To the Editor: In organ-transplant recipients, the standard two-dose vaccination strategy for coronavirus disease 2019 (Covid-19) has suboptimal immunogenicity.1 Both patients and health care providers have questioned whether a third-dose booster in transplant recipients would be safe and enhance immune response.2 We performed a double-blind, randomized, controlled trial of a third dose of mRNA-1273 vaccine (Moderna) as compared with placebo (the protocol is available with the full text of this letter at NEJM.org; ClinicalTrials.gov number, NCT04885907).
Transplant recipients who had received two doses of mRNA-1273 were randomly assigned in a 1:1 ratio to receive either a third dose of mRNA-1273 vaccine or saline placebo 2 months after the second dose of mRNA-1273 (dosing schedule: 0, 1, and 3 months). The primary outcome was a serologic response characterized by an anti–receptor-binding domain (RBD) antibody level of at least 100 U per milliliter at month 4 (measured with an Elecsys Anti-SARS-CoV-2 immunoassay [Roche]). This outcome was prespecified and was based on the protective anti-RBD titer in a challenge study involving nonhuman primates3; it was further corroborated in a large clinical cohort as the upper boundary of the estimated level required to confer 50% protective neutralization.4 Secondary outcomes included the percent neutralization, as measured with a validated surrogate virus neutralization assay (Genscript), and the polyfunctional T-cell response (see the Supplementary Appendix, available at NEJM.org).
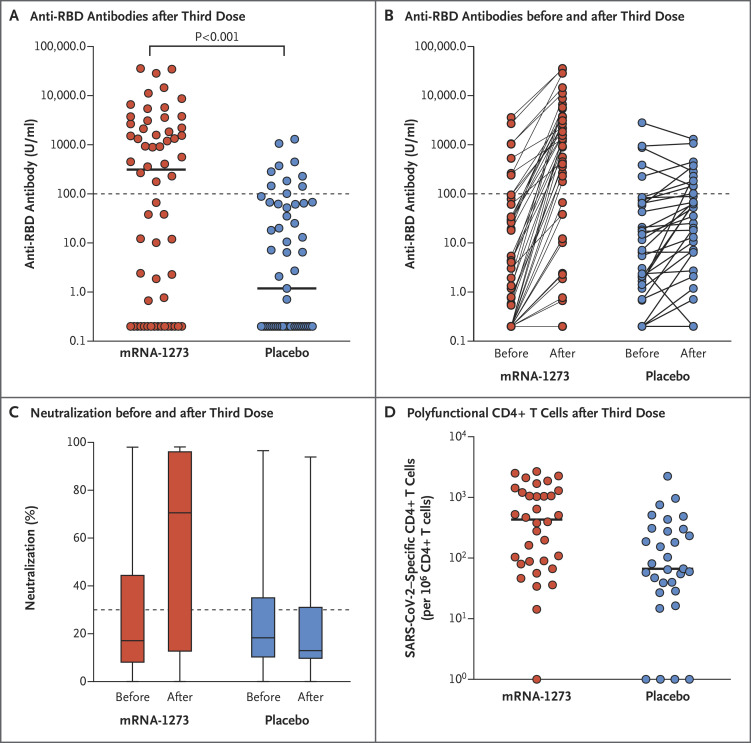
We enrolled 120 organ-transplant recipients (Fig. S1 in the Supplementary Appendix). No patient had a previous diagnosis of Covid-19. The baseline characteristics were similar in the two groups (Table S1), as were the preintervention anti-RBD antibody levels and neutralizing antibody levels (Figure 1B, 1C, and 1D). The median age of the patients was 66.6 years (interquartile range, 63.3 to 71.4), and the median time from transplantation to the third dose was 3.16 years (interquartile range, 1.71 to 6.12). The time from transplantation was slightly shorter in the placebo group than in the mRNA-1273 group; however, the types, doses, and levels of immunosuppression were very similar in the two groups, as were the lymphocyte counts. Covid-19 developed in 1 patient (placebo group; preinfection anti-RBD antibody level, 75 U per milliliter), and 2 patients did not provide follow-up blood specimens.
Figure 1. Immune Responses in Transplant Recipients Who Received a Third Dose of mRNA-1273 or Placebo.
Panel A shows the anti–receptor-binding domain (RBD) antibody levels in the mRNA-1273 group (60 patients) and the placebo group (57 patients) after the third dose. Each point represents an individual patient, and horizontal lines indicate the median. The dotted line indicates the threshold value of 100 U per milliliter. Values below the detection limit are plotted as 0.2 U per milliliter. The relative risk of being above the threshold in the mRNA-1273 group as compared with the placebo group was 3.1 (95% confidence interval [CI], 1.7 to 5.8; P<0.001). Panel B shows the anti-RBD antibody levels before and after the third dose. Panel C shows box-and-whisker plots of the percent neutralization before and after the third dose. The whiskers indicate the range, the top and bottom of the boxes indicate the interquartile range, and the horizontal line within each box indicates the median. The dotted line indicates the 30% threshold for neutralizing antibody positivity. For percent neutralization, the 95% CI for the between-group difference was 11 to 76 percentage points. The relative risk of being above the 30% threshold in the mRNA-1273 group as compared with the placebo group was 2.4 (95% CI, 1.5 to 4.0). Panel D shows the polyfunctional CD4+ T-cell response (i.e., cells producing both interleukin-2 and interferon-γ) before and after the third dose in the mRNA-1273 group (34 patients) and the placebo group (31 patients). Horizontal lines indicate the median (95% CI for the between-group difference, 46 to 986). The widths of the confidence intervals have not been adjusted for multiplicity and cannot be used to infer treatment effects for secondary end points.
At month 4, an anti-RBD antibody level of at least 100 U per milliliter was present in 33 of 60 patients (55%) in the mRNA-1273 group and in 10 of 57 patients (18%) in the placebo group (relative risk, 3.1; 95% confidence interval [CI], 1.7 to 5.8; P<0.001) (Figure 1A and Table S2). The changes in anti-RBD antibody level from before to after the third dose are shown in Figure 1B. After the third dose, the median percent virus neutralization was 71% in the mRNA-1273 group and 13% in the placebo group (95% CI for the between-group difference, 11 to 76 percentage points), and the percentage of patients above the 30% threshold for neutralizing antibody positivity was 60% and 25%, respectively (relative risk, 2.4; 95% CI, 1.5 to 4.0) (Figure 1C and Table S2). Median severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific T-cell counts were greater after the third dose in the mRNA-1273 group than in the placebo group (432 vs. 67 cells per 106 CD4+ T cells; 95% CI for the between-group difference, 46 to 986) (Figure 1D). There was a minimal polyfunctional CD8+ T-cell response in both groups. In the safety evaluation, local and systemic events were slightly more common after the third dose of mRNA-1273 than after the dose of placebo (Fig. S3), but no grade 3 or 4 events and no cases of acute rejection occurred.
A third dose of mRNA vaccine in transplant recipients had substantially higher immunogenicity than placebo, as determined in our analysis of both primary and secondary trial end points. This trial had short follow-up and was not powered to detect differences in clinical outcomes. We also acknowledge that the cutoff value of 100 U per milliliter for the anti-RBD antibody level is arbitrary and is not necessarily predictive of resistance to infection. A third dose was safe when risk versus benefit was considered. We note that a small subgroup of patients who received placebo did have modest increases in antibody levels (Figure 1B). This may reflect ongoing mRNA vaccine–induced B-cell stimulation, as recently described,5 and highlights the importance of evaluating a control group. We conclude that a third-dose booster Covid-19 vaccine should be considered, in conjunction with regulatory approval, for transplant recipients who have received two doses of mRNA-1273.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This letter was published on August 11, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by the Ajmera Transplant Centreand the Di Poce Transplant Fund, University Health Network, University of Toronto. Vaccine was provided by the University Health Network pharmacy. Moderna had no role in funding the trial or in the design, conduct, analysis, or any other aspect of the trial.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021;325:2204-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med 2021;385:661-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021;590:630-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205-1211. [DOI] [PubMed] [Google Scholar]
- 5.Turner JS, O’Halloran JA, Kalaidina E, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021. June 28 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.