Abstract
OBJECTIVE
The Dapagliflozin and Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) study demonstrated risk reduction for kidney and cardiovascular outcomes with dapagliflozin versus placebo in participants with chronic kidney disease (CKD) with and without diabetes. We compared outcomes according to baseline glycemic status.
RESEARCH DESIGN AND METHODS
We enrolled participants with CKD, estimated glomerular filtration rate (eGFR) 25–75 mL/min/1.73 m2, and urinary albumin-to-creatinine ratio 200–5,000 mg/g. The primary composite end point was sustained eGFR decline ≥50%, end-stage kidney disease, or kidney or cardiovascular death.
RESULTS
Of 4,304 participants, 738 had normoglycemia, 660 had prediabetes, and 2,906 had type 2 diabetes. The effect of dapagliflozin on the primary outcome was consistent (P for interaction = 0.19) in normoglycemia (hazard ratio [HR] 0.62 [95% CI 0.39, 1.01]), prediabetes (HR 0.37 [0.21, 0.66]), and type 2 diabetes (HR 0.64 [0.52, 0.79]). We found no evidence for effect modification on any outcome. Adverse events were similar, with no major hypoglycemia or ketoacidosis in participants with normoglycemia or prediabetes.
CONCLUSIONS
Dapagliflozin safely reduced kidney and cardiovascular events independent of baseline glycemic status.
Introduction
In the Dapagliflozin and Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) trial of participants with chronic kidney disease (CKD) with or without type 2 diabetes, the sodium–glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin led to a 39% relative risk reduction in the primary composite outcome of sustained decline in the estimated glomerular filtration rate (eGFR) of ≥50%, end-stage kidney disease, or death from kidney or cardiovascular causes (1). In this prespecified analysis, we report the efficacy and safety of dapagliflozin in participants with normal glucose status, prediabetes, and type 2 diabetes.
Research Design and Methods
DAPA-CKD was a multicenter, double-blind, placebo-controlled, randomized trial. Participants had CKD defined as an eGFR of 25–75 mL/min/1.73 m2 and a urinary albumin-to-creatinine ratio (UACR) of 200–5,000 mg/g. We randomized participants in a 1:1 ratio to dapagliflozin, 10 mg/day, or placebo, and monitored participants for a median of 2.4 years. The trial was stopped early for overwhelming efficacy on recommendation from the Independent Data Monitoring Committee (1).
We classified patients by baseline glycemic status: normoglycemia was defined as HbA1c <5.7% (39 mmol/mol), prediabetes as HbA1c of at least 5.7% (39 mmol/mol) and <6.5% (48 mmol/mol), and type 2 diabetes as a history of diabetes or HbA1c of at least 6.5% (48 mmol/mol).
The primary end point was the composite of time to the first occurrence of a sustained decline in the eGFR ≥50%, onset of end-stage kidney disease, or death from kidney or cardiovascular causes. Secondary end points were the time to a kidney-specific composite outcome (which included the same components as the primary outcome except cardiovascular death), a composite cardiovascular end point (hospitalization for heart failure or cardiovascular death), and death from any cause (all-cause mortality).
A Cox proportional hazards regression model stratified by baseline glycemic status, with UACR as the stratification factor and adjusted for the baseline eGFR, was used to estimate the hazard ratio (HRs) and 95% CIs for dapagliflozin compared with placebo within each glycemic subgroup. We tested for heterogeneity by adding interaction terms between glycemic subgroup and randomized treatment assignment. We calculated annualized incidence rates (events per 100 patient-years). Absolute risk reductions were calculated by subtracting the annualized incidence rate in the dapagliflozin group from the placebo group, and heterogeneity in absolute treatment effects was estimated using fixed-effects meta-analysis.
We examined the effect of treatment according to continuous HbA1c using a linear interaction model.
Results
Of the 4,304 participants enrolled, 738 had normoglycemia, 660 had prediabetes, and 2,906 had type 2 diabetes at baseline (Supplementary Table 1).
The difference in HbA1c between dapagliflozin and placebo during follow-up was –0.1% (95% CI –0.1, 0.0; P = 0.0018; –0.9 mmol/mol [95% CI –1.5, 0.3]). The between-group difference in HbA1c during follow-up in participants with normoglycemia and prediabetes was 0.0% (95% CI –0.2, 0.2; P = 0.8597; 0.2 mmol/mol [95% CI –1.8, 2.2]) and –0.0% (95% CI –0.2, 0.2; P = 0.8764; –0.2 mmol/mol [95%CI –2.3, 1.9]), respectively. In participants with type 2 diabetes, the HbA1c difference was –0.1% (95% CI –0.2, 0.0; P = 0.0378; –1.1 mmol/mol [95% CI –2.1, 0.0]) (Supplementary Fig. 1).
Rates of the primary composite end point of a ≥50% eGFR decline, end-stage kidney disease, or death from cardiovascular or kidney causes were higher in participants with type 2 diabetes relative to participants with prediabetes or normoglycemia at baseline (Fig. 1A). The relative risk reduction by dapagliflozin for the primary composite outcome (HR 0.61 [95% CI 0.51, 0.72]) was consistent across subgroups by baseline glycemic status (P for interaction = 0.19) (Fig. 1A). In continuous analysis, the benefit of dapagliflozin on the primary composite outcome was apparent across a range of HbA1c levels (P for interaction = 0.62) (Fig. 1B).
Figure 1.
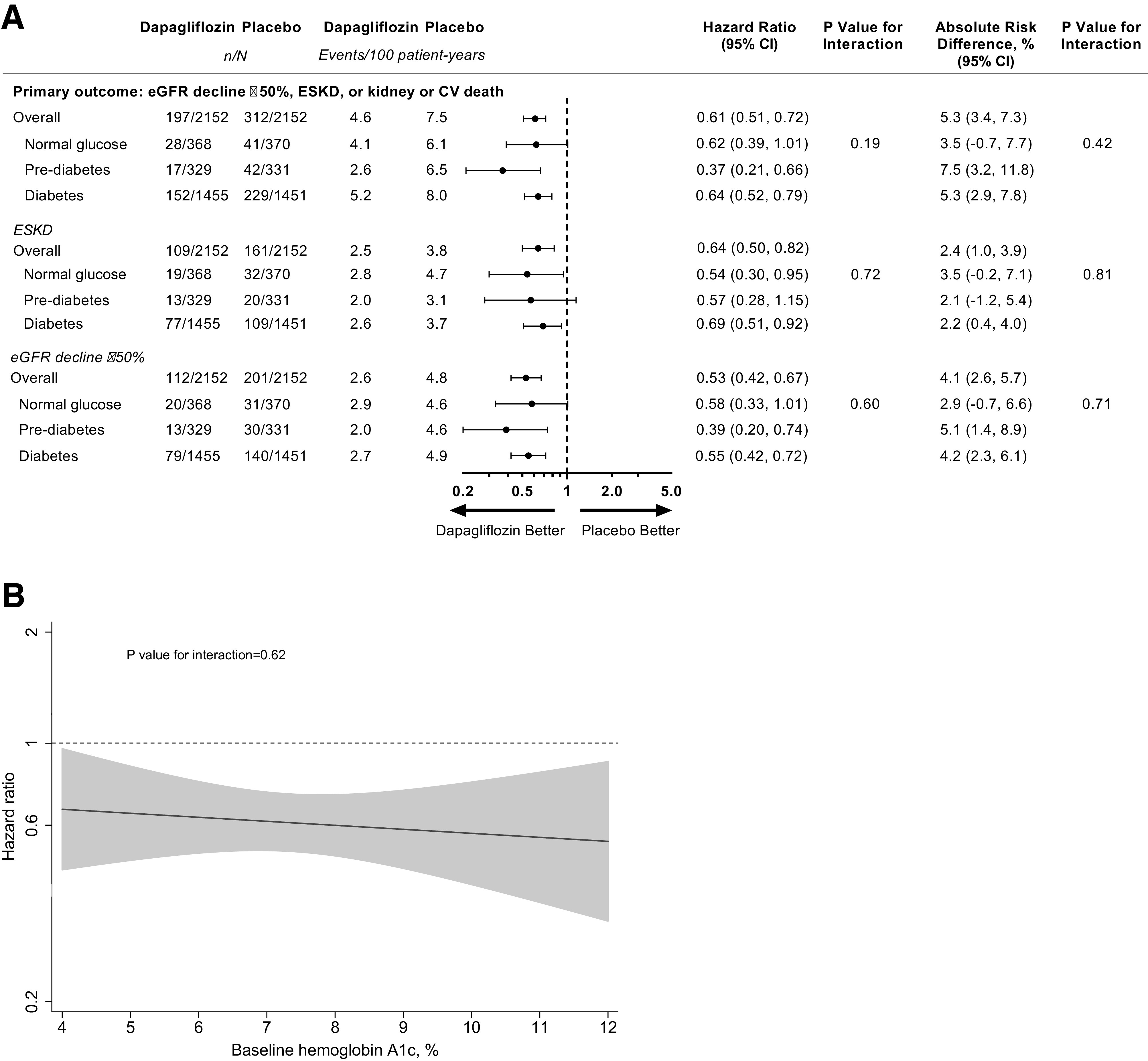
A: Forest plot of the primary composite outcome of ≥50% eGFR decline, end-stage kidney disease (ESKD), or death from cardiovascular (CV) or kidney causes with dapagliflozin compared with placebo by glycemic status at baseline. B: The treatment effect of dapagliflozin compared with placebo as a function of baseline HbA1c (continuous) for the primary outcome. The solid black line represents the HR of the treatment effect. The gray shaded area represents the 95% CI around the treatment effects. The dotted horizontal line represents a HR of 1 (i.e., no difference between randomized groups).
We observed consistent effects for the secondary kidney-specific composite end point of a ≥50% eGFR decline, end-stage kidney disease, or death from kidney causes (P for interaction = 0.42) (Supplementary Fig. 2), and the prespecified exploratory outcome of maintenance dialysis, kidney transplantation, or death from kidney causes (P for interaction = 0.88 (Supplementary Fig. 2). For the composite outcome of heart failure hospitalization or cardiovascular death, the 29% (HR 0.71 [95% CI 0.55, 0.92]) relative risk reduction was consistent across glycemic subgroups (P for interaction = 0.43) (Supplementary Fig. 2). The 31% relative risk reduction for allcause mortality was also consistent (P for interaction = 0.25 (Supplementary Fig. 2). In continuous analysis, the benefit of dapagliflozin on the composite of heart failure hospitalization or cardiovascular death and on all-cause death was apparent across a range of HbA1c levels (Supplementary Fig. 3).
The proportion of participants experiencing a serious adverse event was similar between dapagliflozin and placebo within each glycemic subgroup (P for interaction = 0.18) (Supplementary Table 2). No case of diabetic ketoacidosis occurred in the dapagliflozin group, whereas two cases occurred in the placebo group in participants with type 2 diabetes at baseline (Supplementary Table 2). No dapagliflozin-treated participants with normoglycemia or prediabetes at baseline experienced major hypoglycemia during the study. Notably, in dapagliflozin-treated participants with type 2 diabetes, there was a lower rate of major hypoglycemia compared with placebo (14 vs. 28 participants) (Supplementary Table 2). For other events of special interest (based on predefined lists of preferred terms), there were no between-treatment or glycemia subgroup differences in the number of fractures, amputations, or kidney-related events, and no interaction between glycemic subgroups regarding events of volume depletion.
Conclusions
In this prespecified analysis of the DAPA-CKD trial, we demonstrate that the effects of dapagliflozin on kidney failure, heart failure, and mortality outcomes were consistent regardless of the glycated hemoglobin subgroups. Major hypoglycemia or ketoacidosis events did not occur in participants with normoglycemia or prediabetes, providing reassurance that dapagliflozin can be safely used in these individuals.
Our findings from a dedicated kidney outcome trial substantiate the findings from the Canagliflozin and Renal Events in Diabetes With Established Nephropathy Clinical Evaluation (CREDENCE) study (2), suggesting that the kidney benefits seen with SGLT2 inhibition appear to be independent of their glucose-lowering effects, and extend these results further to those with prediabetes and normoglycemia at baseline. The findings reflect those of the Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) and Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction (EMPEROR-Reduced) trials, where dapagliflozin and empagliflozin, respectively, reduced the risk of worsening heart failure or cardiovascular death in participants with heart failure with reduced ejection fraction, irrespective of diabetes status (3,4).
Few studies have investigated SGLT2 inhibition in prediabetes. During a 13-week randomized comparison between dapagliflozin, metformin, exercise, or control intervention, Færch et al. (5) found that dapagliflozin treatment led to improved glycemic variability, with minor reductions in HbA1c (0.1% or 1.3 mmol/mol) and fasting plasma glucose (0.1 mmol/L or 1.8 mg/dL).
In participants with normoglycemia or prediabetes, dapagliflozin reduced the risk of kidney outcomes without improving glycemic control. These data are in keeping with an analysis of the CANagliflozin cardioVascular Assessment Study (CANVAS) trial (6), where markers of glycemia did not explain the effect of canagliflozin on kidney outcomes. Instead, albuminuria, hemoglobin, and hematocrit were identified as important mediators, pointing to a potential reduction in fluid overload. The recognized effect of SGLT2 inhibitors on hemoglobin and hematocrit may reflect improvement in renal hypoxia and restoration in the hypoxia-inducible factor 1α/2α balance, stimulating erythropoiesis and reducing inflammation (7). Glucose-independent effects may include osmotic diuretic and natriuretic effects as observed in individuals with type 2 diabetes and CKD (8).
Because the DAPA-CKD trial was stopped early, this may have limited the statistical power to examine other end points. Our findings may not be generalizable to lower levels of albuminuria or an eGFR <25 mL/min/1.73 m2.
In conclusion, dapagliflozin prevented the progression of CKD in individuals with normoglycemia, prediabetes, and type 2 diabetes, with similar safety across these subgroups. These data support the favorable benefit-to-risk ratio of dapagliflozin in patients with CKD independent of glycemic status.
Article Information
Acknowledgments. All participants and investigators in the DAPA-CKD trial are acknowledged. The authors would also like to thank Nicola Truss of inScience Communications for assistance in editing and the preparation of figures; this support was funded by AstraZeneca.
Funding. G.M.C. has received research grants from the National Institute of Diabetes and Digestive and Kidney Diseases.
Duality of Interest. The DAPA-CKD trial was funded by AstraZeneca, which was the sponsor of the study and was involved in the study design, analysis, interpretation of data, writing of the report, and the decision to submit the paper for publication. F.P. reports having received research grants from AstraZeneca and lecture fees from AstraZeneca, Merck Sharp & Dohme, Janssen, Eli Lilly, Boehringer Ingelheim, Novo Nordisk A/S, and Novartis, as well as being a consultant/advisory board member for AstraZeneca, Bayer, Amgen, and Merck. P.R. has served as a consultant for AstraZeneca, Astellas, Bayer, Boehringer Ingelheim, Gilead, Merck, Mundipharma, Vifor, Sanofi, and Novo Nordisk A/S (all honoraria to his institution), and received research grants from AstraZeneca and Novo Nordisk A/S. G.M.C. has received fees from AstraZeneca for the DAPA-CKD trial steering committee, research grants Amgen, is on the board of directors for Satellite Healthcare, has received fees for advisory boards for Ardelyx, Baxter, CloudCath, Cricket, DiaMedica, Durect, DxNow, Outset, and Reata, and holds stock options for Ardelyx, CloudCath, Durect, DxNow, and Outset, has received fees from Akebia, Gilead, Sanifit, and Vertex for trial steering committees, and has received fees for Data Safety Monitoring Board service from Angion, Bayer, and ReCor. F.F.H. has received honoraria from AstraZeneca as a member of the executive member of the DAPA-CKD study and received honoraria from AbbVie for participation in a steering committee. J.J.V.M. has received support to his institution, Glasgow University, for work on clinical trials, consulting, and other activities from AbbVie, Alnylam, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Cardurion, Cyclerion, Cytokinetics, DalCor, GSK, Kidney Research UK, Merck, Novartis, Pfizer, Servier, Theracos, and Vifor-Fresenius, and has received personal lecture fees from Abbott, Hickman, Sun Pharmaceuticals, and Servier. R.C.-R. has received fees from AstraZeneca for the DAPA-CKD trial steering committee, speaker fees from Boehringer Ingelheim, Amgen, and Janssen, research support from GlaxoSmithKline and Novo Nordisk, and honoraria for advisory boards from Boehringer Ingelheim, Novo Nordisk, and Medtronic. H.S.B. has received speaking honoraria from Eli Lilly and Novo Nordisk and research funding paid to LMC Healthcare from Amgen, AstraZeneca, Boehringer Ingelheim, Ceapro, Eli Lilly, Gilead, Janssen, Kowa Pharmaceuticals Co. Ltd., Madrigal Pharmaceuticals, Merck, Pfizer, Novo Nordisk, Sanofi, and Tricida. B.V.S. and A.M.L. are employees and stockholders of AstraZeneca. R.D.T. has received support from AstraZeneca as a member of the executive committee for DAPA-CKD, is a consultant for Boehringer Ingelheim, has participated on advisory boards for Bayer and Relypsa, and has served on data monitoring committees for Akebia and Reata Pharmaceuticals, on an executive committee for Amgen, and as a faculty associate for Quest Diagnostics. D.C.W. provides ongoing consultancy services to AstraZeneca and has received honoraria and/or consultancy fees from Amgen, Astellas, Boehringer Ingelheim, Bayer, GlaxoSmithKline, Janssen, Napp, Mundipharma, Merck Sharp & Dohme, Reata, Tricida, and Vifor Fresenius. H.J.L.H. has received support from AstraZeneca to his institution for the DAPA-CKD trial, fees to his institution for his participation in advisory boards for Merck, Mitsubishi Tanabe, Janssen, and Mundipharma, as a consultant for AbbVie, Retrophin, Boehringer Ingelheim, and Novo Nordisk, for participation in steering committees for Janssen, Gilead, Bayer, Chinook, and CSL Pharma, and research support from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. F.P., P.R., and H.J.L.H. researched the data and wrote the first draft of the manuscript. P.V., G.M.C., F.F.H., N.J., J.J.V.M., R.C.-R., H.S.B., B.V.S., R.D.T., A.M.L., and D.C.W. provided input to a revised draft manuscript. All authors approved the final version of the submitted manuscript. H.J.L.H. is the guarantor of this work and, as such had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Publication. Parts of this study were submitted in abstract form to the 81st Scientific Sessions of the American Diabetes Association, 25–29 June 2021.
Footnotes
Clinical trial reg. no. NCT03036150, clinicaltrials.gov.
This article contains supplementary material online at https://doi.org/10.2337/figshare.14465844.
References
- 1.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446 [DOI] [PubMed] [Google Scholar]
- 2.Cannon CP, Perkovic V, Agarwal R, et al. Evaluating the effects of canagliflozin on cardiovascular and renal events in patients with type 2 diabetes mellitus and chronic kidney disease according to baseline HbA1c, Including those with HbA1c <7%: results from the CREDENCE Trial. Circulation 2020;141:407–410 [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJV, Solomon SD, Inzucchi SE, et al.; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008 [DOI] [PubMed] [Google Scholar]
- 4.Anker SD, Butler J, Filippatos G, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR-Reduced Trial. Circulation 2021;143:337–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Færch K, Blond MB, Bruhn L, et al. The effects of dapagliflozin, metformin or exercise on glycaemic variability in overweight or obese individuals with prediabetes (the PRE-D Trial): a multi-arm, randomised, controlled trial. Diabetologia 2021;64:42–55 [DOI] [PubMed] [Google Scholar]
- 6.Li J, Neal B, Perkovic V, et al. Mediators of the effects of canagliflozin on kidney protection in patients with type 2 diabetes. Kidney Int 2020;98:769–777 [DOI] [PubMed] [Google Scholar]
- 7.Packer M. Mechanisms leading to differential hypoxia-inducible factor signaling in the diabetic kidney: modulation by SGLT2 inhibitors and hypoxia mimetics. Am J Kidney Dis 2021;77:280–286 [DOI] [PubMed] [Google Scholar]
- 8.Eickhoff MK, Dekkers CCJ, Kramers BJ, et al. Effects of dapagliflozin on volume status when added to renin-angiotensin system inhibitors. J Clin Med 2019;8:779. [DOI] [PMC free article] [PubMed] [Google Scholar]


