Abstract
Vector-borne diseases (VBDs) are important contributors to the global disease burden and are a key factor in perpetuating economic inequality. Although environmental changes are often cited as drivers of VBDs, the link between deforestation and VBD occurrence remains unclear. Here, we examined this relationship in detail using the spread of visceral leishmaniasis (VL) in São Paulo state (Brazil) as the case study. We used a two-step approach to estimate the causal effects (overall, direct, and indirect) of deforestation on the occurrence of the VL vector, canine visceral leishmaniasis (CVL), and human visceral leishmaniasis (HVL). We first estimated the parameters via a double Metropolis–Hastings algorithm and then estimated the causal effects through a Gibbs sampler. We observed that the odds of vector, CVL, and HVL occurrence were 2.63-, 2.07-, and 3.18-fold higher, respectively, in deforested compared with forested municipalities. We also identified a significant influence of the presence of vector, CVL, and HVL in one municipality on disease occurrence in previously naive neighbouring municipalities. Lastly, we found that a hypothetical reduction in deforestation prevalence from 50 to 0% across the state would reduce vector, CVL, and HVL occurrence by 11%, 6.67%, and 29.87%, respectively. Our results suggest that implementing an eco-friendly development strategy that considers trade-offs between agriculture, urbanization, and conservation could be an effective mechanism of controlling VL.
Keywords: interference, environmental change, Lutzomyia longipalpis, American visceral leishmaniasis, One Health, counterfactual
1. Introduction
Vector-borne diseases (VBDs), such as malaria, dengue, and leishmaniasis, are among the most important public health burdens worldwide. More than half of the global population is at risk of contracting VBDs, which are responsible for more than 700 000 deaths annually [1,2]. The accelerating rate of environmental change has been recognized to contribute to the emergence, re-emergence, and spread of VBDs into new geographical territories [3]. In particular, deforestation caused by urbanization and increased agricultural, mining, and construction activities could affect the distribution of vector and host reservoirs through diverse mechanisms, including generating new favourable habitats for vector reproduction and promoting closer proximity between components of the transmission cycle. Indeed, several studies have linked deforestation to increased VBD occurrence worldwide [4–6].
The potential impact of deforestation on VBD prevalence is the central issue of the present study. We focus on visceral leishmaniasis (VL), a neglected tropical disease that poses a persistent hazard to public health, especially in low- and middle-income countries [7,8]. The disease is endemic in more than 60 countries, with more than 90% of VL cases occurring in just seven countries (Brazil, Ethiopia, India, Kenya, Somalia, South Sudan, and Sudan) [9,10]. The two most common types of VL are due to Leishmania donovani (syn Leishmania archibaldi) infection that is anthroponotic and occurs in Asia and Africa, and to Leishmania infantum (syn. L. chagasi) infection that is zoonotic and occurs in Europe and Latin America. The pathogen is transmitted to humans and other mammals through the bite of sandflies, primarily Lutzomyia longipalpis, and domestic dogs are the main source of human infection in urban areas [9–11]. In Brazil, VL was historically known as a rural endemic disease, but since the 1980s, it has become more widespread and it is now endemic in many large cities [12,13].
The Brazilian state of São Paulo illustrates the geographical expansion of both canine and human VL (CVL and HVL, respectively), which is primarily associated with the colonization of new areas by Lu. longipalpis [14–16]. In 1998, Lu. longipalpis was detected in only two of the 645 municipalities of São Paulo state, but by 2019, it had spread to 203 municipalities (31.47%). The first cases of CVL and HVL were diagnosed in 1998 and 1999, respectively, in the two municipalities where the vector had previously been identified [15,17,18]. Since then, São Paulo state has recorded, until 2019, 3119 cases of HVL, of which 311 were fatal [17], and the disease is now considered endemic in some municipalities (107 out of 645).
Previous studies linked VL expansion in São Paulo state to the Bolivia–Brazil gas pipeline. Its construction between 1997–1998 promoted deforestation and attracted thousands of workers from endemic areas (e.g. Brazil's northeastern states and Mato Grosso do Sul state) through a railroad and a highway [14–16]. Moreover, deforestation led to increased contact between humans, the VL vector, and sylvatic reservoirs, thereby maintaining the cycle of infection. Other factors often cited as drivers of VL occurrence and expansion include socioeconomic condition (e.g. gross domestic product (GDP)), climatic variables (e.g. temperature), and additional environmental variables (e.g. land use) [19,20]. Despite the growing number of studies on deforestation and infectious diseases, we still have a poor understanding of their relationship. In part, this is due to limitations in most current approaches to exploring the interactions, which tend to be qualitative or to ignore the complex and often nonlinear relationship between variables and sample units (e.g. individuals) [21–23]. In particular, more appropriate analytical techniques for causal inference in such complex systems and large-scale settings are underused [21,24,25].
In most studies, data are assumed to be independent and identically distributed (iid). However, this assumption is implausible in some disciplines, such as epidemiology, where one individual exposure to a treatment (e.g. vaccination) may affect other individuals' outcome (e.g. infection), the so-called dependent happenings. Only fairly recently methods to allow for such dependence in causal inference contexts became available [26–33].
In this study, we estimated the causal effects of deforestation on VL by focusing on the presence/absence of the vector, CVL, and HVL. More specifically, we estimated the expected change in the risk of VL under three hypothetical scenarios: (i) changes in the statewide risk when the prevalence of deforestation in the state is increased; for example, from 50 to 100% (overall effect), (ii) changes in a single municipality's risk when that municipality's status shifts from non-deforested to deforested while the statuses of all other municipalities remain constant (direct effect), and (iii) changes in a single non-deforested municipality's risk when the statuses of its neighbours shift from non-deforested to deforested (indirect effect).
2. Methods
(a) . Study area and sample
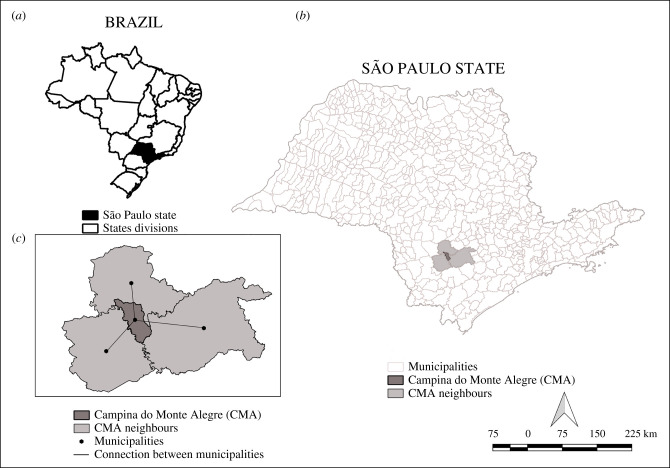
The state of São Paulo is located in Southeast Brazil and has 645 municipalities [34]. The study sample comprised the 620 municipalities that were VL free (i.e. concomitant absence of vector, CVL, and HVL) in the baseline year of 2000. São Paulo (figure 1a,b) is the country's wealthiest state, produces one-third of Brazil's GDP, and is home to approximately 22% of its population [34]. The tropical Atlantic rainforest surrounds urban and livestock farming areas, and its coverage has decreased from 80 to 3% of area of São Paulo state in the last century [35].
Figure 1.
(a) Brazil, (b) São Paulo state, and (c) an example of the network between municipalities.
To account for the interconnection among municipalities, we first built a neighbourhood matrix that allowed the construction of a network in which nodes correspond to a municipality, and edges represent the connectivity between municipalities (i.e. border sharing). An example of this Campina do Monte Alegre municipality is illustrated in figure 1c, and the full network is presented in electronic supplementary material, figure S1. The network was generated using digitized municipal boundary maps available at the Instituto Brasileiro de Geografia e Estatística (Brazilian Institute of Geography and Statistics (IBGE)) website [36] and using R statistical software (v. 3.6.1) [37] with packages maptools [38] and spdep [39].
(b) . Outcomes
Our study considers three outcomes: (i) presence/absence of the VL vector Lu. longipalpis (referred to as VEC), (ii) presence/absence of CVL, and (iii) presence/absence of HVL. Data on VEC were obtained from the São Paulo State Secretary of Health [18] and were derived from surveillance activities, as described in Casanova et al. [15]. Data on CVL cases were also obtained from surveys carried out by the São Paulo State Secretary of Health [18], and data on HVL cases were obtained from the Brazilian Ministry of Health website [17]. Notification of HVL is compulsory in Brazil. All data are freely available and were collected annually from 2000 to 2018.
(c) . Exposure
The exposure was deforestation (DEF) defined as the area of forest clearing over time. To estimate this, we employed land use and land cover data from the Mapbiomas Project [40]. The high-resolution maps (30 m pixel resolution) are based on machine learning algorithms and Landsat satellite imagery, available through the Google Earth Engine Platform. Specifically, we collected data from São Paulo state from 2000 and 2018 and calculated the natural forest percentage by municipality and year. We then calculated the change in forested area in each municipality by subtracting the forested area percentage at the end of the study period (2018) from the percentage at the baseline year (2000). A negative value from this calculation was considered deforestation (DEF = 1) of the municipality; otherwise, the municipality was considered non-deforested (DEF = 0). These analyses were performed with R [37] packages raster [41], tidyverse [42], and sp [43].
(d) . Covariates
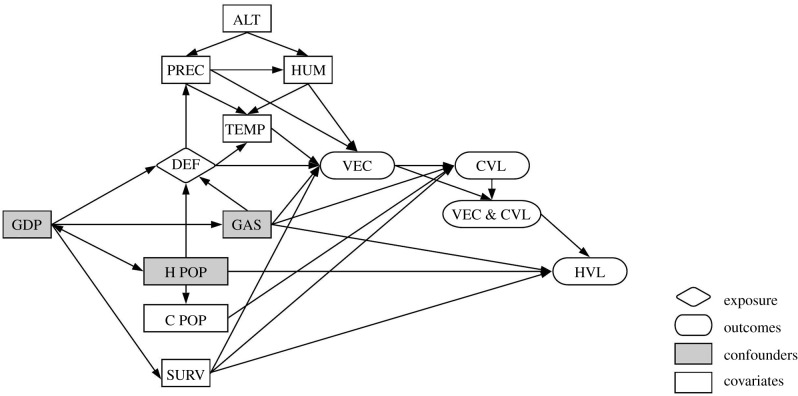
Potential pathways affecting VL occurrence (VEC, CVL, and HVL) were expressed through a directed acyclic graph (DAG) (figure 2). Based on the literature and our DAG, the confounders (i.e. common causes of exposure and the outcome through a causal path) were GDP per capita and the presence of the Bolivia–Brazil gas pipeline (Gas) for the VEC model; and GDP per capita, Gas, and human population size (H Pop) for both the CVL and HVL models.
Figure 2.
Directed acyclic graph depicting the relationship between exposure (deforestation) and outcomes (VEC, CVL, and HVL), and covariates. ALT, altitude; C POP, canine population; CVL, canine visceral leishmaniasis; DEF, deforestation; GDP, gross domestic product; GAS, presence of the Bolivia–Brazil gas pipeline; H POP, human population; HVL, human visceral leishmaniasis; HUM, humidity; PREC, precipitation; SURV, VL surveillance activities; TEMP, temperature; VEC, presence of vector; VEC & CVL, presence of both VEC and CVL.
Data on population and GDP per capita were collected from Fundação Seade (Statewide System for Data Analysis Foundation) and are available through their website [44]. Municipalities were assigned, following the definition by IBGE, to one of seven human population categories: (less than 5000, 5001–10 000, 10 001–20 000, 20 001–50 000, 50 001–100 000, 100 001–500 000, and greater than 500 000 inhabitants), and to one of six GDP per capita categories (less than 10 000, 10 001–15 000, 15 001–20 000, 20 001–25 000, 25 001–30 000, and greater than 30 001 Brazilian reais) that are converted to US dollars (less than 1980, 1980–2970, 2971–3960, 3961–4951, 4952–5941, greater than 5941) [45]. We assigned the municipalities to a binary variable according to the presence of the Bolivia–Brazil gas pipeline (0, absence; 1, presence), using the data available on the Brazilian Ministry of Transport website [46].
(e) . Data analyses
Initially, we assessed the appropriateness of the confounders through correlation and multicollinearity tests. Bivariate correlations between GDP, Gas, and Population were assessed using Pearson's correlation coefficient, and multicollinearity through the variance inflation factor (VIF).
Causal mechanisms have been studied within the framework of causal inference in the presence of interference, allowing for partial interference between units [28–30]. More recently, methods have been developed to allow for full interference by assuming that the data can be described as a network [26,31–33], and representing the data as chain graphs [47,48] that allow inferences about the parameters of the joint distribution of the observed data. A chain graph is a mixed graph that enables a network to be constructed from both the directed relationship of the variables (as in figure 2), and the undirected relationship of the study units (i.e. municipalities connection). The chain graph thus allows inferences to be made about the network causal effects. Figure 3 shows an example of a chain graph, where (E) represents exposure, (Y) outcome, and (C) confounder, and the subscripts represent the network units (i.e. municipalities).
Figure 3.
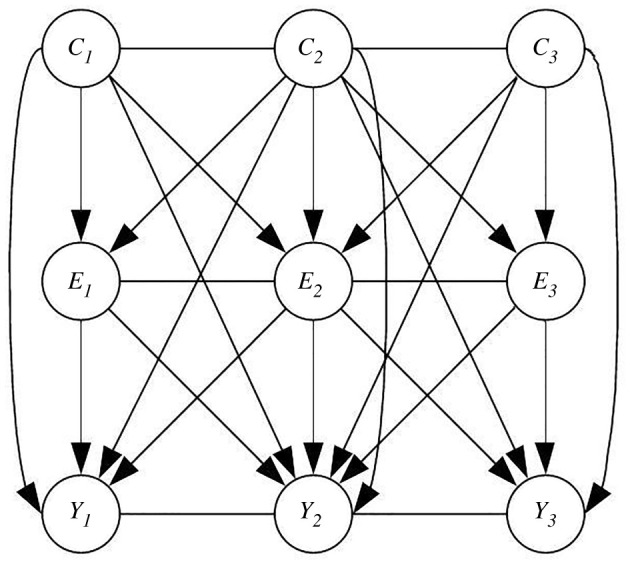
Chain graph representing data from a network of three interconnected units.
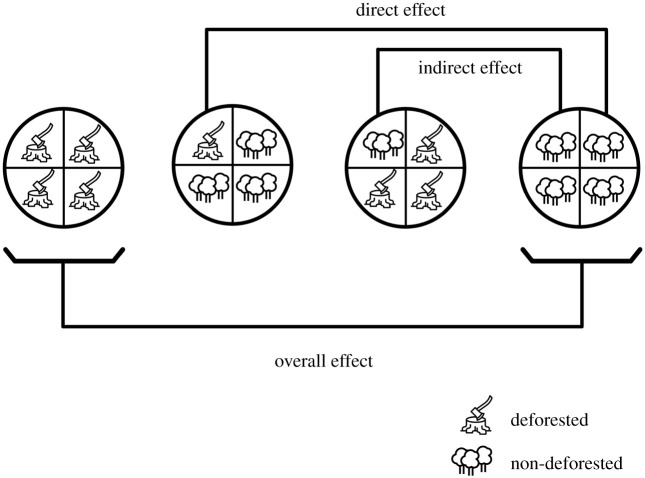
Under this framework, we defined overall, direct, and indirect causal effects as quantities of interest in a network analysis. It was estimated using the observed data (i.e. factual) to simulate what would occur under different exposure (i.e. deforestation) prevalence scenarios and comparing the potential outcomes (i.e. counterfactual) of interest (VEC, CVL, and HVL occurrence). Thus, the direct effect is the expected change in VL risk in a single municipality upon changing its exposure status (non-deforested to deforested) while keeping the other municipality exposure levels constant (i.e. non-deforested). The indirect effect is the expected change in VL risk in a single municipality upon changing the deforestation status (non-deforested to deforested) of the network (i.e. remaining municipalities in São Paulo state) while keeping the given municipality's exposure status constant (i.e. non-deforested). The overall effect was defined as the expected change in the VL risk in the entire state upon changing the prevalence of deforestation in the whole state. These effects are illustrated in figure 4.
Figure 4.
Types of causal effects based on comparisons of different scenarios. Circles represent hypothetical exposure level (deforestation) in the sample (state), with each circle subdivision representing a sample unit (municipality).
Estimation of the network causal effects was conducted in two steps. We first estimated the parameter values for the covariates and the outcomes on the odds ratio (OR) scale through a Double Metropolis–Hastings algorithm, as described by Liang [49]. Then, using the posterior distribution of step one, we ran a Bayesian auto-g-computation algorithm of network effects by network g-formula combined with the Gibbs sampling algorithm of Tchetgen et al. [31,32] resulting in the causal effects (overall, direct, and indirect) of deforestation. For parameter estimation (step 1), we used the agcParam function in the autognet [31,50] R package, with non-informative priors, 75 000 interactions and four independent chains, a burn-in of 7500 and thinned every seventh iteration. For the second step, we ran the auto-g-computation algorithm following Fulcher [31] and Tchetgen et al. [32] under deforestation prevalences of 0, 20, 50, 80, and 100%, using the agcEffect function in the autognet package [50]. The code used in our analysis and respective outputs are presented in the electronic supplementary material.
3. Results
Of the 620 municipalities included in the analysis, most were VEC free (68.71%), CVL free (84.20%), and HVL free (84.51%); deforested (69.67%); and not crossed by the gas pipeline (89.20%). The most common GDP per capita was 1.980–2.970 US dollars (31.13%) and the most common municipality size was less than 5000 inhabitants (24.52%) (table 1). Bivariate correlations between the three confounders were weak (0.06–0.16), and the VIFs showed multicollinearity in acceptable values (1.05–1.25) (table 2). Therefore, all three variables were included for adjustment in the models.
Table 1.
Distribution of variables in the study sample (N = 620 municipalities) between 2000 and 2018. GDP, gross domestic product.
| variable | proportion (%) | n |
|---|---|---|
| Lu. longipalpis (vector) | ||
| presence | 31.29 | 194 |
| absence | 68.71 | 426 |
| canine visceral leishmaniasis | ||
| presence | 15.80 | 98 |
| absence | 84.20 | 522 |
| human visceral leishmaniasis | ||
| presence | 15.49 | 96 |
| absence | 84.51 | 524 |
| deforestation | ||
| yes | 69.67 | 432 |
| no | 30.33 | 188 |
| gas pipeline | ||
| yes | 10.80 | 67 |
| no | 89.20 | 553 |
| GDP per capita (in US dollars) | ||
| <1.980 | 15.9 | 99 |
| 1.980–2.970 | 31.13 | 193 |
| 2.971–3.960 | 23.06 | 143 |
| 3.961–4.951 | 11.30 | 70 |
| 4.952–5.941 | 6.13 | 38 |
| >5.941 | 12.42 | 77 |
| population | ||
| <5000 | 24.52 | 152 |
| 5001–10 000 | 18.87 | 117 |
| 10 001–20 000 | 19.03 | 118 |
| 20 001–50 000 | 18.55 | 115 |
| 50 001–100 000 | 7.74 | 48 |
| 100 001–500 000 | 9.84 | 61 |
| >500 000 | 1.45 | 9 |
Table 2.
Bivariate correlations between covariates and variance inflation factors. GDP, gross domestic product; VIF, variance inflation factor.
| covariate | bivariate correlation (p-value) |
VIF | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| 1. GDP per capita | 1.00 | 0.06 (0.13) | 0.16 (0.00) | 1.05 |
| 2. gas pipeline | 1.00 | 0.12 (0.00) | 1.06 | |
| 3. population | 1.00 | 1.25 | ||
Estimated ORs and credible intervals are given in table 3. Due to scaling by the number of network connections (i.e. neighbours), the outcome and covariate influence of neighbours can be interpreted as the average effect of the covariate value among network connections. The odds of VEC, CVL, and HVL presence were 2.63-, 2.07-, and 3.18-fold higher, respectively, in deforested compared with non-deforested municipalities. Nevertheless, the deforestation status of the network (among neighbours) was significantly associated with a municipality risk only for HVL (OR = 1.59, 95% CI = 1.05–2.70) conditional on the neighbours' outcomes. None of the potential confounders was significantly associated with a municipality risk of VEC, CVL, or HVL. The risk of VEC, CVL, and HVL presence in a municipality was more likely when they were also present in neighbouring municipalities (median ORs = 6.67, 4.26, and 4.27, respectively). The trace plots are given in electronic supplementary material.
Table 3.
Odds ratio estimates of the presence of vector (Lu. longipalpis), canine visceral leishmaniasis (CVL), and human visceral leishmaniasis (HVL).
| variable | outcome |
||
|---|---|---|---|
| vector |
CVL |
HVL |
|
| median (95% credible interval) | |||
| deforestation | 2.63 (2.11–3.33)a | 2.07 (1.61–2.74)a | 3.18 (2.26–4.26)a |
| gas pipeline | 2.17 (0.45–8.58) | 1.04 (0.58–3.06) | 1.55 (0.60–3.60) |
| GDP per capita | 0.86 (0.81–0.92) | 0.92 (0.85–0.99) | 0.54 (0.50–0.58) |
| population | — | 1.01 (0.94–1.09) | 0.89 (0.82–0.96) |
| vector neighbours | 6.67 (3.92–12.19)a | — | — |
| CVL neighbours | — | 4.26 (1.68–7.71)a | — |
| HVL neighbours | — | — | 4.27 (1.79–6.90)a |
| deforestation neighbours | 1.72 (0.98–2.67) | 1.51 (0.93–2.14) | 1.59 (1.05–2.70)a |
| gas pipeline neighbours | 1.64 (0.61–5.84) | 1.32 (0.80–2.50) | 1.13 (0.46–2.05) |
| GDP per capita neighbours | 1.05 (0.91–1.18) | 1.23 (0.99–1.42) | 0.63 (0.53–0.71) |
| population neighbours | — | 1.66 (1.37–1.92)a | 1.27 (1.07–1.43)a |
aStatistically significant (95% credible interval does not contain 1).
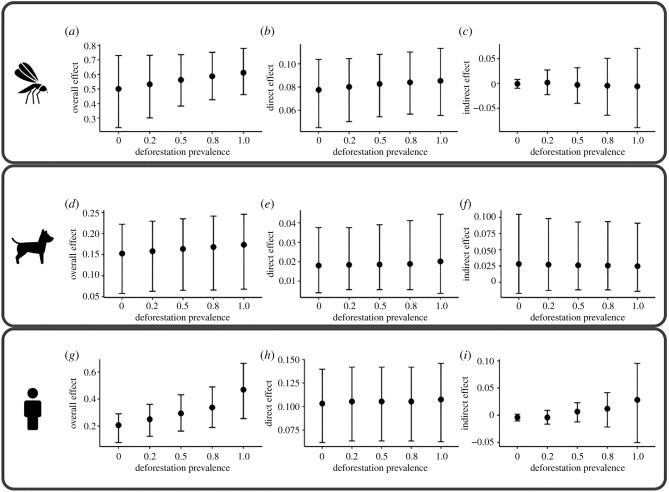
The overall, direct, and indirect effects of deforestation on VEC presence are given in figure 5a–c. The expected statewide prevalence of Lu. longipalpis decreased by 11% (0.56–0.5) when deforestation prevalence was reduced from 50 to 0%; conversely, statewide vector prevalence increased by 8.78% (0.56–0.61) when deforestation increased from 50% to 100% (figure 5a). The direct effect of deforestation on VEC was 0.08 when 50% of the state was deforested, and this effect was increased by 3.16% when deforestation was 100% and decreased by 6.20% when deforestation was 0% (figure 5b). However, the indirect effects on VEC presence were negligible for all deforestation distributions (figure 5c).
Figure 5.
Network causal effect estimates under different degrees of deforestation. Data are presented as the median (circle) and 95% credible intervals. Each panel shows the overall (left), direct (middle), and indirect (right) effects of different degrees of deforestation on the occurrence of the vector Lu. longipalpis (a–c), CVL (d–f), and HVL (g–i) in the network (São Paulo state).
The effect estimates for CVL are presented in figure 5d–f. The statewide CVL prevalence decreased by 6.67% (0.16– 0.15) when deforestation prevalence was reduced from 50 to 0%, whereas the prevalence increased by 6.10% (0.16–0.17) when deforestation prevalence was increased from 50 to 100% (figure 5d). The direct effect of deforestation on CVL was reduced by 2.60% when deforestation prevalence decreased from 50 to 0% and increased by 6.10% when deforestation prevalence was increased from 50 to 100% (figure 5e). There was no evidence of a significant indirect effect of deforestation on CVL occurrence (figure 5f).
The effects of deforestation on HVL occurrence are given in figure 5g–i. The expected statewide HVL prevalence decreased by 29.87% when deforestation was reduced from 50 to 0% and increased by 59.75% when deforestation prevalence was increased from 50 to 100% (figure 5g). The direct effect on HVL, which was 0.10 when deforestation prevalence was 50%, increased by 2.02% when deforestation increased to 100% and decreased by the same value (2.02%) when deforestation was reduced to 0% (figure 5h). No significant indirect effect of deforestation on HVL occurrence was detected (figure 5i).
It is important to highlight that the credible intervals of the overall and direct effects for all outcomes, independently of the distribution of deforestation prevalence, did not contain zero. Therefore, altering the deforestation status of a municipality significantly increased the likelihood of the outcome occurrence.
4. Discussion
In the present study, we observed that the odds of vector, CVL, and HVL occurrence were 2.63-, 2.07-, and 3.18-fold higher, respectively, in deforested compared with forested municipalities. We also identified a significant influence of the presence of vector, CVL, and HVL in one municipality on disease occurrence in previously naive neighbouring municipalities. Lastly, we found that a hypothetical reduction in deforestation prevalence from 50 to 0% across the state would reduce vector, CVL, and HVL.
Specifically, in the São Paulo state, the VL expansion occurred along a major axis extending from the northwest to the southeast towards the Bauru region, following the Bolivia–Brazil gas pipeline construction along the paths of the Novoeste railway and the Marechal Rondon highway [14–16]. The latter crosses São Paulo from the northwest corner to the state capital in the southeast. The same area presents the highest forest clearing from the state [35,51]. Conversely, in the regions where the Brazilian Atlantic Rainforest is preserved, such as the coastal area of São Paulo state, the disease is not endemic, occurring in only a few sporadic cases in humans and dogs, all not related to Lu. longipalpis presence, but to two other sandflies species (Pintomyia fischeri and Migonemyia migonei) [14–18].
Two mechanisms may explain the importance of deforestation on the disease occurrence. First, anthropogenic changes alter the functioning of the ecosystem and the structure of the community [52–54]. These changes occur through a chain of events initiated by the death or migration of the vector's predators and competitors, leading to ecological release and freedom of the vector from natural controls [55]. The vector population can then swiftly expand and disperse throughout the new environment [54,56]. An extension of this concept has been proposed to explain the invasion of Aedes spp. and Rattus spp. in anthropized areas [57,58]. Second, deforestation and its outcomes (e.g. urbanization, agricultural, and livestock expansion) not only directly affect the ecology of the vector but also promote closer proximity between the vector and new reservoirs (e.g. dogs) [5]. Deforestation causes fragmentation of the natural biome to create a mix of different habitats, leading to forest edges characterized by biophysical factors (e.g. elevated species richness) and intense biological activity [57–59]. Expansion of forest edges within a matrix of habitats has been suggested to alter disease niches by bringing together the vector, reservoirs, and humans, thereby increasing the contact rate and risk of transmission. Contact is a key feature of disease dynamics, and the contact rate is one of the main components of the basic reproduction number (R0), a measure of the potential for disease emergence [60].
To the best of our knowledge, this is the first study to apply causal inference methods to estimate the effects of deforestation on VL. Despite growing awareness that the emerging occurrence and dynamics of infectious diseases may be associated, at least in part, with deforestation, relatively few studies have investigated whether and how deforestation might play such a role [21]. Our poor understanding of the relationship between infectious diseases and anthropogenic environmental changes could be due to several factors. One is the high cost of data collection inherent to studies of natural ecosystems. A second factor is the poor quality and/or dearth of data about the prevalence of the various components of infectious disease cycles, including vector, human, and wild host reservoirs; and misdiagnosis, delayed diagnosis, and underreporting of disease cases. A third, and likely most important, obstacle to understanding the impact of deforestation on infectious disease occurrence is the use of methodologies that do not benefit from recent advances in causal inference for complex and large-scale systems [21,25,57]. This obstacle has resulted in the recurrent use of reductionist methods through hypothesis testing, thereby separating the problems into elements and focusing on the elements in isolation, which ignores the complex, probably nonlinear and non-independent effects between the elements [61]. Consequently, although deforestation is frequently implicated in the emerging of infectious diseases, there is still no consensus about the precise roles played by deforestation. For example, there is evidence both for and against an association between leishmaniasis occurrence in Brazil: some studies observed a positive association between VL and greener areas [62–64], some observed no association [65,66], and others found a positive association between urbanized areas and Lu. longipalpis, CVL, and HVL presence [67].
Our study has several strengths that should be highlighted. The first was the use of spatial and temporal data; namely, data from the entire state of São Paulo spanning almost two decades. Second, we integrated data on all VL cycle components; the vector and two infected hosts, allowing a better understanding of the complexity of VL dynamics. Third, we took advantage of an innovative methodology that provided clear large-scale evidence that deforestation increases VL. Alongside these positives, we acknowledge that our study has some limitations. First, although data categorization permits a better convergence of the models, it halts the ability to observe any dose–response effect of deforestation on the outcomes. Second, our model assumes no directionality between the units (i.e. outcomes of a pair of connected municipalities are observed contemporaneously). Although that situation may not occur for an infectious disease, it serves as a reasonable model, given the lack of precise data on person-to-person infection.
In Brazil, the main measures to control VL occurrence and dispersion are the use of insecticides and culling of seropositive dogs, both of which have weak evidence of effectiveness and high operational cost, in combination with the rapid diagnosis and early treatment of human cases [12]. Nevertheless, these measures have not been effective, given that the incidence and mortality rate of VL have not been reduced in recent years, and the disease has spread to all regions of the country and beyond [17].
Our results point to a significant effect of deforestation on the surge of VL, and when we compare a hypothetical scenario where the statewide deforestation dropped, VL occurrence decreases. So, given that deforestation is a causal factor for VL occurrence, we emphasize that decision-makers need to place control of VL and infectious diseases more broadly within a framework that encompasses ecologically correct development and viable solutions for the trade-offs between urbanization, agriculture, and conservation. Forested areas are relevant not only to biodiversity conservation but also to public health, preventing the emergence and re-emergence of infectious diseases [67–69]. Future work should look to more integrated and multidisciplinary approaches, such as the One Health strategies, a widely endorsed concept that recognizes that human health is directly linked to the health of animals and the environment, and that changes in one field inevitably impact upon the others [70].
Supplementary Material
Acknowledgements
We are grateful to Cláudio Casanova, Fredy Galvis-Ovallos, Osias Rangel, and José Tolezano for their valuable help at the beginning of the study.
Data accessibility
The data that support the findings of our study are available in Casanova et al. [15] at [https://doi.org/10.1371/journal.pntd.0003620], and from the following resources available in the public domain: (i) Fundação SEADE [http://www.imp.seade.gov.br/frontend/#/], (ii) Ministério da Saúde da Saúde do Brasil [http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinanwin/cnv/leishvSP.def], (iii) Secretaria de Estado de Saúde de São Paulo [http://www.saude.sp.gov.br/cve-centro-devigilancia-epidemiologica-prof.-alexandre-vranjac/ areas-de-vigilancia/doencas-de-transmissao-porvetores-e-zoonoses/agravos/leishmaniose-visceral/], (iv) MapBiomas project [https://mapbiomas.org/], (v) Ministério dos Transportes do Brasil [https://www.tbg.com.br/traçado-do-gasoduto], (vi) Instituto Brasileiro de Geografia e Estatística (IBGE). Cidades e Estados. 2020. [https://cidades.ibge.gov.br/brasil/sp/sao-paulo/panorama]
The data are provided in electronic supplementary material [71].
Authors' contributions
C.V.B.d.S.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, validation, visualization, writing—original draft; A.d.P.S.: conceptualization, data curation, formal analysis, investigation, resources, supervision, validation, writing—review, and editing; G.L.W.: conceptualization, investigation, methodology, supervision, validation, visualization, writing—review, and editing; C.J.S.: conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—review, and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by the Conselho Nacional de Desenvolvimento Tecnológico e Pesquisa (CNPq), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
References
- 1.World Health Organization. 2020Vector-borne diseases. See who.int/news-room/fact-sheets/detail/vector-borne-diseases
- 2.World Health Organization. 2014A global brief on vector-borne diseases. World Health Organization. See http://apps.who.int/iris/bitstream/10665/111008/1/WHO_DCO_WHD_2014.1_eng.pdf [Google Scholar]
- 3.Jones KE, et al. 2008Global trends in emerging infectious diseases. Nature 451, 990-993. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilcox BA, Gubler DJ. 2005Disease ecology and the global emergence of zoonotic pathogens. Environ. Health Prev. Med. 10, 263-272. ( 10.1007/BF02897701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patz JA, Graczyk TK, Geller N, Vittor AY. 2000Environmental changes and parasitic diseases. Int. J. Parasitol. 30, 1395-1405. ( 10.1016/S0020-7519(00)00141-7) [DOI] [PubMed] [Google Scholar]
- 6.Walsh JF, Molyneux DH, Birley MH. 1993Deforestation effects on vector-borne disease. Parasitology 106, S55-S75. ( 10.1017/S0031182000086121) [DOI] [PubMed] [Google Scholar]
- 7.Alvar J, Yactayo S, Bern C. 2006Leishmaniasis and poverty. Trends Parasitol. 22, 552-557. ( 10.1016/j.pt.2006.09.004) [DOI] [PubMed] [Google Scholar]
- 8.Alvar J, et al. 2012Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7, e35671. ( 10.1371/journal.pone.0035671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. 2020Leishmaniasis. Leishmaniasis fact sheet. See https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis.
- 10.Alvar J, Cañavate C, Molina R, Moreno J, Nieto J. 2004Canine leishmaniasis. Adv. Parasitol. 57, 1-88. ( 10.1016/S0065-308X(04)57001-X) [DOI] [PubMed] [Google Scholar]
- 11.Desjeux P. 2004Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 27, 305-318. ( 10.1016/j.cimid.2004.03.004) [DOI] [PubMed] [Google Scholar]
- 12.Ministério da Saúde. 2014. Manual de vigilância e controle da leishmaniose visceral, 1st ed. Brasília (BR): Ministério da Saúde. [Google Scholar]
- 13.Werneck GL. 2008Forum: geographic spread and urbanization of visceral leishmaniasis in Brazil. Introduction. Cad. Saude Publica. 24, 2937-2940. ( 10.1590/S0102-311X2008001200023) [DOI] [PubMed] [Google Scholar]
- 14.Cardim MFM, Rodas LAC, Dibo MR, Guirado MM, Oliveira AM, Chiaravalloti-Neto F. 2013Introduction and expansion of human American visceral leishmaniasis in the state of Sao Paulo, Brazil, 1999–2011. Rev. Saude Publica. 47, 691-700. ( 10.1590/S0034-8910.2013047004454) [DOI] [PubMed] [Google Scholar]
- 15.Casanova C, Colla-Jacques FE, Hamilton JGC, Brazil RP, Shaw JJ. 2015Distribution of Lutzomyia longipalpis chemotype populations in São Paulo state, Brazil. PLoS Negl. Trop. Dis. 9, 1-14. ( 10.1371/journal.pntd.0003620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sevá A da P, Mao L, Galvis-Ovallos F, Tucker Lima JM, Valle D. 2017Risk analysis and prediction of visceral leishmaniasis dispersion in São Paulo state, Brazil. PLoS Negl. Trop. Dis. 11, e0005353. ( 10.1371/journal.pntd.0005353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministério da Saúde/SVS—Sistema de Informação de Agravos de Notificação (SINAN). 2019Leishmaniose visceral—Casos confirmados notificados no Sistema de Informação de Agravos de Notificação—São Paulo. See http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinanwin/cnv/leishvSP.def.
- 18.Secretaria do Estado de Saúde de São Paulo. 2019Dados estatísticos da Leishmaniose Visceral Americana de 1999–2018. See http://www.saude.sp.gov.br/cve-centro-de-vigilancia-epidemiologica-prof.-alexandre-vranjac/areas-de-vigilancia/doencas-de-transmissao-por-vetores-e-zoonoses/agravos/leishmaniose-visceral/dados-estatisticos (accessed 7 December 2019).
- 19.Belo VS, et al. 2013Factors associated with visceral leishmaniasis in the Americas: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 7, e2182. (doi:10.1371/journal.pntd.0002182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valero NNH, Uriarte M. 2020Environmental and socioeconomic risk factors associated with visceral and cutaneous leishmaniasis: a systematic review. Parasitol. Res. 119, 365-384. ( 10.1007/s00436-019-06575-5) [DOI] [PubMed] [Google Scholar]
- 21.Plowright RK, Sokolow SH, Gorman ME, Daszak P, Foley JE. 2008Causal inference in disease ecology: investigating ecological drivers of disease emergence. Front. Ecol. Environ. 6, 420-429. ( 10.1890/070086) [DOI] [Google Scholar]
- 22.Tucker Lima JM, Vittor A, Rifai S, Valle D. 2017Does deforestation promote or inhibit malaria transmission in the Amazon? A systematic literature review and critical appraisal of current evidence. Phil. Trans. R. Soc. B 372, 20160125. ( 10.1098/rstb.2016.0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patz JA, et al. 2004Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ. Health Perspect. 112, 1092-1098. ( 10.1289/ehp.6877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolhouse MEJ, Gowtage-Sequeria S. 2005Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 11, 1842-1847. ( 10.3201/eid1112.050997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allenby B. 2005Technology at the global scale: integrative cognitivism and Earth systems engineering management. In Scientific and technological thinking (eds M Gorman, R Tweney, D Gooding, A Kincannon), pp. 303-344. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 26.Sherman E, Shpitser I. 2018Identification and estimation of causal effects from dependent data. Adv. Neural Inf. Process Syst. 2018, 9446-9457. [PMC free article] [PubMed] [Google Scholar]
- 27.Halloran ME, Struchiner CJ. 1991Study designs for dependent happenings. Epidemiology 2, 331-338. ( 10.1097/00001648-199109000-00004) [DOI] [PubMed] [Google Scholar]
- 28.Hudgens MG, Halloran ME. 2008Toward causal inference with interference. J. Am. Stat. Assoc. 103, 832-842. ( 10.1198/016214508000000292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobel ME. 2006What do randomized studies of housing mobility demonstrate? Causal inference in the face of interference. J. Am. Stat. Assoc. 101, 1398-1407. ( 10.1198/016214506000000636) [DOI] [Google Scholar]
- 30.Tchetgen EJT, Vanderweele TJ. 2012On causal inference in the presence of interference. Stat. Methods Med. Res. 21, 55-75. ( 10.1177/0962280210386779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulcher IR. 2019Statistical inference for causal mechanisms: mediation and interference. University of Harvard. [Google Scholar]
- 32.Tchetgen TEJ, Fulcher IR, Shpitser I. 2020Auto-G-computation of causal effects on a network. J. Am. Stat. Assoc. 116, 833-884. ( 10.1080/01621459.2020.1811098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogburn EL, Shpitser I, Lee Y. 2020Causal inference, social networks and chain graphs. J. R. Statist Soc A. 183, 1659-1676. ( 10.1111/rssa.12594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Instituto Brasileiro de Geografia e Estatística (IBGE). 2020Cidades e Estados. See https://cidades.ibge.gov.br/brasil/sp/sao-paulo/panorama (accessed 6 January 2020).
- 35.Victor MAM, Cavalli AC, Guillaumon JR, Filho RS. 2005Cem anos de devastação, p. 72. Brasília, Brazil: Ministério do Meio Ambiente. [Google Scholar]
- 36.IBGE. 2020Mapas político-administrativos estaduais. See https://mapas.ibge.gov.br/politico-administrativo/estaduais (accessed 10 January 2020). [Google Scholar]
- 37.R Core Team. 2019R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria. Available from: https://www.r-project.org/. [Google Scholar]
- 38.Bivand R, et al. 2017maptools: Tools for Handling Spatial Objects. R Package version 0.9-4. Retrieved from: http://maptools.r-forge.r-project.org/, http://r-forge.r-project.org/projects/maptools/. [Google Scholar]
- 39.Bivand R. 2017Package ‘spdep’. See https://r-forge.r-project.org/projects/spdep/. [Google Scholar]
- 40.MapBiomas. 2019Coleção 4 da série anual de mapas de cobertura e uso de solo do Brasil. Proj MapBiomas. 33. See https://mapbiomas.org/ (accessed 3 October 2019).
- 41.Hijmans RJ, et al. 2020Package ‘raster’. Cran. 2020, pp. 1-249. See https://cran.r-project.org/web/packages/raster/raster.pdf.
- 42.Wickham H. 2019Package ‘tidyverse’, pp. 1–5. See http://tidyverse.tidyverse.org.
- 43.Pebesma AE, Bivand R. 2013Package ‘sp’. See http://rspatial.r-forge.r-project.org/.
- 44.Seade F. 2019Estatísticas de demografia. See https://imp.seade.gov.br/frontend/#/.
- 45.Cavararo R. 2014Pesquisa Nacional Por Amostra De Domicílios, vol. 39, pp. 1-63. Instituto Brasileiro de Geografia e Estatística. See http://biblioteca.ibge.gov.br/visualizacao/livros/liv81830.pdf. [Google Scholar]
- 46.Ministério dos Transportes. 2020Eixo Dutoviário. See https://www.tbg.com.br/traçado-do-gasodutootherinfo (accessed 10 January 2020).
- 47.Richardson SL. 2007Chain graph models and their causal interpretations. J. R. Stat. Soc.64, 321-361. [Google Scholar]
- 48.Lauritzen SL. 1996Graphical models. Oxford, UK: Oxford University Press. [Google Scholar]
- 49.Liang F. 2010A double Metropolis-Hastings sampler for spatial models with intractable normalizing constants. J. Stat. Comput. Simul. 80, 1007-1022. ( 10.1080/00949650902882162) [DOI] [Google Scholar]
- 50.Fulcher IR. 2020autognet. See https://github.com/isabelfulcher/autognet.
- 51.Oliveira AM, Guirado MM, Dibo MR, Rodas LAC, Bocchi MR, Chiaravalloti-Neto F. 2016Occurrence of Lutzomyia longipalpis and human and canine cases of visceral leishmaniasis and evaluation of their expansion in the Northwest region of the state of São Paulo, Brazil. Rev. Soc. Bras. Med. Trop. 49, 41-50. ( 10.1590/0037-8682-0353-2015) [DOI] [PubMed] [Google Scholar]
- 52.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. 1997Human domination of Earth's ecosystems. Science 277, 494-499. ( 10.1126/science.277.5325.494) [DOI] [Google Scholar]
- 53.Sala OE, et al. 2000Global biodiversity scenarios for the year 2100. Science 287, 1770-1774. ( 10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 54.Begon M, Townsend C, Harper J. 2005ECOLOGY—from individuals to ecosystems, 4th edn. Oxford, UK: Blackwell Publishing. [Google Scholar]
- 55.Dangles O, Malmqvist B. 2004Species richness-decomposition relationships depend on species dominance. Ecol. Lett. 7, 395-402. ( 10.1111/j.1461-0248.2004.00591.x) [DOI] [Google Scholar]
- 56.Richard N, Mack DS, Lonsdale WM, Harry EMC, Bazzaz FA. 2000Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10, 689-710. ( 10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2) [DOI] [Google Scholar]
- 57.Hassell JM, Begon M, Ward MJ, Fèvre EM. 2017Urbanization and disease emergence: dynamics at the wildlife–livestock–human interface. Trends Ecol. Evol. 32, 55-67. ( 10.1016/j.tree.2016.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Despommier D, Ellis BR, Wilcox BA. 2006The role of ecotones in emerging infectious diseases. Ecohealth 3, 281-289. ( 10.1007/s10393-006-0063-3) [DOI] [Google Scholar]
- 59.Macdonald AJ, Mordecai EA. 2020Amazon deforestation drives malaria transmission, and malaria burden reduces forest clearing. Proc. Natl Acad. Sci. USA 117, 20 335-20 335. ( 10.1073/pnas.1901734117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lloyd-Smith JO, et al. 2009Epidemie dynamics at the human-animal interface. Science 326, 1362-1367. ( 10.1126/science.1177345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hilborn R, Mangel M. 1997The ecological detective. Princeton, NJ: Princeton University Press. [Google Scholar]
- 62.Cerbino NJ, Werneck GL, Costa CHN. 2009Factors associated with the incidence of urban visceral leishmaniasis: an ecological study in Teresina, Piauí State, Brazil. Cad Saude Pública. 25, 1543-1551. ( 10.1590/S0102-311X2009000700012) [DOI] [PubMed] [Google Scholar]
- 63.Werneck GL, Costa CHN, Walker AM, David JR, Wand M, Maguire JH. 2007Multilevel modelling of the incidence of visceral leishmaniasis in Teresina, Brazil. Epidemiol. Infect. 135, 195-201. ( 10.1017/S0950268806006881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Werneck GL, Costa CHN, Walker AM, David JR, Wand M, Maguire JH. 2002The urban spread of visceral leishmaniasis: clues from spatial analysis. Epidemiology 13, 364-367. ( 10.1097/00001648-200205000-00020) [DOI] [PubMed] [Google Scholar]
- 65.Araújo VEM. 2011Análise da distribuiçao espaço-temporal da leishmaniose visceral e perfil clınico-epidemiológico dos casos e óbitos, Belo-Horizonte, Minas Gerais, 1994 a 2009. Doctorate Thesis, Universidade Federal de Minas Gerais. [Google Scholar]
- 66.de Araújo VEM, et al. 2013Relative risk of visceral leishmaniasis in Brazil: a spatial analysis in urban area. PLoS Negl. Trop. Dis. 7, e2540. ( 10.1371/journal.pntd.0002540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saccaro NL, Mation LF, Sakowski PAM. 2016Impacts of deforestation on the incidence of diseases in the Brazilian Amazon. Discussion paper/Institute for Applied Economic Research (IPEA). Brasília, Brazil: Rio de Janeiro. See https://www.econstor.eu/bitstream/10419/220300/1/dp_212.pdf.
- 68.Chaves LF, Cohen JM, Pascual M, Wilson ML. 2008Social exclusion modifies climate and deforestation impacts on a vector-borne disease. PLoS Negl. Trop. Dis. 2, 1-8. ( 10.1371/journal.pntd.0000176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vandermeer J, Perfecto I. 2007The agricultural matrix and a future paradigm for conservation. Conserv. Biol. 21, 274-277. ( 10.1111/j.1523-1739.2006.00582.x) [DOI] [PubMed] [Google Scholar]
- 70.American Veterinary Medical Association. 2009One Health: a new professional imperative. See https://www.avma.org/sites/default/files/resources/onehealth_final.pdf (accessed 18 February 2020).
- 71.Santos CVBd, Sevá AdP, Werneck GL, Struchiner CJ. 2021Does deforestation drive visceral leishmaniasis transmission? A causal analysis. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Santos CVBd, Sevá AdP, Werneck GL, Struchiner CJ. 2021Does deforestation drive visceral leishmaniasis transmission? A causal analysis. Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data that support the findings of our study are available in Casanova et al. [15] at [https://doi.org/10.1371/journal.pntd.0003620], and from the following resources available in the public domain: (i) Fundação SEADE [http://www.imp.seade.gov.br/frontend/#/], (ii) Ministério da Saúde da Saúde do Brasil [http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinanwin/cnv/leishvSP.def], (iii) Secretaria de Estado de Saúde de São Paulo [http://www.saude.sp.gov.br/cve-centro-devigilancia-epidemiologica-prof.-alexandre-vranjac/ areas-de-vigilancia/doencas-de-transmissao-porvetores-e-zoonoses/agravos/leishmaniose-visceral/], (iv) MapBiomas project [https://mapbiomas.org/], (v) Ministério dos Transportes do Brasil [https://www.tbg.com.br/traçado-do-gasoduto], (vi) Instituto Brasileiro de Geografia e Estatística (IBGE). Cidades e Estados. 2020. [https://cidades.ibge.gov.br/brasil/sp/sao-paulo/panorama]
The data are provided in electronic supplementary material [71].