Abstract
This study was conducted to evaluate the effect of supplementing hydroxy selenomethionine (OH-SeMet) on performance, selenium (Se) deposition in the breast muscle, quality and oxidative stability, and expression of selenoprotein encoding genes of breast meat of the native slow-growing yellow-feathered broiler birds. A total of 375 one-day-old local yellow male birds were randomly assigned into 5 dietary treatments, supplemented with Se 0.0, 0.2, 0.4, 0.6, and 0.8 mg/kg in the form of OH-SeMet. Each treatment consisted of 5 replicates and each replicate had 15 birds, the birds were fed on basal diet containing corn and soybean meal, and the experiment lasted for 63 d. The results showed that dietary Se supplementation linearly increased (P < 0.001) Se contents in both serum and muscle, no significant changes (P > 0.05) were observed on growth performance, yield of breast, meat color, and intramuscular fat deposition of the breast muscle. Dietary Se addition improved water-holding capacity, the pH24h value, and tenderness of breast muscle, evidenced by a linear decreases of shear force (P < 0.05), accompanied by lower thiobarbituric acid reactive substances and higher glutathione reductase activity. The mRNA abundance of selenoprotein encoding genes also responded to dietary Se levels. It is concluded that, dietary supplementation with OH-SeMet improved muscular Se deposition and meat quality of the native yellow birds, with enhanced antioxidant capability and regulation in selenogenome.
Key words: hydroxy selenomethionine, meat quality, oxidation resistance, native broiler
INTRODUCTION
Poultry meat is the second largest meat source with excellent quality of proteins and essential micronutrients for human nutrition. The native yellow-feathered broiler birds, despite slow growth, is an outstanding and much-liked delicacy in China that possesses desirable characteristics, such as resistance to certain diseases, meat flavor, and taste. It is established that the meat quality traits such as pH, color, water-holding capacity and tenderness are closely related to the oxidation status in the muscles (Asghar et al., 1991; Morrissey et al., 1998; Huff-Lonergan and Lonergan, 2005; King and Whyte, 2006). In this regard, the oxidation of phospholipids in the cell membranes would result in alterations in cell permeability and/or increase in proteolysis and even protein oxidation, thus decrease muscle water-holding capacity (Asghar et al., 1991; Huff-Lonergan and Lonergan, 2005). The oxidation status of myoglobin also predominately determines the meat color (King and Whyte, 2006; Oliveira et al., 2014). Therefore, increased antioxidant capacity may be the direct path to improve meat quality and extend their shelf-life.
Selenium (Se) has become an interesting nutrient in animal production as it improves the nutritional value and quality characteristics of meat products (Surai, 2006). Se deficiency causes various types of muscular dystrophy, such as exudative diathesis in chick, mulberry heart in pig, and white muscle disease in lamb and calf (Rederstorff et al., 2006). Se exerts most of its biological functions through incorporation into the 24/25 selenoproteins in the form of the 21st amino acid, selenocysteine (Sec) (Kryukov et al., 2003). Consequently, Sec exhibits a specific effect in different selenoenzymes as an active center for their catalytic activity. Glutathione peroxidases (GSH-Px) and melanoproteins provide a defense against oxidative stress (Hardy and Hardy, 2004). Interestingly, these antioxidant properties of Se have been found to persist in muscle tissues during the postmortem period (Mahan et al., 2014). For this, various dietary strategies in animal feeding have been developed to provide Se-enriched meat for human Se nutrition (Zhang et al., 2010). Concerning poultry, nutritional Se requirements have been given as 0.15 mg/kg for broilers (NRC, 1994), whereas many studies have shown that diets with more than 0.15 mg/kg Se supplementation have a beneficial effect on meat quality of broiler (Cai et al., 2012; Markovic et al., 2018 and Bakhshalinejad et al., 2019), suggesting that the dose of dietary Se for meat quality should be seriously reconsidered. Se is commonly added to bird diets as sodium selenite, an inorganic form, while Nano-Se has attracted widespread attention because of its high catalytic efficiency, strong adsorbing ability, and lower toxicity relative to sodium selenite (Sarkar et al., 2015). In addition, organic forms are also preferable to sodium selenite as Se sources in animal nutrition due to their excellent bioavailability and lower toxicity (Briens et al., 2013), especially the newly introduced hydroxy selenomethionine (OH-SeMet). Emerging evidences have demonstrated that OH-SeMet exhibits high bioavailability and efficiency in Se deposition in pigs (Jlali et al., 2014) and laying hens (Jlali et al., 2013). The Se deposition efficiency in muscle tissues are as following: OH-SeMet > Yeast-Se > Nano-Se ≈ sodium selenite in broiler (Zhao et al., 2021). However, our search indicates no study has been published on the effect of graded supplementation with OH-SeMet on the meat quality of native broilers.
Therefore, this investigation was to use native slow-growing yellow feather broiler to compare 1) the effects of supplementing OH-SeMet at different levels on growth performance, tissue Se concentration, antioxidant capacity, and meat quality, as well as 2) to define the relationship of the selenogenome in breast muscle and dietary Se manipulation.
MATERIALS AND METHODS
Animal procedures were approved by the Animal Care Office of Sichuan Agricultural University for the humane care and use of animals in research (approval No. SCAUAC201804-02).
Animals and Diets
A totally 375, one-day-old, local yellow male broilers were individually weighed and divided to 5 dietary groups, each group consisting of 5 replicates of 15 chicks per floor pen (1.0 m × 1.5 m), fed on corn-soybean meal-based diets (0.09 ± 0.006 mg Se/kg), supplemented with Se 0.0 (Control), 0.2, 0.4, 0.6 and 0.8 mg/kg in the form of OH-SeMet (Selisseo 2%, Adisseo France S.A.S.), which reflected a −100.0, 33.3, 166.7, 300.0, and 433.3% increment compared to the requirement level of Se (0.15 mg/kg) for this type of birds during 1 to 63 d based on the standards for yellow-feathered broilers (NY/T 33-2004, 2012), respectively. The basal diet was formulated to meet nutrient requirements of yellow broiler except for Se according to NY/T 33-2004 and was showed in Table 1. Diets were fed from 1 to 63 d including starter (1–21 d) and finisher (22–63 d) diets. The actual analyzed Se contents of the 5 experimental diets were 0.09, 0.31, 0.52, 0.73, and 1.04 mg/kg for the starter phase (1–21 d) and 0.15, 0.31, 0.51, 0.77, and 1.19 mg/kg for the finisher phase (22–63 d; Table 2). These data confirmed the proper preparation of experimental diets in this study.
Table 1.
Dietary formulation and composition in the experiment (dry matter basis).
| Ingredients and analysis | 1–21 d | 22–63 d |
|---|---|---|
| Ingredients, % | ||
| Corn | 56.77 | 62.82 |
| Soybean meal | 34.4 | 28.6 |
| Wheat barn | 2.0 | 2.0 |
| Soy-bean oil | 2.5 | 2.5 |
| Limestone | 1.50 | 1.28 |
| Dicalcium phosphate | 1.7 | 1.6 |
| L-Lysine-HCL | 0.00 | 0.08 |
| DL-Methionine | 0.26 | 0.18 |
| L-Threonine | 0.00 | 0.07 |
| Sodium chloride | 0.37 | 0.37 |
| Premix1 | 0.50 | 0.50 |
| Total | 100.0 | 100.0 |
| Calculated analysis, % | ||
| AME, Kcal/kg | 2901.5 | 2956.4 |
| CP2 | 20.09 | 17.98 |
| Dig. Lysine | 1.06 | 0.98 |
| Dig. Methionine | 0.55 | 0.44 |
| Calcium2 | 0.98 | 0.87 |
| Total P2 | 0.72 | 0.69 |
| Non-phytate P | 0.43 | 0.39 |
Abbreviations: AME, apparent metabolizable energy; CP, crude protein; Dig, digestible.
Provided per kilogram of diet: 1–21 d: Vitamin A, 5,000 IU; Vitamin D3, 1,000 IU; Vitamin E, 10 mg/kg; Vitamin K, 0.5 mg/kg; Vitamin B1, 1.8 mg/kg; Vitamin B2, 3.6 mg/kg; Pantothenic acid, 10 mg/kg; Nicotinic acid, 30 mg/kg; Vitamin B6, 3.5 mg/kg; Folic acid, 0.5 mg/kg; Vitamin B12, 10 ug/kg; Choline chloride, 1,000 mg/kg; Cu (CuSO4•5H2O), 8 mg; Fe (FeSO4•7H2O), 80 mg; Zn (ZnSO4•7H2O), 60 mg; Mn (MnSO4•H2O), 80 mg; I (KI), 0.35 mg. 22-63d: Vitamin A, 5,000 IU; Vitamin D3, 1,000 IU; Vitamin E, 10 mg/kg; Vitamin K, 0.5 mg/kg; Vitamin B1, 1.8 mg/kg; Vitamin B2, 3.6 mg/kg; Pantothenic acid, 10 mg/kg; Nicotinic acid, 30 mg/kg; Vitamin B6, 3.5 mg/kg; Folic acid, 0.5 mg/kg; Vitamin B12, 10 ug/kg; Choline chloride, 750 mg/kg; Cu (CuSO4•5H2O), 8 mg; Fe (FeSO4•7H2O), 80 mg; Zn (ZnSO4•7H2O), 60 mg; Mn (MnSO4•H2O), 80 mg; I (KI), 0.35 mg.
The analyzed values.
Table 2.
Se contents of the experimental diets supplemented with different levels of hydroxy selenomethionine (OH-SeMet)1.
| Experimental diets | Total analyzed Se, mg/kg |
|---|---|
| 1–21 d | |
| Basal diet | 0.09 ± 0.006 |
| 0.2 mg/kg Se diet | 0.31 ± 0.008 |
| 0.4 mg/kg Se diet | 0.52 ± 0.004 |
| 0.6 mg/kg Se diet | 0.73 ± 0.003 |
| 0.8 mg/kg Se diet | 1.04 ± 0.011 |
| 22–63 d | |
| Basal diet | 0.15 ± 0.009 |
| 0.2 mg/kg Se diet | 0.31 ± 0.007 |
| 0.4 mg/kg Se diet | 0.50 ± 0.007 |
| 0.6 mg/kg Se diet | 0.77 ± 0.003 |
| 0.8 mg/kg Se diet | 1.19 ± 0.016 |
Results are presented as means±standard deviations, n = 4.
All samples were tested in triplicate within each assay.
Selenium content was analyzed by the hydride generation-atomic fluorescence spectrometer.
All chicks were given ad libitum access to feed and water. The birds were housed in a climate-controlled facility with the initial ambient temperature set at approximately 32°C and then gradually reduced based on normal management practices to 22°C. The light schedule was 23L:1D and 18L:6D (18 L from 4:00 am to 10:00 pm) during 1 to 7 d and beyond, respectively. To determine growth performance, body weight (BW) and feed intake (FI) were recorded for each replicate and were used to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed to gain ratio (F: G).
Sample Collection
At the end of the 63 days of dietary treatment, after fasting for 8 h, one bird of approximately average weight was selected from each pen. Blood samples were collected in anticoagulant-free tubes from via bleeding from the wing vein, and kept on the ice and centrifuged at 3,000 × g for 10 min at 4°C, then the serum was collected immediately and stored at −20°C for later analysis. The birds were euthanized by cervical dislocation after bleeding; the whole breast muscles were immediately removed and weighed. Subsequently, the entire left breast muscles were dissected and stored at 4°C to measure meat quality traits. At the same location of left breast muscles, the samples were obtained and immediately snap-frozen in liquid nitrogen, and stored at −80°C for determination of antioxidant activity and subsequent mRNA analyses.
Diet, Serum, and Breast Muscle Se Determination
Selenium contents in diets, serum, and breast muscle were determined by inductively coupled plasma mass spectrometry using the hydride generation-atomic fluorescence spectrometer (AFS-230E, Beijing Haiguang instrument, China) against a standard reference of Se (GB/T 13883-2008 and GB 5009.93-2010, National Research Centre for Certified Reference Materials, Beijing, China) as described previously (Li et al., 2011). Briefly, approximately 1.0 g of diet or muscle sample, and 1.0 mL of serum was mineralized by microwave-assisted mineralization in 8 mL of 15.2 mol/L HNO3 and 2 mL of 12.3 mol/L HClO4 within a conical flask and the samples were left to digest overnight. Afterward, the mixtures were heated at 340°C by electric heating plate until the solutions ran clear (2 mL solution left). After cooled, the solutions were combined with 5.0 mL HCl (6.0 mol/L) and subjected to continued heating until the solutions ran clear with emission of white smoke from the samples. Then the solutions were cooled and transferred to 50 mL volumetric flasks and fixed to a volume of 50 mL after rinsing 3 times using ultrapure water. The blank control was treated using the same method. All reagents were analytical-regent grade.
Meat Quality Measurements
Meat Color
Meat color including lightness (L*), redness (a*) and yellowness (b*) was determined using a digital Minolta Chromameter (CR-310, Minolta Camera, Japan) at the 45 min postmortem. Measurements were performed in triplicate for each breast meat sample, and the values were averaged.
pH Measurement
The pH of meat was determined at 45 min and 24 h postmortem using an automated pH probe (pH-STAR, SFK-Technology, Denmark) according to the instructions (GB/T 9695.5-2008), which was calibrated before and immediately after each session according to the manufacturer's instructions. The average pH of each muscle sample was based on 3 recordings.
Water-Holding Capacity
The water-holding capacity was measured via drip loss and cooking loss. A weight of approximately 10 g of fresh breast meat sample was dissected and trimmed, the sample about 5 g (W1) with similar shape was kept in a chiller at 4°C for 24 h, after which the samples were taken out of the bags and dried gently, then weighed (W2). Drip loss was calculated as:
In addition, at 24 h postmortem, around 50 g of meat sample was weighed (W1) at room temperature, and placed in a plastic bag and cooked in an 80°C water bath for 20 min. After cooking, the breast meat sample was cooled at room temperature and then weighed as (W2). Cooking loss was calculated as:
Tenderness Measurement
The tenderness analysis was measured through the Warner-Bratzler shear force using Texture Analyzer (TA. XT. plus. Stable Micro systems, UK). Specifically, the meat was cooked to an internal temperature of 70°C in water bath, followed by cooling to 4°C. Then, cooked samples were cut into subsamples and were sheared perpendicular to the longitudinal route of the fibers equipped with a maximum 50- Newton (N) load cell and a crosshead speed of 1 mm/s. The samples’ shear forces were noted as the average of all subsamples’ values, and the results were expressed as N.
Determination of Antioxidant Capacity
Meat samples was mixed with distilled water, homogenized, centrifuged, and supernatant was obtained for measuring GSH-Px, total antioxidant (T-AOC), catalase activity, total superoxide dismutase activity (T-SOD), and thiobarbituric acid reactive substances (TBARS). The activities of GSH-Px, T-AOC, and T-SOD were assessed using the corresponding commercial test kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), and results of parameters were calculated as unit per milligram protein (U/g protein) following the manufacturer's instructions. The concentration of TBARS was determined using the Bioassay Systems Commercial kit and protocols (Bioassay Systems, Hayward, CA). For each measurement, the compared samples were run on the same plate and experiments were performed in triplicate.
Gene Expression Assays
Total RNA was extracted from breast muscle using the Trizol reagent (Takara, Dalian, China). The extracted RNA was quantified and reverse transcribed into cDNA after assessing RNA quantity. The mRNA expressions of genes of interest were assessed by QuantStudio 6 Flex Real-Time PCR detection system (Applied Biosystems, Foster City, CA) in a final volume of 10 μL using the SYBR Premix Ex TaqTM Ⅱ kit (TaKaRa, Dalian, China). The relative mRNA abundances of 19 selenoprotein genes and 5 meat quality-related genes were assayed. Primers were designed using Primer Express 3.0 (Applied Biosystems, Foster City, CA) as shown in Table 3. The standard curve method was used to estimate reaction efficiency (slope). Relative gene expression was quantified by normalizing to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin mRNA.
Table 3.
Primers used for the RT-qPCR of the target and reference genes.
| Gene | Accession number | Primer pairs forward/ reverse (5′ to 3′ direction) |
|---|---|---|
| Iodothyronine deiodinase | ||
| DIO1 | NM_001097614.1 | gggcgaaaagagcagaatga/gtgggaccccagttttcgt |
| DIO2 | NM_204114.3 | ggctgactgcatggacaaca/tgcacactcgctcaaatgaaac |
| DIO3 | NM_001122648.1 | gaccggagggctacaagatct/tctggagccgggttttgtac |
| Thioredoxin reductase | ||
| TXNRD1 | NM_001030762.2 | tacgcctctgggaaattcgt/cttgcaaggcttgtcccagta |
| TXNRD2 | NM_001122691.1 | gctcttaaagatgcccagcactac/gaacagcttgagccatcacaga |
| TXNRD3 | NM_001122777.1 | cctggcaaaacgctagttgtg/cgcaccattactgtgacatctagac |
| Lipid metabolism | ||
| FABP | NM_204290.1 | cagaagtgggatggcaaagag/ccagcaggttcccatcca |
| LPL | NM_205282.1 | tcccgaagctgagatgaatttt/ggctctgcaggtgttcttaagg |
| RRAR-β | AF_163810.1 | tgccgcttccagaaatgc/gcggccaaagcggatt |
| PPAR-γ | KT_899870.1 | gttttggcccgttaattttgg/caaaggaatgcatatgatcatcca |
| PRKAG3 | NM_001031258.2 | cacatcttcggctccaccat/acagctcctgcactgttttctttag |
| Selenoprotein | ||
| GPX1 | NM_001277853.1 | acggcgcatcttccaaag/tgttcccccaaccatttctc |
| MSRB1 | NM_001135558.1 | tggcaagtgtggcaatgg/gaatttgagcgagctgctgaat |
| SELENOF | NM_001012926.2 | acttggcttctccagtaacttgct/gcctacagaatggatccaactga |
| SELENOI | NM_001031528.2 | tgccagcctctgaactggat/tgcaaacccagacatcaccat |
| SELENOK | NM_001025441.2 | gaagagggcctccaggaaat/cagccattggtggtggactag |
| SELENOM | NM_001277859.1 | acatcccgctgtaccataacc/ttctcctcccgggtcatgtc |
| SELENON | NM_001114972.1 | caggatccatgctgagttcca/gagaggacgatgtaacccgtaaac |
| SELENOO | NM_001115017.1 | ccagcgttaaccggaatgat/atgcgcctcctggatttct |
| SELENOP | NM_001031609.2 | ccaagtggtcagcattcacatc/atgacgaccaccctcacgat |
| SELENOS | NM_001024734.2 | ccgacatggtggtaagaagaca/gcttgtgcattcaactcctcttg |
| SELENOT | NM_001006557.3 | aggagtacatgcgggtcatca/gacagacaggaaggatgctatgtg |
| SELENOU | NM_001193518.1 | ttggagcatcgtgagaaagaatt/cggcagcttcaaggacaga |
| SELENOW | NM_001166327.1 | tggtgtgggtctgctttacg/ccaaagctggaaggtgcaa |
| Housekeeping genes | ||
| β-Actin | NM_205518.1 | acctgagcgcaagtactctgtct/catcgtactcctgcttgctgat |
| GAPDH | NM_204305.1 | ttggcattgtggagggtctt/gggccatccaccgtcttc |
Abbreviations: DIO, iodothyronine deiodinase; FABP, fatty acid-binding protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenases; GPX1, glutathione peroxidase 1; LPL, lipoprotein lipase; MSRB1, methionine sulfoxide reductase B1; PPAR, peroxisome proliferator-activated recepto; SELENO, selenoprotein; TXNRD, thioredoxin reductaser.
Statistical Analysis
All data were expressed as the mean ± standard deviation (SD). The statistical power of 0.80 (80%) was obtained in this study when the minimally detectable effect size was 1.0 and the significance level was 0.05. Data were checked for normal distribution and equal variance using the Shapiro-Wilk and Levene's tests, respectively. For all variables, except antioxidant biomarkers, normal distribution and unequal variances were confirmed. Subsequently a one-way ANOVA with Tukey's post hoc test was performed to clarify the effect of dietary Se levels using SAS statistical software (version 9.2, SAS Institute, Cary, NC). In addition, polynomial contrasts and the linearity of response to analyzed dietary Se level were examined using linear and quadratic regression. In this study, broken-line regression analysis was used to estimate the recommended level of dietary Se supplementation using the nonlinear regression (NLIN) procedure of SAS (SAS Institute). Differences were considered significant when P < 0.05.
RESULTS
Dietary Se Supplementation Increased Tissue Se Concentration, but Did Not Change Growth Performance
Data of growth performance show that BW, ADG, ADFI, and feed conversion expressed as F: G did not differ significantly among treatments (P > 0.05; Table 4). Accordingly, there were no differences in breast muscle rate response to dietary Se treatment (Table 4).
Table 4.
Effect of dietary graded Se supplementation on growth performance and breast yield of broilers.
| Dietary supplemented Se level, mg/kg |
P-value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | 0.0 | 0.2 | 0.4 | 0.6 | 0.8 | SEM | ANOVA | Linear | Quadratic |
| BW, g/bird | |||||||||
| 1 d | 32.8 | 33.3 | 33.6 | 33.5 | 33.0 | 0.22 | 0.120 | 0.520 | 0.226 |
| 21 d | 373.4 | 382.0 | 378.2 | 383.7 | 367.7 | 7.38 | 0.249 | 0.594 | 0.059 |
| 63 d | 2007.1 | 2067.2 | 2005.1 | 2008.9 | 1985.9 | 27.86 | 0.336 | 0.270 | 0.355 |
| ADG, g/d/bird | |||||||||
| 1–21 d | 16.2 | 16.6 | 16.4 | 16.7 | 15.9 | 0.25 | 0.274 | 0.570 | 0.163 |
| 22–63 d | 38.9 | 40.1 | 38.7 | 38.7 | 38.5 | 0.60 | 0.363 | 0.269 | 0.452 |
| 1–63 d | 31.3 | 32.3 | 31.3 | 31.4 | 31.0 | 0.44 | 0.337 | 0.267 | 0.359 |
| ADFI, g/d/bird | |||||||||
| 1–21 d | 28.9 | 29.2 | 29.5 | 29.3 | 28.5 | 0.45 | 0.623 | 0.602 | 0.276 |
| 22–63 d | 100.8 | 103.2 | 101.1 | 99.9 | 100.0 | 1.37 | 0.479 | 0.274 | 0.434 |
| 1–63 d | 76.8 | 78.5 | 77.2 | 76.4 | 76.2 | 1.01 | 0.510 | 0.282 | 0.389 |
| F: G, g: g | |||||||||
| 1–21 d | 1.78 | 1.76 | 1.79 | 1.76 | 1.79 | 0.010 | 0.052 | 0.863 | 0.677 |
| 22–63 d | 2.59 | 2.57 | 2.61 | 2.58 | 2.60 | 0.018 | 0.714 | 0.752 | 0.952 |
| 1–63 d | 2.45 | 2.43 | 2.47 | 2.44 | 2.46 | 0.015 | 0.495 | 0.786 | 0.935 |
| Breast yield % | |||||||||
| 63 d | 11.01 | 10.80 | 10.42 | 10.87 | 10.32 | 0.16 | 0.617 | 0.246 | 0.514 |
Abbreviations: ADG, average daily gain; ADFI, average daily feed intake; BW, body weight; F: G, the ratio of feed intake to gain.
As illustrated in Figure 1, the results of Se content indicated that dietary Se supplementation linearly (R2 = 0.804) and quadratically (R2 = 0.875) increased the Se contents in serum (both P < 0.001), and also linearly (R2 = 0.970) and quadratically (R2 = 0.973) facilitated Se deposition in breast muscle (both P < 0.001).
Figure 1.
Se content in serum (A) and breast muscle (B) responses to dietary graded Se supplementation in broilers. a-e Mean values with unlike letters were significantly different among diets with 0.0, 0.2, 0.4, 0.6, and 0.8 mg/kg of Se (one-way ANOVA, P < 0.05, Tukey's post hoc test).
Dietary Se Supplementation Enhanced Oxidation Resistance of Meat
Direct assessment of TBARS and antioxidase suggested that TBARS levels were linearly (R2 = 0.688; P < 0.01) and quadratically (R2 = 0.821; P < 0.05) decreased as the level of dietary Se increased. Lower TBARS content was observed in the birds receiving 0.2 to 0.8 mg/kg of Se compared with the Control group (Table 5). Considering the oxidation resistance of breast muscle, a linear (R2 = 0.769) and quadratic (R2 = 0.796) responses were observed in GSH-Px to dietary Se supplementation (P < 0.01). Similarly, dietary Se manipulation also linearly (R2 = 0.579) and quadratically (R2 = 0.992) increased T-AOC activity (P < 0.01). The birds supplemented with Se 0.6 and 0.8 mg/kg showed increased GSH-Px activity compared with those receiving Se 0.0, 0.2 and 0.4 mg/kg, but only significantly higher T-AOC activity was observed in the birds receiving Se 0.8 mg/kg compared with the other 4 treatments. Dietary Se supplementation did not affect T-SOD activity among the 5 treatments (Table 5).
Table 5.
Effect of dietary graded Se supplementation on meat antioxidant biomarkers of birds.
| Dietary supplemented Se level, mg/kg |
P-value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | 0.0 | 0.2 | 0.4 | 0.6 | 0.8 | SEM | ANOVA | Linear | Quadratic |
| TBARS | 0.22a | 0.13b | 0.15b | 0.10b | 0.11b | 0.02 | 0.002 | <0.001 | <0.001 |
| GSH-Px | 23.49c | 33.47c | 37.99bc | 65.03a | 53.55ab | 3.86 | 0.037 | <0.001 | <0.001 |
| T-AOC | 0.08b | 0.06b | 0.06b | 0.10b | 0.19a | 0.01 | 0.042 | 0.002 | <0.001 |
| T-SOD | 56.29 | 58.15 | 56.51 | 67.61 | 50.45 | 2.86 | 0.671 | 0.914 | 0.576 |
Abbreviations: GSH-Px, glutathione peroxidase; T-AOC, total antioxidant capacity; TBARS, thiobarbituric acid reactive substances; T-SOD, total superoxide dismutase. a-c Mean values with unlike letters were significantly different among diets with 0.0, 0.2, 0.4, 0.6, and 0.8 mg/kg of Se (one-way ANOVA, P < 0.05, Tukey's post hoc test).
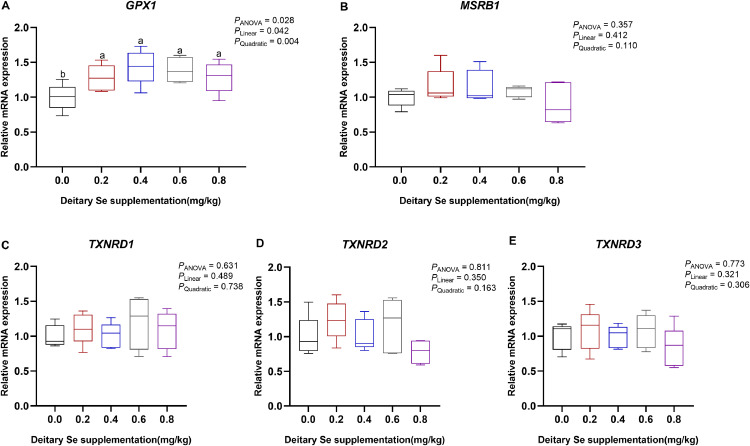
Next, we examined if dietary Se treatment could alter the transcription level of well-known antioxidase, and the results are presented in Figure 2. Dietary Se supplementation had significant linear and quadratic effects on the mRNA level of GPX1 in breast muscle. The birds receiving Se 0.4 to 0.8 mg/kg showed increased GPX1 mRNA abundance compared with the control (Figure 2A). No significant differences (P > 0.05) were found in the expression of Methionine-R-sulfoxide reductase B1 (MSRB1), Thioredoxin reductase 1 (TXNRD1), TXNRD2, and TXNRD3 in breast muscle across all the treatments on 63 d (Figures 2B–2E).
Figure 2.
Effect of dietary Se supplementation on (A) glutathione peroxidase (GPX1), (B) methionine sulfoxide reductase B1(MSRB1), and (C–E) thioredoxin reductase (TXNRD). a-c Mean values with unlike letters were significantly different among diets with 0.0, 0.2, 0.4, 0.6, and 0.8 mg/kg of Se (one-way ANOVA, P < 0.05, Tukey's post hoc test).
Dietary Se Addition Improved Meat Quality of Breast Muscle
Concerning meat quality of breast muscle, there were no differences in meat color, cooking loss, and pH45min due to treatment (P > 0.05; Table 6). However, dietary Se administration linearly (R2 = 0.718) and quadratically (R2 = 0.884) decreased drip loss, that is, the birds receiving Se 0.4, 0.6, and 0.8 mg/kg treatments showed lower drip loss in breast muscle after slaughtering compared to birds from Se 0.0 and 0.2 mg/kg (P < 0.05). In addition, supplementing different levels of dietary Se had a linear (R2 = 0.744) and quadratic (R2 = 0.705) effect on the pH24h value in muscle (P < 0.001; Table 6). The birds receiving Se 0.6 and 0.8 mg/kg exhibited increased pH24h value of breast muscle compared with those consuming Se 0.0, 0.2, and 0.4 mg/kg. With regard to the tenderness of breast muscle, a linear response (R2 = 0.854; P = 0.002) was observed in shear force. The birds receiving Se 0.8 mg/kg showed remarkably lower shear force compared with the control, but no differences were observed among those fed on Se 0.2, 0.4, 0.6, and 0.8 mg/kg (Table 6).
Table 6.
Effects of dietary graded Se supplementation on meat color, water-holding capacity, pH value, and shear force in broilers.
| Dietary supplemented Se level, mg/kg |
P-value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | 0 | 0.2 | 0.4 | 0.6 | 0.8 | SEM | ANOVA | Linear | Quadratic |
| Color | |||||||||
| L* | 39.82 | 39.52 | 39.2 | 38.72 | 38.92 | 0.31 | 0.392 | 0.235 | 0.476 |
| a* | 2.77 | 2.67 | 2.56 | 2.84 | 2.55 | 0.16 | 0.596 | 0.810 | 0.927 |
| b* | 5.70 | 6.98 | 6.91 | 6.08 | 6.08 | 0.22 | 0.266 | 0.932 | 0.179 |
| Water-holding capacity | |||||||||
| Drip loss, % | 1.54a | 1.63ab | 1.48b | 1.46b | 1.3b | 0.06 | 0.023 | 0.002 | 0.003 |
| Cooking loss, % | 25.18 | 23.34 | 24.37 | 25.28 | 23.55 | 0.74 | 0.894 | 0.804 | 0.970 |
| pH | |||||||||
| pH45min | 6.27 | 6.57 | 6.44 | 6.58 | 6.51 | 0.04 | 0.091 | 0.118 | 0.204 |
| pH24h | 5.73b | 5.86b | 5.79b | 6.38a | 6.30a | 0.06 | 0.018 | <0.001 | <0.001 |
| Shear force, kg | 2.49a | 2.19ab | 2.22ab | 2.09ab | 1.67b | 0.11 | 0.038 | 0.002 | 0.071 |
Mean values with unlike letters were significantly different among diets with 0.0, 0.2, 0.4, 0.6 and 0.8 mg/kg of Se (one-way ANOVA, P < 0.05, Tukey’s post hoc test).
Dietary Se Administration is Not Associated With Intramuscular Fat Deposition
To evaluate intramuscular fat deposition in breast muscle that may be induced by dietary Se, lipoprotein lipase (LPL) and a few key regulatory factors that play critical roles in controlling lipid metabolism were examined using RT-PCR. As shown in Figure 3, no significant differences (P > 0.05) were found in fatty acid-binding proteins (FABP), peroxisome proliferator-activated receptor (PPAR) and adenosine monophosphate-activated protein kinase γ3 (PRKAG3) in breast muscle across all the treatments (Figures 3A–3C). Furthermore, dietary Se supplementation did not apparently change (P > 0.05) the transcription of LPL in the meat sample (Figure 3D).
Figure 3.
Effect of dietary Se supplementation on intramuscular fat deposition of breast muscle. (A) Fatty acid-binding proteins (FABP), (B, C) peroxisome proliferator-activated receptor (PPAR), (D) lipoprotein lipase (LPL), and (E) adenosine monophosphate-activated protein kinase γ3 (PRKAG3). Data were analyzed to a one-way ANOVA with the Tukey's post hoc test. Differences were considered significant when P < 0.05.
Analysis of Selenoprotein in Breast Muscle
The close relationship between Se status and selenoprotein prompted us to examine the alteration of selenoprotein encoding genes which could have a potential effect on meat quality. Here, dietary Se linearly and quadratically upregulated expression of iodothyronine deiodinase 2 (DIO2) and DIO3, and diets with Se 0.4 to 0.8 mg/kg or 0.6 to 0.8 mg/kg exhibited a higher mRNA abundance in terms of DIO2 and DIO3 relative to the control diet, respectively (Figures 4A–4C). Focused on the expression of other 11 selenoprotein encoding genes in breast muscle of the birds, a linear and quadratic downregulation was observed in selenoprotein I (SELENOI), SELENOK, SELENOS, and SELENOT response to the graded Se levels. However, both SELENOU and SELENOW mRNA levels were linearly and quadratically increased as the level of dietary Se increased. Notably, the Se supplementation had significant quadratic effects on the mRNA level of SELENON and SELENOO, and the birds supplemented with Se 0.4 mg/kg and 0.2 mg/kg showed higher SELENON and SELENOO abundance compared with the control group (Figure 4D).
Figure 4.
(A-C) Iodothyronine deiodinase (DIO) and (D) Selenoprotein (SELENO) expression in breast muscle responses to dietary graded Se supplementation in broilers. a-c Mean values with unlike letters were significantly different among diets with 0.0, 0.2, 0.4, 0.6 and 0.8 mg/kg of Se (one-way ANOVA, P < 0.05, Tukey's post hoc test).
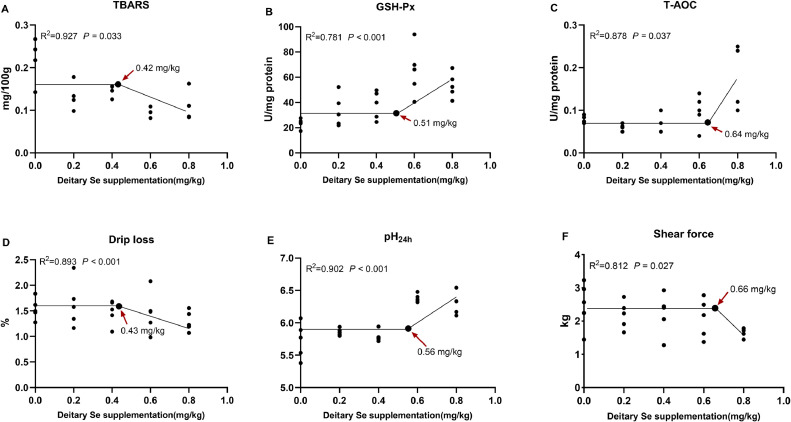
The Recommended Level of Dietary Se Based on Broken-Line Analysis
The recommended levels of Se using one-slope broken-line analysis are shown in Figure 5. From the perspective of antioxidant biomarkers, TBARS level and the activities of both GSH-Px and T-AOC of breast meat were decreased and increased with dietary Se supplementation, individually, where the recommended level of dietary Se based on TBARS, GSH-Px, and T-AOC were 0.42, 0.51, and 0.64 mg/kg, respectively (Figures 5A–5C). As far as meat quality is concerned, the broken-line analysis revealed that the recommended level of dietary Se were 0.43, 0.56, and 0.66 mg/kg based on drip loss, pH24h value, and shear force, respectively (Figures 5D–5F).
Figure 5.
The recommended level of dietary Se based on Broken-line analysis of (A) thiobarbituric acid reactive substances (TBARS), (B) glutathione peroxidase (GSH-Px), (C) total antioxidant (T-AOC), (D) drip loss, (E) pH24h value, and (F) shear force.
DISCUSSION
In the present study, our results revealed that supplementing Se 0.2 to 0.8 mg/kg in the form of OH-SeMet had no effect on the growth performance of the native birds. This finding was consistent with numerous other studies on lambs (Vignola et al., 2009), veal (Skrivanova et al., 2007), rodent (Zeng et al., 2012), and pigs (Zhou et al., 2009 and Liu et al., 2012). Chicken is more sensitive to Se status than mammalian and the broiler fed with supranutritional Se (3.0 mg Se/kg in form of selenite) has lower feed intake and body weight gain (Huang et al., 2016) and depresses immunity (Tang et al., 2017). Dietary Se deficiency (<0.02–0.03 mg/kg) depresses the growth performance of birds and causes nutritional pancreatic atrophy (Xu et al., 2017). Accordingly, the Se content in the basal diet (0.09–0.15 mg/kg) appeared to be sufficient to maintain normal growth of the birds in this study. These observations are consistent with those reported by Oliveira et al. (2014), who found dietary supplementation with 0.15 to 0.60 mg/kg of Se not noticeably enhanced the performance of the broilers. Of note, we can explain the reasons why the diet with 0.80 mg/kg Se failed to affect the performance. Contrast to our outcomes, a few early reports that Se supplementation resulted in increased growth performance in chickens (Dlouhá et al., 2008; Skřivan et al., 2011), possibly due to increased protein digestibility and energy utilization (Saleh, 2014). The discrepancies may result from differences in the nutritional status of Se and vitamin E at hatch, the dosages and chemical forms and/or the chicken breeds used.
Meat is an important natural Se source for human nutrition. Early studies have revealed that organic Se had a higher deposition in muscle than inorganic Se (Choct et al., 2004; Payne and Southern, 2005). Surai (2006) pointed out only SeMet can be effectively transferred from diets to the muscles. Briens et al. (2013) demonstrated the new molecule OH-SeMet showed significantly higher efficiency than Se-yeast for muscle Se enrichment. Analogously, the previous results showed that OH-SeMet effectively enhanced Se deposition in the breast muscle and exhibited a linear dose response. Inclusion with Se 0.2 and 0.4 mg/kg would produce chicken meat enriched with Se (200–500 ng/g), which could contribute to enhanced selenoprotein expression, as reflected by the increases in both GSH-PX activity and GPX1 mRNA level (Finley, 2006; Vignola et al., 2009). Baltić et al. (2015) reported a significant relationship between GSH-Px activity and Se concentration in plasma in ducks. Emerging evidences found that serum GSH-Px activity was increased with increasing supplemental Se in pigs (Adkins and Ewan, 1984), ducks (Baltić et al., 2015), and broilers (Cai et al., 2012; Markovic et al., 2018). In this study, dietary Se supplementation linearly and quadratically increased GSH-Px activity and GPX1 mRNA abundance in breast muscle, and the total antioxidant capacity, with greater T-AOC and lower TBARS concentration. Our findings support Cai et al. (2012) who reported increases in serum total antioxidant capacity along with decreased content of malondialdehyde (a product of lipid peroxidation) in both serum and liver, following dietary Se (Nano-form) increase from 0.0 to 2.0 mg/kg. Likewise, Li et al. (2011) found dietary Se levels (0.3 vs. 3.0 mg/kg in form of Se-yeast) contributed to higher activities of GSH-Px in the muscle and the lower content of TBARS in liver and muscle in pigs. Moreover, it has been recognized that the selenoprotein enzymes related to antioxidant functions include MSRB1 and TXNRD, responsible for reducing oxidized methionine (R)-sulfoxide back to methionine (Fomenko et al., 2009) and regenerating reduced thioredoxin (Su et al., 2005), respectively. In the present study, however, no association was observed between dietary Se level and the mRNA expression of MSRB1 and TXNRDs, further studies are needed to understand their link. pH value is an important parameter for meat quality, which has a positive correlation with water holding capacity, redness, and tenderness (Le Bihan-Duval et al., 2008) and a negative correlation with the lightness (Zhang et al., 2012) and drip loss (Wang et al., 2009). Our results showed supplementation with the Se tended to increase the pH45min, and Se addition promotes the pH24h value in the breast muscles with age, in agreement with report by Markovic et al. (2018) for broiler chickens and Li et al. (2011) for the longissimus dorsi muscles of pigs. However, other studies indicate that the Se levels have no effects on pH24h value in the breast or thigh meats of broiler chickens (Oliveira et al., 2014 ). Previous studies in broilers showed that after slaughtering, oxidative stress, especially excessive hydrogen peroxide accumulation, speeds up pH drop (Zhang et al., 2011; Chen et al., 2017). Thus, a higher ultimate pH value (pH24h) in the breast muscle may be related to the strong antioxidant effect, increasing dietary Se could reduce lactic acid production by attenuating the oxidative stress to prevent the induction of low pH, as muscle glycogen level at slaughter largely determines the ultimate pH (Le Bihan-Duval et al., 2008).
Our study found the Se supplementation promoted water holding capacity of the beast muscle, which is in line with the findings of Cai et al. (2012) on Nano-Se for broiler, Li et al (2011) for pigs, and Bakhshalinejad et al. (2019) who tested selenomethionine. The mechanism can be attributed to the capacity of selenoprotein enzymes to stabilize membrane integrity postmortem, and/or to retard proteolysis and even protein oxidation through increased antioxidant capacity (Wang et al., 2021 ). In addition, meat color as an essential quality attribute for consumers is closely related to pH, nitrites, water-holding capacity, and especially myoglobin oxidation (King and Whyte, 2006). Some studies have shown that increasing Se supplementation has a beneficial effect on meat color of broiler (Bakhshalinejad et al., 2019 ) and finishing pig (Chen et al., 2019a), and further speculated that the positive role of Se manipulation was associated with enhanced oxidation resistance, by preventing myoglobin or oxymyoglobin from being oxidized to metmyoglobin, consequently deepen the muscle chroma and improve meat color (Oliveira et al., 2014). However, our results demonstrated Se supplementation enhanced the antioxidant capacity, but did not change the color of breast muscles, suggesting that, apart from antioxidant function, other potential mechanisms may exist on Se modifying meat color. Among them, a candidate is Se supplementation might contribute to nitrite degradation, and thus may have potential influence on meat color (Chen et al., 2019b).
Our results showed Se supplementation decreased the shear force that is an indication of tenderness of the breast muscle, a crucial sensory quality that influences acceptance by consumer (Maltin et al., 1997). Similarly, Chen et al. (2019a) reported that dietary supplementation with Se 0.5 mg/kg to moderately-reduced energy and protein diet tended to decrease shear force of loin muscle of pigs. Baowei et al. (2011) reported supplementing Se 0.3 mg/kg as Se-yeast reduced hardness of breast muscles in goose. A possible explanation, as explained by Gault (1985), is the improved water-holding capacity may benefit tenderness of the cooked meat regardless of the amount of connective tissue in the muscles. According to Carne et al. (2014), tenderness is also determined by the structural properties of various proteins and fats in the muscle. Moseti et al. (2016) considered the PPAR-β and PPAR-γ are the key early regulators of adipogenesis, while FABP and LPL are responsible for the formation of mature adipocytes and the hydrolysis, respectively. Vedran Bertić et al. (2013) explains PRKAG3 gene encodes a muscle-specific isoform of the adenosine monophosphate-activated protein kinase gamma subunit, and is closely related to meat quality. However, comparable transcription abundance of FABP, PPAR-β, PPAR-γ, PRKAG3, and LPL of breast muscle indicated that intramuscular fat deposition might not be predominantly contributor to dietary Se favorited tenderness of breast muscle in the present study. In addition, tenderness also depends on myofibrillar protein degradation implicated by the calpain family (µ-calpain, m-calpain, and calpain3) and a specific inhibitor, the calpastatin (Morton et al., 1999). Wang et al. (2020) reported dietary Se could interfere with the expression of calpain and calpastatin in rainbow trout, suggesting that the calpain family may change the tenderness of breast muscle due to Se treatment.
To unravel the interference of melanoproteins with the meat quality, the response of selenoprotein encoding genes to dietary Se supplementation was further examined in breast muscle. Of the 11 selenoprotein genes response to Se levels, SELENON that encoded by the gene SELENON mutation resulted in rigid spine muscular dystrophy in humans (Ferreiro et al., 2002). SELENOU and SELENOW can protect cells from oxidative stress (Jiang et al., 2015; Loflin et al., 2006), and the increasing expression of SELENOW is associated with enhancing the water-holding capacity of meat (Li et al., 2011) and improving meat quality (Zhang et al., 2020). Therefore, the upregulation of SELENON, SELENOU and SELENOW may contribute to enhance the meat quality of yellow feather broilers. Furthermore, we observed a pronounced upregulation for DIO2 and DIO3 transcription response to increasing dietary Se level, both are responsible for thyroid hormone regulation and consequently enhancing metabolism (Darras and Herck, 2012). Of note, we also noticed the downregulation in the mRNA level of SELENOK, SELENOS, and SELENOT with the supplementation of Se, suggesting that oxidation and/or immunological stress may be eased by dietary Se as the proteins encoded by these genes localized to the endoplasmic reticulum and involved in the inflammatory response (Verma et al., 2011; Ye et al., 2004). Collectively, it is reasonable to assume that higher DIOs expression along with a lower transcription of SELENOK, SELENOS, and SELENOT might be a positive effect in physical well-being of broiler. In addition, although SELENOI is crucial for the phospholipid biosynthesis (Horibata and Hirabayashi, 2007), and decreased SELENOI expression indicates they probably do not influence meat quality.
A limitation of the study was that the statistical assessment. Unequal variances and non-normal distribution were observed in antioxidant biomarkers, which contribute to an insufficient number of samples. Therefore, we admit the possibility that some of our conclusions may include overestimation or underestimation of roles of dietary Se in meat quality, oxidation resistance, and selenoprotein expression.
CONCLUSIONS
In conclusion, similar to the fast-growing broiler, this study confirmed dietary supplementation with the chemically synthesized organic Se source (OH-SeMet) effectively increased Se deposition in the breast muscles and improved meat quality of the native slow-growing yellow feather broilers. Specifically, the dietary graded Se addition increased the pH24h, and decreased the drip loss and shear force of the breast muscles, the possible mechanism appears to be the increased Se adsorption and deposition that enhances selenoprotein synthesis and consequently enhanced antioxidant capacity. The study results add evidence to use OH-SeMet as a nutrition strategy to improve meat quality for the native broiler production. Most important, the recommend supplemental dosage of OH-SeMet for the birds is between 0.42 and 0.66 mg Se/kg based on antioxidant biomarkers and meat quality.
Acknowledgments
ACKNOWLEDGMENTS
This work was supported partly by the National Natural Science Foundation of China (No. 31272468), the Special Research Funding for Discipline Construction inSichuan Agricultural University (No. 03570126), and the Sichuan Longda Animal Husbandry Science and Technology Co., Ltd (No. 2015SCLD001)
Author contributions: Hua Zhao and Jiayong Tang designed the research; Jiayong Tang and Zhen He conducted the experiments; Gang Jia, Guangmang Liu, Xiaoling Chen, Gang Tian, Jingyi Cai, Bo Kang and Hua Zhao collected sample and analyzed the data; Hua Zhao, Yonggang Liu and Jiayong Tang wrote the paper; and Hua Zhao had primary responsibility for the final content. All authors read and approved the final manuscript.
DISCLOSURES
The authors declare that there is no conflict of interest, financial or otherwise.
REFERENCES
- Adkins R.S., Ewan R.C. Effect of selenium on performance, serum selenium concentration and glutathione peroxidase activity in pigs. J. Anim. Sci. 1984;58:346–350. doi: 10.2527/jas1984.582346x. [DOI] [PubMed] [Google Scholar]
- Asghar A., Gray J.I., Miller E.R., Ku P.K., Booren A.M. Influence of supranutritional vitamin E supplementation in the feed on swine growth performance and deposition in different tissues. J. Sci. Food Agric. 1991;57:19–29. [Google Scholar]
- Bakhshalinejad R., Hassanabadi A., Swick R.A. Dietary sources and levels of selenium supplements affect growth performance, carcass yield, meat quality and tissue selenium deposition in broilers. Anim. Nutr. 2019;5:256–263. doi: 10.1016/j.aninu.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltić M.Ž., Dokmanović Starčević M., Bašić M., Zenunović A., Ivanović J., Marković R., Janjić J., Mahmutović H. Effects of selenium yeast level in diet on carcass and meat quality, tissue selenium distribution and glutathione peroxidase activity in ducks. Anim. Feed Sci. Tech. 2015;210:225–233. [Google Scholar]
- Baowei W., Guoqing H., Qiaoli W., Bin Y. Effects of yeast selenium supplementation on the growth performance, meat quality, immunity, and antioxidant capacity of goose. J. Anim. Physiol. Anim. Nutr. (Berl.) 2011;95:440–448. doi: 10.1111/j.1439-0396.2010.01070.x. [DOI] [PubMed] [Google Scholar]
- Briens M., Mercier Y., Rouffineau F., Vacchina V., Geraert P.A. Comparative study of a new organic selenium source vs. seleno-yeast and mineral selenium sources on muscle selenium enrichment and selenium digestibility in broiler chickens. Br. J. Nutr. 2013;110:617–624. doi: 10.1017/S0007114512005545. [DOI] [PubMed] [Google Scholar]
- Cai S.J., Wu C.X., Gong L.M., Song T., Wu H., Zhang L.Y. Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult. Sci. 2012;91:2532–2539. doi: 10.3382/ps.2012-02160. [DOI] [PubMed] [Google Scholar]
- Carne A., Bekhit A., Carne A., Ha M., Franks P. Physical interventions to manipulate texture and tenderness of fresh meat: a review. Int. J. Food Prop. 2014;17:433–453. [Google Scholar]
- Chen J., Tian M., Guan W., Wen T., Yang F., Chen F., Zhang S., Song J., Ren C., Zhang Y., Song H. Increasing selenium supplementation to a moderately-reduced energy and protein diet improves antioxidant status and meat quality without affecting growth performance in finishing pigs. J. Trace Elem. Med. Biol. 2019;56:38–45. doi: 10.1016/j.jtemb.2019.07.004. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang L., Li J., Gao F., Zhou G. Hydrogen peroxide-induced change in meat quality of the breast muscle of broilers is mediated by ROS generation, apoptosis, and autophagy in the NF-κB signal pathway. J. Agr. Food Chem. 2017;65:3986–3994. doi: 10.1021/acs.jafc.7b01267. [DOI] [PubMed] [Google Scholar]
- Chen Y., Li Q., Xia C., Yang F., Xu N., Wu Q., Hu Y., Xia L., Wang C., Zhou M. Effect of selenium supplements on the antioxidant activity and nitrite degradation of lactic acid bacteria. World J. Microbiol. Biotechnol. 2019;35:61. doi: 10.1007/s11274-019-2609-x. [DOI] [PubMed] [Google Scholar]
- Choct M., Naylor A.J., Reinke N. Selenium supplementation affects broiler growth performance, meat yield and feather coverage. Br. Poult. Sci. 2004;45:677–683. doi: 10.1080/00071660400006495. [DOI] [PubMed] [Google Scholar]
- Darras V.M., Herck S.L.V. Iodothyronine deiodinase structure and function: from ascidians to humans. J. Endocrinol. 2012;215:189–206. doi: 10.1530/JOE-12-0204. [DOI] [PubMed] [Google Scholar]
- Dlouhá G., Ševčíková S., Dokoupilová A., Zita L., Heindl J., Skřivan M. Effect of dietary selenium sources on growth performance, breast muscle selenium, glutathione peroxidase activity and oxidative stability in broilers. Czech J. Anim. Sci. 2008;53:265–269. [Google Scholar]
- Ferreiro A., Quijano-Roy S., Pichereau C., Moghadaszadeh B., Goemans N., Bönnemann C., Jungbluth H., Straub V., Villanova M., Leroy J.P., Romero N.B., Martin J.J., Muntoni F., Voit T., Estournet B., Richard P., Fardeau M., Guicheney P. Mutations of the selenoprotein N gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multiminicore disease: reassessing the nosology of early-onset myopathies. Am. J. Hum. Genet. 2002;71:739–749. doi: 10.1086/342719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley J.W. Bioavailability of selenium from foods. Nutr. Rev. 2006;64:146–151. doi: 10.1111/j.1753-4887.2006.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Fomenko D.E., Novoselov S.V., Natarajan S.K., Lee B.C., Koc A., Carlson B.A., Lee T.H., Kim H.Y., Hatfield D.L., Gladyshev V.N. MsrB1 (methionine-R-sulfoxide reductase 1) knock-out mice: roles of MsrB1 in redox regulation and identification of a novel selenoprotein form. J. Biol. Chem. 2009;284:5986–5993. doi: 10.1074/jbc.M805770200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault N.F. The relationship between water-holding capacity and cooked meat tenderness in some beef muscles as influenced by acidic conditions below the ultimate pH. Meat Sci. 1985;15:15–30. doi: 10.1016/0309-1740(85)90071-3. [DOI] [PubMed] [Google Scholar]
- GB/T . China Agricultural Industry Standards; Beijing, China: 2008. Meat and Meat Products-Measurement of pH (GB/T 9695.5-2008) [Google Scholar]
- Hardy G., Hardy I. Selenium: the Se-XY nutraceutical. Nutrition (Burbank, Los Angeles County, Calif.) 2004;20:590–593. doi: 10.1016/j.nut.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Horibata Y., Hirabayashi Y. Identification and characterization of human ethanolaminephosphotransferase1. J. Lipid Res. 2007;48:503–508. doi: 10.1194/jlr.C600019-JLR200. [DOI] [PubMed] [Google Scholar]
- Huang X., Tang J., Xu J., Jia G., Liu G., Chen X., Cai J., Shang H., Zhao H. Supranutritional dietary selenium induced hyperinsulinemia and dyslipidemia via affected expression of selenoprotein genes and insulin signal-related genes in broiler. RSC Adv. 2016;6:84990–84998. [Google Scholar]
- Huff-Lonergan E., Lonergan S.M. Mechanisms of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Sci. 2005;71:194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Jiang Y.Y., Huang J.Q., Lin G.C., Guo H.Y., Ren F.Z., Zhang H. Characterization and expression of chicken selenoprotein U. Biol. Trace Elem. Res. 2015;166:216–224. doi: 10.1007/s12011-015-0257-z. 166. [DOI] [PubMed] [Google Scholar]
- Jlali M., Briens M., Rouffineau F., Mercerand F., Geraert P.A., Mercier Y. Effect of 2-hydroxy-4-methylselenobutanoic acid as a dietary selenium supplement to improve the selenium concentration of table eggs. J. Anim. Sci. 2013;91:1745–1752. doi: 10.2527/jas.2012-5825. [DOI] [PubMed] [Google Scholar]
- Jlali M., Briens M., Rouffineau F., Geraert P.A., Mercier Y. Evaluation of the efficacy of 2-hydroxy-4-methylselenobutanoic acid on growth performance and tissue selenium retention in growing pigs. J. Anim. Sci. 2014;92:182–188. doi: 10.2527/jas.2013-6783. [DOI] [PubMed] [Google Scholar]
- King N., Whyte R. Does it look cooked? A review of factors that influence cooked meat color. J. Food Sci. 2006;71:R31–R40. [Google Scholar]
- Kryukov G.V., Castellano S., Novoselov S.V., Lobanov A.V., Zehtab O., Guigó R., Gladyshev V.N. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- Le Bihan-Duval E., Debut M., Berri C.M., Sellier N., Santé-Lhoutellier V., Jégo Y., Beaumont C. Chicken meat quality: genetic variability and relationship with growth and muscle characteristics. BMC Genetics. 2008;9:53. doi: 10.1186/1471-2156-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.G., Zhou J.C., Zhao H., Lei X.G., Xia X.J., Gao G., Wang K.N. Enhanced water-holding capacity of meat was associated with increased Sepw1 gene expression in pigs fed selenium-enriched yeast. Meat Sci. 2011;87:95–100. doi: 10.1016/j.meatsci.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhao H., Zhang Q., Tang J., Li K., Xia X.J., Wang K.N., Li K., Lei X.G. Prolonged dietary selenium deficiency or excess does not globally affect selenoprotein gene expression and/or protein production in various tissues of pigs. J. Nutr. 2012;142:1410–1416. doi: 10.3945/jn.112.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loflin J., Lopez N., Whanger P.D., Kioussi C. Selenoprotein W during development and oxidative stress. J. Inorg. Biochem. 2006;100:1679–1684. doi: 10.1016/j.jinorgbio.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Mahan D.C., Azain M., Crenshaw T.D., Cromwell G.L., Dove C.R., Kim S.W., Lindemann M.D., Miller P.S., Pettigrew J.E., Stein H.H., van Heugten E. Supplementation of organic and inorganic selenium to diets using grains grown in various regions of the United States with differing natural Se concentrations and fed to grower-finisher swine. J. Anim. Sci. 2014;92:4991–4997. doi: 10.2527/jas.2014-7735. [DOI] [PubMed] [Google Scholar]
- Maltin C.A., Warkup C.C., Matthews K.R., Grant C.M., Porter A.D., Delday M.I. Pig muscle fibre characteristics as a source of variation in eating quality. Meat Sci. 1997;47:237–248. doi: 10.1016/s0309-1740(97)00055-7. [DOI] [PubMed] [Google Scholar]
- Markovic R., Ciric J., Drljacic A., Sefer D., Jovanovic I., Jovanovic D., Milanovic S., Trbovic D., Radulovic S., Baltic M.Z., Starcevic M. The effects of dietary Selenium-yeast level on glutathione peroxidase activity, tissue Selenium content, growth performance, and carcass and meat quality of broilers. Poult. Sci. 2018;97:2861–2870. doi: 10.3382/ps/pey117. [DOI] [PubMed] [Google Scholar]
- Morrissey P.A., Sheehy P.J., Galvin K., Kerry J.P., Buckley D.J. Lipid stability in meat and meat products. Meat Sci. 1998;49s1:S73–S86. [PubMed] [Google Scholar]
- Morton J.D., Bickerstaffe R., Kent M.P., Dransfield E., Keeley G.M. Calpain-calpastatin and toughness in M. longissimus from electrically stimulated lamb and beef carcasses. Meat Sci. 1999;52:71–79. doi: 10.1016/s0309-1740(98)00150-8. [DOI] [PubMed] [Google Scholar]
- Moseti D., Regassa A., Kim W.K. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int. J. Mol. Sci. 2016;17:124. doi: 10.3390/ijms17010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- NY/T 33-2004 . China Agricultural Industry Standards; Beijing, China: 2012. Nutrient Requirement of Broilers. [Google Scholar]
- Oliveira T.F.B., Rivera D.F.R., Mesquita F.R., Braga H., Ramos E.M., Bertechini A.G. Effect of different sources and levels of selenium on performance, meat quality, and tissue characteristics of broilers. J. Appl. Poult. Res. 2014;23:15–22. [Google Scholar]
- Payne R.L., Southern L.L. Comparison of inorganic and organic selenium sources for broilers. Poult. Sci. 2005;84:898–902. doi: 10.1093/ps/84.6.898. [DOI] [PubMed] [Google Scholar]
- Rederstorff M., Krol A., Lescure A. Understanding the importance of selenium and selenoproteins in muscle function. Cell. Mol. Life Sci. 2006;63:52–59. doi: 10.1007/s00018-005-5313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A.A. Effect of dietary mixture of Aspergillus probiotic and selenium nano-particles on growth, nutrient digestibilities, selected blood parameters and muscle fatty acid profile in broiler chickens. Anim. Sci. Pap. Rep. 2014;32:65–79. [Google Scholar]
- Sarkar B., Bhattacharjee S., Daware A., Tribedi P., Krishnani K.K., Minhas P.S. Selenium nanoparticles for stress-resilient fish and livestock. Nanoscale Res. Lett. 2015;10:371. doi: 10.1186/s11671-015-1073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skřivan M., Englmaierová M., Dlouhá G., Bubancová I., Skřivanová V. High dietary concentrations of methionine reduce the selenium content, glutathione peroxidase activity and oxidative stability of chicken meat. Czech J. Anim. Sci. 2011;56:398–405. [Google Scholar]
- Skřivanová E., Marounek M., De Smet S., Raes K. Influence of dietary selenium and vitamin E on quality of veal. Meat Sci. 2007;76:495–500. doi: 10.1016/j.meatsci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Su D., Novoselov S.V., Sun Q.A., Moustafa M.E., Zhou Y., Oko R., Hatfield D.L., Gladyshev V.N. Mammalian selenoprotein thioredoxin-glutathione reductase. Roles in disulfide bond formation and sperm maturation. J. Biol. Chem. 2005;280:26491–26498. doi: 10.1074/jbc.M503638200. [DOI] [PubMed] [Google Scholar]
- Surai P.F. Nottingham University Press; Nottingham, UK: 2006. Selenium Nutrition and Health. [Google Scholar]
- Tang J., Huang X., Wang L., Li Q., Xu J., Jia G., Liu G., Chen X., Shang H., Zhao H. Supranutritional dietary selenium depressed expression of selenoprotein genes in three immune organs of broilers. Anim Sci J. 2017;88:331–338. doi: 10.1111/asj.12645. [DOI] [PubMed] [Google Scholar]
- Vedran Bertić Z.B., Đurkin I., Lukić B., Jovanovac S., Kušec G. PRKAG3 gene and meat quality of hybrid pigs. Agr. Conspectus Sci. 2013;78:159–162. [Google Scholar]
- Verma S., Hoffmann F.W., Kumar M., Huang Z., Roe K., Nguyen-Wu E., Hashimoto A.S., Hoffmann P.R. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J. Immunol. (Baltimore, Md.: 1950) 2011;186:2127–2137. doi: 10.4049/jimmunol.1002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignola G., Lambertini L., Mazzone G., Giammarco M., Tassinari M., Martelli G., Bertin G. Effects of selenium source and level of supplementation on the performance and meat quality of lambs. Meat Sci. 2009;81:678–685. doi: 10.1016/j.meatsci.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Wang J.P., Wan C.P., Zhao S.J., Yang Z.Q., Celi P., Ding X.M., Bai S.P., Zeng Q.F., Mao X.B., Xu S.Y., Zhang K.Y., Li M.X. Differential analysis of gut microbiota and the effect of dietary Enterococcus faecium supplementation in broiler breeders with high or low laying performance. Poult Sci. 2021;100:1109–1119. doi: 10.1016/j.psj.2020.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang D., Li S., Wang L., Yin J., Xu Z., Zhang X. Dietary selenium promotes somatic growth of rainbow trout (Oncorhynchus mykiss) by accelerating the hypertrophic growth of white muscle. Biol. Trace Elem. Res. 2020;199:2000–2011. doi: 10.1007/s12011-020-02282-w. [DOI] [PubMed] [Google Scholar]
- Wang R.R., Pan X.J., Peng Z.Q. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poult. Sci. 2009;88:1078–1084. doi: 10.3382/ps.2008-00094. [DOI] [PubMed] [Google Scholar]
- Xu J.Y., Wang L.Q., Tang J.Y., Jia G., Liu G.M., Chen X.L., Cai J.Y., Shang H.Y., Zhao H. Pancreatic atrophy caused by dietary selenium deficiency induces hypoinsulinemic hyperglycemia via global down-regulation of selenoprotein encoding genes in broilers. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Shibata Y., Yun C., Ron D., Rapoport T.A. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature. 2004;429:841–847. doi: 10.1038/nature02656. [DOI] [PubMed] [Google Scholar]
- Zeng M.S., Li X., Liu Y., Zhao H., Zhou J.C., Li K., Huang J.Q., Sun L.H., Tang J.Y., Xia X.J., Wang K.N., Lei X.G. A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radic. Biol. Med. 2012;52:1335–1342. doi: 10.1016/j.freeradbiomed.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.W., Tang J.Y., Jia G., Tian G., Liu G.M., Chen X.L., Cai J.Y., Kang B., Zhao H. Effect of selenium sources on growth performance, serum and muscle selenium content, antioxidation capacity and meat quality of broilers. Chin. J. Anim. Nutr. 2021;33:2024–2032. [Google Scholar]
- Zhang W., Xiao S., Lee E.J., Ahn D.U. Consumption of oxidized oil increases oxidative stress in broilers and affects the quality of breast meat. J. Agr. Food Chem. 2011;59:969–974. doi: 10.1021/jf102918z. [DOI] [PubMed] [Google Scholar]
- Zhang W., Xiao S., Samaraweera H., Lee E.J., Ahn D.U. Improving functional value of meat products. Meat Sci. 2010;86:15–31. doi: 10.1016/j.meatsci.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Zhang Z.Y., Jia G.Q., Zuo J.J., Zhang Y., Lei J., Ren L., Feng D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012;91:2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]
- Zhang K., Zhao Q.Y., Zhan T.F., Han Y.S., Tang C.H., Zhang J.M. Effect of different selenium sources on growth performance, tissue selenium content, meat quality, and selenoprotein gene expression in finishing pigs. Biol. Trace Elem. Res. 2020;196:463–471. doi: 10.1007/s12011-019-01949-3. [DOI] [PubMed] [Google Scholar]
- Zhou J.C., Zhao H., Li J.G., Xia X.J., Wang K.N., Zhang Y.J., Liu Y., Zhao Y., Lei X.G. Selenoprotein gene expression in thyroid and pituitary of young pigs is not affected by dietary selenium deficiency or excess. J. Nutr. 2009;139:1061–1066. doi: 10.3945/jn.109.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]