Abstract
OBJECTIVE
Short- and long-term glycemic variability are risk factors for diabetes complications. However, there are no validated A1C target ranges or measures of A1C stability in older adults. We evaluated the association of a patient-specific A1C variability measure, A1C time in range (A1C TIR), on major adverse outcomes.
RESEARCH DESIGN AND METHODS
We conducted a retrospective observational study using administrative data from the Department of Veterans Affairs and Medicare from 2004 to 2016. Patients were ≥65 years old, had diabetes, and had at least four A1C tests during a 3-year baseline period. A1C TIR was the percentage of days during the baseline in which A1C was in an individualized target range (6.0–7.0% up to 8.0–9.0%) on the basis of clinical characteristics and predicted life expectancy. Increasing A1C TIR was divided into categories of 20% increments and linked to mortality and cardiovascular disease (CVD) (i.e., myocardial infarction, stroke).
RESULTS
The study included 402,043 veterans (mean [SD] age 76.9 [5.7] years, 98.8% male). During an average of 5.5 years of follow-up, A1C TIR had a graded relationship with mortality and CVD. Cox proportional hazards models showed that lower A1C TIR was associated with increased mortality (A1C TIR 0 to <20%: hazard ratio [HR] 1.22 [95% CI 1.20–1.25]) and CVD (A1C TIR 0 to <20%: HR 1.14 [95% CI 1.11–1.19]) compared with A1C TIR 80–100%. Competing risk models and shorter follow-up (e.g., 24 months) showed similar results.
CONCLUSIONS
In older adults with diabetes, maintaining A1C levels within individualized target ranges is associated with lower risk of mortality and CVD.
Introduction
Diabetes treatment often focuses on lowering A1C to prevent complications. Older patients with diabetes frequently have comorbidities and limited life expectancy, and this can affect the balance between benefits and harms of lower A1C levels. Many clinical practice guidelines (1–4) recommend higher A1C levels for older adults on the basis of patient-level factors such as life expectancy and treatment risks. However, A1C treatment goals that include only an upper limit (e.g., <8% or 64 mmol/mol) may place more weight on the risks of hyperglycemia and less on the risks of potential overtreatment, leading to a wide range of A1C levels considered acceptable.
Emerging evidence suggests that both short-term and long-term glycemic variability are linked to diabetes complications (5–16). Target ranges for day-to-day glucose measurements and thresholds for time in range (TIR) have been proposed to minimize risks of hypo- and hyperglycemia and microvascular complications (7). Many older adults with diabetes, and particularly those who are not treated with insulin, may derive limited benefits from periodic or continuous glucose monitoring (17–20), and their glucose control will be tracked by A1C levels. Long-term glycemic variability as measured by fluctuations in A1C over time also predicts diabetes complications, including micro- and macrovascular disease and mortality (8–16). Yet, there are no validated A1C target ranges that associate TIR with diabetes complications. Studies have focused on measures of A1C variability, such as SD or coefficient of variation, but have not linked A1C stability within specific ranges with adverse outcomes.
We developed a patient-level measure that captures A1C stability over time, termed A1C TIR, that is based on A1C target ranges adjusted to each patient’s comorbidities, complications, and life expectancy. To demonstrate the usefulness of this measure as a predictor of adverse outcomes, we assessed the effects of A1C TIR on mortality and incident cardiovascular disease (CVD) in a large nationwide sample of Medicare-eligible veterans.
Research Design and Methods
Study Population
The study was reviewed and approved by the institutional review board at the Department of Veterans Affairs (VA) Boston Healthcare System. We followed criteria by Miller et al. (21) to establish a diabetes diagnosis, which was based on at least one inpatient and/or two outpatient diagnosis codes and/or diabetes medications. Veterans aged ≥65 years who met these criteria were eligible for the study.
Study Timing
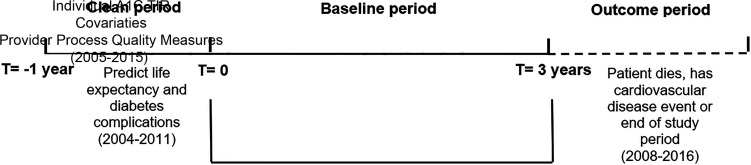
We used administrative and health care utilization data from VA and Medicare from 2004 through 2016. Each patient had a 1-year clean period, a 3-year baseline period, and an outcome period that lasted until they experienced an outcome or the end of 2016, whichever came first. Since outcomes could take many years to develop, we followed patients for as many years as we had available data (through 2016).
The 1-year clean period was used to determine the presence of diabetes complications and to predict life expectancy before the baseline period. This information was used to set the individual’s initial A1C target range during the baseline (see Calculating A1C TIR for more details).
To determine A1C TIR, we required that patients have at least four A1C tests during the 3-year baseline period, with tests no more than 1 year apart. VA performance metrics during the study period required annual A1C testing for patients with diabetes (22). Requiring four A1C tests during the 3-year baseline period identified patients who were more reliant on the VA for their health care, increasing the reliability of the A1C TIR because laboratory results were only available from VA data. Patients could enter the clean period from 2004 to 2011 and start the baseline period between 2005 and 2012, with follow-up through 2016 or when a participant experienced an outcome (Fig. 1).
Figure 1.
Study design. T, time.
The final sample size for the main analyses was 402,043 for mortality models after excluding patients with missing covariate data and those living outside the U.S. For incident myocardial infarction (MI) and stroke models, the sample size was 388,515 after excluding patients who had these diagnoses during the clean and baseline periods (Supplementary Appendix A).
Calculating A1C TIR
Patient-level A1C TIR was calculated as the percentage of days during the 3-year baseline period that a patient’s A1C level was within an individualized target range. We used the VA/Department of Defense Diabetes Clinical Practice Guideline (2) to define individual A1C target ranges. This guideline proposes upper and lower bounds for A1C values and proposes different A1C target ranges on the basis of comorbidities, diabetes complications, and life expectancy.
To determine life expectancy, we predicted <5, 5–10, and ≥10 year mortality risk on the basis of 20 comorbidities or procedures, demographics, diabetes medications, biomarker and laboratory tests, and inpatient and outpatient health care utilization (23). The algorithm predicting life expectancy included several biomarkers that were recommended for annual screening (e.g., serum cholesterol, creatinine), so we used the 1-year clean period to minimize missing data on these biomarkers (24). To assess diabetes complications, we applied the Diabetes Complications Severity Index (DCSI) (25,26), with scores of 0–1, 2–3, or ≥4 considered to indicate absent/mild, moderate, or advanced diabetes complications, resp ectively. All predictors of life expectancy and diabetes complications were examined in the 1-year clean period to set the initial A1C target range. A1C target ranges were then updated annually to account for increasing age, new comorbidities, and development of diabetes complications.
To create the A1C TIR measure, we used linear interpolation and extrapolation between A1C levels and test dates to calculate monthly A1C values for the entire baseline period. We then computed A1C TIR as a percentage of time that A1C levels were within the target range (see Supplementary Appendix B for a detailed example). We tested A1C TIR for normality, graphically and statistically, and found that it was not normally distributed. Consequently, it was divided into categories of 20% increments (i.e., 0 to <20%, 20 to <40%, 40 to <60%, 60 to <80%, 80–100%).
Covariates
Baseline covariates included demographics, measures of ability to obtain VA services, indicator variables for the calendar quarter in which a patient entered the outcome period to control for time trends, and VA medical center to control for facility variation. To examine whether A1C TIR was independent from A1C levels and other measures of glycemic variability, we included each individual’s average A1C level and A1C SD throughout the baseline period. We also included the number of A1C tests during baseline since A1C TIR may be affected by the frequency of tests.
Comorbidities were taken from the Elixhauser comorbidity index (27) and were computed separately for each baseline year. Diabetes severity was based on the highest DCSI score (range 0–13) during baseline. We also modeled whether the individual was prescribed several categories of diabetes medications during baseline (e.g., insulin, metformin, sulfonylurea). Medication adherence was measured as a dichotomous indicator using the proportion of days covered by ≥80% for all prescribed diabetes medications.
Other laboratory and clinical measures included serum creatinine, albumin, urine albumin-to-creatinine ratio, BMI, blood lipids (HDL, LDL, triglycerides), and blood pressure. These measures were separated into three categories (i.e., low, normal, high) using clinical criteria, with a separate category if the measure was missing.
Clinical provider characteristics that may correlate with diabetes care were collected. These variables were computed at the clinician level for all other patients cared for by the same provider during the baseline period and included percentage of blood pressure readings >140/90 mmHg, percentage of LDL cholesterol levels >100 mg/dL, and percentage of A1C levels >9% (75 mmol/mol) (28). Also included were clinician type (physician, nurse practitioner, physician assistant, other) and whether the clinician was a primary care provider.
Outcomes
Outcomes included death and incident MI or stroke. The Medicare Vital Status File, which determines the date of death from VA, Medicare, and Social Security Administration data, was used to determine all-cause mortality (29). We combined MI (24,30–32) and stroke (33) into a composite CVD outcome defined as the first occurrence of either event. MI included both ST elevation MI and non–ST elevation MI, and stroke included ischemic and hemorrhagic stroke.
Analyses
We estimated the effects of A1C TIR on the risks of mortality and CVD using separate Cox proportional hazards models for each outcome. Each model included patient A1C TIR as the main explanatory variable as well as all covariates. In the primary analyses, the outcome period extended through 2016 unless the individual experienced a censoring event. In the mortality model, individuals were censored upon death or at the end of 2016. In the CVD model, individuals were censored after experiencing MI or stroke, upon death, or at the end of the study period.
Several sensitivity analyses were conducted. We used separate models to predict mortality and CVD, but individuals are at risk for both outcomes at the same time, and this could lead to biased results (34–36). For the CVD outcome, we used Fine and Gray’s competing risk Cox proportional hazards model using SAS 9.4 statistical software. We also tested whether results held with a shorter follow-up period limited to 24 months. Finally, we included A1C TIR as a continuous variable to test the robustness of the results. All other analyses were conducted using Stata 15 software (37).
Results
Patients in the sample had a mean age 76.9 years and were predominantly White (86.3%) and male (98.8%); 26.4% had DCSI scores >5, and 24.4% were using insulin (Table 1). Fifty-two percent of the sample died, and 21% experienced a stroke or MI during an average outcome period of 5.5 years. Fifteen percent of the sample was followed for <2 years, and 47% were followed for ≥6 years.
Table 1.
Selected descriptive demographic and comorbidity statistics at baseline (N = 402,043)
| Parameter | Mean (SD) or n (%) |
|---|---|
| Demographics | |
| Age at the start of the outcome period (years) | |
| 68–72 | 83,144 (20.7) |
| 73–76 | 98,120 (24.4) |
| 77–81 | 109,330 (27.2) |
| 82–105 | 111,449 (27.7) |
| Sex | |
| Male | 397,009 (98.8) |
| Female | 5,034 (1.3) |
| Race/ethnicity | |
| White | 346,909 (86.3) |
| Black | 43,065 (10.7) |
| Hispanic | 6,164 (1.5) |
| Asian | 1,431 (0.4) |
| Other | 4,474 (1.1) |
| Marital status | |
| Married | 274,368 (68.2) |
| Divorced/separated | 53,872 (13.4) |
| Widowed | 54,560 (13.6) |
| Other | 19,243 (4.8) |
| Years of follow-up | |
| Up to 2 years | 58,800 (14.6) |
| 2 to <4 years | 75,091 (18.7) |
| 4 to <6 years | 75,365 (18.8) |
| 6 to <8 years | 70,310 (17.5) |
| ≥8 years | 122,477 (30.5) |
| Clinical characteristics | |
| A1C during baseline | |
| % | 7.0 (1.0) |
| mmol/mol | 53 |
| A1C SD during baseline | 0.56 (0.5) |
| Number of A1C tests | 6.3 (2.4) |
| DCSI (highest score during the baseline period) | |
| 0 | 29,173 (7.3) |
| 1–2 | 103,321 (25.7) |
| 3–5 | 163,691 (40.7) |
| 6–8 | 87,483 (21.8) |
| ≥9 | 18,375 (4.6) |
| Average BMI during the baseline period (kg/m2) | |
| <18.5 | 647 (0.2) |
| 18.5–24.9 | 51,442 (12.8) |
| 25–29.9 | 152,662 (38.0) |
| 30–39.9 | 162,025 (40.3) |
| ≥40 | 17,962 (4.5) |
| Missing | 17,305 (4.3) |
| Cardiovascular comorbidities | |
| Cardiac arrhythmias | 169,215 (42.1) |
| Cardiovascular | 277,401 (69.0) |
| Cerebrovascular | 121,562 (30.2) |
| Congestive heart failure | 120,914 (30.1) |
| Hypertension | 385,495 (95.9) |
| Medicationsa | |
| Sulfonylureas | 214,792 (53.4) |
| Biguanides | 198,435 (49.4) |
| Insulin | 98,086 (24.4) |
| Thiazolidinediones | 63,362 (15.8) |
| α-Glucosidase inhibitors | 7,694 (1.9) |
| Other medicationb | 6,569 (1.6) |
| Adherence | |
| Any diabetes medications with proportion of days covered ≥80% | 228,430 (56.8) |
| Any diabetes medications with proportion of days covered <80% | 173,613 (43.2) |
A1C data are means (SD); otherwise, data are presented as n (%).
All medications taken by a patient; hence, the percentage represents prevalence within each medication category.
Other medications include amylin analog, bile acid sequestrants, dipeptidyl peptidase inhibitor, dopamine receptor agonist, glucagon-like peptide, meglitinides, and sodium–glucose cotransporter inhibitor.
Target A1C Levels To Determine TIR
Patients were assigned to different A1C target ranges on the basis of life expectancy and diabetes complications, and these varied from 14% with an A1C target range of 6.0–7.0% (42–53 mmol/mol) to 17% with an A1C target range of 8.0–9.0% (64–75 mmol/mol) (Table 2).
Table 2.
A1C target ranges for the study sample (N = 402,403)
| Diabetes complications | |||
|---|---|---|---|
| Life expectancya | Absent or mild (DCSI = 0–1) | Moderate (DCSI = 2–3) | Advanced (DCSI ≥4) |
| >10 years | |||
| A1C range, % (mmol/mol) | 6.0–7.0 (42–53) | 7.0–8.0 (53–64) | 7.5–8.5 (58–69) |
| n (%) | 56,124 (14.0) | 73,968 (18.4) | 82,764 (20.6) |
| 5–10 years | |||
| A1C range, % (mmol/mol) | 7.0–8.0 (53–64) | 7.5–8.5 (58–69) | 7.5–8.5 (58–69) |
| n (%) | 13,069 (3.3) | 31,293 (7.8) | 76,873 (19.1) |
| <5 years | |||
| A1C range, % (mmol/mol) | 8.0–9.0 (64–75) | 8.0–9.0 (64–75) | 8.0–9.0 (64–75) |
| n (%) | 3,926 (1.0) | 12,278 (3.1) | 51,748 (12.9) |
Life expectancy was based on predicting the likelihood of mortality in <5 years, 5–10 years, and ≥10 years in the 3rd year of baseline.
A1C TIR and Clinical Outcomes
The average baseline A1C for the study sample was 7.0% (53 mmol/mol). Among the A1C TIR categories, mean A1C was lowest for the groups with A1C TIR 0 to <20% and 80–100%. A1C SD decreased with increasing A1C TIR (Supplementary Appendix C).
Sixty-eight percent of the sample was in the A1C TIR 0 to <20% or 20 to <40% category, with the remaining 32% having A1C TIR ≥40% during the baseline period (Table 3). There was a graded relationship between lower A1C TIR and higher mortality and CVD outcomes. In unadjusted analyses, patients with A1C TIR 0 to <20% had a 58.2% mortality rate during the outcome period compared with 31.3% with A1C TIR 80–100%. For CVD events, these clinical outcomes were 22.6% and 14.7%, respectively (data not shown).
Table 3.
HRs of A1C TIR predicting mortality and CVD
| Cox proportional hazards model | HR | 95% CI | P value |
|---|---|---|---|
| Mortality (n = 402,043) | |||
| Individual A1C TIR (reference 80–100%, n = 36,642) | |||
| 60 to <80% (n = 41,042) | 1.10 | 1.07–1.12 | <0.001 |
| 40 to <60% (n = 51,609) | 1.11 | 1.09–1.14 | <0.001 |
| 20 to <40% (n = 76,801) | 1.14 | 1.12–1.16 | <0.001 |
| 0 to <20% (n = 195,949) | 1.22 | 1.20–1.25 | <0.001 |
| A1C SD during baseline | 1.14 | 1.13–1.16 | <0.001 |
| A1C average during baseline | 1.04 | 1.03–1.05 | <0.001 |
| Myocardial infarction and stroke (n = 388,515) | |||
| Individual A1C TIR (reference 80–100%, n = 36,309) | |||
| 60 to <80% (n = 40,181) | 1.06 | 1.02–1.10 | <0.001 |
| 40 to <60% (n = 50,015) | 1.07 | 1.04–1.11 | <0.001 |
| 20 to <40% (n = 73,980) | 1.08 | 1.04–1.11 | <0.001 |
| 0 to <20% (n = 188,030) | 1.14 | 1.11–1.19 | <0.001 |
| A1C SD during baseline | 1.03 | 1.01–1.05 | <0.001 |
| A1C average during baseline | 1.12 | 1.11–1.13 | <0.001 |
Model also includes all covariates listed in Supplementary Appendix D when predicting the outcomes.
In Cox proportional hazards models that controlled for covariates, including mean A1C level, A1C SD, and number of A1C tests, A1C TIR retained a significant relationship with mortality and CVD (Table 3). The risk of mortality increased with decreasing A1C TIR. Compared with A1C TIR 80–100%, the hazard ratio (HR) was 1.10 (95% CI 1.07–1.12) for A1C TIR 60 to <80%, 1.11 (95% CI 1.09–1.14) for A1C TIR 40 to <60%, 1.14 (95% CI 1.12–1.16) for A1C TIR 20 to <40%, and 1.22 (95% CI 1.20–1.25) for A1C TIR 0 to <20%.
Similar results emerged for the CVD outcome. Compared with A1C TIR 80–100%, the HR was 1.06 (95% CI 1.02–1.10) for A1C TIR 60 to <80%, 1.07 (95% CI 1.04–1.11) for A1C TIR 40 to <60%, 1.08 (95% CI 1.04–1.11) for A1C TIR 20 to <40%, and 1.14 (95% CI 1.11–1.19) for A1C TIR 0 to <20%.
Other covariates also had significant relationships with mortality and CVD. Older veterans, higher DCSI scores, insulin use, elevated urine albumin-to-creatine ratio, and congestive heart failure had increased risk of both outcomes. Biguanide and thiazolidinedione use were associated with lower risk of both outcomes (Supplementary Appendix D).
Sensitivity Analyses
Sensitivity models further supported the relationship between A1C TIR and adverse outcomes (Supplementary Appendix E). In models that assessed a shorter outcome period (i.e., 24 months) lower A1C TIR was associated with a higher risk of mortality (A1C TIR 0 to <20%: HR 1.28 [95% CI 1.22–1.34]) and CVD events (A1C TIR 0 to <20%: HR 1.19 [95% CI 1.12–1.26]). Similarly, in a competing risk model that predicted CVD events with the competing risk of mortality, patients with higher A1C TIR had a lower risk of these outcomes (A1C TIR 0 to <20%: HR 1.11 [95% CI 1.08–1.14]). In models that included A1C TIR in linear form, there was a significant negative relationship between higher TIR and mortality and CVD outcomes (data not shown).
Conclusions
We show that in older adults with diabetes, maintaining stability of A1C levels within individualized target ranges over a 3-year period is associated with reduced risk of mortality and CVD outcomes. A1C TIR was based on comorbidities, complications, and life expectancy, and outcomes were measured over an average of 5.5 years and up to 9 years. The results were robust, with models that controlled for several relevant covariates, including mean A1C levels, A1C SD, and number of A1C tests.
These findings advance diabetes care in two important ways. First, A1C goal setting in older adults often balances reducing risks of acute hyperglycemia and microvascular complications with minimizing potential burdens and harms from hypoglycemia and polypharmacy. Clinical practice guidelines recognize these exigencies and propose treatment goals, often with higher A1C levels, that account for patients’ unique characteristics and goals of care (1–4). However, A1C treatment goals that include only an upper limit may infer that a wide range of levels, including normal levels, may be acceptable. Our findings suggest that examining the time spent within specific A1C ranges with upper and lower bounds may have important implications. Patients with lower A1C TIR were more likely to experience mortality and CVD outcomes. Second, several studies have shown that increased short-term and long-term glycemic variability are risk factors for diabetes complications (5,6,8–16). Measuring short-term glycemic variability requires frequent glucose testing with continuous glucose monitoring or conventional fingerstick methods. There are limited data on the benefits of daily glucose monitoring in older adults, although a recent study suggests that continuous glucose monitoring may identify patients with increased risk of all-cause and CVD mortality (18,19,38). Periodic A1C levels remain a mainstay for monitoring glucose control, and A1C TIR may be useful to risk stratify such patients.
Future studies may consider the influence of A1C TIR on other adverse events, such as hypoglycemia and microvascular complications, since increasing A1C variability is associated with each of these outcomes (39,40). One key question is whether individuals who are consistently above their A1C target range will experience different risks for microvascular or macrovascular complications than individuals who are consistently below their A1C target range.
Strengths and Limitations
Our study has several strengths. We used a large nationwide sample of veterans with an extended follow-up period. The study design used a baseline period followed by an outcome period to minimize the possibility of reverse causation. The results were robust, and sensitivity analyses with a shorter outcome period corroborated the main results. Because it is unlikely that a randomized trial could be conducted to test the effects of A1C TIR on adverse outcomes, observational studies are necessary to define such associations. We used data that are regularly included in electronic health records, so it is also possible to present A1C TIR as a measure of long-term glycemic stability to clinicians at the point of care.
Our study also has limitations. The study population was ≥65 years of age and predominantly male. Results may not generalize to females or younger individuals. Medicare data do not include laboratory test results, so we may be missing some A1C or other laboratory tests that were performed outside the VA. Diabetes complications tend to track with longer duration of disease, but we were unable to reliably determine duration of diabetes because this is not coded in administrative data. Our risk-adjusted A1C target ranges and the impact of greater A1C TIR on health outcomes derive from population data. Ultimately, risk assessments are best applied at the individual level. Treating older adults with diabetes should also consider individual circumstances beyond comorbidities and complications. Maintaining A1C stability over time may be affected by frailty or financial or social instability, and these are not always captured in coded health data.
This is an observation study. Unobserved factors, such as nutrition and self-management, may confound the apparent association among A1C TIR, mortality, and CVD outcomes, and we cannot affirm causality. While there are different clinical strategies for maintaining A1C stability, our results cannot assert that prospectively targeting A1C TIR as a treatment goal will reduce risks of adverse outcomes.
In summary, we show that in older adults with diabetes, maintaining A1C levels within specific and unique ranges over time is associated with a lower risk of mortality and CVD outcomes. These results support using both a personalized approach to A1C goal setting and A1C stability over time when treating older patients with diabetes.
Article Information
Acknowledgments. The authors are indebted to Rebecca Lamkin at VA Boston Healthcare System for invaluable administrative support.
Funding. This work was supported by the U.S. Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development (IIR 15-116), and the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-114098). Data were obtained with support from the VA Information Resource Center, VA/Centers for Medicare & Medicaid Services Data for Research Project (SDR 02-23).
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the VA or the U.S. Government, Boston University, or University of Utah.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.C.P., D.C.M., L.Z., D.L., and A.L. contributed to the statistical analyses. J.C.P., D.C.M., L.Z., D.L., A.L., R.E.N., and P.R.C. contributed to the interpretation of the data and drafting and critical revision of the manuscript. J.C.P. and P.R.C. contributed to the design of the study. L.Z., D.L., and A.L. contributed to the acquisition of data. J.C.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14515170.
References
- 1.American Diabetes Association . 6. Glycemic targets. Diabetes Care 2017;40:S48–S56 [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Veterans Affairs . VA/DOD Clinical Practice Guidelines: Management of Diabetes Mellitus in Primary Care. Accessed 17 April 2020. Available from https://www.healthquality.va.gov/guidelines/CD/diabetes/
- 3.LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of diabetes in older adults: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab 2019;104:1520–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ; Clinical Guidelines Committee of the American College of Physicians . Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018;168:569–576 [DOI] [PubMed] [Google Scholar]
- 5.Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019;42:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018;41:2370–2376 [DOI] [PubMed] [Google Scholar]
- 7.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penno G, Solini A, Bonora E, et al.; Renal Insufficiency and Cardiovascular Events Study Group . HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: the Renal Insufficiency and Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care 2013;36:2301–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takao T, Matsuyama Y, Yanagisawa H, Kikuchi M, Kawazu S. Association between HbA1c variability and mortality in patients with type 2 diabetes. J Diabetes Complications 2014;28:494–499 [DOI] [PubMed] [Google Scholar]
- 10.Hirakawa Y, Arima H, Zoungas S, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care 2014;37:2359–2365 [DOI] [PubMed] [Google Scholar]
- 11.Zoppini G, Verlato G, Targher G, Bonora E, Trombetta M, Muggeo M. Variability of body weight, pulse pressure and glycaemia strongly predict total mortality in elderly type 2 diabetic patients. The Verona Diabetes Study. Diabetes Metab Res Rev 2008;24:624–628 [DOI] [PubMed] [Google Scholar]
- 12.Prentice JC, Pizer SD, Conlin PR. Identifying the independent effect of HbA1c variability on adverse health outcomes in patients with type 2 diabetes. Diabet Med 2016;33:1640–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes A, Murrells T, Mulnier H, Sinclair AJ. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol 2018;6:476–486 [DOI] [PubMed] [Google Scholar]
- 14.Critchley JA, Carey IM, Harris T, DeWilde S, Cook DG. Variability in glycated hemoglobin and risk of poor outcomes among people with type 2 diabetes in a large primary care cohort study. Diabetes Care 2019;42:2237–2246 [DOI] [PubMed] [Google Scholar]
- 15.Sheng CS, Tian J, Miao Y, et al. Prognostic significance of long-term HbA1c variability for all-cause mortality in the ACCORD trial. Diabetes Care 2020;43:1185–1190 [DOI] [PubMed] [Google Scholar]
- 16.Gorst C, Kwok CS, Aslam S, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care 2015;38:2354–2369 [DOI] [PubMed] [Google Scholar]
- 17.Young LA, Buse JB, Weaver MA, et al.; Monitor Trial Group . Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med 2017;177:920–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malanda UL, Welschen LM, Riphagen II, Dekker JM, Nijpels G, Bot SD. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev 2012;1:CD005060. [DOI] [PubMed] [Google Scholar]
- 19.Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 2012;35:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park C, Le QA. The effectiveness of continuous glucose monitoring in patients with type 2 diabetes: a systemic review of literature and meta-analysis. Diabetes Technol Ther 2018;20:613–621 [DOI] [PubMed] [Google Scholar]
- 21.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care 2004;27:B10–B21 [DOI] [PubMed] [Google Scholar]
- 22.Kerr EA, Fleming B. Making performance indicators work: experiences of US Veterans Health Administration. BMJ 2007;335:971–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith KN, Prentice JC, Mohr DC, Conlin PR. Predicting 5- and 10-year mortality risk in older adults with diabetes. Diabetes Care 2020;43:1724–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prentice JC, Conlin PR, Gellad WF, Edelman D, Lee TA, Pizer SD. Capitalizing on prescribing pattern variation to compare medications for type 2 diabetes. Value Health 2014;17:854–862 [DOI] [PubMed] [Google Scholar]
- 25.Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care 2008;14:15–23 [PMC free article] [PubMed] [Google Scholar]
- 26.Glasheen WP, Renda A, Dong Y. Diabetes Complications Severity Index (DCSI)-update and ICD-10 translation. J Diabetes Complications 2017;31:1007–1013 [DOI] [PubMed] [Google Scholar]
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27 [DOI] [PubMed] [Google Scholar]
- 28.National Committee for Quality Assurance . The State of Health Care Quality 2011: Continuous Improvement and the Expansion of Quality Measurement. Washington, DC, National Committee for Quality Assurance, 2011 [Google Scholar]
- 29.Arnold N, Maynard C, Hynes DM. VIReC Technical Report 2: VANDI Mortality Data Merge Project. Edward Hines, Jr. VA Hospital, Hines, IL: VA Information Resource Center, April 9, 2006. https://vaww.virec.research.va.gov/References/Techn icalReports/VIReCTechnicalReports.htm
- 30.Petersen LA, Wright S, Normand SL, Daley J. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med 1999;14:555–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J 2004;148:99–104 [DOI] [PubMed] [Google Scholar]
- 32.Patel AB, Hude Q, Welsh RC, et al. Validity and utility of ICD-10 administrative health data for identifying ST- and non-ST-elevation myocardial infarction based on physician chart review. CMAJ Open 2015;3:E413–E418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothendler JA, Rose AJ, Reisman JI, Berlowitz DR, Kazis LE. Choices in the use of ICD-9 codes to identify stroke risk factors can affect the apparent population-level risk factor prevalence and distribution of CHADS2 scores. Am J Cardiovasc Dis 2012;2:184–191 [PMC free article] [PubMed] [Google Scholar]
- 34.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999;18:695–706 [DOI] [PubMed] [Google Scholar]
- 35.Southern DA, Faris PD, Brant R, et al.; APPROACH Investigators . Kaplan-Meier methods yielded misleading results in competing risk scenarios. J Clin Epidemiol 2006;59:1110–1114 [DOI] [PubMed] [Google Scholar]
- 36.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res 2007;13:559–565 [DOI] [PubMed] [Google Scholar]
- 37.StataCorp . Stata Statistical Software: Release 15. College Station, TX, StataCorp LLC, 2017 [Google Scholar]
- 38.Lu J, Wang C, Shen Y, et al. Time in range in relation to all-cause and cardiovascular mortality in patients with type 2 diabetes: a prospective cohort study. Diabetes Care 2021;44:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong VW, Juhaeri J, Cole SR, et al. HbA1C variability and hypoglycemia hospitalization in adults with type 1 and type 2 diabetes: a nested case-control study. J Diabetes Complications 2018;32:203–209 [DOI] [PubMed] [Google Scholar]
- 40.Zhao MJY, Prentice JC, Mohr DC, Conlin PR. Association between hemoglobin A1c variability and hypoglycemia-related hospitalizations in veterans with diabetes mellitus. BMJ Open Diabetes Res Care 2021;9:e001797. [DOI] [PMC free article] [PubMed] [Google Scholar]