Abstract
Toxic shock syndrome (TSS) is an acute, toxin-mediated disease process which is commonly caused by Staphylococcus aureus or Streptococcus pyogenes. A high level of clinical suspicion is imperative, with prompt antibiotic therapy with a penicillinase-resistant penicillin (vancomycin in areas with increased methicillin-resistant Staphylococcus aureus) and clindamycin, given the high morbidity and mortality. Here, a case is reported of streptococcal-mediated TSS in a 37-year-old woman with a history of endometriosis, four days after a laparoscopic cystectomy; an intrauterine device (IUD) was left in situ at the time of uterine manipulation and not removed until hospital day 3 of the patient’s readmission. Although no specific guidelines exist for removing IUDs, it is a foreign body and therefore it is recommended that early removal be considered regardless of the level of suspicion that it is the source of sepsis.
Keywords: Toxic shock syndrome, Streptococcus, Uterine manipulation, Sepsis, Intrauterine device, IUD
Highlights
-
•
An intrauterine device should be considered a potential source of infection in septic patients after uterine manipulation.
-
•
In toxic shock syndrome, all foreign bodies should be removed and antibiotics started promptly.
-
•
Criteria for toxic shock syndrome overlap with other causes of sepsis.
1. Introduction
Toxic shock syndrome (TSS) is an acute, toxin-mediated illness most commonly caused by Staphylococcus aureus or Streptococcus pyogenes [[1], [2], [3]]. Typical symptoms of streptococcal TSS include hypotension, multi-organ dysfunction and an erythematous rash[2,3]. Although diagnostic criteria exist, key criteria can be determined only late in the course of disease. A high index of suspicion is therefore needed for prompt treatment, which is important given the high morbidity and mortality of TSS. Few reports discuss TSS in association with an intrauterine device (IUD). Here, a case is reported of streptococcal TSS in the setting of recent gynecologic surgery using a uterine manipulator and a levonorgestrel IUD in situ.
2. Case Presentation
A 37-year-old woman, G0, presented for a laparoscopic left ovarian cystectomy for a 6 cm endometrioma and pelvic pain. Her history was significant for endometriosis, prior right oophorectomy for a large endometrioma, complex regional pain syndrome of the left upper extremity, anxiety, depression, hyperlipidemia, cervical disc disease, and neuropathy. She used a levonorgestrel IUD for contraception, which had been placed approximately one year prior. She underwent an uncomplicated left ovarian cystectomy with the use of a Hulka uterine manipulator, and the IUD was left in situ. She was admitted overnight for pain management, but was discharged on post-operative day (POD) 1 without further incident. On POD 4, she presented to the emergency department with worsening abdominal pain and decreased urine output. Physical examination revealed tachycardia, hypothermia to 35.5 °C rectally, tachypnea with a respiratory rate of 25, and severe abdominal distension with guarding and rebound. Bedside ultrasound demonstrated a significant amount of free fluid in the abdomen. Initial labs were significant for a lactic acid of 6 mmol/L, which trended down to 3.3 mmol/L after intravenous hydration was provided. Her chemistry was notable only for creatinine 2.3 mg/dL. She had no leukocytosis, and her hematocrit was stable compared with her pre-operative labs. She was empirically treated with vancomycin and piperacillin-tazobactam, as per the emergency department sepsis protocol. Initial CT scan revealed large-volume ascites, thickened small bowel with mucosal hyperenhancement suspicious for shock bowel, and wall thickening of the sigmoid colon (possibly reactive in the setting of recent left adnexal surgery). There was no evidence of bladder or ureteral injury. Gynecology, urology and general surgery teams were consulted, given her recent surgery and concern for possible post-operative complications, including ureteral or bowel injury. The patient was brought urgently to the operating room for diagnostic laparoscopy by gynecology and general surgery.
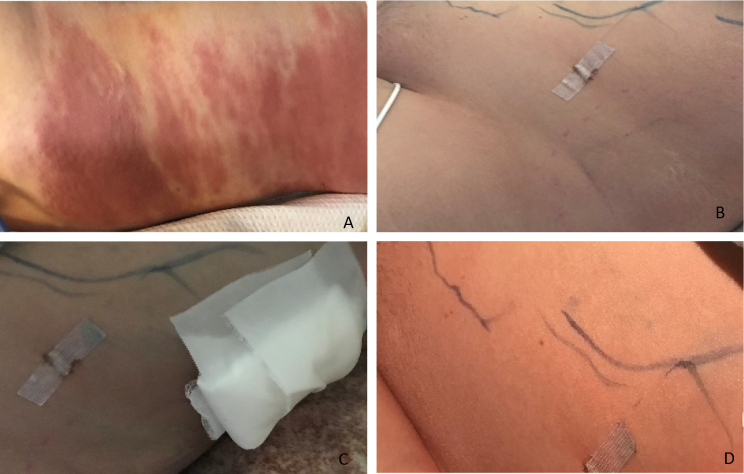
Intraoperatively, copious dark serosanguinous fluid was noted filling the abdomen and pelvis. The procedure was then converted to exploratory laparotomy in order to facilitate full bowel inspection. In total, over a liter of the ascitic fluid was evacuated. The previous surgical site at the left ovary was explored; no hematoma or active bleeding was noted. The full length of the bowel was examined and while there was evidence of inflammation and fibrinous exudate, no overt injury was detected. Angiography demonstrated normal perfusion of the small bowel. Immediately post-operatively, new skin changes were noted over the patient's entire left flank, which was erythematous and ecchymotic. Laboratory workup demonstrated only anemia, with normal coagulation. Over the next few hours, the left-flank ecchymosis spread and became more pronounced (Fig. 1). Hematology was consulted for possible bleeding diathesis, and the patient received two units of fresh frozen plasma and one unit of packed red blood cells. The patient remained intubated and was moved to the surgical ICU, where she remained in guarded condition.
Fig. 1.
Rash
A. HD#2; B. HD#4 pre-drainage; C. HD#4 post drainage; D. HD#5
In the ICU she remained on broad-spectrum antibiotics (vancomycin, meropenem and metronidazole) and diflucan. She continued to be tachycardic, with new-onset atrial fibrillation, and the flank rash continued to spread up the axilla and down to the patient's hip. The area also became edematous and firm to touch. She became febrile to 38.6 °C, now with labs revealing leukocytosis and lactic acidosis. CT scan revealed a simple fluid collection between the internal and external oblique, which was drained and cultured. On hospital day 4, admission blood cultures grew S. pyogenes. Antibiotics were then switched to intravenous clindamycin and penicillin, after consultation with the infectious disease team. In addition, the patient's IUD was removed and cultured, ultimately growing rare S. pyogenes. All other cultures (urine, vaginal, sputum, hospital day 4 repeat blood, and wound) were negative for bacteria. Vaginal and sputum cultures grew rare Candida albicans. Her clinical status then improved. She was extubated on hospital day 6 and discharged from the hospital one week later on intravenous antibiotics and close follow-up.
3. Discussion
Toxic shock syndrome (TSS) is a toxin-mediated illness with high morbidity, and a mortality rate ranging from 30% to 80% if not promptly treated [2]. TSS is most commonly caused by Staphylococcus aureus and Streptococcal pyogenes (Group A Strep). The pathophysiology has been thoroughly described, although the diagnosis and confirmation of the source of infection often remain challenging. It is known that super-antigens activate T-cells, interfering with antigen-mediated immune responses. This interference leads to massive cytokine release, which causes regulatory dysfunction throughout multiple organ systems. Common causes cited for streptococcal TSS include viral infections, pharyngitis, local soft-tissue infections, and deep infections (e.g. penetrating injuries or necrotizing fasciitis). Unlike in the case presented, IUDs and postsurgical cases of TSS are more commonly associated with staphylococcal-induced TSS [2,3].
In 2010, the CDC published diagnostic criteria for streptococcal TSS [4]. These criteria include hypotension, multi-organ involvement (at least 2 out of 5), including renal impairment, coagulopathy, liver involvement, acute respiratory distress, erythematous rash, and evidence of soft-tissue necrosis (Table 1). Laboratory criteria include evidence of Group A Streptococcus (GAS) isolated from a non-sterile or sterile site. The patient described above meets criteria for a confirmed case of streptococcal TSS with positive blood cultures, and meeting 3 out of 5 clinical criteria (erythematous rash, renal impairment, and generalized edema). However, it should be noted that the published diagnostic criteria for TSS, while helpful for retrospective analysis of a case for research and academic purposes, are difficult to use in a clinical context. While some clinical markers may be noted early in the disease process, others, such as appearance of a rash, can take days to present. It is therefore critical to have a high index of suspicion for TSS in patients with risk factors, so that prompt treatment can be initiated.
Table 1.
Streptococcal toxic shock syndrome (STSS) (Streptococcus pyogenes) 2010 CDC case definition4
| Clinical Criteria |
1. Hypotension defined by a systolic blood pressure ≤ 90 mmHg for adults or less than the fifth percentile by age for children aged ≤16 years. 2. Multi-organ involvement characterized by two or more of the following: a. Renal impairment: Creatinine ≥2 mg/dL (≥177 μmol/L) for adults or ≥ twice the upper limit of normal for age. In patients with preexisting renal disease, ˃ twofold elevation over the baseline level. b. Coagulopathy: Platelets ≤100,000/mm3 (≤100 × 106/L) or disseminated intravascular coagulation, defined by prolonged clotting times, low fibrinogen level, and the presence of fibrin degradation products. c. Liver involvement: Alanine aminotransferase, aspartate aminotransferase, or total bilirubin levels ≥twice the upper limit of normal for the patient's age. In patients with preexisting liver disease, a ˃ twofold increase over the baseline level. d. Acute respiratory distress syndrome: defined by acute onset of diffuse pulmonary infiltrates and hypoxemia in the absence of cardiac failure or by evidence of diffuse capillary leak manifested by acute onset of generalized edema, or pleural or peritoneal effusions with hypoalbuminemia. e. Generalized erythematous macular rash: may desquamate. f. Soft-tissue necrosis: including necrotizing fasciitis or myositis, or gangrene. |
| Laboratory Criteria | Isolation of group A Streptococcus |
|
Probable: A case that meets the clinical case definition in the absence of another identified etiology for the illness and with isolation of group A Streptococcus from a non-sterile site. Confirmed: A case that meets the clinical case definition and with isolation of group A Streptococcus from a normally sterile site (e.g. blood or cerebrospinal fluid or, less commonly, joint, pleural, or pericardial fluid). | |
Once the diagnosis is made, treatment with appropriate antibiotics and source control is imperative. Typical initial antibiotic coverage includes a penicillinase-resistant penicillin or first-generation cephalosporin. In areas with an increased prevalence of methicillin-resistant S. aureus, vancomycin or linezolid should also be considered [2]. Clindamycin has also been found to inhibit super-antigen production and has better tissue penetration than penicillin, improving outcomes when added to the treatment regimen. However, clindamycin should not be used as single-agent treatment, as its mechanism of action is bacteriostatic only, not bactericidal [3]. In the patient described above, broad-spectrum antibiotics were appropriately initiated on arrival, as per the local sepsis protocol, and narrowed to penicillin and clindamycin once blood cultures isolated GAS.
While appropriate antibiotic coverage is crucial in the treatment of TSS, source control is of equal importance in effectively treating these critically ill patients. All patients thought to have TSS should be thoroughly evaluated for a possible source. This includes a thorough skin examination, pan-cultures, as well as removal of all foreign bodies. Without removal of foreign bodies, infection can continue to worsen as the object acts as a reservoir for bacteria. In the present case, the patient's IUD was not removed until hospital day 3, on the same day that admission blood cultures came back positive for GAS. Whether the IUD or the uterine manipulation acted as a nidus for the following septicemia is arguable, given that the overall risk of pelvic infection with IUDs is low [5] and the lack of literature regarding the safety of uterine manipulators with IUDs in situ. There are case reports, however, in which IUDs have been considered the source of TSS [1,6,7] as well as uterine manipulators [8].
Given that the risk of IUD removal is infinitesimally low when compared with the risk of maintaining a possible reservoir of infection, in retrospect, strong consideration should be given to the removal of IUDs at the time of first presentation of sepsis, when any new disruption (uterine manipulation, endometrial biopsy, LEEP, IUD placement) has occurred. This is regardless of the level of suspicion that the IUD is the source. This case illustrates the need to consider TSS early in the differential diagnosis of patients who present with sepsis after gynecologic procedures, as well as the need for further investigation into the safety of IUDs left in situ during gynecologic surgeries requiring uterine manipulation.
Contributors
Michelle Gruttadauria was involved in patient care, participated in the conception of the case report, drafted the manuscript, revised the article critically for important intellectual content and approved the final submitted version.
Jennifer Pollard was involved in patient care, revised the article critically for important intellectual content and approved the final submitted version.
Sara Kim revised the article critically for important intellectual content and approved the final submitted version.
Xun Lian was involved in patient care, participated in the conception of the case report, revised the article critically for important intellectual content and approved the final submitted version.
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Provenance and peer review
This article was not commissioned and was peer reviewed
Contributor Information
Michelle Gruttadauria, Email: michelle.gruttadauria@stonybrookmedicine.edu.
Sara Kim, Email: sara.kim@stonybrookmedicine.edu.
Xun Lian, Email: Xun.Lian@stonybrookmedicine.edu.
References
- 1.Cho E.E., Fernando D. Fatal streptococcal toxic shock syndrome from an intrauterine device. J. Emerg. Med. 2013;44(4):777–780. doi: 10.1016/j.jemermed.2012.03.020[published. Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 2.Gottlieb M., Long B., Koyfman A. The evaluation and management of toxic shock syndrome in the emergency department: a review of the literature. J. Emerg. Med. 2018;54(6):807–814. doi: 10.1016/j.jemermed.2017.12.048[published. Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 3.Wilkins A.L., Steer A.C., Smeesters P.R., Curtis N. Toxic shock syndrome - the seven Rs of management and treatment. J. Inf. Secur. 2017;74(Suppl. 1) doi: 10.1016/s0163-4453(17)30206-2[published. S147-s52. Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 4.Streptococcal Toxic Shock Syndrome (STSS) (Streptococcus pyogenes) Center for Surveillance, Epidemiology, and Laboratory Services (CSELS), Division of Health Informatics and Surveillance (DHIS); 2021. 2010 Case Definition: Centers for Disease Control and Prevention, Office of Public Health Scientific Services (OPHSS) [Google Scholar]
- 5.Committee Opinion No 672 Clinical challenges of long-acting reversible contraceptive methods. Obstet. Gynecol. 2016;128(3) doi: 10.1097/aog.0000000000001644[published. e69–77. Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 6.Klug C.D., Keay C.R., Ginde A.A. Fatal toxic shock syndrome from an intrauterine device. Ann. Emerg. Med. 2009;54(5):701–703. doi: 10.1016/j.annemergmed.2009.05.030[published. Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 7.Wu C.M., Noska A. Intrauterine device infection causing concomitant streptococcal toxic shock syndrome and pelvic abscess with Actinomyces odontolyticus bacteraemia. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-213236[published. Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyirjesy P., Jones R.S., Chatwani A., Zinner E.S., Axelrod P. Streptococcal toxic shock-like syndrome as an unusual complication of laparoscopic tubal ligation. A case report. J. Reprod. Med. 1994;39(8):649–651. [PubMed] [Google Scholar]