Abstract
BACKGROUND
There is substantial evidence that adults with type 1 diabetes have reduced bone mineral density (BMD); however, findings in youth are inconsistent.
PURPOSE
To perform a systematic review and meta-analysis of BMD in youth with type 1 diabetes using multiple modalities: DXA, peripheral quantitative computed tomography (pQCT), and/or quantitative ultrasound (QUS).
DATA SOURCES
PubMed, Embase, Scopus, and Web of Science from 1 January 1990 to 31 December 2020, limited to humans, without language restriction.
STUDY SELECTION
Inclusion criteria were as follows: cross-sectional or cohort studies that included BMD measured by DXA, pQCT, or QUS in youth (aged <20 years) with type 1 diabetes and matched control subjects.
DATA EXTRACTION
We collected data for total body, lumbar spine, and femoral BMD (DXA); tibia, radius, and lumbar spine (pQCT); and phalanx and calcaneum (QUS). Weighted mean difference (WMD) or standardized mean difference was estimated and meta-regression was performed with age, diabetes duration, and HbA1c as covariates.
DATA SYNTHESIS
We identified 1,300 nonduplicate studies; 46 met the inclusion criteria, including 2,617 case and 3,851 control subjects. Mean ± SD age was 12.6 ± 2.3 years. Youth with type 1 diabetes had lower BMD: total body (WMD −0.04 g/cm2, 95% CI −0.06 to −0.02; P = 0.0006), lumbar spine (−0.02 g/cm2, −0.03 to −0.0; P = 0.01), femur (−0.04 g/cm2, −0.05 to −0.03; P < 0.00001), tibial trabecular (−11.32 g/cm3, −17.33 to −5.30; P = 0.0002), radial trabecular (−0.91 g/cm3, −1.55 to −0.27; P = 0.005); phalangeal (−0.32 g/cm3, −0.38 to −0.25; P < 0.00001), and calcaneal (standardized mean difference −0.69 g/cm3, −1.11 to −0.26; P = 0.001). With use of meta-regression, total body BMD was associated with older age (coefficient −0.0063, −0.0095 to −0.0031; P = 0.002) but not with longer diabetes duration or HbA1c.
LIMITATIONS
Meta-analysis was limited by the small number of studies with use of QUS and pQCT and by lack of use of BMD z scores in all studies.
CONCLUSIONS
Bone development is abnormal in youth with type 1 diabetes, assessed by multiple modalities. Routine assessment of BMD should be considered in all youth with type 1 diabetes.
Introduction
Type 1 diabetes is associated with abnormal bone health and increased fracture risk in adults with type 1 diabetes. This was first described in 1927 (1), with more recent studies, including two meta-analyses, demonstrating significantly increased risk of fracture (6 studies, 35,925 adults with type 1 diabetes), lower bone mineral density (BMD) (16 studies, 966 adults with type 1 diabetes), and osteoporosis (2–4).
In contrast, the evidence for an association between type 1 diabetes and lower BMD in children and adolescents with type 1 diabetes is more limited. Evaluation of bone health in youth is hampered by the fact that their bones are still mineralizing, lengthening, and acquiring bone mass, and puberty has a major influence. Bone health may be more vulnerable in those who develop type 1 diabetes earlier in life because childhood and adolescence are critical periods for skeletal development (5). Vascular complications are significantly associated with longer diabetes duration and higher HbA1c, but the relationship of these factors with abnormal BMD is not well established across the life span in type 1 diabetes (6,7).
A systematic review in 2014 (8) demonstrated lower total body BMD (five studies) and femoral BMD (four studies), but not lumbar spine BMD (eight studies), in youth aged <20 years with type 1 diabetes. Only raw BMD values from use of DXA were reported, whereas z scores are more appropriate in youth to account for age and pubertal status. Moreover, the review did not include studies using other measures of BMD, such as peripheral quantitative computed tomography (pQCT), which measures trabecular and cortical bone, thereby providing additional information regarding bone content. pQCT is safe, with the total effective dose of a single pQCT scan <0.1 μSv, which is less than for DXA (9).
Therefore, we performed a systematic review and meta-analysis of BMD, bone mineral content (BMC), and cross-sectional area (CSA) in youth with type 1 diabetes using multiple modalities, including DXA, pQCT, and quantitative ultrasound (QUS). The latter two methods may be more appropriate for assessing a maturing skeleton, as they are less influenced by bone size (10–12) and use less radiation. We performed subgroup analysis by age and sex and meta-regression for age, diabetes duration, and HbA1c.
Method
Data Sources and Searches
We performed a systematic search using PubMed, Embase, Scopus, and Web of Science (from 1 January 1990 to 31 December 2020) with Medical Subject Headings (MeSH) terms “type 1 diabetes mellitus” AND “bone density or bone health or bone geometry” and limited the results to human studies.
Study Selection
Inclusion criteria were as follows: cross-sectional, cohort, or case-control studies that provided data on BMD, BMC, or CSA, as measured by DXA, pQCT, or QUS, in children and adolescents with type 1 diabetes and healthy control subjects (aged <20 years). Only published studies or abstracts were considered.
Case reports, case series, review articles, qualitative studies, and animal studies were excluded. Publications in all languages were considered. In addition, we supplemented electronic searches by hand searching reference lists of relevant articles and reviews. The titles and abstracts of the studies were reviewed by three authors (P.L., K.N., and M.E.C.). Disagreements about final study inclusion were resolved by consensus.
Data Extraction and Quality Assessment
The following data were extracted for each eligible study and included in the review: author(s), year of publication, title, journal, study location, study design, participant age, number of case and control subjects, diabetes duration, method of BMD measurement, BMD measurement sites, mean ± SD of BMD measurements in case and control subjects, z score for age and sex, BMC, volumetric BMD (vBMD), CSA, HbA1c, and presence or absence of celiac autoantibodies/celiac disease. If repeated measurements were available, the latest measurements were extracted for cross-sectional analysis.
We sent email requests to authors of 11 studies or published conference abstracts seeking data necessary for inclusion. One author responded and provided data that were included in the review (13).
Data Synthesis and Analysis
Data from included studies were pooled on the basis of site and method selected for bone assessment. The weighted mean difference (WMD) estimate was calculated between case and control subjects. Standardized mean difference was used for one outcome measure (QUS, calcaneal) because different measurement scales were used between studies. Statistical heterogeneity was tested with the I2 statistic, which provides the percentage of variance of the summary effect attributable to heterogeneity between studies.
The Joanna Briggs Institute Critical Appraisal tool, Checklist for Analytical Cross Sectional Studies, was used for assessment of study quality (14). The checklist gives a score out of 8, and good quality was defined as a score ≥6 (reported in Supplementary Table 1).
We conducted a sensitivity analysis by excluding lower-quality trials (score <6) and one study with some participants aged >20 years. Prespecified subgroup analyses were planned for age (<11, 11–14, and >14 years), sex, and coexisting celiac disease in youth with type 1 diabetes.
All analyses were conducted with the use of Review Manager, version 5 (RevMan; The Cochrane Collaboration, Oxford, U.K.). Meta-regression was performed with Stata 14.2 to estimate the effects of duration of illness, age, sex, and HbA1c on BMD.
Results
Characteristics of Included Studies
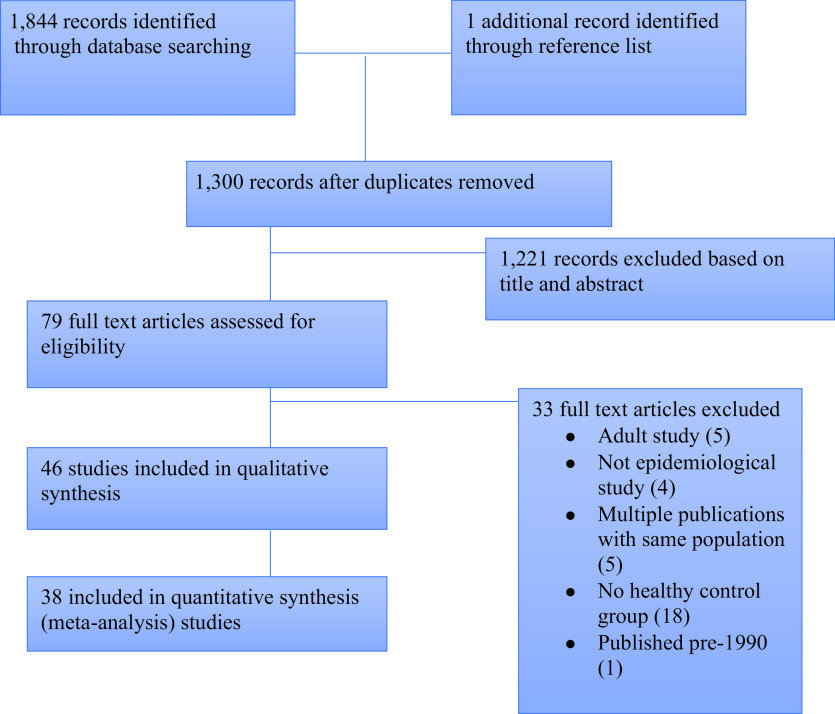
With the initial search strategy we identified 1,844 studies (PubMed search, 496, and Embase, 198, with 544 duplicates). One additional study was identified from hand searching the reference lists. There were 1,221 records excluded based on titles and abstracts. A total of 79 full text articles were subsequently assessed for eligibility; 46 met the inclusion criteria, and 38 were included in the meta-analysis (Fig. 1). The 46 included studies, from 24 countries, involved 2,617 case subjects (1,144 male, 1,245 female, and 228 unspecified) and 3851 control subjects (1,760 male, 1,826 female, 265 unspecified). Five studies only included females (15–18), and one study only included males (19). Characteristics of included studies are summarized in Supplementary Table 1.
Figure 1.
Flow diagram of study selection and search.
Two studies (16,20) included the same participants; in each case, the later studies were included (16). Four studies (two abstracts and two articles) reported on the same group (21–24); the article with the greater number of bone outcome measures was included (21). This also was the case for two other studies (18,25).
In all studies BMD was measured in youth with type 1 diabetes and healthy control subjects with use of DXA, QUS, or pQCT. Mean ± SD age of the study participants was 12.6 ± 2.3 years. One study (26) included youth up to 24.8 years of age but was included by consensus, as the median age was ∼18 years (below the inclusion criteria of 20 years). One study was not included in the meta-analysis because the method of bone measurement was not specified and attempts to contact the authors were unsuccessful.
Study Quality
The majority of studies (44 of 46) were of good quality. One study did not include a representative sample of case subjects, and control subjects were poorly matched (27). The other study did not specify inclusion and exclusion criteria, potential confounders were not identified, and the method for outcome measurement was not specified (19).
DXA Results
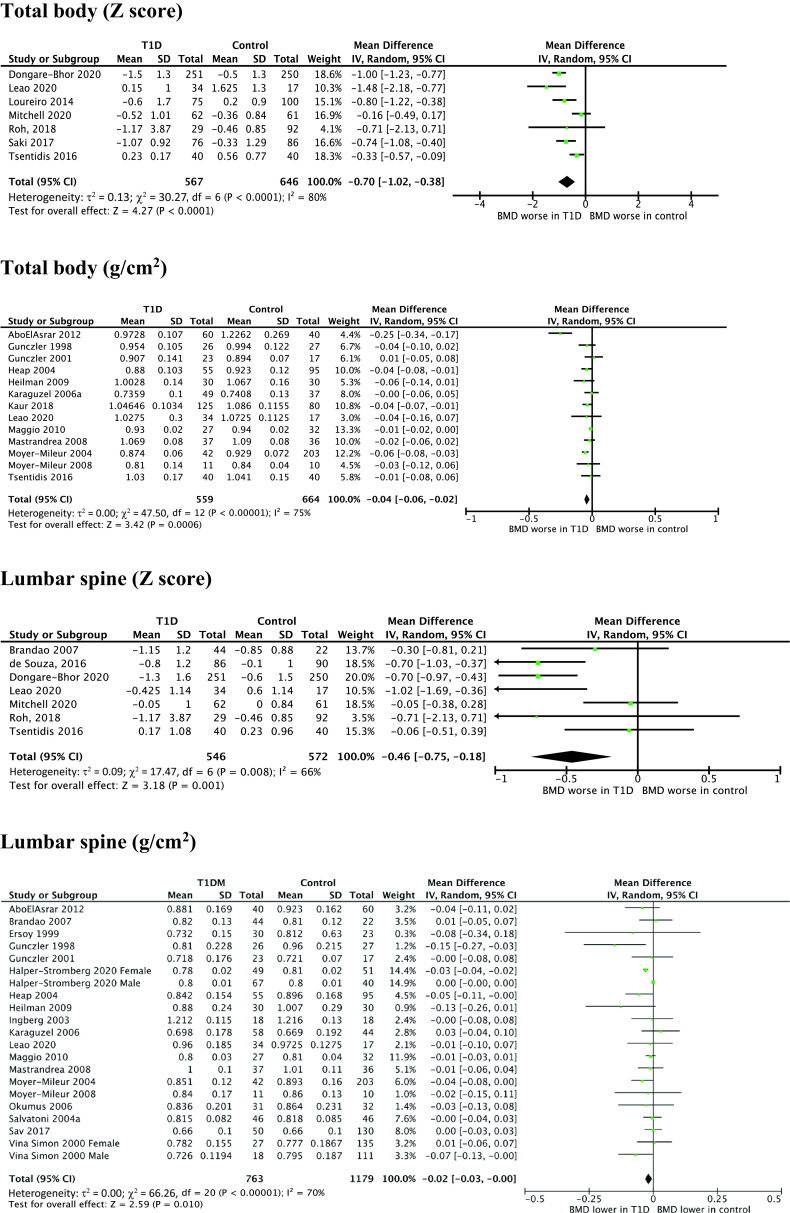
Figure 2 shows DXA results for total body, lumbar spine, and femur BMD and z scores for total body and lumbar spine BMD.
Figure 2.
WMD for DXA measurements of BMD in youth with type 1 diabetes vs. healthy control subjects. IV, inverse variance; T1D, type 1 diabetes.
Total Body BMD
Total body BMD was not different between case and control subjects (14 studies, WMD −0.03 g/cm2, 95% CI −0.06 to 0.01; P = 0.10). When the study of low quality was removed (27), case subjects had lower total body BMD (WMD −0.04 g/cm2, −0.06 to −0.02; P = 0.0006 [Fig. 2]). There was significant study heterogeneity (I2 = 75%). The forest plot demonstrated one outlying study (28), which included a disproportionately large number of female subjects in the case group; when this study was removed, the effect estimate was largely unchanged (WMD −0.03 g/cm2, −0.04 to −0.01). Total body BMD z score was lower in case subjects than in control subjects (seven studies, WMD −0.70, −1.02 to −0.38; P < 0.0001). There was significant heterogeneity (I2 = 80%).
In subgroup analysis by sex, BMD was lower in female case subjects (four studies, WMD −0.03 g/cm2, −0.06 to −0.01; P = 0.005). BMD was not different between male case and control subjects (two studies, WMD −0.01 g/cm2, 95% CI −0.06 to 0.03; P = 0.54). In subgroup analysis by age, the difference was only significant for studies with a mean age of >14 years: pooled WMD for studies with a mean age of <11 years was −0.01 g/cm2 (two studies, 95% CI −0.02 to 0.0; P = 0.07), 11–14 years −0.03 g/cm2 (three studies, −0.07 to 0.01; P = 0.24), and >14 years −0.07 g/cm2 (four studies, −0.12 to −0.03; P = 0.005). BMC was not different (six studies, WMD 55.72 g, −73.58 to 185.03; P = 0.4). This was unchanged following sensitivity analysis (WMD −85.76 g, −220.04 to 38.51; P = 0.18).
Lumbar Spine BMD
BMD of the lumbar spine was lower in case subjects (19 studies, WMD −0.02 g/cm2, 95% CI −0.03 to −0.0; P = 0.010 [Fig. 2]), with high study heterogeneity (I2 = 70%). There was asymmetry in the funnel plot, indicating publication bias. When the outlying studies were removed, the effect estimate was reduced but remained significant (WMD −0.01 g/cm2, −0.02 to −0.0; P = 0.02). Lumbar spine z score was lower in case subjects (seven studies, WMD −0.46, −0.75 to −0.18; P = 0.001). There was moderate heterogeneity (I2=66%).
In subgroup analysis by sex, lumbar spine BMD in female subjects was lower in the female case subjects (five studies, WMD −0.03 g/cm2, 95% CI −0.04 to −0.02; P < 0.00001). Lumbar spine BMD was not different between groups (two studies, WMD −0.03 g/cm2, −0.09 to −0.04; P = 0.43).
One study reported lumbar spine BMC in male and female subjects separately (9). BMC was lower in male subjects (MD −0.53, 95% CI −0.88 to −0.18; P = 0.003) but not female (−0.14, −0.34 to 0.06; P = 0.17). In subgroup analysis by age, BMD was lower in case subjects aged 11–14 years (nine studies, WMD −0.07 g/cm2, −0.1 to −0.02; P = 0.0004) and >14 years (11 studies, WMD −0.03 g/cm2, −0.04 to −0.01, P = 0.002) but not in case subjects aged <11 years (four studies, WMD −0.01 g/cm2, −0.03 to 0.01; P = 0.22).
Femur BMD
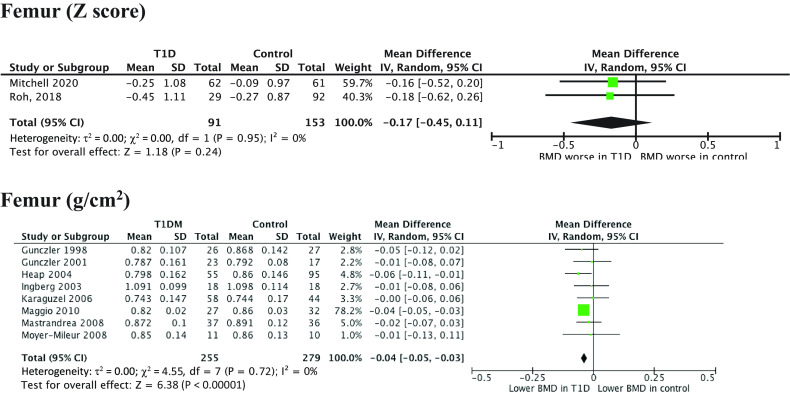
BMD was lower in case subjects (eight studies, WMD −0.04 g/cm2, 95% CI −0.05 to −0.03; P < 0.00001). There was low heterogeneity (I2 = 0%) and no publication bias suggested by forest plot. Two studies reported z score and did not show a significant difference (WMD −0.17, −0.45 to 0.11; P = 0.24).
In subgroup analysis by age, there was lower BMD for case subjects with a mean age of <11 years (two studies, WMD −0.04 g/cm2, 95% CI −0.05 to −0.03; P < 0.00001) and a trend toward lower BMD for case subjects aged >14 years (three studies, WMD −0.03 g/cm2, −0.07 to −0.00; P = 0.05) but not for those aged 11–14 years (three studies, WMD −0.02 g/cm2, −0.06 to 0.02; P = 0.03).
pQCT
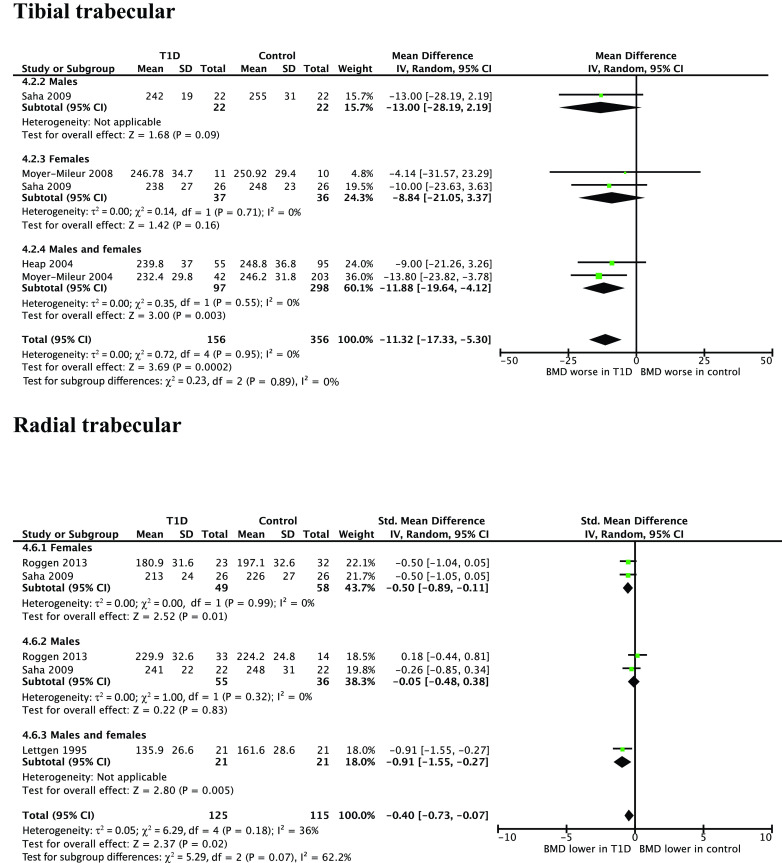
Figure 3 shows pQCT results of BMD in youth in trabecular bone.
Figure 3.
WMD for pQCT measurements of trabecular BMD in youth with type 1 diabetes vs. healthy control subjects. IV, inverse variance; T1D, type 1 diabetes.
Trabecular
vBMD was lower in subjects with type 1 diabetes (four studies, WMD −11.32 g/cm3, 95% CI −17.33 to −5.30; P = 0.0002). Three studies reported vBMD in the trabecular bone of the radius (WMD −0.40 g/cm3, −0.73 to −0.07; P = 0.02). One study reported vBMD in the trabecular bone of the lumbar spine (MD −0.52 g/cm3, −0.92 to −0.11; P = 0.01). One study reported the z score for trabecular bone of the radius and found significantly lower vBMD in subjects with type 1 diabetes (MD −0.9, −1.09 to −0.71; P < 0.00001).
In subgroup analysis by sex, there was no significant difference in tibial trabecular BMD, though there were few studies included (female subjects, two studies, WMD −8.84 g/cm3, 95% CI −21.05 to 3.37; P = 0.16; male subjects, one study, MD –13.00 g/cm3, −28.19 to 2.19; P = 0.09). There was a significant difference in radial trabecular BMD in females (two studies, WMD −0.50 g/cm3, −0.89 to −0.11; P = 0.01) but not males (two studies, WMD −0.05 g/cm3, −0.48 to 0.38; P = 0.83).
Cortical
There was no significant difference in cortical vBMD in the tibia between case and control subjects (four studies, WMD 0.36 g/cm3, 95% CI −5.79 to 6.50; P = 0.91) (Supplementary Fig. 1). Three of these studies also reported BMC and demonstrated significantly lower BMC in subjects with type 1 diabetes (WMD −19.12 g, −33.74 to −4.49; P = 0.01). Cortical vBMD of the radius was not different (three studies, MD 0.10 g/cm3, −0.24 to 0.43; P = 0.56) (Supplementary Fig. 1). Cortical vBMD z score of the radius was significantly greater in children with type 1 diabetes (one study, mean difference [MD] 0.46, 0.25–0.67; P < 0.00001). Cortical vBMD of lumbar spine was not different (one study, −0.36 g/cm3, −0.76 to 0.05; P = 0.08).
In subgroup analysis by sex, there was no difference in cortical vBMD of the tibia or radius in male and female subjects. There was, however, smaller CSA of the tibia by sex in case subjects (female subjects, two studies, WMD −63.17 cm2, 95% CI −84.8 to −41.52; P < 0.00001; male subjects, two studies, WMD −33 cm2, −54.1 to −11.9; P = 0.005). The tibial cortical CSA was also smaller in the one study that reported it in male subjects but not in female subjects. This parameter was not statistically significant in the radius of either sex.
QUS
Four studies reported QUS measurements, including 267 case and 461 control subjects. There were significantly lower z scores measured by QUS (amplitude-dependent speed of sound) in the proximal phalanges of the nondominant hand of the case group (two studies, WMD −0.32, 95% CI −0.38 to −0.25; P < 0.00001).
Two studies measured BMD at the left calcaneum and reported either broadband ultrasound attenuation z scores (29) or speed of sound z score (30) and therefore are not directly comparable. BMD was lower in youth with type 1 diabetes (standardized mean difference −0.69, 955 CI −1.11 to −0.26; P = 0.001). This information is displayed in Supplementary Fig. 2.
Meta-Regression
Meta-regression analysis revealed a significant correlation between age and effect size of total body BMD (coefficient −0.0063, 95% CI −0.0095 to −0.0031; P = 0.002). This was following exclusion of the one outlying study (28) with a disproportionately large number of female subjects in the case group. Due to the relationship between sex and pubertal age, this study was excluded from the meta-regression analysis, as we felt it distorted the results. In meta-regression models with examination of age and effect size on DXA measurements of the lumbar spine and femoral neck, results were not significant.
There was a trend toward an inverse relationship between effect estimate of diabetes duration and total body BMD measured by DXA (coefficient −0.0074, 95% CI −0.019 to 0.004; P = 0.2). This was not replicated at other sites.
Meta-regression analysis did not show a significant relationship between HbA1c and effect size of total body BMD (coefficient −0.0312, −0.11 to 0.04; P = 0.39). Similarly, HbA1c was not associated with the effect estimate for lumbar spine and femoral neck measured by DXA. There were insufficient data to establish a relationship between age, diabetes duration, HbA1c, and BMD as measured by pQCT and QUS.
Discussion
This systematic review and meta-analysis of 46 studies involving 6,468 participants demonstrates lower BMD in youth with type 1 diabetes than in healthy control subjects, based on studies using DXA, pQCT, and/or QUS. This is the first meta-analysis to examine multiple modalities measuring BMD and to report z scores, with adjustment for age and sex. We demonstrate lower total body, spine, and femoral neck BMD, as well as lower total body BMD and lumbar spine BMD z scores (by DXA) and lower phalangeal and calcaneal BMD (QUS). pQCT demonstrated a differential effect of type 1 diabetes on the trabecula and cortical components of bone. Age was significantly associated with BMD in meta-regression but not with longer diabetes duration or HbA1c. Our findings support routine monitoring of BMD in youth with type 1 diabetes, since lower BMD and smaller CSA may increase the risks of fractures and osteoporosis, although these outcomes were not examined in our review.
Quantifying the effect of type 1 diabetes on BMD in youth is challenged by outcome assessment involving multiple sites, methods, and equipment, which limit generalizability. DXA has been used traditionally to examine BMD in children and adolescents, while more recent modalities such as pQCT and QUS remain largely research tools. The smaller previous systematic review (10 studies, 341 youth and 624 adults with type 1 diabetes) demonstrated significantly lower absolute total body and femoral BMD (8) and lower lumbar spine BMD in males aged <20 years (3). While DXA is currently the gold standard for measuring BMD in youth, it may be imprecise in growing bones because it is an areal measurement rather than a true volumetric density. Therefore, lower BMD measured by DXA could be due to smaller bone size and not be a reflection of abnormal bone development. This may be particularly important in type 1 diabetes because affected girls may have smaller bones (31).
In contrast, pQCT measures vBMD and provides information surrounding bone geometry and mineral distribution within the bone CSA. It also separates the measurement of trabecular and cortical bone compartments, which may allow for earlier detection of changes in bone (12). We found significantly lower trabecular vBMD, irrespective of site examined, and higher radial cortical vBMD z score (one study) in case compared with control subjects. Trabecular bone is more metabolically active and therefore more susceptible to the effects of chronic hyperglycemia (26,32), which may also explain the reduced trabecular vBMD, while the high material density in cortical bone may be due to reduced bone turnover or abnormities in collagen glycosylation.
BMD was significantly lower in youth with type 1 diabetes in the four studies that reported QUS measurements. The benefits of QUS for assessment of BMD in youth include its ease of use, low cost, and safety profile, particularly the absence of ionizing radiation. It is less dependent on bone size compared with DXA, therefore potentially improving accuracy (10). Studies in adults have suggested similar prognostic values for osteoporotic fractures and also in detecting low BMD in youth with fragility fractures (33).
The impact of HbA1c on BMD has been controversial in previous studies. In meta-regression, we did not find a significant relationship between BMD and HbA1c. However, we could only include HbA1c results that were either a single measurement at the time of bone examination or a result averaged over the previous 12 months. It would have been preferable to include HbA1c averaged over the disease course, and this is recommended in future studies. Indeed, there was a significant correlation between BMD and average HbA1c over disease course, and the last 12 months, but not for the acute measurement at the time of BMD measurement (34). It has been hypothesized that chronic hyperglycemia leads to both altered osteoblast differentiation and maturation (35), and also alteration of osteoclast activity, therefore characterizing type 1 diabetes as a state of low bone turnover (22).
There was no consistent association between sex and BMD. In some studies, control subjects were not well matched, with a sex disparity between case and control groups. Conflicting data have been observed in other studies: no sex difference (36,37), male subjects with lower BMD (38,39), and female subjects with lower BMD (40).
The strength of this review is that it included multiple modalities for measuring BMD (DXA, pQCT, and QUS). It also is the first meta-analysis to include studies that reported BMD by z score and to examine the effect of putative effect-modifying variables such as HbA1c, diabetes duration, and age using meta-regression. The significant sources of heterogeneity between included studies resulted from varied inclusion criteria of each study, in age, pubertal stage, race, diabetes duration, and HbA1c, but only age was significant in meta-regression. Weaknesses of the review include the overall sample size, with few studies evaluating cortical BMD by pQCT or QUS, which, although beyond our control, may have influenced the ability to detect differences in all outcome measures.
In conclusion, we found evidence of abnormal bone development in youth with type 1 diabetes, assessed by multiple modalities. Future studies, ideally longitudinal in design, should evaluate lifetime HbA1c, glycemic variability, sex, measurement of celiac antibodies, vitamin D status, and reversibility of adverse bone measures. We recommend that future studies report z scores for all bone measurements, as well as consistency in the sites and methods of assessment. The utility of pQCT and QUS remains unclear, along with the predictive role and functional effects of early detection of lower trabecular bone mineral density in youth with type 1 diabetes. Further research is also required to establish the mechanism of abnormal bone development in type 1 diabetes so as to inform recommendations for primary and secondary prevention. In the absence of diabetes-specific data, recommendations for youth with type 1 diabetes should align with general population guidelines, including lifestyle modification, such as regular weight-bearing exercise and a diet sufficient in calcium.
Article Information
Acknowledgments. The authors thank Viral Shah, University of Colorado School of Medicine, who provided data.
Funding. M.C. is supported by a National Health and Medical Research Council Practitioner Fellowship (APP1136735).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. P.L. performed the literature search, screened abstracts, extracted data, performed statistical analysis, interpreted the data, and wrote the manuscript. K.N. performed the literature search, screened abstracts, extracted and interpreted data, and revised the manuscript. C.F.M. reviewed data and edited the manuscript. M.E.C. conceived and designed the review, extracted data, evaluated and interpreted data, and edited the manuscript. M.E.C. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14618409.
References
- 1.Morrison LB. Bogan IK. Bone development in diabetic children; roentgen study. Am J Med Sci 1927;274:313–319 [Google Scholar]
- 2.Thong EP, Herath M, Weber DR, et al. Fracture risk in young and middle-aged adults with type 1 diabetes mellitus: a systematic review and meta-analysis. Clin Endocrinol (Oxf) 2018;89:314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah VN, Harrall KK, Shah CS, et al. Bone mineral density at femoral neck and lumbar spine in adults with type 1 diabetes: a meta-analysis and review of the literature. Osteoporos Int 2017;28:2601–2610 [DOI] [PubMed] [Google Scholar]
- 4.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007;166:495–505 [DOI] [PubMed] [Google Scholar]
- 5.Williams KM. Update on bone health in pediatric chronic disease. Endocrinol Metab Clin North Am 2016;45:433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton EJ, Rakic V, Davis WA, et al. Prevalence and predictors of osteopenia and osteoporosis in adults with type 1 diabetes. Diabet Med 2009;26:45–52 [DOI] [PubMed] [Google Scholar]
- 7.Heilman K, Zilmer M, Zilmer K, Tillmann V. Lower bone mineral density in children with type 1 diabetes is associated with poor glycemic control and higher serum ICAM-1 and urinary isoprostane levels. J Bone Miner Metab 2009;27:598–604 [DOI] [PubMed] [Google Scholar]
- 8.Pan H, Wu N, Yang T, He W. Association between bone mineral density and type 1 diabetes mellitus: a meta-analysis of cross-sectional studies. Diabetes Metab Res Rev 2014;30:531–542 [DOI] [PubMed] [Google Scholar]
- 9.Saha MT, Sievänen H, Salo MK, Tulokas S, Saha HH. Bone mass and structure in adolescents with type 1 diabetes compared to healthy peers. Osteoporos Int 2009;20:1401–1406 [DOI] [PubMed] [Google Scholar]
- 10.Chobot AP, Haffke A, Polanska J, et al. Bone status in adolescents with type 1 diabetes. Diabetologia 2010;53:1754–1760 [DOI] [PubMed] [Google Scholar]
- 11.Bechtold S, Dirlenbach I, Raile K, Noelle V, Bonfig W, Schwarz HP. Early manifestation of type 1 diabetes in children is a risk factor for changed bone geometry: data using peripheral quantitative computed tomography. Pediatrics 2006;118:e627–e634 [DOI] [PubMed] [Google Scholar]
- 12.Stagi S, Cavalli L, Cavalli T, de Martino M, Brandi ML. Peripheral quantitative computed tomography (pQCT) for the assessment of bone strength in most of bone affecting conditions in developmental age: a review. Ital J Pediatr 2016;42:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaur H, Joshee P, Franquemont S, et al. Bone mineral content and bone density is lower in adolescents with type 1 diabetes: a brief report from the RESISTANT and EMERALD studies. J Diabetes Complications 2018;32:931–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moola S, Munn Z, Tufanaru C, et al. Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis. Adelaide, Australia, Joanna Briggs Institute, 2017 [Google Scholar]
- 15.Ingberg CM, Särnblad S, Palmér M, Schvarcz E, Berne C, Aman J. Body composition in adolescent girls with type 1 diabetes. Diabet Med 2003;20:1005–1011 [DOI] [PubMed] [Google Scholar]
- 16.Mastrandrea LD, Wactawski-Wende J, Donahue RP, Hovey KM, Clark A, Quattrin T. Young women with type 1 diabetes have lower bone mineral density that persists over time. Diabetes Care 2008;31:1729–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyer-Mileur LJ, Slater H, Jordan KC, Murray MA. IGF-1 and IGF-binding proteins and bone mass, geometry, and strength: relation to metabolic control in adolescent girls with type 1 diabetes. J Bone Miner Res 2008;23:1884–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell DM, Caksa S, Joseph T, Bouxsein ML, Misra M. Elevated HbA1c is associated with altered cortical and trabecular microarchitecture in girls with type 1 diabetes. J Clin Endocrinol Metab 2020;105:e1648–e1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tahir A, Shoaib M, Akhtar B, Khalid A, Shahid A, Qureshi HJ. Effect of type 1 diabetes mellitus on bone status of children. Pak J Med Health Sci 2013;7:251–253 [Google Scholar]
- 20.Liu EY, Wactawski-Wende J, Donahue RP, Dmochowski J, Hovey KM, Quattrin T. Does low bone mineral density start in post-teenage years in women with type 1 diabetes? Diabetes Care 2003;26:2365–2369 [DOI] [PubMed] [Google Scholar]
- 21.Tsentidis C, Gourgiotis D, Kossiva L, et al. Higher levels of s-RANKL and osteoprotegerin in children and adolescents with type 1 diabetes mellitus may indicate increased osteoclast signaling and predisposition to lower bone mass: a multivariate cross-sectional analysis. Osteoporos Int 2016;27: 1631–1643 [DOI] [PubMed] [Google Scholar]
- 22.Tsentidis C, Gourgiotis D, Kossiva L, Marmarinos A, Doulgeraki A, Karavanaki K. Sclerostin distribution in children and adolescents with type 1 diabetes mellitus and correlation with bone metabolism and bone mineral density. Pediatr Diabetes 2016;17:289–299 [DOI] [PubMed] [Google Scholar]
- 23.Tsentidis C, Gourgiotis D, Kossiva L, et al. Increased osteoclast activity in children and adolescents with type 1 diabetes mellitus indicated by higher levels of osteoprotegerin and s-RANKL may predispose to lower bone mass. Horm Res Paediatr 2014;1:89–90 [Google Scholar]
- 24.Tsentidis C, Gourgiotis D, Kossiva L, et al. Correlation of sclerostin levels with bone metabolism markers and bone mineral density in children and adolescents with type 1 diabetes mellitus (T1DM). Horm Res Paediatr 2014;1:89 [Google Scholar]
- 25.Joseph TV, Caksa S, Misra M, Mitchell DM. Hip structural analysis reveals impaired hip geometry in girls with type 1 diabetes. J Clin Endocrinol Metab 2020;105:dgaa647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roggen I, Gies I, Vanbesien J, Louis O, De Schepper J. Trabecular bone mineral density and bone geometry of the distal radius at completion of pubertal growth in childhood type 1 diabetes. Horm Res Paediatr 2013;79:68–74 [DOI] [PubMed] [Google Scholar]
- 27.Abd El Dayem SM, El-Shehaby AM, Abd El Gafar A, Fawzy A, Salama H. Bone density, body composition, and markers of bone remodeling in type 1 diabetic patients. Scand J Clin Lab Invest 2011;71:387–393 [DOI] [PubMed] [Google Scholar]
- 28.AboElAsrar MA, Elbarbary NS, Elshennawy DE, Omar AM. Insulin-like growth factor-1 cytokines cross-talk in type 1 diabetes mellitus: relationship to microvascular complications and bone mineral density. Cytokine 2012;59:86–93 [DOI] [PubMed] [Google Scholar]
- 29.Galluzzi F, Stagi S, Salti R, et al. Osteoprotegerin serum levels in children with type 1 diabetes: a potential modulating role in bone status. Eur J Endocrinol 2005;153:879–885 [DOI] [PubMed] [Google Scholar]
- 30.Khoshhal KI, Sheweita SA, Al-Maghamsi MS, Habeb AM. Does type 1 diabetes mellitus affect bone quality in prepubertal children? J Taibah Univ Med Sci 2015;10:300–305 [Google Scholar]
- 31.Tylleskär K, Tuvemo T, Gustafsson J. Diabetes control deteriorates in girls at cessation of growth: relationship with body mass index. Diabet Med 2001;18:811–815 [DOI] [PubMed] [Google Scholar]
- 32.Lettgen B, Hauffa B, Möhlmann C, Jeken C, Reiners C. Bone mineral density in children and adolescents with juvenile diabetes: selective measurement of bone mineral density of trabecular and cortical bone using peripheral quantitative computed tomography. Horm Res 1995;43:173–175 [DOI] [PubMed] [Google Scholar]
- 33.Vierucci F, Del Pistoia M, Erba P, Federico G, Saggese G. Usefulness of phalangeal quantitative ultrasound in identifying reduced bone mineral status and increased fracture risk in adolescents with Turner syndrome. Hormones (Athens) 2014;13:353–360 [DOI] [PubMed] [Google Scholar]
- 34.Camurdan MO, Ciaz P, Bideci A, Demirel F. Role of hemoglobin A(1c), duration and puberty on bone mineral density in diabetic children. Pediatr Int 2007;49:645–651 [DOI] [PubMed] [Google Scholar]
- 35.Goodman WG, Hori MT. Diminished bone formation in experimental diabetes. Relationship to osteoid maturation and mineralization. Diabetes 1984;33:825–831 [DOI] [PubMed] [Google Scholar]
- 36.Pascual J, Argente J, Lopez MB, et al. Bone mineral density in children and adolescents with diabetes mellitus type 1 of recent onset. Calcif Tissue Int 1998;62:31–35 [DOI] [PubMed] [Google Scholar]
- 37.Valerio G, del Puente A, Esposito-del Puente A, Buono P, Mozzillo E, Franzese A. The lumbar bone mineral density is affected by long-term poor metabolic control in adolescents with type 1 diabetes mellitus. Horm Res 2002;58:266–272 [DOI] [PubMed] [Google Scholar]
- 38.Rakic V, Davis WA, Chubb SAP, Islam FMA, Prince RL, Davis TME. Bone mineral density and its determinants in diabetes: the Fremantle Diabetes Study. Diabetologia 2006;49:863–871 [DOI] [PubMed] [Google Scholar]
- 39.Viña Simón E, Bueno Lozano G, Armadá Maresca MI, et al. Bone mineral density in juvenile-onset diabetes mellitus. An Esp Pediatr 2000;52:507–515[in Spanish] [PubMed] [Google Scholar]
- 40.Neumann T, Sämann A, Lodes S, et al. Glycaemic control is positively associated with prevalent fractures but not with bone mineral density in patients with type 1 diabetes. Diabet Med 2011;28:872–875 [DOI] [PubMed] [Google Scholar]