Levels of microRNAs (miRNAs) within extracellular vesicles (EVs) have been shown to be useful diagnostic and prognostic biomarkers in a number of disease states [1–3]. However, EVs miRNAs have never been investigated in COVID-19.
We recently demonstrated that miR-24 is expressed in human brain endothelial cells (ECs) and targets Neuropilin-1 [4], a co-factor needed for SARS-CoV-2 internalization that has been linked to cerebrovascular (CBV) manifestations of COVID-19 [5]. Henceforth, we hypothesized an association between plasma levels of endothelial EV miR-24 and the onset of CBV events in patients hospitalized for COVID-19. CBV events were defined by the presence of ischemic or hemorrhagic stroke (confirmed by imaging), migraine, or transient ischemic attack (no findings at imaging evaluation).
We obtained plasma from 369 patients hospitalized for COVID-19, consecutively enrolled from November 2020 to April 2021 at the “Ospedali dei Colli”. We excluded 48 patients with a history of CBV disease, cancer, atrial fibrillation, deep vein thrombosis, or unavailability of admission blood samples; thus, the study was conducted in 321 subjects. As a control age- and sex-matched COVID-19 negative population, we obtained plasma from 57 healthy donors and 37 patients with CBV disorders. A SARS-CoV-2 test (RT-qPCR) was performed in all subjects to confirm or rule out the COVID-19 diagnosis. EC-EVs were extracted from the plasma collected from these patients via serial centrifugation and CD31+ magnetic isolation [1], and EC-EVs miR-24 levels were quantified as described [1, 4, 6].
Clinical parameters of our population are reported in Table 1. CBV events were diagnosed in 58 COVID-19 patients. No significant differences in comorbidities and in therapeutic management were observed. We found that EC-EV miR-24 levels were significantly reduced in patients with vs without CBV disorders among COVID-19 patients, but not when examining subjects without COVID-19 (Table 1). These results were confirmed when subdividing our population according to the presence of ischemic or hemorrhagic findings at imaging evaluation (Fig. 1). Strikingly, using a stepwise multiple regression analysis, adjusting for age, hypertension, dyslipidemia, diabetes, and D-dimer, the association between EC-EV miR-24 and CBV disease in COVID-19 patients was confirmed [Wald: 17.723; Exp(B): 0.955, C.I. 95%: 0.935–0.976, P < 0.001].
Table 1.
Main characteristics of our population
| COVID-19 negative | COVID-19 positive | |||||
|---|---|---|---|---|---|---|
| NO CBV | CBV | P | NO CBV | CBV | P | |
| (57) | (37) | (263) | (58) | |||
| Age (years) | 59.4 ± 14.78 | 65.37 ± 12.75* | 0.04 | 61.5 ± 14.2 | 63.6 ± 14.6 | 0.292 |
| Sex (male, %) | 50.9 | 59.5 | 0.42 | 54.7 | 55.1 | 0.954 |
| BMI (kg/m2) | 25.62 ± 3.83 | 25.44 ± 2.9 | 0.8 | 24.93 ± 3.59 | 25.01 ± 2.9 | 0.865 |
| SBP (mmHg) | 133.88 ± 16.1 | 143.08 ± 19.3* | 0.014 | 137.76 ± 19.5 | 142.2 ± 19.3 | 0.114 |
| DBP (mmHg) | 79.93 ± 9.5 | 84.38 ± 8.8* | 0.025 | 84.46 ± 9.47 | 86.3 ± 14.9 | 0.239 |
| Hypertension (%) | 26.3 | 51.3* | 0.013 | 39.9 | 44.8 | 0.493 |
| Glycemia (mg/dl) | 104.9 ± 22.2 | 112.2 ± 27.8 | 0.163 | 109.39 ± 28.2 | 112.48 ± 42.7 | 0.497 |
| Diabetes (%) | 7.0 | 21.6* | 0.039 | 12.5 | 17.2 | 0.344 |
| Dyslipidemia (%) | 24.5 | 43.2 | 0.059 | 30.8 | 34.4 | 0.586 |
| Smoking (current/past, %) | 14/22.8 | 21.6/37.8* | 0.032 | 18.2/26.6 | 12.1/34.4# | 0.081 |
| D-dimer (µg/ml) | 2.35 ± 1.73 | 3.52 ± 0.95* | 0.001 | 2.80 ± 1.68 | 3.18 ± 1.83 | 0.120 |
| IL-6 (pg/ml) | 1.7 ± 1.1 | 4.0 ± 2.8* | 0.002 | 7.5 ± 4.0# | 8.4 ± 5.5# | 0.121 |
| TNFα (pg/ml) | 4.5 ± 2.3 | 6.0 ± 4.7* | 0.035 | 6.5 ± 4.2 | 5.8 ± 4.7 | 0.271 |
| hs-CRP (µg/ml) | 2.15 ± 1.1 | 2.6 ± 1.16 | 0.07 | 3.6 ± 3.2# | 4.2 ± 2.9# | 0.144 |
| EC-EV miR-24 (copies/10 nl) | 30.5 ± 14.6 | 29.85 ± 15.5 | 0.827 | 26.64 ± 20.9# | 15.41 ± 14.7*,# | 0.001 |
All P values in the table are reported in italic
Data on quantitative parameters are expressed as mean ± standard deviation; data on qualitative characteristics are expressed as percentage values or absolute numbers. BMI: Body mass index; CBV: cerebrovascular (events); DBP: diastolic blood pressure; EC-EV miR-24: level of miR-24 shuttled by endothelial (CD31+) extracellular vesicles; hs-CRP: high-sensitivity C-reactive protein; IL-6: interleukin-6; SBP: systolic blood pressure; and TNFα: tumor necrosis factor α. Following verification of normality (Shapiro–Wilk’s test) and equal variance (Bartlett’s test), continuous variables were compared using ANOVA followed by Tukey–Kramer test for independent samples, whereas variables not normally distributed were compared via the Kruskal–Wallis test; categorical data were compared using the χ2 test; *P < 0.05 versus NO CBV; #P < 0.05 versus COVID-19 negative
Fig. 1.
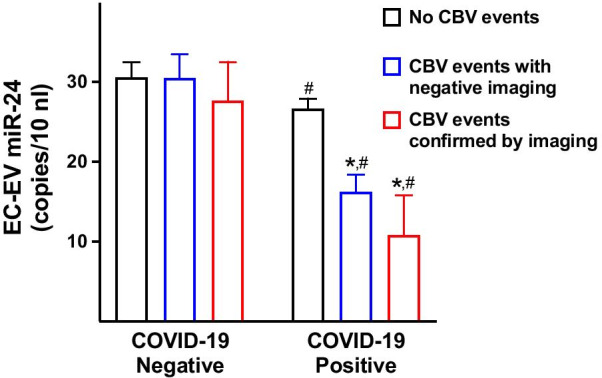
miR-24 levels were measured within endothelial extracellular vesicles (EC-EV), identified by the endothelial marker CD31. Cerebrovascular events (CBV) were divided in events with no findings at imaging evaluation, which included transient ischemic attacks (TIA) and migraine (blue bars), and ischemic or hemorrhagic stroke confirmed by imaging (red bars). Data are represented as mean ± SE; *P < 0.05 versus NO CBV; #P < 0.05 versus COVID-19 Negative
To our knowledge, this is the first study showing an association between EC-EV non-coding RNA and clinical outcome in COVID-19 patients.
The main limitation of the present study is the relatively small size of our population; moreover, our findings, which are limited to Caucasian individuals, refer to subjects that have been hospitalized for COVID-19 and therefore cannot be generalized to patients with a mild disease.
We identified a significant association linking EC-EV miR-24 and CBV disorders, which could be valuable to understand the mechanisms underlying the pathophysiology of CBV complications in COVID-19. Indeed, low levels of EC-EV miR-24 suggest an increased expression of Neuropilin-1 in ECs [4]. Further analyses in larger groups are warranted to ratify our results, confirm their prognostic value, and investigate the role of miR-24 in other COVID-19-related neurologic events.
Acknowledgements
We thank Drs. Stanislovas S. Jankauskas, Daniela Sorriento, Xujun Wang, and Anna Annunziata for helpful discussion.
Authors' contributions
JG, AC, RI, and GF obtained data. JG, BT, and GS analyzed and discussed the data. JG and GS wrote the manuscript. All authors read and approved the final manuscript.
Funding
The Santulli Lab is supported in part by the National Institutes of Health (NIH: R01-DK123259, R01-DK033823, R01-HL146691, R01-HL159062, R56-AG066431, and T32-HL144456 to G.S.), by the Irma T. Hirschl and Monique Weill-Caulier Trusts (to G.S.), and by the AHA (20POST35211151) to J.G.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and was approved by the local Institutional Review Board, and informed consent was obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prattichizzo F, De Nigris V, Sabbatinelli J, Giuliani A, Castano C, Parrizas M, Crespo I, Grimaldi A, Baranzini N, Spiga R, et al. CD31(+) extracellular vesicles from patients with type 2 diabetes shuttle a miRNA signature associated with cardiovascular complications. Diabetes. 2021;70(1):240–254. doi: 10.2337/db20-0199. [DOI] [PubMed] [Google Scholar]
- 2.Mori MA, Ludwig RG, Garcia-Martin R, Brandao BB, Kahn CR. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. 2019;30(4):656–673. doi: 10.1016/j.cmet.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santulli G. microRNA: Medical Evidence - From Molecular Biology to Clinical Practice. Hardcover Book (Springer International Publishing, Switzerland), 2015; ISBN: 978-3-319-22670-5. 10.1007/978-3-319-22671-2 .
- 4.Mone P, Gambardella J, Wang X, Jankauskas SS, Matarese A, Santulli G. miR-24 Targets the Transmembrane Glycoprotein Neuropilin-1 in Human Brain Microvascular Endothelial Cells. Noncoding RNA. 2021;7(1):9. doi: 10.3390/ncrna7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayi BS, Leibowitz JA, Woods AT, Ammon KA, Liu AE, Raja A. The role of Neuropilin-1 in COVID-19. PLoS Pathog. 2021;17(1):e1009153. doi: 10.1371/journal.ppat.1009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santulli G, Wronska A, Uryu K, Diacovo TG, Gao M, Marx SO, Kitajewski J, Chilton JM, Akat KM, Tuschl T, et al. A selective microRNA-based strategy inhibits restenosis while preserving endothelial function. J Clin Invest. 2014;124(9):4102–4114. doi: 10.1172/JCI76069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


