Abstract
Over about 10 million years, the ancestors of whales transformed from herbivorous, deer-like, terrestrial mammals into carnivorous and fully aquatic cetaceans. Protocetids are Eocene whales that represent a unique semiaquatic stage in that dramatic evolutionary transformation. Here, we report on a new medium-sized protocetid, Phiomicetus anubis gen. et sp. nov., consisting of a partial skeleton from the middle Eocene (Lutetian) of the Fayum Depression in Egypt. The new species differs from other protocetids in having large, elongated temporal fossae, anteriorly placed pterygoids, elongated parietals, an unfused mandibular symphysis that terminates at the level of P3, and a relatively enlarged I3. Unique features of the skull and mandible suggest a capacity for more efficient oral mechanical processing than the typical protocetid condition, thereby allowing for a strong raptorial feeding style. Phylogenetic analysis nests Phiomicetus within the paraphyletic Protocetidae, as the most basal protocetid known from Africa. Recovery of Phiomicetus from the same bed that yielded the remingtonocetid Rayanistes afer provides the first clear evidence for the co-occurrence of the basal cetacean families Remingtonocetidae and Protocetidae in Africa. The discovery of Phiomicetus further augments our understanding of the biogeography and feeding ecology of early whales.
Keywords: whales, evolution, biogeography, phylogeny, Fayum
1. Introduction
Protocetidae is a paraphyletic assemblage of semiaquatic whales that inhabited a niche midway between their semi-terrestrial predecessors of the earlier Cenozoic Era, such as Pakicetus, and the ocean-going whales, such as Basilosaurus, which is first documented a few million years later [1–6]. Protocetids have the ancestral crown placental dental formula and cheek teeth that preserve modified trigonids and talonids bearing protoconid and hypoconid cusps, respectively. With some exceptions, these teeth have no accessory denticles [4–6]. Nevertheless, by possessing large supraorbital processes, protocetids resemble more derived cetaceans, such as basilosaurids and early members of Neoceti [5]. Protocetidae emerged during the early middle Eocene in the Indo-Pakistan region, where they rapidly diversified and dispersed to other parts of the world [6–10]. Protocetidae initially included Protocetus and Eocetus from the middle Eocene of Egypt [11], but with more recent discoveries has expanded into a large paraphyletic family of archaeocetes that includes three subfamilies: Protocetinae, Makaracetinae, and Georgiacetinae [12].
The Egyptian protocetids were the first protocetids to be described [13], even before the family ‘Protocetidae’ was named. Protocetus atavus and Eocetus schweinfurthi (which was originally considered to be a protocetid) were discovered from the Mokattam Limestone of Lutetian age, in the Mokattam Formation of Gebel Mokattam near Cairo [13]. In 2011, another protocetid, Aegyptocetus tarfa [8] was discovered when a large block of marbleized limestone from the Khashm El Raqaba quarry, located on the northern flank of Wadi Tarfa, was commercially exported to Italy. Prior to this study, the most recently named protocetid species from Egypt was Aegicetus gehennae, which was collected from the Gehannam Formation of the Wadi El-Hitan World Heritage Site of the Fayum Depression and represents the latest-surviving protocetid known to date, surviving until ca 38 Ma [10].
The greatest concentration of cetacean-bearing fossil localities in Egypt is in the Fayum Depression of the Egyptian Western Desert [14–16]. The Fayum area is not only famous for prehistoric whales; it is also an important region for Palaeogene fish, sharks, and land mammals [17–19]. Here, we report on a new protocetid whale, Phiomicetus anubis, gen. et sp. nov., from the middle Lutetian part of the Midawara Formation in the Fayum Depression of Egypt. The holotype specimen, Mansoura University Vertebrate Paleontology Center (MUVP) 500, consists of a cranium, mandibles, cervical and thoracic vertebrae, and rib fragments. Not only does this new species shed much-needed light on early whale evolution in Africa during the middle Eocene, but our associated phylogenetic and palaeobiogeographic analyses also provide definitive evidence for the coexistence of basal cetacean families in Africa. The new species is based on a partial skeleton, revealing the most basal protocetid whale known from Africa. Moreover, the new specimen further shows that early protocetid whales were more diversified in their anatomy and feeding behaviour than was previously thought.
2. Methods
(a) . Phylogenetic analyses
We coded Phiomicetus into the matrix of Lambert et al. [9], supplemented with additional taxa, characters, and some coding modifications (see electronic supplementary material, data). With the addition of Phiomicetus, the updated matrix includes 44 taxa and 190 characters, with 39 ordered characters. We analysed the matrix in MrBayes 3.2.5 using a Bayesian tip-dating approach with the fossilized-birth-death (FBD) prior. In order to obtain a prior for the clockratepr parameter needed for the clock analysis, we first ran a non-clock (standard Bayesian) analysis; for this non-clock analysis, the matrix was run for 5 million Markov chain Monte Carlo generations, with two runs and four chains and a temperature of 0.02, sampling every 1000 generations. The first 25% of the samples were discarded as burn-in. The average standard deviation of split frequencies was 0.0062 in the final generation, consistent with convergence of the runs. Using the allcompat tree derived from the non-clock analysis and the midpoints of the uniform age priors for included fossil taxa, we used the R script of Gunnell et al. [20] to derive a lognormal (−1.44, 0.91) clockratepr setting for the clock analysis. The treeagepr parameter was set based on the oldest possible bound on an included fossil taxon, i.e. 48.6 Ma; we set the parameter as truncated normal (48.6, 48.61, 1.0). The species sampled in the matrix derive from the last common ancestor of all non-camelid artiodactyls, given the outgroup sampling of two distantly cetacean relatives (Hippopotamidae and Sus scrofa). The sampleprob parameter was therefore set based on the 352 extant species in the clade that includes non-camelid artiodactyls; with two extant species sampled, the sampleprob value was set as 2/352 = 0.006. We enabled the FBD prior by calling prset brlenspr = clock: fossilization and used the independent gamma rates model for the clockvarpr parameter, flat priors for the fossilizationpr and extinctionpr parameters, and set samplestrat to ‘fossiltip’. The clock analysis was run for 25 million generations, with 2 runs of 8 chains and a temperature of 0.02. The average standard deviation of split frequencies was 0.0042 in the final generation, and the minimum effective sample size for all parameters was 1853.72; both diagnostics provide strong evidence for convergence. The first 25% of samples were discarded as burn-in, and the remaining trees were summarized with an ‘allcompat’ (majority-rule plus compatible groups) consensus tree.
Parsimony analyses were run in PAUP* 4b10. Sus and Hippopotamus were set as outgroups for all analyses. All characters were equally weighted, and 40 characters were treated as ordered. A heuristic search was run for 10 000 replicates with random addition sequence, Tree Bisection and Re-connection (TBR) branch swapping, and a 30 s time limit on each replicate. All recovered trees were summarized by a strict consensus (see electronic supplementary material, data). Support for individual nodes was estimated via bootstrap analysis, also run for 10 000 pseudoreplicates.
3. Systematic palaeontology
Mammalia Linnaeus, 1758
Cetacea Brisson, 1762
Protocetidae Stromer, 1908
Phiomicetus anubis, n. gen. et sp.
(a) . Etymology
The generic name Phiom is derived from the Fayum Depression in the Western Desert of Egypt, the type locality of many ancient whale fossils, and cetus, whale (L., masc.). The species name ‘anubis’ is the Greek name of the ancient Egyptian God of death, mummification, the afterlife, and the Underworld. Anubis is usually represented by a figure of a man with the head of a jackal, and we have chosen it due to the superficial similarity of the skulls of protocetids to modern canids.
(b) . Holotype
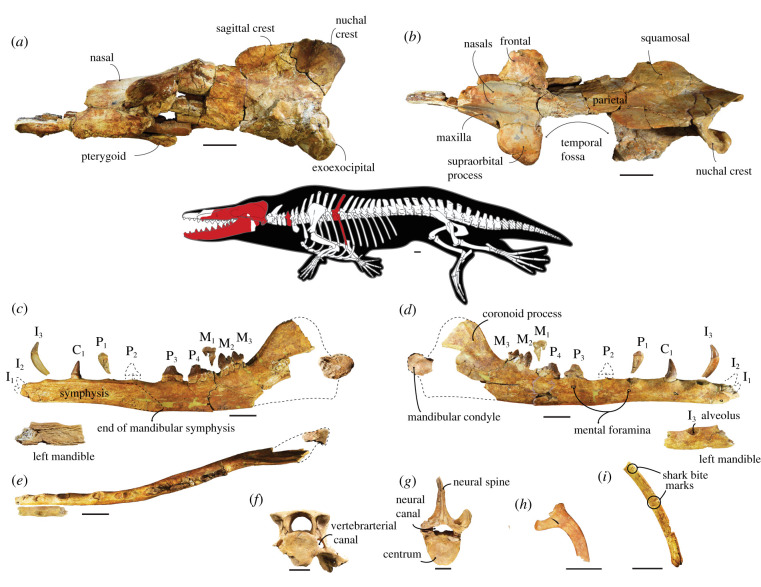
MUVP 500, an associated partial skeleton of a single individual including the cranium, the right mandible, incomplete left mandible, isolated teeth, the fifth cervical, and the sixth thoracic vertebrae and ribs (figure 1; electronic supplementary material). The holotype is the only known specimen.
Figure 1.
The holotype of Phiomicetus anubis, n. gen. et sp., MUVP 500. Skull in lateral (a) and dorsal (b) views; the mandibles and associated teeth in medial (c), lateral (d), and occlusal (e) views; (f) the fifth cervical vertebra in cranial view; (g) the sixth thoracic vertebra in cranial view; (h) the sixth left rib in cranial view; (i) shaft of a right rib in caudal view. Scale bar equals 5 cm. (Online version in colour.)
(c) . Type locality and horizon
The type locality is Al Amaim, just west of Mastabet El-Ruwayan which is on the southern margin of Wadi Al-Ruwayan in the Fayum Depression of the Western Desert in Egypt (electronic supplementary material, figure S1). The holotype, MUVP 500, was collected from the upper-middle part of the Midawara Formation that includes the fourth and fifth Mokattamian stages (MK4–5) [21], suggesting a middle Lutetian age, ca 43.0–42.0 Ma [22].
(d) . Diagnosis
Phiomicetus differs from all known protocetids in exhibiting a number of autapomorphies, including the presence of a relatively large, elongated temporal fossa; an anteriorly placed pterygoid, located at the anterior edge of the temporal fossa, just below the level of the postorbital process of the frontal; an elongated parietal; a tongue-shaped frontal shield whose anterolateral margin is more rounded in outline than those of most other protocetids, leaving the orbit small and circular in lateral view; an unfused and elongated mandibular symphysis, measuring 51% of total preserved dentary length; a high ratio (approx. 2.9) between the height of the mandible at the coronoid process versus the height at the anterior margin of P4; an enlarged I3 rather than I2 as in other protocetids; and a minor difference between the length-to-width ratios of the centrum in the preserved cervical vertebra and the same vertebra in most other protocetids (extended diagnosis and comparisons in the electronic supplementary material).
(e) . Comparisons
Phiomicetus is similar to Rodhocetus kasrani [23] in size, retaining a sutured and open mandibular symphysis and having a single-rooted P1, but differs in the position of the pterygoids, and in having the mandibular symphysis terminate at P3. Phiomicetus differs from Protocetus atavus (SMNS 11084) in retaining a small, single-rooted P1, the presence of nuchal tubercles, shorter external auditory meatus, and in being significantly larger based on the cranial measurements. Phiomicetus differs from Takracetus (GSP-UM 3041) in having smaller orbits, a narrower base of the rostrum, and in having a larger, triple-rooted M3. Phiomicetus differs from Togocetus traversei (KPG-M 1 and ULDG KPO1) in lacking a salient metaconid on the trigonid of M1. Phiomicetus also differs from Kharodacetus sahnii and Dhedacetus hyaeni [24] in having relatively smaller premolars and molars. Phiomicetus differs from Pappocetus lugardi (NHML 11086) in being significantly smaller, and in lacking the step-like notch on the ventral side of the dentary. It further differs from Georgiacetus vogtlensis (GSM 350) in lacking small accessory cusps on the cheek teeth. Phiomicetus differs from Indocetus ramani (LUVP 11034) in having a wider cranium (24.2 cm versus about 22 cm in width, measured across lateral margins of the exoccipitals). Phiomicetus differs from Qaisracetus arifi [3] in having a relatively narrow cranium and a proportionally larger temporal fossa. Phiomicetus differs from Maiacetus inuus [7], Aegyptocetus tarfa [8], P. lugardi (NHML 11086), and Babiacetus indicus (GSP-UM 3005) in having an open and sutured mandibular symphysis. It further differs from Peregocetus pacificus [9], Rodhocetus balochistanensis (GSP-UM 3485), Maiacetus inuus [7], A. tarfa [8], and Babiacetus (GSP-UM 3005) in having a longer mandibular symphysis. Phiomicetus differs from A. tarfa [8] and Makaracetus bidens [12] in lacking clinorhynchy (downward deflection of the rostrum and frontal shield relative to the braincase). Phiomicetus differs from B. indicus (GSP-UM 3005), G. vogtlensis (GSM 350), and Aegicetus gehennae [10] in having a single-rooted P1. Phiomicetus differs from Aegicetus [10] in having laterally, rather than ventrolaterally, directed exoccipital processes.
4. Results and discussion
(a) . Phylogenetic relationships
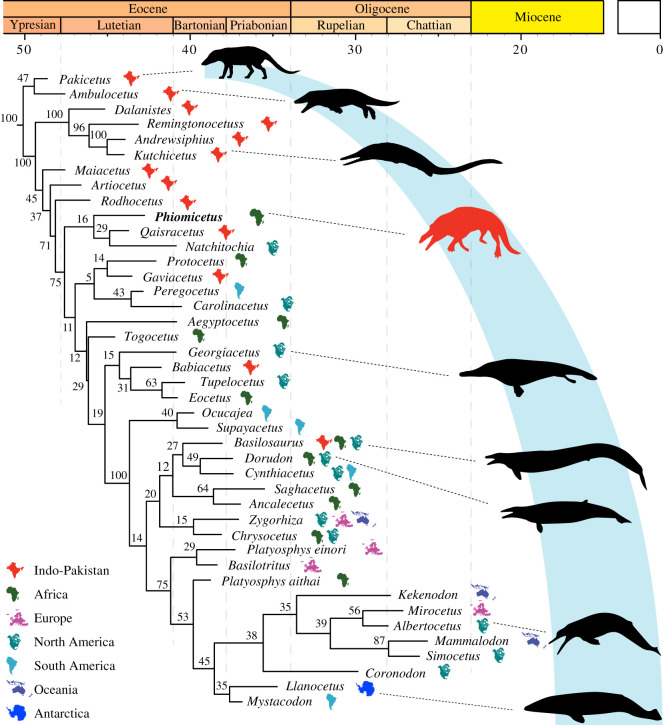
Phylogenetic analysis of our data matrix using Bayesian tip-dating analysis and FBD parameterization (figure 2; electronic supplementary material) recovered Protocetidae as paraphyletic. This consensus configuration is similar to what has been found in previous studies [9,25]. The Bayesian tip-dating analysis confirms that the most basal protocetids are the Indo-Pakistani protocetids Maiacetus, Artiocetus, and Rodhocetus, followed by sequentially more crownward genera. The arrangement of taxa along the pectinate stem at the base of Protocetidae in the allcompat tree is supported by posterior probabilities (PPs) in the range of 0.37–0.71. The ‘allcompat’ consensus (majority-rule plus compatible groups) tree places Phiomicetus in a basal position among protocetids, being in a more crownward position than Maiacetus, Artiocetus, and Rodhocetus and forming a weakly supported (PP = 0.16) clade with Qaisracetus and Natchitochia (figure 2). There are notable differences between the relationships recovered by our analysis relative to those of Lambert et al. [9] which can be explained by the different method (Bayesian tip-dating versus parsimony), and the larger amount of information being considered in the tip-dating analysis (ages of taxa, rates of morphological evolution, and the underlying model of evolution). Protocetus and Gaviacetus form a weakly supported clade (PP = 0.14), and this clade is placed as the sister clade, but with very weak support (PP = 0.05), of a moderately supported (PP = 0.43) clade containing Peregocetus and Carolinacetus from the western Hemisphere. The African protocetids Aegyptocetus and Togocetus are adjacent branches of a pectinate series of stem taxa that are consecutive sister taxa to more derived protocetids and a well-supported (PP = 1.0) Pelagiceti clade. The North American protocetid Tupelocetus occupies the most advanced position among protocetids and forms a moderately supported clade (PP = 0.63) with Eocetus, with Babiacetus as its sister taxon, but with weak support (PP = 0.31). Georgiacetus is the basal-most member of that weakly supported (PP = 0.15) clade. Our analyses provide no support for the monophyly of the proposed protocetid subfamilies Protocetinae or Georgiacetinae [12].
Figure 2.
Phylogenetic relationships and biogeography of Phiomicetus and other extinct cetaceans. ‘Allcompat’ consensus (majority-rule plus compatible groups) tree from the Bayesian tip-dating analysis of the 190-character matrix in MrBayes 3.2.5 with the implementation of the FBD prior. Numerical values to the left of nodes represent posterior probabilities (PPs) (×100). Icons to the right of taxon names reflect the geographic location of each fossil. (Online version in colour.)
Parsimony analysis (see electronic supplementary material) similarly places Phiomicetus in a basal position among protocetids (electronic supplementary material, figure S19)—i.e. more closely related to crownward cetaceans than Artiocetus, Maiacetus, Rodhocetus, Peregocetus, and Qaisracetus, but more basal than all other protocetids, basilosaurids, and members of Neoceti. Such a basal position among Protocetidae is consistent with the results of the Bayesian tip-dating analysis, with both analyses suggesting that Phiomicetus is the most basal known protocetid from Africa.
(b) . Geographic overlap of the earliest cetaceans
Phiomicetus anubis provides the first clear evidence for co-occurrence of members of Remingtonocetidae and Protocetidae in middle to upper Lutetian strata of Africa, as the Midawara Formation of Egypt has also yielded remains of the remingtonocetid Rayanistes afer [26]. The association of remingtonocetids with protocetids is also documented in the Domanda Formation of central Pakistan and the Harudi Formation of northwestern India [27]. In addition, the Castle Hayne Formation of southeastern North America has produced a tooth that has been provisionally identified as remingtonocetid [28] alongside the protocetid Crenatocetus rayi [29]. The Domanda Formation has produced the remingtonocetids Remingtonocetus domandaensis and Dalanistes ahmedi and the protocetids Rodhocetus kasrani, Takracetus simus, Gaviacetus razai, Qaisracetus arifi, and Makaracetus bidens [3,12,27,30]. The Harudi Formation has produced the remingtonocetids Remingtonocetus harudiensis and Andrewsiphius sloani and the protocetids Babiacetus indicus, Indocetus ramani, Kharodacetus sahnii, and Dhedacetus hyaeni [23–24,27].
An abundance of echinoids in the middle part of the Midawara Formation suggests a carbonate marine environment [26], thereby indicating that Phiomicetus and Rayanistes occupied coastal marine waters that had no freshwater influence. Phiomicetus was recovered from the green shale of the Midawara Formation, indicating an even more deep-water setting. Phiomicetus constitutes the earliest record and the first evidence of protocetid whales from the middle Eocene of the Fayum Depression, as the only other named protocetid from Fayum, Aegicetus, was recovered from late Eocene (Priabonian [10]) deposits. The discovery of the primitive protocetid Phiomicetus confirms quite a high degree of dispersal ability early in cetacean evolution, raises new questions about early cetacean biogeography, and raises the prospect that further exploration of Lutetian formations in Egypt may uncover even more primitive archaeocetes such as those known from the phylogenetically more basal families of Archaeoceti (Pakicetidae and Ambulocetidae). Future exploration of such Lutetian strata in Egypt, and other areas in Africa, will provide an important test of the hypothesis that basal archaeocetes were restricted to Indo-Pakistan.
(c) . Craniomandibular morphology and musculature
One of the key features seen in the cranium of Phiomicetus is the enlarged temporal fossa, occupying most of the lateral surface of the neurocranium. The size of the temporal fossa is a powerful predictor of the size and efficiency of the temporalis musculature [31–34]. A relatively large temporal fossa in Phiomicetus would have been occupied by relatively large muscles of mastication with larger cross-sectional areas and increased capacity for powerful bite force, presumably representing an adaptation for raptorial feeding on larger prey [31–33]. The large temporal fossa coupled with an elongate and tall sagittal crest and prominent and posterodorsally flaring nuchal crests suggest a large attachment area for both the superficial and deep portions of the temporalis muscle [32,35]. The mandible of Phiomicetus retained a high coronoid process preserving two well-developed muscular fossae—one bounded laterally by a low ridge on the ventral margin (for insertion of the superficial temporalis), and the other medially for insertion of the deep temporalis. The high coronoid process and its well-developed fossae improve the mechanical advantage of the temporalis muscle [35]. This arrangement suggests that Ph. anubis had the capacity for both strong bite force and posterior retraction of the mandible, allowing for more powerful handling (oral mechanical processing) of large prey items [36]. Another well-developed muscular fossa is located on the condyloid crest of the mandible for the insertion of m. masseter pars profundus (deep masseter) [32,35].
Phiomicetus retained an unfused mandibular symphysis that terminated at the level of P3, with a large symphyseal articulation. An unfused symphysis is a primitive mammalian feature, serving principally to rapidly transfer chewing forces [37]. Another striking feature in Ph. anubis is the elongation of I3, which suggests that incisors and canines were used to catch, debilitate, and retain faster and more elusive prey items (e.g. fish) before they were moved to the cheek teeth to be chewed into smaller pieces and swallowed.
(d) . Feeding ecology
The most notable anatomical features of Phiomicetus are all linked to its mode of feeding. Dental wear strongly resembles that of modern pinnipeds and to a lesser extent odontocetes [38]. There are two main types of tooth wear in Ph. anubis: apical and surficial wear. Apical wear is light and almost flat on the lower anterior teeth of Ph. anubis. Wear on I3 is present from the apex toward the root. On the lower premolars, apical wear is most extreme on P2 and decreases from P2 to P4. Apical wear on the molars is most extreme on M1, and there is no apical wear present on any of the cusps of M2 or M3. Extreme apical wear in Orcinus, the killer whale, is found in individuals that feed on large prey such as seals [39]. Surficial wear, the type of wear found on the relatively flat surfaces of the crowns of the cheek teeth, is located in MUVP 500 on the labial sides of lower cheek teeth and lingual sides of upper cheek teeth. Surficial wear facets are extended vertically on P3, reflecting the direction that the jaw moved during oral processing of food; in MUVP 500, the enamel is completely worn, exposing dentin. Apical wear on the anterior teeth and the cheek teeth suggests that these teeth were being used for prey capture, as proposed for some other archaeocetes [5,7,15], and for feeding on armoured fish/sea turtles or sharks [39,40]. These results suggest that the diet of Phiomicetus was mixed (both piscivorous and carnivorous) and included various invertebrates and vertebrates. They further suggest that prey items were usually too large to be swallowed whole and had to be sheared into smaller parts. Possible prey items include large fish, chelonians, and even smaller cetaceans [7,40] and invertebrates such as nautiloids [39]. Phiomicetus may have used the same mechanism that modern crocodilians and sharks use in hunting large prey items (e.g. large fish or small cetaceans) by pulling them onto land or tearing them by seizing a part of the prey with powerful jaws then rolling and twisting the entire body [39]. Overall skeletal anatomy, musculature, and dental anatomy of Phiomicetus indicate high efficiency in capturing elusive prey.
(e) . Body weight, postcranial morphology, and sign of scavenging
Based on a comparison of cranial measurements for Phiomicetus with other protocetids [3,8,10,23,41], the individual represented by MUVP 500 is estimated to have weighed ca 600 kg in life. As in other protocetids, the C5 centrum is short, indicating a shortened neck [3]. The sixth thoracic vertebra of Phiomicetus preserves high neural spines, large and flat zygapophyses, and robust processes resembling those found in mesonychids, but differ in their larger neural canals, less posteriorly inclined neural spines, and proportionately shorter and taller centra. The presence of long neural spines on anterior thoracic vertebrae is a feature common in land mammals that is found in protocetids [3,23] and distinguishes these species from basilosaurids. Long neural spines on anterior thoracic vertebrae are part of the suspensory system seen in land mammals [42], and suggests that Phiomicetus was capable of supporting its weight on land like most other protocetids. This is consistent with the interpretation of Phiomicetus and other protocetids as having been semiaquatic [2]. Finally, the ribs of Phiomicetus show evidence of postmortem scavenging. The morphology and the position of the bite marks on the ribs of Phiomicetus suggest that they were made by small sharks; such species were likely not large enough to hunt Phiomicetus, and rather were only capable of scavenging on the carcasses of dead individuals.
Supplementary Material
Acknowledgements
We are especially grateful to P. Gingerich for his constructive comments and valuable assistance in the excavation of the holotype skeletal remains of Phiomicetus. We thank J. Graph for his assistance in excavating and preparing the specimen. We would like to thank M. Talaat, Egyptian Environmental Affairs Agency, for the access to the Wadi El-Hitan and Wadi El Rayan Protected Areas and for encouraging this research. We are thankful to the park rangers and staff of the Wadi El-Hitan and Wadi El Rayan Protected Areas, in particular A. Nada, for their tireless assistance. We are grateful to members of the Sallam Lab for their enthusiastic support and help with preparation and photography of the specimen. We also thank the Intrinsic Specialized Hospital of Mansoura University for performing the computed tomographic scanning and data acquisitions.
Data accessibility
The LSID (life science identifier) for Phiomicetus is urn:lsid:zoobank.org:act:49366EFE-802C-4694-9F21-4F6699A63E62, and for Phiomicetus anubis is urn:lsid:zoobank.org:act:9E9CF4EF-3089-46ED-B89F-BC889791CAB9. Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.k3j9kd57t [43].
The data are provided in the electronic supplementary material [44].
Authors' contributions
A.S.G.: conceptualization, methodology, writing-original draft, writing-review, and editing; M.S.A.: investigation, supervision, writing-review, and editing; R.W.B.: conceptualization, writing-review, and editing; D.A.S.: supervision, writing-review, and editing; S.E.: methodology, writing-review, and editing; E.S.: methodology, software, writing-review, and editing; I.S.Z.: data curation and methodology; H.M.S.: conceptualization, supervision, writing-review, and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Gingerich PD, Ul-Haq M, Zalmout IS, Khan IH, Malkani MS. 2001Origin of whales from early artiodactyls: hands and feet of Eocene Protocetidae from Pakistan. Science 293, 2239-2242. ( 10.1126/science.1063902) [DOI] [PubMed] [Google Scholar]
- 2.Gingerich PD. 2003Land-to-sea transition of early whales: evolution of Eocene Archaeoceti (Cetacea) in relation to skeletal proportions and locomotion of living semiaquatic mammals. Paleobiology 29, 429-454. ( 10.1666/0094-8373(2003)0292.0.CO;2) [DOI] [Google Scholar]
- 3.Gingerich PD, Ul-Haq M, Khan IH, Zalmout IS. 2001Eocene stratigraphy and archaeocete whales (Mammalia, Cetacea) of Drug Lahar in the eastern Sulaiman Range, Balochistan (Pakistan). Cont. Mus. Paleontol. Univ. Mich. 30, 269-319. [Google Scholar]
- 4.Thewissen JG, Williams EM, Roe LJ, Hussain ST. 2001Skeletons of terrestrial cetaceans and the relationship of whales to artiodactyls. Nature 413, 277-281. ( 10.1038/35095005) [DOI] [PubMed] [Google Scholar]
- 5.Uhen MD. 2010The origin(s) of whales. Annu. Rev. Earth Planet. Sci. 38, 189-219. ( 10.1146/annurev-earth-040809-152453) [DOI] [Google Scholar]
- 6.Kellogg R. 1936A review of the Archaeoceti. Carnegie Inst. Washingt. 482, 1-366. [Google Scholar]
- 7.Gingerich PD, Ul-Haq M, von Koenigswald W, Sanders WJ, Smith BH, Zalmout IS. 2009New protocetid whale from the middle Eocene of Pakistan: birth on land, precocial development, and sexual dimorphism. PLoS ONE 4, e4366. ( 10.1371/journal.pone.0004366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianucci G, Gingerich PD. 2011Aegyptocetus tarfa, n. gen. et sp. (Mammalia, Cetacea), from the middle Eocene of Egypt: clinorhynchy, olfaction, and hearing in a protocetid whale. J. Vertebr. Paleontol. 31, 1173-1188. ( 10.1080/02724634.2011.607985) [DOI] [Google Scholar]
- 9.Lambert O, Bianucci G, Salas-Gismondi R, Di Celma C, Steurbaut E, Urbina M, de Muizon C. 2019An amphibious whale from the Middle Eocene of Peru reveals early South Pacific dispersal of quadrupedal cetaceans. Curr. Biol. 29, 1352-1359. ( 10.1016/j.cub.2019.02.050) [DOI] [PubMed] [Google Scholar]
- 10.Gingerich PD, Antar MSM, Zalmout IS. 2019Aegicetus gehennae, a new late Eocene protocetid (Cetacea, Archaeoceti) from Wadi Al Hitan, Egypt, and the transition to tail-powered swimming in whales. PLoS ONE 14, e0225391. ( 10.1371/journal.pone.0225391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichenbach SE. 1908Die Archaeoceti des ägyptischen Eozäns. Beiträge zur Paläontologie und Geologie Österreich-Ungarns und des Orients, Vienna 21, 106-178. [Google Scholar]
- 12.Gingerich PD, Zalmout IS, Ul-Haq M, Bhatti MA. 2005Makaracetusbidens, a new protocetid archaeocete (Mammalia, Cetacea) from the early Middle Eocene of Balochistan (Pakistan). Contrib. Mus. Paleontol. Univ. Mich. 31, 197-210. [Google Scholar]
- 13.Fraas E. 1904Neue Zeuglodonten aus dem unteren Mitteleocän vom Mokattam bei Cairo. Geologische und Paläontologische Abhandlungen, Jena 6, 197-220. ( 10.5962/bhl.title.39828) [DOI] [Google Scholar]
- 14.Gingerich PD. 1992Marine mammals (Cetacea and Sirenia) from the Eocene of Gebel Mokattam and Fayum, Egypt: stratigraphy, age, and paleoenvironments. Univ. Michigan Pap. Paleontol. 30, 1-84. [Google Scholar]
- 15.Uhen MD. 2004Form, function, and anatomy of Dorudon atrox (Mammalia, Cetacea): an archaeocete from the Middle to Late Eocene of Egypt. Univ. Michigan Pap. Paleontol. 34, 1-222. [Google Scholar]
- 16.Gingerich PD. 2010Cetacea. In Cenozoic mammals of Africa (eds Werdelin L, Sanders WJ), pp. 873-899. Berkeley, CA: University of California Press. [Google Scholar]
- 17.Peters S, Antar M, Zalmout I, Gingerich P. 2009Sequence stratigraphic control on preservation of Late Eocene whales and other vertebrates at Wadi Al-Hitan, Egypt. Palaios 24, 290-302. ( 10.2110/palo.2008.p08-080r) [DOI] [Google Scholar]
- 18.Sallam HM, Seiffert ER. 2016New phiomorph rodents from the latest Eocene of Egypt, and the impact of Bayesian ‘clock’-based phylogenetic methods on estimates of basal hystricognath relationships and biochronology. PeerJ 4, e1717. ( 10.7717/peerj.1717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Sayed SE, Kora MA, Sallam HM, Claeson KM, Seiffert ER, Antar MS. 2017A new genus and species of marine catfishes (Siluriformes; Ariidae) from the upper Eocene Birket Qarun Formation, Wadi El-Hitan, Egypt. PLoS ONE 12, e0172409. ( 10.1371/journal.pone.0172409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunnell GF, et al. 2018Fossil lemurs from Egypt and Kenya suggest an African origin for Madagascar's aye-aye. Nat. Commun. 9, 3193. ( 10.1038/s41467-018-05648-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strougo A. 2008The Mokattamian stage: 125 years later. Earth Sci. Ser. Middle East Res. Center Ain Shams Univ. Cairo 22, 47-108. [Google Scholar]
- 22.Luterbacher HP, et al. 2004The Paleogene Period. In A geologic time scale 2004 (eds Gradstein FM, Ogg JG, Smith AG), pp. 384-408. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Gingerich PD, Raza SM, Arif M, Anwar M, Zhou X. 1994New whale from the Eocene of Pakistan and the origin of cetacean swimming. Nature 368, 844-847. ( 10.1038/368844a0) [DOI] [Google Scholar]
- 24.Bajpai S, Thewissen JGM. 2014Protocetid cetaceans (Mammalia) from the Eocene of India. Palaeontol. Electron. 17, 1-19. ( 10.26879/459) [DOI] [Google Scholar]
- 25.Geisler JH, Sanders AE, Luo ZX. 2005A new protocetid whale (Cetacea: Archaeoceti) from the Late Middle Eocene of South Carolina. Am. Mus. Novit. 3480, 1-65. ( 10.1206/0003-0082(2005)480[0001:ANPWCA]2.0.CO;2) [DOI] [Google Scholar]
- 26.Bebej RM, Zalmout IS, Abed El-Aziz AA, Antar MSM, Gingerich PD. 2015First remingtonocetid archaeocete (Mammalia, Cetacea) from the middle Eocene of Egypt with implications for biogeography and locomotion in early cetacean evolution. J. Paleontol. 89, 882-893. ( 10.1017/jpa.2015.57) [DOI] [Google Scholar]
- 27.Williams EM. 1998Synopsis of the earliest cetaceans. In The emergence of whales (ed. Thewissen JGM), pp. 1-28. New York, NY: Plenum Press. [Google Scholar]
- 28.Uhen MD, Peredo CM. 2021The first possible remingtonocetid stem whale from North America. Acta Palaeontol. Pol. 66, 77-83. ( 10.4202/app.00799.2020) [DOI] [Google Scholar]
- 29.McLeod SA, Barnes LG. 2008A new genus and species of Eocene protocetid archaeocete whale (Mammalia, Cetacea) from the Atlantic Coastal Plain. In Geology and vertebrate paleontology of western and southern North America: contributions in honor of David P. Whistler, vol. 41 (eds Wang X, Barnes LG), pp. 73-98. Los Angeles, CA: Natural; History Museum of Los Angeles County Science Series. [Google Scholar]
- 30.Gingerich PD, Arif M, Clyde WC. 1995New Archaeocetes (Mammalia, Cetacea) from the middle Eocene Domanda formation of the Sulaiman Range, Punjab (Pakistan). Contrib. Mus. Paleontol. Univ. Mich. 29, 291-330. [Google Scholar]
- 31.Galatius A, Racicot R, McGowen M, Olsen MT. 2020Evolution and diversification of Delphinid skull shapes. iScience 23, 101543. ( 10.1016/j.isci.2020.101543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snively E, Fahlke JM, Welsh RC. 2015Bone-breaking bite force of Basilosaurus isis (Mammalia, Cetacea) from the Late Eocene of Egypt estimated by finite element analysis. PLoS ONE 10, e0118380. ( 10.1371/journal.pone.0118380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambert O, Bianucci G, Muizon C. 2017Macroraptorial sperm whales (Cetacea, Odontoceti, Physeteroidea) from the Miocene of Peru. Zool. J. Linn. Soc. 179, 404-474. ( 10.1111/zoj.12456) [DOI] [Google Scholar]
- 34.Heithaus MR, Dill L. 2009Feeding strategies and tactics. In The encyclopedia of marine mammals (eds Perrin WF, Würsig B, Thewissen HGM), pp. 414-423, 2nd edn. San Diego, CA: Academic Press. [Google Scholar]
- 35.Turnbull WD. 1970Mammalian masticatory apparatus. Fieldiana 18, 1-209. [Google Scholar]
- 36.Fahlke JM. 2012Bite marks revisited— evidence for middle-to-late Eocene Basilosaurus isis predation on Dorudon atrox (both Cetacea, Basilosauridae). Palaeontol. Electron. 15, 1-16. ( 10.26879/341) [DOI] [Google Scholar]
- 37.Scott JE, Hogue AS, Ravosa MJ. 2012The adaptive significance of mandibular symphyseal fusion in mammals. J. Evol. Biol. 4, 661-673. ( 10.1111/j.1420-9101.2012.02457.x) [DOI] [PubMed] [Google Scholar]
- 38.Adam PJ, Berta A. 2002Evolution of prey capture strategies and diet in the Pinnipedimorpha (Mammalia, Carnivora). Oryctos 4, 83-107. [Google Scholar]
- 39.Werth AJ. 2000Feeding in marine mammals. In Feeding: form, function and evolution in tetrapod vertebrates (ed. Schwenk K), pp. 487-526. San Diego, CA: Academic Press. [Google Scholar]
- 40.Voss M, Antar MSM, Zalmout IS, Gingerich PD. 2019Stomach contents of the archaeocete Basilosaurus isis: apex predator in oceans of the late Eocene. PLoS ONE 14, e0209021. ( 10.1371/journal.pone.0209021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gingerich PD. 2015Body weight and relative brain size (encephalization) in Eocene Archaeoceti (Cetacea). J. Mamm. Evol. 23, 17-31. ( 10.1007/s10914-015-9304-y) [DOI] [Google Scholar]
- 42.Pabst DA. 1990Axial muscles and connective tissues of the bottlenose dolphin. In The bottlenose dolphin (eds Leatherwood S, Reeves R), pp. 51-67. San Diego, CA: Academic Press. [Google Scholar]
- 43.Gohar AS, Antar MS, Boessenecker RW, Sabry DA, El-Sayed S, Seiffert ER, Zalmout IS, Sallam HM. 2021Data from: A new protocetid whale offers clues to biogeography and feeding ecology in early cetacean evolution. Dryad Digital Repository. ( 10.5061/dryad.k3j9kd57t) [DOI] [PMC free article] [PubMed]
- 44.Gohar AS, Antar MS, Boessenecker RW, Sabry DA, El-Sayed S, Seiffert ER, Zalmout IS, Sallam HM. 2021A new protocetid whale offers clues to biogeography and feeding ecology in early cetacean evolution. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gohar AS, Antar MS, Boessenecker RW, Sabry DA, El-Sayed S, Seiffert ER, Zalmout IS, Sallam HM. 2021Data from: A new protocetid whale offers clues to biogeography and feeding ecology in early cetacean evolution. Dryad Digital Repository. ( 10.5061/dryad.k3j9kd57t) [DOI] [PMC free article] [PubMed]
- Gohar AS, Antar MS, Boessenecker RW, Sabry DA, El-Sayed S, Seiffert ER, Zalmout IS, Sallam HM. 2021A new protocetid whale offers clues to biogeography and feeding ecology in early cetacean evolution. Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The LSID (life science identifier) for Phiomicetus is urn:lsid:zoobank.org:act:49366EFE-802C-4694-9F21-4F6699A63E62, and for Phiomicetus anubis is urn:lsid:zoobank.org:act:9E9CF4EF-3089-46ED-B89F-BC889791CAB9. Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.k3j9kd57t [43].
The data are provided in the electronic supplementary material [44].