To the Editor: The Centers for Disease Control and Prevention recently reported cases of myocarditis and pericarditis in the United States after coronavirus disease 2019 (Covid-19) messenger RNA (mRNA) vaccination.1 In recently published reports, diagnosis of myocarditis was made with the use of noninvasive imaging and routine laboratory testing.2-5 Here, we report two cases of histologically confirmed myocarditis after Covid-19 mRNA vaccination.
Patient 1, a 45-year-old woman without a viral prodrome, presented with dyspnea and dizziness 10 days after BNT162b2 vaccination (first dose). A nasopharyngeal viral panel was negative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), influenza A and B, enteroviruses, and adenovirus (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). A serum polymerase-chain-reaction (PCR) assay and serologic tests showed no evidence of active parvovirus, enterovirus, human immunodeficiency virus, or infection with SARS-CoV-2. At presentation, she had tachycardia; ST-segment depression detected on electrocardiography, which was most prominent in the lateral leads (Fig. S1); and a troponin I level of 6.14 ng per milliliter (reference range, 0 to 0.30). A transthoracic echocardiogram showed severe global left ventricular systolic dysfunction (ejection fraction, 15 to 20%) and normal left ventricular dimensions. Right heart catheterization revealed elevated right- and left-sided filling pressures and a cardiac index of 1.66 liters per minute per square meter of body-surface area as measured by the Fick method. Coronary angiography revealed no obstructive coronary artery disease. An endomyocardial biopsy specimen showed an inflammatory infiltrate predominantly composed of T-cells and macrophages, admixed with eosinophils, B cells, and plasma cells (Figure 1A and Figs. S2 through S4). She received inotropic support, intravenous diuretics, methylprednisolone (1 g daily for 3 days), and, eventually, guideline-directed medical therapy for heart failure (lisinopril, spironolactone, and metoprolol succinate). Seven days after presentation, her ejection fraction was 60%, and she was discharged home.
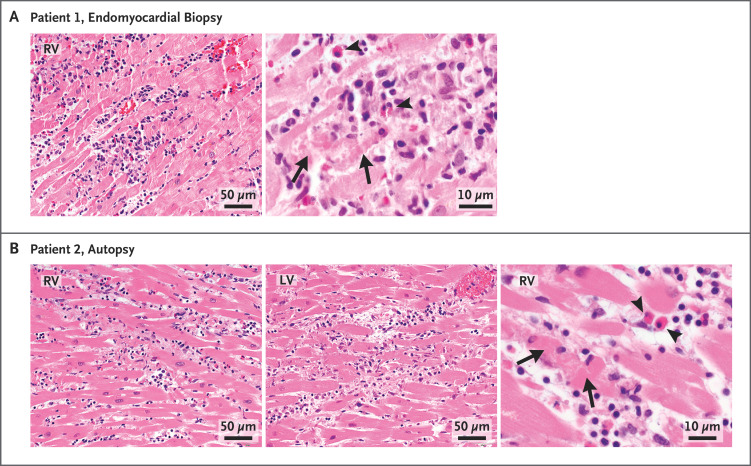
Figure 1. Histopathological Findings from Endomyocardial Biopsy and Autopsy.
Hematoxylin–eosin stains of heart-tissue specimens obtained by means of endomyocardial biopsy in patient 1 (Panel A) and autopsy in patient 2 (Panel B) showed myocarditis in both patients, with multifocal cardiomyocyte damage (arrows) associated with mixed inflammatory infiltration. Scattered eosinophils were noted (arrowheads). The images of the hematoxylin–eosin stains were obtained with 10× eyepieces and 40× or 60× objectives. Additional information is provided in the Supplementary Appendix. RV denotes right ventricle, and LV left ventricle.
Patient 2, a 42-year-old man, presented with dyspnea and chest pain 2 weeks after mRNA-1273 vaccination (second dose). He did not report a viral prodrome, and a PCR test was negative for SARS-CoV-2 (Table S1). He had tachycardia and a fever, and his electrocardiogram showed diffuse ST-segment elevation (Fig. S1). A transthoracic echocardiogram showed global biventricular dysfunction (ejection fraction, 15%), normal ventricular dimensions, and left ventricular hypertrophy. Coronary angiography revealed no coronary artery disease. Cardiogenic shock developed in the patient, and he died 3 days after presentation. An autopsy revealed biventricular myocarditis (Figure 1B and Figs. S5 and S6). An inflammatory infiltrate admixed with macrophages, T-cells, eosinophils, and B cells was observed, a finding similar to that in Patient 1.
In these two adult cases of histologically confirmed, fulminant myocarditis that had developed within 2 weeks after Covid-19 vaccination, a direct causal relationship cannot be definitively established because we did not perform testing for viral genomes or autoantibodies in the tissue specimens. However, no other causes were identified by PCR assay or serologic examination.
Supplementary Appendix
Disclosure Forms
This letter was published on August 18, 2021, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Myocarditis and pericarditis following mRNA COVID-19 vaccination. Centers for Disease Control and Prevention, June 2021. (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html).
- 2.Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in seven adolescents following Pfizer–BioNTech COVID-19 vaccination. Pediatrics 2021. June 4 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 3.Larson KF, Ammirati E, Adler ED, et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation 2021. June 16 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muthukumar A, Narasimhan M, Li Q-Z, et al. In depth evaluation of a case of presumed myocarditis following the second dose of COVID-19 mRNA vaccine. Circulation 2021. June 16 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosner CM, Genovese L, Tehrani BN, et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation 2021. June 16 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.