GSDMD and IL-1β/18 to β-actin was present as a different colour and (b) protein expression related to pyroptosis measured by blotting.
3.5. Relative mRNA expression of genes related to pyroptosis in the brain
The mRNA expression levels of pyroptosis-related factors were further detected by qRT-PCR in this research. As shown in the electronic supplementary material, table S3, the mRNA expression level of NRRP3 was markedly increased (p < 0.05) in a time-dependent manner following PRV treatment. Moreover, the mRNA expression levels of Caspase-1 in the PRV inoculation groups were also upregulated (p < 0.01) compared to that among groups. Besides, the GSDMD mRNA expression level was upregulated in an increased tendency (p < 0.01) compared with that in the control group.
4. Discussion
PRV is a kind of neurophilic α herpesvirus, which belongs to the genus Varicellovirus, family Herpesviridae. Pigs are the only natural hosts of PRV for their survival to infection. However, mice and rats can be naturally infected with PRV and cause a fatal disease. In the laboratory, after intranasal infection in adult mice, PRV enters peripheral nerve cells and spreads to the CNS [11]. Previous studies on PRV mainly focused on pathogenicity and the resulting host immune response. The purpose of this study was to research the kinetics of cytokine secretion in vivo and to clarify whether cytokine storm is involved in the pathogenesis of PRV. In this work, we identified cytokine storm and pyroptosis as the main causes of rapid death in mice infected with PRV.
The innate immune system is the first line of defense against the invasion of pathogens, accompanied by the recruitment of immune cells and inflammatory response [12]. Inflammation is a process to solve microbial infection and a complex process involving the regulation of cytokine production. Dysfunction of these factors can induce cytokine storms and related multiple organ failure [13]. An inflammatory response is usually caused by various pro-inflammatory cytokines, such as TNF, IL-1 and IL-6. These cytokines are the kinds of pleiotropic proteins involved in the regulation of cell death in inflammatory tissue, vascular endothelial cell permeability, attracting the blood cells to inflammatory tissue and acute-phase proteins production [14].
Cytokines play an important role in aspects of the immune response, coordinating the innate and adaptive immune response. Consequently, in most cases, cytokines play a protective role in resisting endogenous and exogenous noxious stimuli, such as tissue injury and microbial invasion. IFNs are recognized as the central factors of antiviral infection, which has a pivotal role in innate immune response [15]. In addition, cytokines play an important role in the pathogenesis of antiviral and viral infections. However, excessive immune activation and excessive release of cytokines could be rather pernicious [16]. For example, over-expression of TNF-α, IL-1 and IL-6 in the immune system could lead to vascular leakage, systemic fatigue, cardiomyopathy and acute-phase protein synthesis [16]. In addition, persistent excessive IFN-α/β may also be harmful to the immune system [15]. In the present study, we found that cytokine storm was induced by PRV in mice brains from 36 to 72 hpi, including the elevated expression levels of Type I IFNs (IFN-α and IFN-β) and Type II IFNs (IFN-γ) as well as pro-inflammatory factors compared to that in the control group (p < 0.01). This storm was consistent with the histopathological changes, including hyperemia and inflammatory cell infiltration in mice brains. Studies have shown that PRV could regulate the expression of cytokines, including Type I and Type II IFN and inflammatory factors, to establish a successful infection [17,18]. Furthermore, IFN-α and IFN-β mediate a positive feedback regulation by binding to IFN-α and IFN-β receptor in an automatic or paracrine manner [19].
PRV infection can induce apoptosis, which has been reported previously in vitro and in vivo [20,21]. However, apoptosis is usually considered as an insoluble programmed cell death (PCD), which is characterized by an active programmed process of cell decomposition to avoid inflammation [22]. The discrepancy found in this research may be induced by a new kind of PCD in the cell death process. Pyroptosis is a new type of pro-inflammatory cell death, which is emerging as the mechanism of antagonizing and clearing pathogen infection and requires the activation of Caspase1/4/5/11 [23]. In this research, an increasing tendency of pyroptotic cells induced by PRV was detected in the brain through flow cytometry by comparing with the control group (p < 0.01). Furthermore, the expression of genes and proteins related to the pyroptosis pathway were elevated by qRT-PCR and blotting methods, respectively. Inflammasomes are cytosolic sensors that could activate Caspase-1 [24]. Once activated, Caspase-1 can process and maturates IL-1β and IL-18 precursors, as well as cleaving GSDMD, resulting in cell membrane channel opening and the pyroptosis [9]. Among the inflammasomes, NLRP3 is currently the most well-known one, which responds to various stimuli. NLRP3 was activated by PRV in this research. In addition, GSDMD, a key executor in pyrotosis [25], was also activated by PRV. This activity of GSDMD leads to the indirect release of IL-1β and IL-18 from membrane pores [25]. The pyroptotic cell-fate decision provides a large amount of inflammatory response at the site of infection. This was consistent with the results from histopathological analysis (figure 1) and immunohistochemistry (figure 4), as well as a full explanation for cytokine storm caused by PRV in mice brains. However, cytokine storms and pyroptosis might cause the rapid death of mice caused by PRV strain. Furthermore, due to the difference in immune systems between mice and pigs, more detailed information about the pathogenesis to pigs and other mammals needs to be further clarified in the future.
5. Conclusion
Cytokine storms and pyroptosis might be the cause of rapid death in mice inoculated with PRV strain. These results provided a new insight for further understanding the pathogenesis caused by PRV.
Supplementary Material
Acknowledgements
Many thanks to Jiangtao Feng for his help in animal administration.
Ethics
This study was approved by Animal Care and Use Committee of Tongren Polytechnic College and Dossy Experimental Animal Corporation (SYXK2014-189). All animal administrations, sample collection and procedures were performed according to the approved guidelines.
Data accessibility
The datasets supporting this article have been uploaded in the form of ‘electronic supplementary material’. In addition, the raw material related to this work are deposited at Dryad (https://doi.org/10.5061/dryad.zw3r2287j) [26]. The data are provided in the electronic supplementary material [27].
Authors' contributions
W.S. conceived this study, revised the manuscript, participated in the data analysis and artwork making; S.L. did the qRT-PCR and Western blotting experiment and participated in the design of this study; R.Y. did the animal experiment. X.H. and J.Y. carried out a final revision of the manuscript.
Competing interests
The authors declare that there is no conflict of interest to this research.
Funding
This research was supported by Guizhou Science and Technology Planning Project (grant no. [2019]1456) and Tongren Science and Technology Planning Project (grant no. [2019]12-6).
References
- 1.Alexander SV, Philipp GD, Marion S, Joachim SH, Anton SEH, Max S, Matthias K, Boris BL, Von BMS. 2018Cytokine release syndrome. J. Immunother Cancer 6, 56. ( 10.1186/s40425-018-0343-9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broz P, Dixit VM. 2016Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407-420. ( 10.1038/nri.2016.58) [DOI] [PubMed] [Google Scholar]
- 3.Cheung AK, Chen Z, Sun Z, Mccullough D. 2000Pseudorabies virus induces apoptosis in tissue culture cells. Arch. Virol. 145, 2193-2200. ( 10.1007/s007050070049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tudor D, Riffault S, Carrat C, Lefèvre F, Bernoin M, Charley B. 2001. Type I IFN modulates the immune response induced by DNA vaccination to Pseudorabies virus glycoprotein C. Virology 286, 197-205. ( 10.1006/viro.2001.0957) [DOI] [PubMed] [Google Scholar]
- 5.Delva JL, Nauwynck HJ, Mettenleiter TC, Favoreel HW. 2020The attenuated Pseudorabies virus vaccine strain Bartha K61: a brief review on the knowledge gathered during 60 years of research. Pathogens 9, 897. ( 10.3390/pathogens9110897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC. 2018The pore-forming protein Gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35-44. ( 10.1016/j.immuni.2017.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan S, Yuan H, Liu L, Li H, Guan H. 2020Pseudorabies virus encephalitis in humans: a case series study. J. Neurovirol. 26, 556-564. ( 10.1007/s13365-020-00855-y) [DOI] [PubMed] [Google Scholar]
- 8.Fink SL, Cookson BT. 2005Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 73, 1907-1916. ( 10.1128/IAI.73.4.1907-1916.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH. 2015Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25, 1285-1298. ( 10.1038/cr.2015.139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorgensen I, Rayamajhi M, Miao EA. 2017Programmed cell death as a defence against infection. Nat. Rev. Immunol. 17, 151-164. ( 10.1038/nri.2016.147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klopfleisch R, Teifke JP, Fuchs W, Kopp M, Klupp BG, Mettenleiter TC. 2004Influence of tegument proteins of pseudorabies virus on neuroinvasion and transneuronal spread in the nervous system of adult mice after intranasal inoculation. J. Virol. 78, 2956-2966. ( 10.1111/j.1600-079X.2008.00631.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klopfleisch R, Klupp BG, Fuchs W, Kopp M, Teifke JP, Mettenleiter TC. 2006Influence of pseudorabies virus proteins on neuroinvasion and neurovirulence in mice. J. Virol. 80, 36. ( 10.1128/JVI.02589-05) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kogut MH, Lee A, Santin E. 2020Microbiome and pathogen interaction with the immune system. Poultry Sci. 99, 1906-1913. ( 10.1016/j.psj.2019.12.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mcnab F, Mayer-Barber K, Sher A, Wack A, O'Garra A. 2015Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87-103. ( 10.1038/nri3787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mettenleiter TC2020Aujeszky's disease and the development of the marker/DIVA vaccination concept. Pathogens 9, 563-569. ( 10.3390/pathogens9070563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller LC, Zanella EL, Waters WR, Lager KM. 2010Cytokine protein expression levels in tracheobronchial lymph node homogenates of pigs infected with pseudorabies virus. Clin. Vaccine Immunol. 17, 728-734. ( 10.1128/CVI.00485-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pomeranz LE, Reynolds AE, Hengartner CJ. 2005Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 69, 462-500. ( 10.1128/MMBR.69.3.462-500.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero CH, Meade PN, Shultz JE, Chung HY, Lollis G. 2015Venereal transmission of pseudorabies viruses indigenous to feral swine. J. Wildlife Dis. 37, 289-296. ( 10.7589/0090-3558-37.2.289) [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi O, Akira S. 2010Innate immunity to virus infection. Immunol. Rev. 227, 75-86. ( 10.1111/j.1600-065X.2008.00737.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi O, Akira S. 2010Pattern recognition receptors and inflammation. Cell 140, 805-820. ( 10.1016/j.cell.2010.01.022) [DOI] [PubMed] [Google Scholar]
- 21.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Katze MG. 2012Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 76, 16-32. ( 10.1128/MMBR.05015-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei J, et al. 2017Alpha/beta interferon receptor deficiency in mice significantly enhances susceptibility of the animals to pseudorabies virus infection. Vet. Microbiol. 203, 234-244. ( 10.1016/j.vetmic.2017.03.022) [DOI] [PubMed] [Google Scholar]
- 23.Wu R, Bai C, Sun J, Chang S, Zhang X. 2013Emergence of virulent pseudorabies virus infection in Northern China. J. Vet. Sci. 14, 363-365. ( 10.4142/jvs.2013.14.3.363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh CJ, Lin PY, Liao MH, Liu HJ, Lee JW, Chiu SJ, Hsu HY, Shih WL. 2008TNF-alpha mediates pseudorabies virus-induced apoptosis via the activation of p38 MAPK and JNK/SAPK signaling. Virology 381, 55-66. ( 10.1016/j.virol.2008.08.023) [DOI] [PubMed] [Google Scholar]
- 25.Zhou Z, He H, Wang K, Shi X, Shao F. 2020Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368, eaaz7548. ( 10.1126/science.aaz7548) [DOI] [PubMed] [Google Scholar]
- 26.Sun W, Liu SS, Huang XF, Yuan R, Yu JS. 2021Data from: Cytokine storms and pyroptosis are primarily responsible for the rapid death of mice infected with pseudorabies virus. Dryad Digital Repository. ( 10.5061/dryad.zw3r2287j) [DOI] [PMC free article] [PubMed]
- 27.Sun W, Liu S, Huang X, Yuan R, Yu J. 2021Data from: Cytokine storms and pyroptosis are primarily responsible for the rapid death of mice infected with pseudorabies virus. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sun W, Liu SS, Huang XF, Yuan R, Yu JS. 2021Data from: Cytokine storms and pyroptosis are primarily responsible for the rapid death of mice infected with pseudorabies virus. Dryad Digital Repository. ( 10.5061/dryad.zw3r2287j) [DOI] [PMC free article] [PubMed]
- Sun W, Liu S, Huang X, Yuan R, Yu J. 2021Data from: Cytokine storms and pyroptosis are primarily responsible for the rapid death of mice infected with pseudorabies virus. Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded in the form of ‘electronic supplementary material’. In addition, the raw material related to this work are deposited at Dryad (https://doi.org/10.5061/dryad.zw3r2287j) [26]. The data are provided in the electronic supplementary material [27].