Abstract
FAdV-4 is the major strain of adenovirus that responsible for hydro-pericardial syndrome (HPS) in poultry. In this study, the virus's specific gene fragments were isolated from clinically suspected cases and amplified by PCR. Finally, after a viral infection to investigate the immune response of the host, the gene expression of MHC (major histo-compatible) molecules (MHCIα, MHCIIβ), Ii (Invariant Chain) gene, inflammatory cytokines (IFN-β, IFN-γ, and IL-1β), and transcription factors (MDA5, STING, IRF7, and NF-kB) were detected by real-time PCR (fluorescence technology). The results of sequence comparison showed that the clinically isolated virus was 100% homologous to a virulent strain of avian adenovirus group C serotype 4 (FAdV-4), which were named AH-FAdV-4. The TCID50 and pathogenicity of the virus were determined that was 106.52/0.1 mL with a mortality rate of 100% in chickens and 0% in ducks. Furthermore, results showed that the expression level of MHCIα, MHCIIβ, and Ii genes in chicken embryo kidney cells significantly (P < 0.01) upregulated (increased) after infection, which was 43, 5.2, and 2.5 times higher than the control group. With the addition of PDTC, an inhibitor of NF-kB, then the expression level of MHCIα, MHCIIβ, and Ii was decreased significantly (P < 0.01) than the control group. The transcription levels of these genes were decreased 0.64, 0.27, and 0.26 respectively. Simultaneously, the expression levels of IFN-β, IFN-γ, and IL-1β were also significantly (P < 0.01) up-regulated (increased) 7.8, 22.7, and 5 times higher than the control group. It was found that up-regulation of STING and NF-κB pathways are directly involved in the regulation of inflammatory cytokines (IFN-β, IFN-γ, and IL-1β), MHC molecules (MHCIα, MHCIIβ), and Ii gene. The results also showed that the gene regulation pathways consecutively increased the expression levels of MDA5, STING, IRF7, and NF-kB. It is conducted that the expression levels of cytokines, MHC molecules, and li gene were increased by STING and NF-kB pathways.
Key words: cytokines, pathogenic, interferon, MHC, transcription factor
INTRODUCTION
The type-I adenovirus is an economically important pathogen for poultry that causes hydropericardium syndrome (HPS) and inclusion body hepatitis (IBH) (Mansoor et al., 2011; Niu et al., 2016). It is a DNA virus which is divided into 5 subgroups (A-E; FAdV-A to FAdV-E) and contains 12 serotypes (1-11; FAdV-1 to 11 but serotype 8 is divided into 2 groups such as 8a and 8b), among them FAdV-4 of C subgroup is the most common serotype that infects chickens, ducks, and geese. FAdV-8b and FAdV-11 are the main serotypes that infect turkeys, so there is a cross-transmission or cross-spreading virus among species (Steer et al., 2009; Marek et al., 2010; Jiang et al., 2019; Shen et al., 2019; Sahindokuyucu et al., 2020; Wei et al., 2019). The mortality rate caused by FAdV-4 is 30 to 100%, and the higher mortality rate depends on virus serotypes and their pathogenicity and depends upon immunosuppressive properties of viruses (Jiang et al., 2019; Yu et al., 2019). The hexon, penton, and fiber are the 3 main components of the capsid (shell) protein of the FAdV-4 virus. There is a difference in the sequence of hexon amino acids in different strains, so it is the main base for virus classification (Marek et al., 2012; San Martin, 2012; Gao et al., 2019). The analysis of the hexon gene of the FAdV-4 showed that China's Shandong FAdV-4 strains were originated from India (Wei et al., 2019). The viral fiber protein has 2 filaments (Fiber-1 and Fiber-2), projected from the capsid's surface that forms the head region (Marek et al., 2012; Grgić et al., 2014). Studies have shown that the fiber proteins, specially Fiber-2 protein is the main area that can cause disease in its host, so it is the ideal domain for detecting the virus and vaccine production (Shao et al., 2019a,b; Tian et al., 2020). There are few studies on the pathogenic mechanism of FAdV-4. Specific and nonspecific immunity plays an essential role in viral-infected animals. The MHC (MHCI, MHCII) molecules are important for antigen-presenting. These molecules assist the chaperone molecule Ii in triggering the cellular and humoral immune response in infected animals (Blees et al., 2017; Dijkstra and Yamaguchi, 2019). At the same time, the virus acts on the receptors of susceptible cells to produce corresponding antiviral cytokines, for example, the cyclic GMP-AMP (cGAMP) synthase coenzyme (cGAS) as an important DNA virus sensor that can induce proinflammatory cytokines and type I interferon through STING pathway (Niu et al., 2019; Gang et al., 2020). Viruses can invade most of the organs of their host, but the lesions mainly occur on the heart, liver, and kidneys, the virus can also cause structural and functional damage of the immune organs through apoptosis and induce severe inflammatory response (Niu et al., 2016; Guan et al., 2018; Schachner et al., 2018). The mechanism of virus damage to the organs is still not clear, but it is possible that the virus may affect the cell's metabolic pathway by cell receptors.
The previous studies have shown that CCT7 (chaperonin containing TCP1, subunit 7 Eta) on the surface of Leghorn male hepatocellular (LMH) cells is an essential target of FAdV-4 (Gao et al., 2019). This virus enters the hepatic cell and replicates through the JNK MAPK pathway. The inhibitor SP600125 can lock the replication of the virus by blocking the JNK MAPK pathway. It inhibits virus replication due to the lack of macrophage receptors, so this inhibitor is not sensitive to FAdV-4 (He et al., 2019).
No study has been done on the cellular pathways by which FAdV-4 induces interferon, MHC, and Ii molecules. This study will provide basic information to understand the chicken body's immune response and help to understand about antiviral (FAdV-4) mechanism, and control.
MATERIALS AND METHODS
Animals and Ethics Statement
The 1-day-old specific pathogen-free (SPF) chicken, chicken embryo, and ducks were purchased from Zhejiang Lihua Agricultural Co., Ltd. (Anhui, China) that were used as experimental birds. The birds were kept under healthy extensive care and controlled environmental conditions according to the age and behavior of birds (28–33°C) for 15 d. We tried our best to provide them with a clean and comfortable environment. All birds care was taken according to the Institutional Animal Care and Use Committee (IACUS) guidelines by the school of animal science and technology, Anhui Agricultural University, Hefei, China (AHAU 2019-011). We are very sorry to do the experiments at the cost of their lives. These chicks made a significant contribution to scientific research to save more chickens in the future and improve the health of the human being.
Source of FAdV-4 and LMH
The liver tissue of HPS infected chicken was collected from Anhui Poultry Diagnostic Center and stored at −20°C to use in experiments. The chicken liver cancer cells (LMH) were taken from the Key Laboratory of Veterinary Pathobiology and Disease Control, College of Animal Science and Technology, Anhui Agricultural University, China.
Primer Designing
FAdV-4 detection primers HF2 and HR2 were synthesized by Nanjing Dynamo Technology Co., Ltd. These primers were used to amplify the conserved region of the hexon gene. The primer sequence of forward was HF2; 5′-CCARATGGCSACSAACTACAA-3′ and reverse was HR2; 5′-TVGCGAAGGCGCGGGAAG-3′. The length of the expected amplified gene fragment was 599bp (Dou et al., 2017).
Identification and Comparative Analysis of FAdV-4
The 1.0 g liver of morbid chicken was taken to isolate the virus according to the already optimized method (Blees et al., 2017; Dou et al., 2017; Yu et al., 2019). The allantoic (sac) fluid was collected from SPF chicken embryos under a sterile environment, twice filtered it through 0.22-μm filter paper, and stored at −80°C. The third generation of viruses was obtained for further experiments.
The kidneys of the morbid chicken embryo were extracted and washed three times with PBS for DNA extraction (Tiangen, Beijing, China). PCR for identification of virus was carried out (Pan et al., 2017a), and obtained DNA fragments were cloned into a pMD18-T vector (Dongsheng, Guangzhou, China) for sequencing (Huada, Shanghai, China). The BLAST sequence of the gene was obtained from NCBI by online comparison analysis. DNA star and MEGA 4.0 were used for evolutionary genetic analysis of isolated and selected strain and Maximum Likelihood (ML) method to draw the phylogenetic tree.
Calculation of FAdV-4 TCID50
LMH cells were cultured in a medium containing 10% FBS and F12. Spread this media in 96-well plates at the ratio of 1 × 104 cells/100 μL, and then placed this plate in an incubator with 5% CO2 overnight at 37°C. The virus was diluted 1/10 with PBS, followed by 8 gradients of 101, 102, 103, 104, 105, 106, 107, and 108 at the total volume of 100 μL/well, and the last 2 wells were added with only PBS as the negative control. After incubating at 37°C for 2 h, culture was replaced with a 0.1-mL complete culture medium. A 0.1-mL complete culture medium and each gradient were repeated in 8 wells and observed continuously for 7 d. Upon the cultural characteristics, when cells showed rounding, aggregation, enhanced refractive index, and typical beading, it was judged as infection. According to the Reed-Muench formula, cells death and time period were recorded as TCID50 (50% Tissue Culture Infective Dose).
Animal Pathogenicity Test of FAdV-4
There were 20 SPF chickens and 20 mallard ducks of 15 days old were divided into 4 groups, 10 in each group. One group from chicken and one group from ducks treated as control negative. The virus was injected by intramuscular (IM) in thigh muscles with the dose rate of 1 × 106.52 TCID50/100 μL for the positive control group but the negative control group was injected only normal saline with the same dose rate. All birds were kept according to standard protocols. All data were collected daily, such as clinical signs and symptoms, and the number of deaths. Swab sampling from spleen and cloaca of dead and live sick birds was done after third day of FAdV-4 infection.
Isolation and Culturing of Chicken Embryo Primary Kidney Cells
According to the Schat method, the chicken embryo primary kidney cells were cultured in a 6-well plate for 24 h at 37°C with 5% CO2 in the incubator. The virus concentration of 1 × 107/well was inoculated according to the virus content of 1 MOI/well, while one well containing only PBS. All cells were washed three times with PBS after 2 h of culturing. The DNA and RNA extraction was done, after that RNA reverse transcription was done for cDNA. RT-PCR was done to detect viruses and cytokines-related genes then this obtained DNA and cDNA were preserved at −20°C for further study.
Detection of Interferon and MHC Molecules by RT-PCR
Reference sequence of signaling pathways regulating molecules (NF-κB, STING), membrane related receptor (MDA5), cytokines (IFN-γ, IFN-β, IL-1β), signal protein (MAVS), MHCIα, MHCIIβ, and Ii genes were used from GenBank for primer designing. The gene names, primer sequences, and the size of the fragments amplified and login ID number are shown in (Table 1). The relative expression was calculated by the 2−ΔΔCT method.
Table 1.
The sequences of primers used in this study.
| Gene names | Primer sequences (5′-3′) | Size of segment | Login ID |
|---|---|---|---|
| MDA5 | F: CGAATGAAAACCTGGGACAG R: TGGTTTTGCCACTGCCTGTA |
177 | AB371640 |
| STING | F: CGGCTGTGACATCTGGGAT R: CCCGAGTCAGGATGGTCTC |
86 | KP893157 |
| IRF7 | F: ACAACGCCAGGAAGGATGTC R: CCAGCAGCATGAACATGTGA |
156 | NM_205372 |
| MAVS | F: GAACGCAAACCACCTTCAAC R: CCAGGAGCAGCACTCAAATC |
84 | NM_001012893 |
| IFN-β | F: TTGCCCACAACAAGACGTGA R: GTGTGCGGTCAATCCAGTGT |
155 |
GU119897/AY974089 |
| IFN-γ | F: GTCAAAGCCGCACATCAAAC R: GGCTTTGCGCTGGATTCTC |
137 | NM_205149.1 |
| NF-κB1 | F: GTGCATCCTGAACTTGGCTATC R: GTACTGCTGCCTGGCGAATA |
94 | NM_205134.1 |
| IL-1β | F: GGCCTGAGTCATGCATCGTT R: ATAAATACCTCCACCCCGACAA |
125 |
NM_204524.1 |
| GAPDH | F: CAGAACATCATCCCAGCGTC R: GGCAGGTCAGGTCAACAAC |
82 | NM_204305 |
| MHCIα | F: AGTCAGGATGCCTACGATGG R: GTACTGCTTCAGCCCCTCAG |
217 | AY234768.1 |
| MHCIIβ | F: GAGCGTGGAGCCCAAGGTG R: GCCAGACGGTCGGTTTCGG |
69 | AY510090.1 |
| Ii | F: GGTGAAAGCCAAGTAGAAG R: TCAGGAAAGCAAGGTAAG |
109 | AY597053.1 |
Pyrrolidine Dithiocarbamate Inhibits the NF-κB Cytokines Production Pathway
As previously described, the chicken embryo primary kidney cells were inoculated into a 6-well culture plate and for virus infection. The medium was changed after 2 h, 100 μmol/L pyrrolidine dithiocarbamate (PDTC) inhibitor was added to the experimental group (experimental wells), and there was nothing in the control group except normal saline (PBS). Cell culture supernatant was discarded and recovered cells after 12, 48, and 72 h and washed these cells 3 times with PBS for mRNA extraction. Then mRNA was reverse-transcribed into cDNA by RT-PCR for the detection of MHCIα, MHCIIβ and Ii genes.
Statistical Analysis
The statistical analysis was performed by SPSS 16.0 (SPSS Inc., Chicago, IL). The results of all experiments were analyzed by one-way analysis of variance (ANOVA), followed by Tukey's HSD (honestly significant differences) for post-hoc testing to compare the significance (P) between the means of different groups. P ˂ 0.05 was considered to indicate a significant difference between the values compared.
RESULT
Characterization of FAdV-4 by RT-PCR
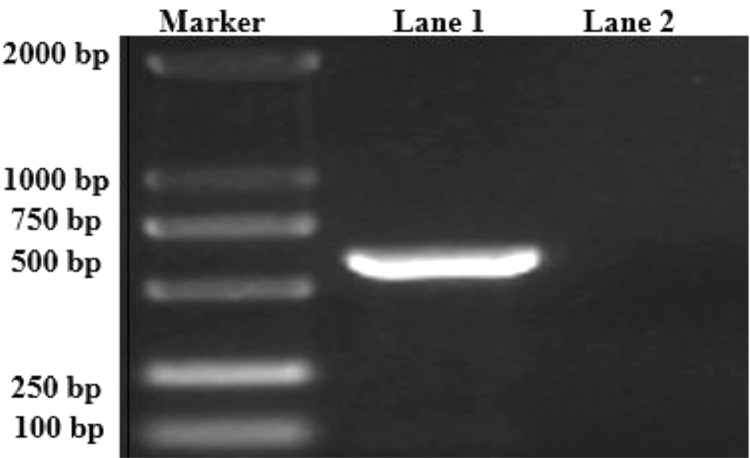
A suspected type 4 avian adenovirus (FAdV-4) was isolated from the diseased tissue. The extracted chicken liver’s DNA was used as a template for PCR determination. The results showed that DNA of the diseased chicken (treated) liver amplification was obtained in a specific band and the size was 599 bp. In contrast, the DNA of the healthy (untreated) chicken liver was not amplified to the target band or strip as shown (Figure 1).
Figure 1.
PCR analysis and identification of FAdV-4 serotype virus, showing in lane 1 and having size 599 bp.
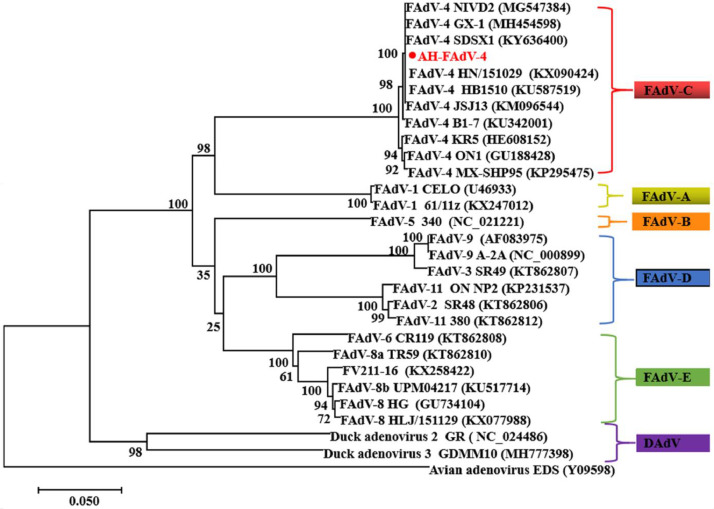
The amplified DNA fragment was sequenced, and its sequence was compared and analyzed with the sequence of avian adenovirus registered in GenBank. It was found that the isolated strain belongs to FAdV-4. Furthermore, 28 avian adenovirus strains (including FAdV-A, B, C, D, E, and DAdV) were randomly selected, including 10 C group strains. The gene sequence of hexon 599 bp has been obtained from the GenBank by the peer-to-peer match of gene BLAST. The use of MEGA 5.0 software to map the systematic evolutionary tree of the hexon gene, the phylogenetic relationship of reference, and studied sequences for genetic evolution showed that the isolated strain had high homology to the group-I of avian adenovirus subgroup-C Serotype-4 (FAdV-4) and homogeneity is about 94.9 to 100%. On the same branch of JSJ13, a strong virulent is named AH-FAdV-4 (Figure 2).
Figure 2.
Genetic evolution of hexon gene between isolated strains and reference strains of different serotypes.
The TCID50 of the FAdV-4
Pathogenic avian adenovirus can cause disease in LMH cells in vitro by 96-well culture plate. For this reason, the TCID50 of this strain was tested. The cultured virus was diluted by 10 times and then used to infect the LMH cells in the 96-well cell culture plate to observe cell changes. After 7 d of culture, the TCID50 of the virus was calculated according to the Reed-Muench method as 106.52/0.1 mL (Table 2).
Table 2.
Calculation of virus TCID50.
| The cumulative number |
|||||
|---|---|---|---|---|---|
| Dilution | No. of positive CPE holes. | No. of negative CPE holes | Positive holes | Negative holes | Percentage of positive CPE holes |
| 10−1 | 8 | 0 | 48 | 0 | 100% |
| 10−2 | 8 | 0 | 40 | 0 | 100% |
| 10−3 | 8 | 0 | 32 | 0 | 100% |
| 10−4 | 8 | 0 | 24 | 0 | 100% |
| 10−5 | 7 | 1 | 16 | 1 | 94% |
| 10−6 | 6 | 2 | 9 | 3 | 75% |
| 10−7 | 3 | 5 | 3 | 8 | 27% |
| 10−8 | 0 | 8 | 0 | 16 | 0% |
Abbreviation: CPE; cytopathic effect.
Necropsy Findings and Gross Pathological Changes
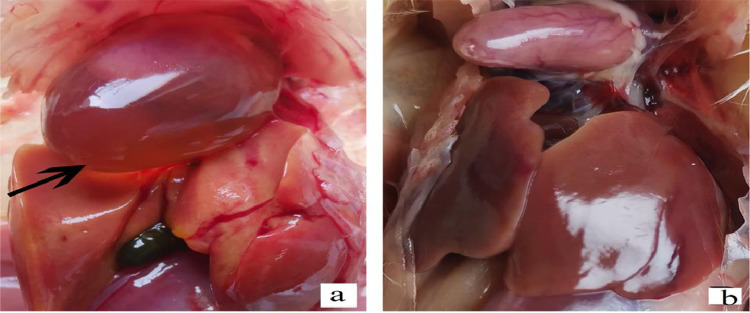
After the challenge with FAdV-4, the experimental birds showed HPS. To determine the pathogenicity of FAdV-4, chickens and ducks were used separately. At the age of day 15th, all birds got the infection, and all infected chickens died (100% mortality) (Table 3) within 3 to 5 d after infection. Necropsies of dead chickens showed severe pericardial fluid accumulation, yellow-brown liver, and brittle texture (Figure 3A), while no obvious lesions were found in the control group. After the challenge, the duck's feed intake was decreased for 3 to 5 d and then became normal without obvious symptoms and death, and even no specific postmortem lesions.
Table 3.
Test results of infected SPF chicken and Muscovy duck.
| Types of birds | Groups | Number of experimental birds | Number of mortality | Mortality (%) |
|---|---|---|---|---|
| SPF chicken | Infected group | 10 | 10 | 100 |
| Control group | 10 | 0 | 0 | |
| SPF Muscovy ducks | Infected group | 10 | 0 | 0 |
| Control group | 10 | 0 | 0 |
Abbreviation: SPF, specific pathogen-free.
Figure 3.
(A) Infected group of chicken showing enlargement of heart due to pericardial fluid accumulation, yellow and cooked appearance of liver, (B) control group showing no grass pathological changes.
After third day of infection, the swabbing samples were taken from the abdominal cavity of chicks and ducks with a sterile cotton swab, and kidneys were also removed. PCR detected the extracted viral DNA. The swabbing samples and kidneys of the infected birds were matched to an amplified FAdV-4 specific band which were positive indications. It indicated that both chickens and ducks were infected. It also showed that the strain had a high mortality rate in chicks. Still, ducks could also be infected with the same strain without any severe pathogenicity and mortality because ducks could detoxify their bodies through fecal discharge.
The FAdV-4 Increases the Gene Expression of MHC Molecules and Its Chaperone Molecule Ii Through NF-kB Pathway
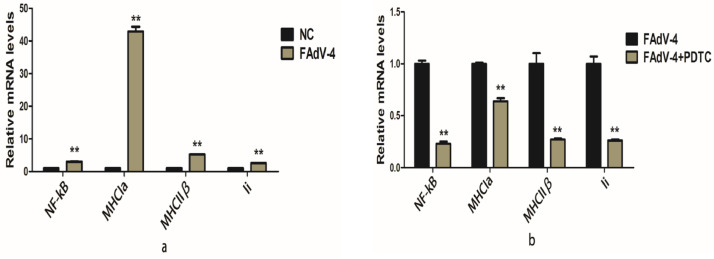
To further understand how the virus affects the expression of MHC molecules and Ii molecules. The expression of the virus was most obvious after the infection on d first, second, and third which were detected on the production base of MHCIα, MHCIIβ, and Ii by qPCR. In this study chicken embryo kidney cells were used for infection with FAdV-4 for the quantitative detection of the huge amount of transcription levels of key genes such as MHCIα, MHCIIβ, and Ii. So the Transcriptional key regulatory NF-kB genes (MHCIα, MHCIIβ, and Ii) were detected. On the first, second, and third day of infection, the NF-kB genes increased significantly, which were 0.94, 1.08, and 3, respectively. On the third day of infection, the expression of these genes was 3 times greater than the control group, so the third day is the best day for genes detection. On the third day of infection the transcription levels of MHCIα, MHCIIβ, and Ii were 43, 5.2, and 2.5, respectively that were greater (P < 0.01) than the control group (Figure 4A). These results indicated that the virus-induced transcriptional immune gene pathway in the cells. To verify that the virus induces this transcription level, the NF-kB inhibitor PDTC (100 umol/L) was used. It was found that the effect of PDTC after 12, 24, 48 h later, there was a linear decrease in the transcription level of NF-kB, which were 0.23, 0.45, and 0.67 respectively as compared to the control group. These results indicate that the effect of early infection was the most obvious. After the 12 h of PDTC addition, the transcription levels of MHCIα, MHCIIβ, and Ii were significantly (P < 0.01) decreased that were 0.64, 0.27, and 0.26 respectively as compared to the control group (Figure 4B). These results suggested that FAdV-4 could induce MHCIα, MHCIIβ, and Ii genes in the cells to enhance the immune response NF-kB pathway.
Figure 4.
Effect of adenovirus (FAdV-4) on the expression levels of MHCIα, MHCIIβ, and Ii genes (A); control group (B); infected Group (PDTC Inhibition group). ∗ Indicates the minimum significant difference (P < 0.05) while ∗∗ Indicates the highly significant difference (P < 0.01).
FAdV-4 Increases the Gene Expression of IFN-β and IFN-γ in Chicken Embryo Kidney Cells
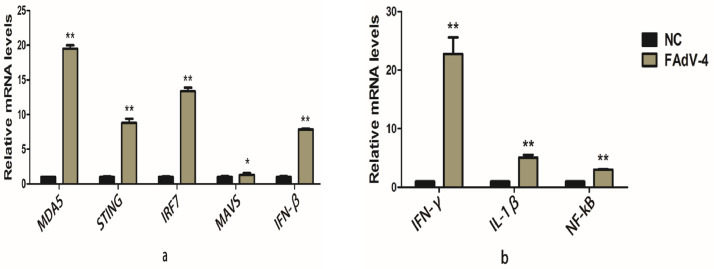
IL-1β is an inflammatory cytokine that causes pathological changes such as edema; IFN-β and IFN-γ are immunomodulatory and antiviral cytokines that play an important role in the body's antiviral response. To understand the transcription levels of these three cytokines in infected cells, IL-1β, IFN-β, and IFN-γ at different periods after infection were tested. The extracted DNA from the cells was used as a template to detect interferon (IFN-β and IFN-γ) gene expression and related pathways using RT-PCR. The results showed that the expression of cytokines increased after the third day of viral infection. The transcription levels of related genes MDA5, STING, IRF7, MAVS, and IFN-β related signaling pathways increased significantly up to 19.5, 8.8, 13.3, 1.3, and 7.8 than the control group. The statistical analysis showed that the expression of other genes except MAVS was significantly different (P < 0.01) (Figure 5A). The transcription level of IFN-γ and IL-1β genes increased by 22.7 and 5 times compared to the control group. The corresponding regulatory molecule NF-κB gene transcription level increased by three times than the control group. The difference was extremely significant (P < 0.01). These results showed that FAdV-4 stimulated the production of IL-1β, IFN-β, and IFN-γ cell's genes and also increased the transcription levels of the corresponding regulatory molecule genes. It is suggested that FAdV-4 infection can cause chicken embryo kidney cells to produce inflammatory and antiviral cytokines to provide immune responses.
Figure 5.
Effect of adenovirus on the expression levels of STING, NF-κB and their related factors pathway (A); STING pathway (B); NF-κB. ∗ Indicates the minimum significant difference (P < 0.05) while ∗∗ Indicates the highly significant difference (P < 0.01).
DISCUSSION
The FAdV-4 is a pathogenic strain that causes a high mortality rate in chickens, but it has no obvious pathological changes in ducks. Since the outbreak of adenovirus in 2015, researchers have identified different strains in different regions and found that the serotypes of chickens are 4, 8a, 8b, and 11, while FAdV-4 was the main serotypes (Li et al., 2018b; Chen et al., 2019). Different strains are susceptible to recombination, especially FAdV-E but FAdV-4 has many hosts (Schachner et al., 2019). The analysis of gene sequence found that the FAdV-4 virus genome sequence of chickens and ducks was homologous more than 94%. Still, no cross-infection, especially FAdV-4 of chicken, did not easily infect ducks, due to cytokine production (Pan et al., 2017a,b; Li et al., 2018a,b). The virus has a 100% mortality rate in chickens, but it was not lethal for ducks. However, the virus can replicate in the duck's body but can be detoxified by the excretion of the virus through the cloaca, indicating that ducks might be virus carriers. The virus can be transmitted in many forms, even by air, which was one reason for the high incidence and mortality of that disease (Li et al., 2019). The main infected organs by FAdV-4 were the liver and heart, the liver of the infected chicken was brittle and yellow while the pericardium of the heart was filled with fluid, which is the main cause of chicken's death (Li et al., 2018a).
Viruses induced interferon production through the STING pathway and regulated the expression of MHC molecules by the NF-kB pathway. RIGs were an important receptor for cells to recognize PAMP in the cytoplasm. There were three main types of RIGs, named RIG-I, MDA5, and LGP2, but in chicken, the deletion of RIG-I makes MDA5 particularly important in natural immunity. Some studies found that MAD5 can identify RNA and DNA viruses, and induced type I interferon production. In this cellular pathway, the DNA virus acted on MDA5 and induce STING pathways to stimulate the expression of IRF7 and MAVS, which regulates the expression of IFN-β (Roth-Cross et al., 2008; Barber et al., 2010). In this study, the expression level of MDA5, STING, IRF7, and MAVS was increased by 19.5, 8.8, 13.3, and 1.3 times respectively, after virus infection. It was indicated that MDA5 acts as a receptor of virus (FAdV-4) in the cytoplasm to regulate the natural (innate) immunity of the cells (Li et al., 2018b, 2019).
The specific immunity of cells (cellular immunity) also played an important role in the antiviral process. After infection virus stimulated the cells by which the expression of antigen-presenting molecules MHCIα, MHCIIβ, and Ii in the cells increased significantly by 43, 5.2, and 2.5 times, respectively. The PDTC inhibited the production of NF-κB and as a result, the expression level of NF-kB and MHC molecules (MHCIα, MHCIIβ, and Ii) was decreased by 0.2, 0.64, 0.27, and 0.26 times respectively.
It showed that viruses could regulate MHC-type molecules’ expression and their partner molecule Ii through NF-κB. Then trigger the body's specific cellular and humoral immunity. The findings of this study have provided in-depth knowledge about the immune system that can be of greater help to prevent and control FAdV-4.
CONCLUSIONS
This studied indicated that the FAdV-4 effects on the transcription level cytokines in the immune response. The results indicated that the strain was highly pathogenic for chickens (100% mortality), but there were no obvious clinical symptoms to ducks. It showed that ducks could detoxify its body through cloaca discharge. FAdV-4 strain activates the STING pathway through MDA5 to stimulate the expression of cytokine IFN-β; simultaneously, the strain can activate the NF-κB transcription factor, which in turn stimulates the transcription of a cytokine such as IFN-γ and IL-Iβ. Through the NF-κB pathway, the strain can also upregulate the transcription levels of MHCIα, MHCIIβ, and Ii genes (P < 0.01). In the control group, it was inhibiting the NF-κB transcription factor downregulated the transcription levels of these 3 genes.
Acknowledgments
ACKNOWLEDGMENTS
Meizhen Li and Muhammad Akmal Raheem have equally contributed to conducting this study. This work was supported by a grant from the National Natural Science Foundation of China [grant numbers 31972728 and 31572496].
Data availability: All materials, data, and associated protocols promptly available to readers without undue qualifications in material transfer agreements.
Author contribution: Meizhen Li: Data curation, Formal analysis, Methodology, Resources, Software, Validation, Writing - original draft, Writing - review & editing. Muhammad Akmal Raheem: Formal analysis, Investigation, Project administration, Resources. Chunyang Han: Data curation, Formal analysis, Methodology, Resources, Validation, Writing – original draft, Writing - review & editing. Fengmei Yu: Data curation, Formal analysis, Methodology, Writing - review & editing. Yin Dai: Software, Validation. Qin Hong: Resources, Validation, Writing - review & editing. Jun zhang: Software, Validation. Jun zhang: Formal analysis, Methodology, Writing - review & editing. Muhammad Imran: Data curation, Formal analysis, Methodology, Resources, Validation, Writing – original draft, Writing - review & editing. Fangfang Chen: Funding acquisition, Investigation, Project administration, Supervision, Writing - review & editing.
DISCLOSURES
All the authors declared that they have no conflict of interest in the conduction of this study.
REFERENCES
- Sahindokuyucu I., Çöven F., Kiliç H., Yilmaz Ö., Kars M., Yaziciouglu Ö., Ertunç E., Yazıcı Z. First report of fowl aviadenovirus serotypes FAdV-8b and FAdV-11 associated with inclusion body hepatitis in commercial broiler and broiler-breeder flocks in Turkey. Arch. Virol. 2020;165:43–51. doi: 10.1007/s00705-019-04449-w. [DOI] [PubMed] [Google Scholar]
- Barber M.R.W., Aldridge J.R., Webster R.G., Magor K.E. Association of RIG-I with innate immunity of ducks to influenza. Proc. Natl. Acad. Sci. 2010;107:5913–5918. doi: 10.1073/pnas.1001755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blees A., Januliene D., Hofmann T., Koller N., Schmidt C., Trowitzsch S., Moeller A., Tampé R. Structure of the human MHC-I peptide-loading complex. Nature. 2017;551:525–528. doi: 10.1038/nature24627. [DOI] [PubMed] [Google Scholar]
- Chen L., Yin L., Zhou Q., Peng P., Du Y., Liu L., Zhang Y., Xue C., Cao Y. Epidemiological investigation of fowl adenovirus infections in poultry in China during 2015–2018. BMC Vet. Res. 2019;15:271. doi: 10.1186/s12917-019-1969-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra J.M., Yamaguchi T. Ancient features of the MHC class II presentation pathway, and a model for the possible origin of MHC molecules. Immunogenetics. 2019;71:233–249. doi: 10.1007/s00251-018-1090-2. [DOI] [PubMed] [Google Scholar]
- Dou Y., Zheng X., Chen H., Yu X., Zhang Y., Zhang M. Isolation and identification of the fowl adenovirus serotype 4 from chicken. Chin. J. Vet. Sci. 2017;37:1036–1040. [Google Scholar]
- Gao J., Zhao M., Duan X., Wang Y., Cao H., Li X., Zheng S.J. Requirement of cellular protein CCT7 for the replication of Fowl adenovirus serotype 4 (FAdV-4) in Leghorn male hepatocellular cells via interaction with the viral hexon protein. Viruses. 2019;11:107. doi: 10.3390/v11020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgić H., Krell P.J., Nagy É. Comparison of fiber gene sequences of inclusion body hepatitis (IBH) and non-IBH strains of serotype 8 and 11 fowl adenoviruses. Virus Genes. 2014;48:74–80. doi: 10.1007/s11262-013-0995-y. [DOI] [PubMed] [Google Scholar]
- Guan R., Tian Y., Han X., Yang X., Wang H. Complete genome sequence and pathogenicity of fowl adenovirus serotype 4 involved in hydropericardium syndrome in Southwest China. Microb. Pathog. 2018;117:290–298. doi: 10.1016/j.micpath.2018.02.012. [DOI] [PubMed] [Google Scholar]
- He Z., Chen X., Fu M., Tang J., Li X., Cao H., Wang Y., Zheng S.J. Inhibition of fowl adenovirus serotype 4 replication in Leghorn male hepatoma cells by SP600125 via blocking JNK MAPK pathway. Vet. Microbiol. 2019;228:45–52. doi: 10.1016/j.vetmic.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Liu M., Wang C., Zhou X., Li F., Song J., Pu J., Sun Y., Wang M., Shahid M., Wei F., Sun H. Characterization of fowl adenovirus serotype 4 circulating in chickens in China. Vet. Microbiol. 2019;238 doi: 10.1016/j.vetmic.2019.108427. [DOI] [PubMed] [Google Scholar]
- Li R., Li G., Lin J., Han S., Hou X., Weng H., Guo M., Lu Z., Li N., Shang Y. Fowl adenovirus serotype 4 SD0828 infections causes high mortality rate and cytokine levels in specific pathogen-free chickens compared to ducks. Front. Immunol. 2018;9:49. doi: 10.3389/fimmu.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Wang J., Chen P., Zhang S., Sun J., Yuan W. Pathogenicity and molecular characterization of a fowl adenovirus 4 isolated from chicken associated with IBH and HPS in China. BMC Vet. Res. 2018;14:1–8. doi: 10.1186/s12917-018-1733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Yu G., Niu Y., Cai Y., Liu S. Airborne transmission of a serotype 4 Fowl adenovirus in chickens. Viruses. 2019;11:262. doi: 10.3390/v11030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor M.K., Hussain I., Arshad M., Muhammad G. Preparation and evaluation of chicken embryo-adapted fowl adenovirus serotype 4 vaccine in broiler chickens. Trop. Anim. Health Prod. 2011;43:331–338. doi: 10.1007/s11250-010-9694-z. [DOI] [PubMed] [Google Scholar]
- Marek A., Günes A., Schulz E., Hess M. Classification of fowl adenoviruses by use of phylogenetic analysis and high-resolution melting-curve analysis of the hexon L1 gene region. J. Virol. Methods. 2010;170:147–154. doi: 10.1016/j.jviromet.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Marek A., Nolte V., Schachner A., Berger E., Schlötterer C., Hess M. Two fiber genes of nearly equal lengths are a common and distinctive feature of Fowl adenovirus C members. Vet. Microbiol. 2012;156:411–417. doi: 10.1016/j.vetmic.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Niu Y., Sun Q., Shi Y., Ding Y., Li Z., Sun Y., Li M., Liu S. Immunosuppressive potential of fowl adenovirus serotype 4. Poult. Sci. 2019;98:3514–3522. doi: 10.3382/ps/pez179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y., Sun W., Zhang G., Qu Y., Wang P., Sun H., Xiao Y., Liu S. Hydropericardium syndrome outbreak caused by fowl adenovirus serotype 4 in China in 2015. J. Gen. Virol. 2016;97:2684–2690. doi: 10.1099/jgv.0.000567. [DOI] [PubMed] [Google Scholar]
- Pan Q., Liu L., Wang Y., Zhang Y., Qi X., Liu C., Gao Y., Wang X., Cui H. The first whole genome sequence and pathogenicity characterization of a fowl adenovirus 4 isolated from ducks associated with inclusion body hepatitis and hydropericardium syndrome. Avian Pathol. 2017;46:571–578. doi: 10.1080/03079457.2017.1311006. [DOI] [PubMed] [Google Scholar]
- Pan Q., Yang Y., Shi Z., Liu L., Gao Y., Qi X., Liu C., Zhang Y., Cui H., Wang X. Different dynamic distribution in chickens and ducks of the hypervirulent, novel genotype fowl adenovirus serotype 4 recently emerged in China. Front. Microbiol. 2017;8:1005. doi: 10.3389/fmicb.2017.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Cross J.K., Bender S.J., Weiss S.R. Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia. J. Virol. 2008;82:9829–9838. doi: 10.1128/JVI.01199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Martin C. Latest insights on adenovirus structure and assembly. Viruses. 2012;4:847–877. doi: 10.3390/v4050847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner A., Gonzalez G., Endler L., Ito K., Hess M. Fowl adenovirus (FAdV) recombination with intertypic crossovers in genomes of FAdV-D and FAdV-E, displaying hybrid serological phenotypes. Viruses. 2019;11:1094. doi: 10.3390/v11121094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner A., Matos M., Grafl B., Hess M. Fowl adenovirus-induced diseases and strategies for their control–a review on the current global situation. Avian Pathol. 2018;47:111–126. doi: 10.1080/03079457.2017.1385724. [DOI] [PubMed] [Google Scholar]
- Shao H., Lu Y., Wang W., Li T., Zhang J., Wan Z., Liang G., Gao W., Qin A., Ye J. Two novel monoclonal antibodies against fiber-1 protein of FAdV-4 and their application in detection of FAdV-4/10. BMC Vet. Res. 2019;15:232. doi: 10.1186/s12917-019-1987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H., Wang P., Wang W., Zhang J., Li T., Liang G., Gao W., Qin A., Ye J. A novel monoclonal antibodies-based sandwich ELISA for detection of serotype 4 fowl adenovirus. Avian Pathol. 2019;48:204–208. doi: 10.1080/03079457.2019.1566595. [DOI] [PubMed] [Google Scholar]
- Shen Z., Xiang B., Li S., Ren X., Hong Y., Liao J., Yu D., Ren T., Liao M., Xu C. Genetic characterization of fowl adenovirus serotype 4 isolates in Southern China reveals potential cross-species transmission. Infect. Genet. Evol. 2019;75 doi: 10.1016/j.meegid.2019.103928. [DOI] [PubMed] [Google Scholar]
- Steer P.A., Kirkpatrick N.C., O'Rourke D., Noormohammadi A.H. Classification of fowl adenovirus serotypes by use of high-resolution melting-curve analysis of the hexon gene region. J. Clin. Microbiol. 2009;47:311–321. doi: 10.1128/JCM.01567-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian K., Guo H.-F., Li N., Zhang Y., Wang Z., Wang B., Yang X., Li Y., Zhao J. Protection of chickens against hepatitis-hydropericardium syndrome and Newcastle disease with a recombinant Newcastle disease virus vaccine expressing the fowl adenovirus serotype 4 fiber-2 protein. Vaccine. 2020;38:1989–1997. doi: 10.1016/j.vaccine.2020.01.006. [DOI] [PubMed] [Google Scholar]
- Wang J., Ba G., Han Y.-Q., Ming S.-L., Wang M.-D., Fu P.-F., Zhao Q.-Q., Zhang S., Wu Y.-N., Yang G.-Y., Chu B.-B. Cyclic GMP-AMP synthase is essential for cytosolic double-stranded DNA and fowl adenovirus serotype 4 triggered innate immune responses in chickens. Int. J. Biol. Macromol. 2020;146:497–507. doi: 10.1016/j.ijbiomac.2020.01.015. [DOI] [PubMed] [Google Scholar]
- Wei Z., Liu H., Diao Y., Li X., Zhang S., Gao B., Tang Y., Hu J., Diao Y. Pathogenicity of fowl adenovirus (FAdV) serotype 4 strain SDJN in Taizhou geese. Avian Pathol. 2019;48:477–485. doi: 10.1080/03079457.2019.1625305. [DOI] [PubMed] [Google Scholar]
- Yu G., Lin Y., Dou Y., Tang Y., Diao Y. Prevalence of fowl adenovirus serotype 4 and co-infection by immunosuppressive viruses in fowl with hydropericardium hepatitis syndrome in Shandong province. Chin. Viruses. 2019;11:517. doi: 10.3390/v11060517. [DOI] [PMC free article] [PubMed] [Google Scholar]