Highlights
-
•
Revascularization for PAD has been characterized by the adaptation of technologies from the treatment of CAD, with few therapies specifically developed for PAD.
-
•
In light of recent increases in all-cause mortality driven by endovascular PAD interventions that employ elution of antiproliferative agents, this review highlights the unmet need to address underlying local inflammation and proposes an alternative therapeutic approach.
-
•
Development of next-generation PAD-specific endovascular interventions will benefit from integration of specialized immunotherapies that target macrophage polarization and focus on vessel healing.
Key Words: drug-eluting balloon, drug-eluting stent, endovascular intervention, macrophage polarization, paclitaxel, peripheral arterial disease, vascular healing, vascular inflammation
Abbreviations and Acronyms: BMS, bare-metal stent; CAD, coronary artery disease; DES, drug-eluting stent; FP, femoropopliteal; IL, interleukin; MI, myocardial infarction; PAD, peripheral artery disease; PTA, percutaneous transluminal angioplasty; SFA, superficial femoral artery; TGF, transforming growth factor; TNF, tumor necrosis factor
Summary
Peripheral artery disease (PAD) has a significant impact on human health, affecting 200 million people globally. Advanced PAD severely diminishes quality of life, affecting mobility, and in its most severe form leads to limb amputation and death. Treatment of PAD is among the least effective of all endovascular procedures in terms of long-term efficacy. Chronic inflammation is a key driver of PAD; however, stents and coated balloons eluting antiproliferative drugs are most commonly used. As a result, neither stents nor coated balloons produce durable clinical outcomes in the superficial femoral artery, and both have recently been associated with significantly increased mortality. This review summarizes the most common clinical approaches and limitations to treating PAD and highlights the necessity to address the underlying causes of inflammation, identifying macrophages as a novel therapeutic target in the next generation of endovascular PAD intervention.
Central Illustration
Peripheral artery disease (PAD) is a major form of cardiovascular disease characterized by impaired blood flow within peripheral blood vessels, occurring most commonly in the lower extremities. PAD is the third most common clinical manifestation of atherosclerosis after coronary artery disease (CAD) and stroke, although it can be due to thrombosis, vasculitis, or degenerative disease (1). An aging population combined with increases in major risk factors including diabetes, hypertension, smoking, obesity, and dyslipidemia make PAD a significant health burden worldwide. Advanced PAD leads to a decline in patient mobility and diminished quality of life. In its most severe form, critical limb-threatening ischemia, rest pain, and tissue necrosis are associated with high rates of limb amputation, morbidity, and mortality. Additionally, PAD sufferers have a 2-fold increased risk of death after multivariate adjustment, even among patients without recognized cardiovascular disease (2).
Current professional society guidelines recommend an endovascular-first revascularization strategy in most symptomatic PAD patients with critical limb-threatening ischemia (3). However, there is no consensus for the default endovascular therapy with all commercially available interventions yielding suboptimal long-term outcomes. The unique biomechanical and biologic conditions of the peripheral arteries predispose them to chronic inflammation, accelerated atherosclerosis, and restenosis following percutaneous intervention. Balloon angioplasty and bare-metal stents (BMS) mechanically reopen vessels but do little to address the underlying drivers of treatment failure. Elution of cytotoxic antiproliferative drugs (eg, paclitaxel) suppresses local cell growth for the period of drug elution, meaning that their effectiveness is limited. In diabetic patients, in whom advanced PAD often manifests below the knee, the downward spiral of chronic limb-threatening ischemia, tissue loss, and amputation is intractable (4). Alarmingly, paclitaxel-eluting devices significantly increase amputation rates and all-cause mortality (5) due to off-target effects, meaning that the most successful approach for revascularization is putting patients’ lives at risk. There is an urgent need to develop better and safer treatments for PAD.
Revascularization Strategies
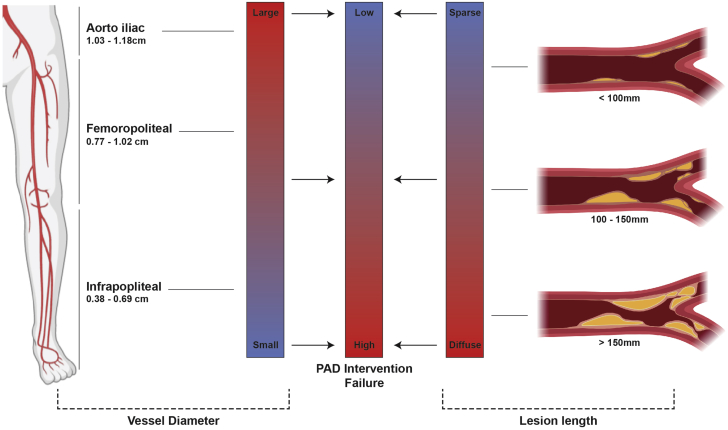
Revascularization of occlusive lesions in PAD becomes more challenging with increasing arterial length and decreasing arterial diameter (Figure 1). With the exception of aortoiliac (common femoral) artery disease, surgical bypass is no longer the recommended frontline treatment for medically refractory PAD in American College of Cardiology/American Heart Association and European Society of Cardiology guidelines (3,6). Endovascular interventions are now the dominant therapy, conducted roughly 4 times more frequently than bypass surgery (7). Stenoses of the superficial femoral artery (SFA) and contiguous popliteal artery, constituting the femoropopliteal (FP) segment, are the most common cause of symptomatic PAD, accounting for more than 70% of cases (8).
Figure 1.
PAD Pathophysiology vs Intervention Failure
Revascularization of occlusive lesions in peripheral artery disease (PAD) becomes more challenging with decreasing arterial diameter (left) and increasing lesion length (right).
The FP arterial segment represents a unique challenge for revascularization, with several important distinctions from the coronary circulation. The FP segment is extremely long and subject to highly dynamic forces of flexion, extension, and torsion associated with leg and knee movement (9). These mechanical deformations predispose the vessel to chronic injury, increased smooth muscle cell proliferation, accelerated atherosclerosis, and restenosis. When self-expanding nitinol stents are deployed, their chronic outward force accentuates these issues and may increase risk of in-stent restenosis (10). Considering the complexity of the anatomy and dynamics of FP disease, clinically durable therapies have proven elusive. Concurrently, there has been a steady rise in PAD interventions, with a 25% increase in procedures in the United States from 2011 to 2016 (11).
Balloon Angioplasty and BMS
The effectiveness of percutaneous transluminal angioplasty (PTA) (balloon angioplasty) is greatest for lesions in the iliac arteries and progressively decreases for more distal vessels. PTA of the superficial femoral and infrapopliteal lesions is associated with high rates of clinical failure, with restenosis in 40% to 60% of treated segments at 1 year (12). In longer and heavily calcified segments, PTA has been superseded by BMS. However, even when using nitinol, stent fracture in the peripheries is relatively common, occurring in up to 37% of patients, dependent on stent design and delivery (13). Stent fracture increases with proximity to joints and length of treated segment and is associated with an increasing number of stents or use of overlapping stents. When fracture is avoided, self-expanding nitinol stents exert a continual outward radial force on the vessels that, along with the increased torsional and anatomical forces in peripheral lesions, drives an exaggerated and lasting inflammatory response. Randomized trials have shown that self-expanding nitinol stents provide superior short-term patency compared with PTA (63% vs 37% restenosis) at 1 year for lesions up to 175 mm (14). However, progressive decline in patency after stenting is consistently reported in studies with 12- to 60-month follow-ups. Data from the 2-year ABSOLUTE (Balloon Angioplasty Versus Stenting With Nitinol Stents in the Superficial Femoral Artery) randomized trial showed that while stenting was associated with lower rates of restenosis (49% vs 74% for PTA), the clinical (symptomatic) benefit was lost at 2 years (15). Owing to the poor long-term outcomes of PTA and BMS, recent endovascular interventions for PAD have been uniformly characterized by adaptation of drug-elution technologies from treatment of CAD, with few therapies specifically developed for PAD.
Drug-Eluting Stents and Balloons
The development of drug-eluting stents (DES) that release antiproliferative agents such as sirolimus and paclitaxel revolutionized management of CAD by achieving dramatic and durable reductions in restenosis. Based on the success of DES in CAD, there was great enthusiasm for the potential of DES to transform the durability of PAD interventions. Refinements in DES to improve safety for coronary applications have included the replacement of paclitaxel with less toxic sirolimus family derivatives (eg, everolimus, zotarolimus) with limited doses and elution times (usually 1 month). However, the benefits observed in the coronaries have failed to translate to the peripheries (Table 1). In the SIROCCO (Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease) randomized trial comparing sirolimus-eluting self-expanding nitinol stents to BMS for SFA disease, restenosis was equivalent at 2 years (22.9% DES vs 21.1% BMS) (16). The nonrandomized STRIDES (Superficial Femoral Artery Treatment with Drug-Eluting Stents) study of everolimus-eluting nitinol stents for SFA disease showed promising early outcomes, with a restenosis rate of 6% at 6 months (17). However, 1-year outcomes were disappointing, with a primary patency rate of only 68.5%, similar to BMS equivalents.
Table 1.
Summary of Outcomes for Major Trials for Paclitaxel-, Sirolimus-, or Everolimus-Eluting or -Noneluting Balloons and Stents in Peripheral Revascularization
| First Author, Year (Ref. #) | Trial | Test Arm, n | Control Arm, n | Lesion | Trial Endpoints | Outcome (Test vs Control) |
|---|---|---|---|---|---|---|
| Schillinger et al, 2006 (14) | ABSOLUTE | Nitinol stent, 51 | PTA, 53 | SFA | In-stent mean luminal diameter stenosis after 12 mo | 74% vs 49% |
| Duda et al, 2006 (48) | SIROCCO | Sirolimus (DES), 47 | BMS, 46 | SFA | In-stent mean luminal diameter stenosis after 24 mo of DES vs BMS | 22.9% vs 21.1% |
| Lammer et al, 2011 (17) | STRIDES | Everolimus (DES), 104 | N/A | SFA | Percentage of patients free from 50% ISR after 12 mo | 68% (single arm) |
| Dake et al, 2016 (18) | ZILVER PTX | Paclitaxel (DES), 236 | PTA, 238 | FPA | Percentage of patients free from 50% ISR 12 mo | 74.8% vs 26.5% |
| Gray et al, 2018 (20) | IMPERIAL | Eluvia Paclitaxel (DES), 309 | Zilver PTX Paclitaxel (DES), 156 | SFA/IPA | Primary patency at 12-mo follow-up | 92.1% vs 81.8% |
| Muller-Hulsbeck et al, 2017 (49) | MAJESTIC | Eluvia Paclitaxel (DES) | N/A | SFA/IPA | Primary patency at 24-mo follow-up | 83.5% (single arm) |
| Zeller et al, 2020 (22) | IN.PACT DEEP | IN.PACT Paclitaxel (DEB), 239 | PTA, 119 | SFA | Primary patency at 5-y follow-up | 74.5% vs 65.3% |
ABSOLUTE = Balloon Angioplasty Versus Stenting With Nitinol Stents in the Superficial Femoral Artery; BMS = bare-metal stent; DEB = drug-eluting balloon; DES = drug-eluting stent; FP = femoropopliteal artery; IN.PACT DEEP = Randomized IN.PACT Amphirion Drug-Coated Balloon vs Standard percutaneous Transluminal Angioplasty for the Treatment of Below-the-Knee Critical Limb Ischemia; IPA = intrapopliteal artery; MAJESTIC = Stenting of the Superficial and/or Proximal Popliteal Artery Project; N/A = not available; PTA = percutaneous transluminal angioplasty; SFA = superficial femoral artery; SIROCCO = Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease; STRIDES = Superficial Femoral Artery Treatment with Drug-Eluting Stents.
To combat the more aggressive restenosis in the peripheries, contemporary DES platforms for PAD elute high-dose paclitaxel for as long as 12 months. In randomized trials, the Zilver PTX DES (Cook Medical, Bloomington, Indiana), which elutes paclitaxel from a polymer-free nitinol stent, achieved higher patency rates than PTA. However, there was little difference in terms of symptoms and walking impairment scores suggesting that the trial design likely overstates the real clinical benefits of the Zilver PTX (18). Furthermore, in a prospective multicenter study of 690 patients with 831 FP lesions treated by the Zilver PTX, the 1-year restenosis rate was 37%, a result akin to BMS (19). More recently, the Eluvia DES (Boston Scientific, Marlborough, Massachusetts), which elutes paclitaxel at a lower dose (∼18× less than the Zilver PTX), but over a longer time period from a biostable fluoropolymer, has shown promising patency and safety results at 1 year in short lesions (92% patency) (20). However, at 3-year follow-up, primary patency appears to decline precipitously, suggesting that their effectiveness is limited to the period of drug elution only. Despite early enthusiasm, a growing concern has been a lack of vessel healing where paclitaxel is present, resulting in uncovered stent struts, necrotic tissue, and ongoing risk of late stent thrombosis and amputation (21). Overall, the adaptation of coronary DES technology to PAD has not significantly enhanced the durability of PAD interventions.
In contrast to DES, drug-eluting balloons (DEBs) that release paclitaxel but leave no permanent implant in place have emerged in recent years as an alternative. The rationale for DEBs was to lessen the potential proinflammatory effects induced by stent polymer coatings and eliminate the chronic outward force on the vessel wall from nitinol stents. However, DEBs have the same shortcomings as traditional PTA, including vessel damage caused by mechanical recoil following balloon removal. In the initial trials of DEBs, treating patients with focal lesions (<100 mm), low incidences of total chronic occlusions and calcification, 5-year outcomes show small but significant improvements when compared with PTA (74.5% vs 65.3%) (22). However, recent studies of DEBs have shown only marginally higher patency than PTA at 12 months (65.2% vs 52.6%) in intermediate lesions (<150 mm) (23). When compared with DES, stenting is favored in longer lesions, total occlusions, advanced PAD (Rutherford 4), and high-risk patients (age over 75 years) (24).
Increased Late Morbidity and Mortality for Paclitaxel-Eluting Devices
Although its use is based on its robust inhibitory activity on microtubule formation and subsequent cell proliferation, paclitaxel is both nonspecific and cytotoxic. There is mounting evidence that the use of paclitaxel comes at a significant cost. The IN.PACT DEEP (Randomized IN.PACT Amphirion Drug-Coated Balloon vs Standard percutaneous Transluminal Angioplasty for the Treatment of Below-the-Knee Critical Limb Ischemia) trial comparing plain balloon angioplasty to paclitaxel DEBs stopped recruiting at 12 months following increased amputation rates in the presence of the drug (8.8% vs 3.6%) (25), an increase that persisted at 5-year follow-up (22).
Further, a recent systematic review and meta-analysis identified a significant increase in all-cause mortality at 5 years in patients treated with a paclitaxel device (14.7% vs 8.1%) (5). The U.S. Food and Drug Association concluded that a late mortality signal associated with the use of paclitaxel-coated devices to treat femoropopliteal PAD is present, while cautioning that further studies on the effect of paclitaxel dose and the determination of a mechanism for the increased mortality are needed. Regardless, current Food and Drug Association recommendations urge close follow-up of patients treated with paclitaxel devices, discussion with patients of the increased risk of death, and the collection of more clinical data. The significantly increased long-term mortality risk associated with paclitaxel-eluting devices in PAD further compounds the shortcomings of eluting antiproliferative therapies for PAD as a whole. There is a clear unmet need to identify novel therapeutic drugs and approaches for better endovascular management of PAD.
Understanding Device Failure in PAD
A large number of longitudinal and cross-sectional studies demonstrate a mechanistic link between inflammation and vessel injury-driven restenosis, the dominant intractable mode of PAD device failure (26). Because all forms of endovascular injury involve distention of the vessel wall, the larger and more elastic the artery is, the better it can conform to distention, resulting in less severe injury. In fact, larger elastic arteries like the carotid exhibit relatively low rates of restenosis in contrast to the muscular arteries of the FP segment (27). This suggests that stiffer vessels like those in the peripheries are subject to more severe injury and potentially exaggerated inflammation. However, the precise mechanisms underlying the more aggressive restenosis and higher failure rates observed for PAD devices as compared with equivalent implants used in the coronary circulation have yet to be fully determined. In part, this is due to the limited number of large preclinical models that adequately replicate restenosis in reasonable time frames.
The current industry-standard preclinical animal model for evaluating PAD interventions is 60-day implantation in the SFA or iliac artery of familial hypercholesterolemic pigs (28). The dominant models of injury include deployment of an oversized noncompliant balloon (29), arterial wall disruption using an isometric cutting or scoring balloon followed by stretching with a compliant balloon (30), or oversized stent implantation (31). Neointimal hyperplasia is measured via angiography and complementary histology, and is usually modest at these short time frames, in the order of 25%. The degree of inflammation has been mainly evaluated through traditional hematoxylin and eosin, which stains all cell types blue and relies on morphology observations to differentiate cells. This gives only a very rudimentary measure of gross inflammation, and detailed studies of immune cell numbers and phenotype using immunohistochemistry have been crucially absent. To date, the focus on clinically relevant outcomes such as restenosis has prevailed over detailed mechanistic studies that facilitate understanding of the nuances of device deployment in PAD and CAD.
Inflammation and Macrophages in PAD
Although it is well established that chronic inflammation is central to the progression of PAD and other cardiovascular diseases, immunotherapies are not currently advised in its clinical management. A growing number of clinical trials such as the CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcome Study) study and the LoDoCo (Low Dose Colchicine for Secondary Prevention of Cardiovascular Disease) trial demonstrate the therapeutic benefits of inflammation lowering therapies. The most common current approaches focus on systemically inhibiting individual proinflammatory cytokines implicated in the pathogenesis of atherosclerosis and restenosis (Table 2). Although cytokine therapy represents a promising new approach for vascular disease, the pleiotropic nature of cytokines and the careful balance of their network of interactions mean that single alterations can lead to impaired global immune responses, an issue compounded by systemic administration routes that lead to downstream off-target effects. Accordingly, the systemic use of therapeutic antibodies or antagonists to target inflammatory cytokines has revealed a range of immune-related side effects including monoclonal antibody–induced infusion reactions, cytokine release syndrome, infections, and autoimmunity deficits (32). As a result, the therapeutic modulation of vascular inflammation will optimally involve the inhibition of a complex network of immune reactions not readily addressed by antagonism of an individual cytokine.
Table 2.
Cytokine-Targeting Drugs Currently Under Preclinical Investigations or Clinical Trials
| First Author, Year (Ref. #) | Trial | Drug, n (Mechanism) | Dose (mg) | Control, n | Efficacy Endpoints | Outcome (Drug vs Control) |
|---|---|---|---|---|---|---|
| Ridker et al, 2017 (50) | CANTOS | Canakinumab, 6,717 (IL-1β antagonist) | 50, 150, 300 | Placebo, 3,344 | Nonfatal MI, stroke, or cardiovascular death at 48 mo | 4.11% (50 mg) 3.86% (100 mg) 3.9% (300 mg), vs 4.5% (placebo) |
| Ikonomidis et al, 2008 (51) | Anakinra ADHF | Anakinra, 23 (IL-1R antagonist) | 150 | Prednisolone, 19 | Nitrooxidative stress and vascular function at 30-d follow-up | Nitrotyrosine (-38% vs -11%) Flow-mediated dilation (45% vs -9%) Coronary flow reserve (29% vs 4%) Aortic distensibility (45% vs 2%) |
| Giles et al, 2019 (52) | ENTRACTE | Tocilizumab, 1,538 (IL-6 antagonist) | 8/kg | Etancercept, 1,542 | Comparison of time to first occurrence of MACE over 3.2 y | Decrease in MACE, hazard ratio 1.05 (95% CI: 0.77-1.43) |
| Klein et al, 2020 (53) | RHAPSODY | Rilonacept, (IL-6 antagonist) | 320 | Placebo, 160 | Time to pericarditis reoccurrence at 24 weeks | Enrolling |
| Chung et al, 2003 (54) | ATTACH | Infliximab, 101 (TNF-α antagonist) | 5/kg 10/kg |
Placebo, 49 | Circulating inflammatory biomarkers and risk of death at 28 weeks | Elevated mortality risk and/or hospitalization, hazard ratio 2.84 (95% CI: 1.01-7.97) |
| Bissonnette et al, 2012 (55) | NCT00940862 | Adalimumab, 20 (TNF-α antagonist) | 80 | Placebo, 10 | Change in carotid and ascending aorta inflammation by PET scan at 15 weeks | Improvement in target-to-background ratio in both aortic and ascending aorta (-0.26 ± 0.11 and -0.32 ± 0.15) |
Anakinra ADHF = Interleukin-1 Blockade in Acute Heart Failure; ATTACH = Anti-TNF Therapy Against Congestive Heart Failure; CANTOS = Canakinumab Anti-Inflammatory Thrombosis Outcome Study; CI = confidence interval; ENTRACTE = Study of Tocilizumab in Comparison to Etanercept in Participants With Rheumatoid Arthritis and Cardiovascular Disease Risk Factors; IL = interleukin; MACE = major adverse cardiovascular event; MI = myocardial infarction; PET = positron emission tomography; RHAPSODY = Rilonacept Inhibition of Interleukin-1 Alpha and Beta for Recurrent Pericarditis: a Pivotal Symptomatology and Outcomes Study; TNF-α = tumor necrosis factor α.
Among the numerous immune cells involved in restenosis, macrophages play a dominant role in sustaining chronic inflammation and may represent a more effective target for comprehensive and controlled resolution of vascular inflammation. Tissue samples collected from angioplasty catheters deployed in peripheral vessels have found a strong positive correlation between macrophage numbers and restenosis severity. A greater density of macrophages was observed more than any other immune cell in restenotic compared with nonrestenotic lesions (33). Further, macrophage polarization state is a key aspect of the regulation of the local inflammatory environment which could potentially be leveraged as a therapeutic target to treat restenosis in PAD. Macrophages exist across a broad spectrum of phenotypes characterized at the extremes by proinflammatory (M1) and inflammation-resolving (M2) states. Within regions of vessel inflammation, macrophages do not exist as pure populations, but rather as ratios of their respective subtypes according to microenvironmental changes. Derived from circulating monocytes, macrophages initially adhere to adhesion proteins expressed on endothelial cells following endovascular injury, which direct their differentiation toward a proinflammatory M1 phenotype through damage-associated molecular signals released during cell death (Figure 2). M1 macrophages serve as the primary source of proinflammatory cytokine secretion, which in turn sustains further endothelial damage and facilitates smooth muscle cell phenotypic switching or proliferation during both atherosclerosis and restenosis (34). Within the overarching M2 phenotype, M2a macrophages are recognized as “wound healing” and classically anti-inflammatory macrophages, triggered by interleukin (IL)-4 and IL-13 to secrete profibrotic factors (fibronectin, insulin-like growth factor 1, transforming growth factor [TGF]-β) that contribute to tissue repair. In contrast, M2b and M2c susbets are characterized as “regulatory macrophages” that also secrete anti-inflammatory cytokines (IL-10, TGF-β) but are activated by separate ligands. M2b are activated by Toll-like receptor ligands or IL-1 receptor antagonists and M2c macrophages are triggered by IL-10 and glucocorticoids. The precise role each subset is not yet known in the context of PAD, and the classification of these subsets is largely restricted to in vitro definition following defined stimulus. In vivo, the focus and the weight of literature so far has been dominated by discussion of the broad M2 phenotype, without particular focus on an individual subset, which collectively stem inflammation. For example, ischemic tissue biopsies from peripheral vessels link elevated M2 markers including the mannose receptor CD206 and CD163 with reduced PAD progression (35).
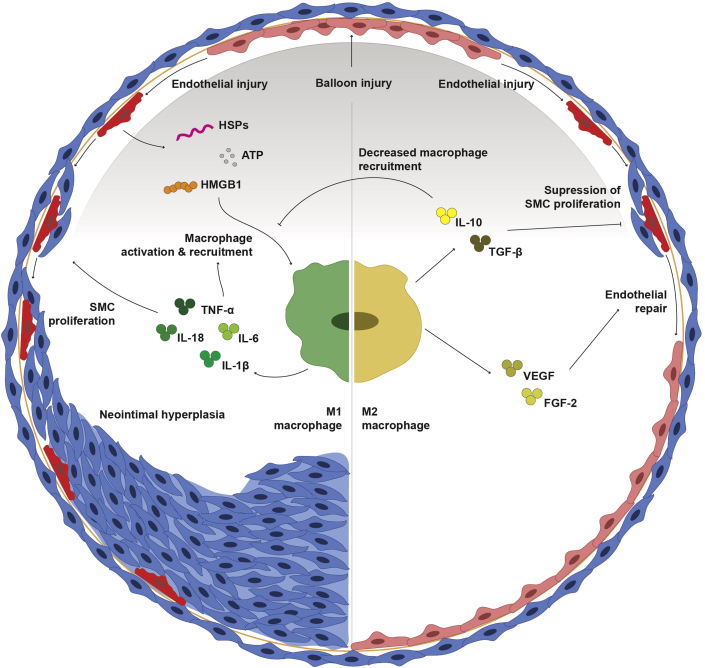
Figure 2.
Mechanisms of Macrophage-Driven Restenosis Following Balloon Injury
Cross-section of balloon injured blood vessel showing M1 macrophages coordinating immune cell recruitment in response to endothelial damage as well as secretion of proinflammatory cytokines that stimulate smooth muscle cell proliferation into the intima. M2 polarization halts immune cell recruitment through the release of anti-inflammatory cytokines and facilitates endothelial repair through the release of proangiogenic factors. ATP = adenosine triphosphate; FGF = fibroblast growth factor; HSP = heat shock protein; IL = interleukin; SMC = smooth muscle cell; TGF = transforming growth factor; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor.
In advanced PAD, restenosis results in ischemia, triggering further inflammation within the muscles of the lower limbs. Gene expression analysis in mice shows a significant shift in muscle macrophage polarization in the quadriceps and gastrocnemius muscles, with a higher proportion of the M1 phenotype during early ischemia, returning to baseline in later stages. In these studies, disruption of the early M1 increase by statins prevented walking impairment driven by ischemia (36). In the gastrocnemius muscles of human patients with PAD, lower M1 (CD206–) macrophage abundance was associated with better walking performance, while an increased M2 (CD206+) phenotype was linked to greater myosatellite cells (muscle stem cells) and muscle fiber size (35). This suggests that M2 macrophages are associated with ongoing reparative processes in the ischemic PAD muscle. Though a direct therapeutic link has not yet been identified, these studies highlight the potential benefits of local interventions that focus on shifting the overall ratios of local macrophage phenotypes (rather than focusing on a specific subset), either reducing the number of M1 macrophages or rapidly polarizing them toward an anti-inflammatory M2 phenotype following endovascular intervention (Central Illustration).
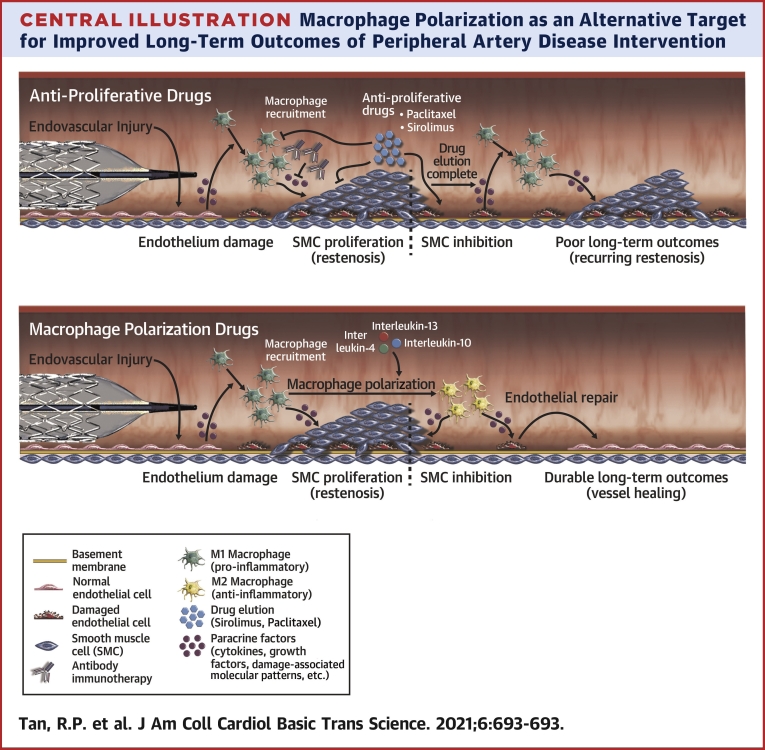
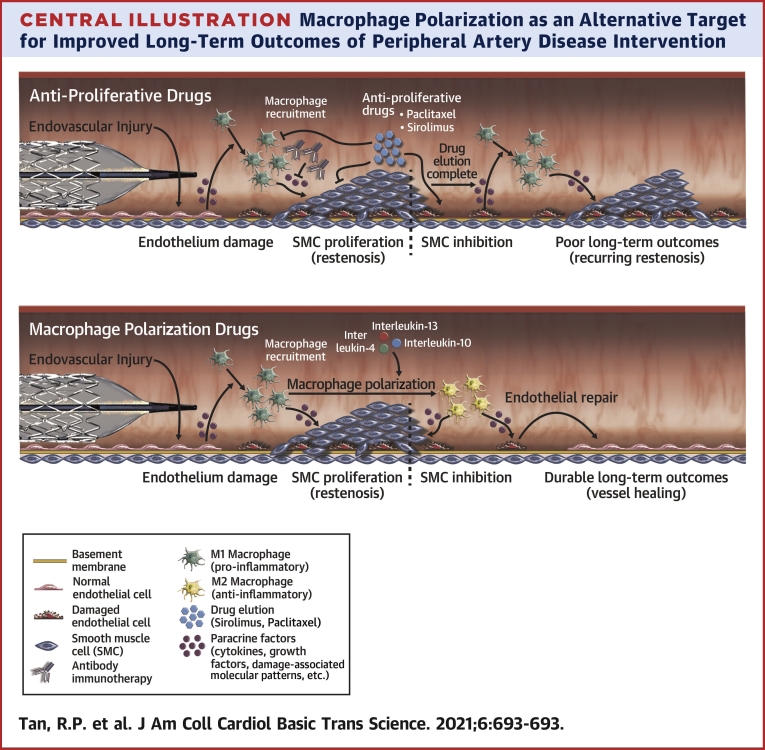
Central Illustration.
Macrophage Polarization as an Alternative Target for Improved Long-Term Outcomes of Peripheral Artery Disease Intervention
Current drug-eluting peripheral artery disease interventions relying on antiproliferative drugs (eg, sirolimus, paclitaxel) suppress restenosis for the duration of drug elution but do not address underlying vessel injury and inflammation driven by M1 macrophages (Top). Additionally, current immunotherapy approaches focus solely on targeting individual cytokines sustaining vascular injury but are similarly only effective as long as the drug is eluted. Polarization of macrophages toward anti-inflammatory/tissue reparative M2 phenotypes represents a potentially more effective and long-lasting approach to suppress vascular inflammation by repairing injured vessels (Bottom).
Toward Macrophage-Targeting PAD Interventions
Therapeutically targeting macrophage polarization has a number of inherent benefits that could lead to long-lasting biological effects. Inflammatory mediators and signaling molecules have additive and reinforcing effects, such that reductions in local inflammation can be propagated and sustained once set in motion. For example, M1 macrophages secrete IL-12 to promote Th1 polarization resulting in secretion of tumor necrosis factor (TNF)-α and interferon γ that further amplify M1 polarization. Similarly, M2 macrophages can secrete IL-4 to promote Th2 polarization resulting in secretion of IL-4 and IL-13 that further amplify M2 polarization (37). As previously discussed, stimulating lasting M2 polarization after PAD intervention may also involve timely delivery, immediately after an endovascular procedure to inhibit early M1 signaling. Additional benefit may also arise from therapies robust enough to simultaneously counteract pre-existing pathological inflammation within the surrounding ischemic PAD muscle to aid in muscle regeneration. This could potentially be achieved by strategic dosing of M2 polarizing therapies combined with new platform technologies for localized delivery to further improve their overall effectiveness and safety. In addition, M2 macrophages participate in endothelial repair pathways through the release of proangiogenic mediators including basic fibroblast growth factor-2, TGF-β, and vascular endothelial growth factor A (38). In this way, rapid M2 polarization would be expected to contribute to enhanced repair of the endothelium and faster formation of this protective cell layer. A therapeutic approach grounded in the local suppression of restenosis coupled with enhanced endothelial regeneration would have significant implications for enhancing the durability of PAD interventions.
Data from bioengineered materials and scaffolds implanted subcutaneously demonstrate the feasibility of polarizing macrophages using locally released signaling molecules. For example, delivery of lentiviral IL-10 gene therapy around implanted polylactic-co-glycolic acid polymer scaffolds reduced proinflammatory (M1) macrophage infiltration by half during the acute foreign body response (39). Similarly, nanocoatings that slowly released IL-4 from polypropylene surgical meshes promoted M2 macrophage polarization leading to reduced fibrotic capsule thickness and improved host vascularization through enhanced proangiogenic function (40). Using these classical cytokine stimuli (IL-10 and IL-4) likely increased the population of M2c and M2a macrophages respectively (though both were referred to as M2 in the studies). Extrapolating from this principle, it would be expected that specific subsets could be enriched by appropriate signaling molecules if required (eg, M2b using IL-1 agonists) but that enhancing the ratio of M2 to M1 macrophages is an appropriate initial therapeutic goal.
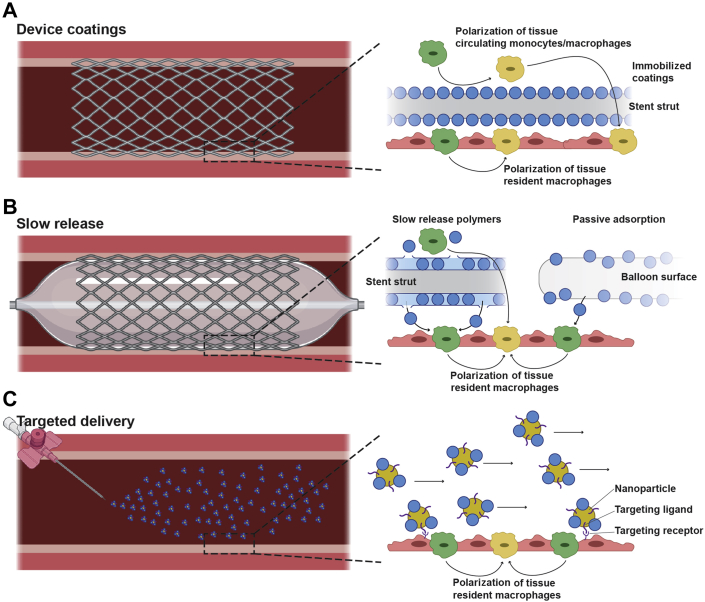
Adapting this approach from soft tissue into the vasculature also requires additional consideration of the effects of blood flow given that local delivery is required to avoid the known systemic effects. Several strategies have emerged to address this, centered around stabilizing M2-promoting cytokines onto material surfaces either targeted to or implanted directly at sites of vessel injury (Figure 3). Polyester nanoparticles loaded with IL-10 and functionalized with peptide sequences designed for high affinity for collagen type IV (Col-IV IL-10 NP22), the abundant matrix protein exposed at sites of endothelial denudation, show targeted accumulation and retention within atherosclerotic plaques following systemic administration (41). Col-IV IL-10 NP22–treated plaques show reduced necrotic cores as a result of decreased macrophage accumulation and a reduction in IL-1β, TNF-α, and nitric oxide. Synthetic vascular grafts fabricated from polycaprolactone coated with a covalently immobilized layer of IL-4 show favorable up-regulation of anti-inflammatory cytokines, IL-10 and TGF-β, and down-regulation of proinflammatory cytokines, TNF-α and IL-1β, in macrophages responding to the graft surface (42). This resulted in IL-4 grafts showing both strikingly impaired neointimal hyperplasia and improved long-term patency. These early findings highlight the promise of localized macrophage immunomodulation, warranting the investigation of similar approaches on stents and balloons for peripheral applications.
Figure 3.
Emerging Localized Delivery Platforms for Peripheral Artery Disease Intervention
The effectiveness of M2 macrophage polarizing agents (blue circles) will rely on localized delivery to injured vessels or damaged endothelium for lasting suppression of restenosis. (A) Device coatings comprise covalently immobilized agents can polarize both circulating monocytes and macrophages as well as tissue resident macrophages. (B) Slow-release platforms consisting of degradable polymers or passively adsorbed can release agents not only locally into the vessel well, but also into systemic circulation. (C) Targeted systemic delivery strategies may involve nanoparticles decorated with targeting ligands to localize agents only to areas of vessel injury. SMC = smooth muscle cell.
Issues that Remain to be Resolved in Targeting Macrophage Polarization
Despite their origin, circulating monocyte-derived macrophages and native tissue–resident macrophages express similar gene expression patterns in response to M2 stimuli (43). This heterogeneity of phenotypes exists even at the monocyte (precursor) level as monocytes can also belong to anti-inflammatory phenotypes driven by classical M2 ligands such as IL-4 (44). This suggests that M2 therapies would broadly affect immune cell recruitment to vascular injury, drastically altering remodeling outcomes. Although, as previously discussed, very little information exists in vivo on the effects of the multiple subsets within the M2 phenotype and how they would respond to treatment. Toward this goal, next-generation sequencing has helped transformed our knowledge of macrophage function in atherosclerosis. For example, single-cell RNA sequencing has recently discovered a new subclass of CX3CR1+ macrophages with never before reported M1-like gene signatures that contribute to plaque progression in mouse aortas (45). Similar studies using atherosclerotic mice have discovered M1-like CD45+/TREM2hi macrophage subsets responsible for lipid metabolism or catabolism and lesion calcification (46). Following these approaches, critical assessment on the role of individual macrophage subphenotype and their respective therapeutic impact on PAD and restenosis will need to be conducted if targeted macrophage therapies for lastingly effective PAD treatment are to be formulated.
As of now there is some evidence that triggering and maintaining robust M2 polarization has a lasting impact in disease in the context of atherosclerosis. Numerous studies have demonstrated that enrichment of M2-associated gene expression in plaque macrophages is associated with plaque regression. These studies are beginning to elucidate the role of metabolic shifts and mitochondrial function in determining phenotype reversal of macrophages in lesions (47). Applying these methods to the study of macrophages in restenosis will likely translate to new therapeutic opportunities to promote robust M2 polarization. Combined with new advances in local delivery technologies with increased precision, targeted M2 therapies could spur the next generation of “immune-regenerative” interventions to promote vessel healing and lastingly mitigate vascular inflammation in PAD. By catering these advances toward current intervention technologies, such therapies could also be delivered in a manner consistent with minimally invasive clinical procedures. These future research endeavors have the promise to transform the global landscape of PAD treatment and potentially other cardiovascular diseases.
Conclusions
Endovascular interventions are the frontline strategy for treating ischemic PAD, dominantly employing DES and DEBs adapted from use in the coronaries. Unlike other blood vessels in the body affected by atherosclerosis, treatment of peripheral arteries with DES or DEBs is not lastingly effective. Patients treated with DES and DEBs frequently return within 2 years for further intervention, when the period of drug elution was complete and restenosis renarrows the artery. Recent meta-analyses showing an significant increase in all-cause mortality from using paclitaxel-eluting devices have redefined the cost-benefit calculation for PAD patients. Now is the time to urgently reimagine PAD intervention, in a manner distinct from traditional drug-eluting approaches. Immunotherapies targeting M2 macrophage-mediated vascular inflammation pose a highly promising alternative approach driven by the strong clinical associations between inflammation and PAD device failure. Successful integration of M2 macrophage immunotherapies with PAD intervention will require technologies that allow the localization of their effects only to sites of disease. Focused research initiatives and advancements in this field will undoubtedly revolutionize the treatment of PAD.
Funding Support and Author Disclosures
This work was supported by the National Health and Medical Research Council (APP1162969 [to Drs. Wise and Ng]), the National Foundation for Medical Research and Innovation (to Dr. Wise), and the financial support of E. Brackenreg. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Robertson L., Paraskevas K.I., Stewart M. Angioplasty and stenting for peripheral arterial disease of the lower limbs: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;2017:CD012542. [Google Scholar]
- 2.Pande R.L., Perlstein T.S., Beckman J.A., Creager M.A. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23. doi: 10.1161/CIRCULATIONAHA.110.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerhard-Herman M.D., Gornik H.L., Barrett C. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. doi: 10.1161/CIR.0000000000000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freisinger E., Malyar N.M., Reinecke H., Lawall H. Impact of diabetes on outcome in critical limb ischemia with tissue loss: a large-scaled routine data analysis. Cardiovasc Diabetol. 2017;16:41. doi: 10.1186/s12933-017-0524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsanos K., Spiliopoulos S., Kitrou P., Krokidis M., Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.011245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tendera M., Aboyans V., Bartelink M.L. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2851–2906. doi: 10.1093/eurheartj/ehr211. [DOI] [PubMed] [Google Scholar]
- 7.Parvar SL, Ngo L, Dawson J, et al. Long-term outcomes following endovascular and surgical revascularization for peripheral artery disease: a propensity score-matched analysis. Eur Heart J. Published online February 24, 2021. https://doi.org/10.1093/eurheartj/ehab116 [DOI] [PubMed]
- 8.Sareen N., Ojha A. IntechOpen; 2018. Peripheral Arterial Disease: A Practical Approach. [Google Scholar]
- 9.Banerjee S. superficial femoral artery is not left anterior descending artery. Circulation. 2016;134:901–903. doi: 10.1161/CIRCULATIONAHA.116.023690. [DOI] [PubMed] [Google Scholar]
- 10.Razzouk L., Aggarwal S., Gorgani F., Babaev A. In-stent restenosis in the superficial femoral artery. Ann Vasc Surg. 2013;27:510–524. doi: 10.1016/j.avsg.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Guez D., Hansberry D.R., Gonsalves C.F. Recent trends in endovascular and surgical treatment of peripheral arterial disease in the medicare population. AJR AM J Roentgenol. 2020;214:962–966. doi: 10.2214/AJR.19.21967. [DOI] [PubMed] [Google Scholar]
- 12.Dormandy J.A., Rutherford R.B. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31:S1–S296. [PubMed] [Google Scholar]
- 13.Babaev A.A., Kotwal A., Zavlunova S., Telis A. Stent fractures in the superficial femoral artery and restenosis: how strong is the association? J Am Coll Cardiol. 2013;61:E1809. [Google Scholar]
- 14.Schillinger M., Sabeti S., Loewe C. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–1888. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 15.Schillinger M., Sabeti S., Dick P. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115:2745–2749. doi: 10.1161/CIRCULATIONAHA.107.688341. [DOI] [PubMed] [Google Scholar]
- 16.Duda S.H., Bosiers M., Lammer J. Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: the SIROCCO II trial. J Vasc Interv Radiol. 2005;16:331–338. doi: 10.1097/01.RVI.0000151260.74519.CA. [DOI] [PubMed] [Google Scholar]
- 17.Lammer J., Bosiers M., Zeller T. First clinical trial of nitinol self-expanding everolimus-eluting stent implantation for peripheral arterial occlusive disease. J Vasc Surg. 2011;54:394–401. doi: 10.1016/j.jvs.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 18.Dake M.D., Ansel G.M., Jaff M.R. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare-metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4:495–504. doi: 10.1161/CIRCINTERVENTIONS.111.962324. [DOI] [PubMed] [Google Scholar]
- 19.Iida O., Takahara M., Soga Y. 1-Year results of the ZEPHYR Registry (Zilver PTX for the Femoral Artery and Proximal Popliteal Artery): predictors of restenosis. J Am Coll Cardiol Intv. 2015;8:1105–1112. doi: 10.1016/j.jcin.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Gray W.A., Keirse K., Soga Y. A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomised, non-inferiority trial. Lancet. 2018;392:1541–1551. doi: 10.1016/S0140-6736(18)32262-1. [DOI] [PubMed] [Google Scholar]
- 21.Kozuki A., Shinke T., Otake H. Optical coherence tomography study of chronic-phase vessel healing after implantation of bare-metal and paclitaxel-eluting self-expanding nitinol stents in the superficial femoral artery. J Cardiol. 2016;67:424–429. doi: 10.1016/j.jjcc.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Zeller T., Micari A., Scheinert D. The IN.PACT DEEP clinical drug-coated balloon trial. 5-year outcomes. J Am Coll Cardiol Intv. 2020;13:431–443. doi: 10.1016/j.jcin.2019.10.059. [DOI] [PubMed] [Google Scholar]
- 23.Jongsma H., van Mierlo-van den Broek P., Imani F., van den Heuvel D., de Vries J.-P.P.M., Fioole B. Randomized comparison of Femoropopliteal Artery Drug-Eluting Balloons and Drug-Eluting Stents (FOREST trial): study protocol for a randomized controlled trial. J Vasc Surg. 2017;66:1293–1298. doi: 10.1016/j.jvs.2017.05.098. [DOI] [PubMed] [Google Scholar]
- 24.Laird J.A., Schneider P.A., Jaff M.R. Long-term clinical effectiveness of a drug-coated balloon for the treatment of femoropopliteal lesions. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.118.007702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeller T., Baumgartner I., Scheinert D. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN.PACT DEEP randomized trial. J Am Coll Cardiol. 2014;64:1568–1576. doi: 10.1016/j.jacc.2014.06.1198. [DOI] [PubMed] [Google Scholar]
- 26.Shah Prediman K. Inflammation, neointimal hyperplasia, and restenosis. Circulation. 2003;107:2175–2177. doi: 10.1161/01.CIR.0000069943.41206.BD. [DOI] [PubMed] [Google Scholar]
- 27.Schillinger M., Minar E. Restenosis after percutaneous angioplasty: the role of vascular inflammation. Vasc Health Risk Manag. 2005;1:73–78. doi: 10.2147/vhrm.1.1.73.58932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granada Juan F., Milewski K., Zhao H. Vascular response to zotarolimus-coated balloons in injured superficial femoral arteries of the familial hypercholesterolemic swine. Circ Cardiovasc Interv. 2011;4:447–455. doi: 10.1161/CIRCINTERVENTIONS.110.960260. [DOI] [PubMed] [Google Scholar]
- 29.Krueger K.D., Mitra A.K., DelCore M.G., Hunter W.J., 3rd, Agrawal D.K. A comparison of stent-induced stenosis in coronary and peripheral arteries. J Clin Pathol. 2006;59:575–579. doi: 10.1136/jcp.2004.025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houbballah R., Robaldo A., Albadawi H., Titus J., LaMuraglia G.M. A novel model of accelerated intimal hyperplasia in the pig iliac artery. Int J Exp Pathol. 2011;92:422–427. doi: 10.1111/j.1365-2613.2011.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castro Júnior C., Pereira A.H., Pasa M.B. Morphometric analysis of the intimal reaction after stent implantation in iliac arteries submitted to angioplasty in pigs. Acta Cir Bras. 2006;21:139–143. doi: 10.1590/s0102-86502006000300004. [DOI] [PubMed] [Google Scholar]
- 32.Rider P., Carmi Y., Cohen I. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int J Cell Biol. 2016;2016:9259646. doi: 10.1155/2016/9259646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M., Cresswell N., Tavora F., Mont E., Zhao Z., Burke A. In-stent restenosis is associated with neointimal angiogenesis and macrophage infiltrates. Pathol Res Pract. 2014;210:1026–1030. doi: 10.1016/j.prp.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Koh T.J., DiPietro L.A. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosmac K., Gonzalez-Freire M., McDermott Mary M. Correlations of calf muscle macrophage content with muscle properties and walking performance in peripheral artery disease. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.118.015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellegrin M., Bouzourène K., Poitry-Yamate C. Experimental peripheral arterial disease: new insights into muscle glucose uptake, macrophage, and T-cell polarization during early and late stages. Physiol Rep. 2014;2 doi: 10.1002/phy2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muraille E., Leo O., Moser M. TH1/TH2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front Immunol. 2014;5:603. doi: 10.3389/fimmu.2014.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 39.Boehler R.M., Kuo R., Shin S. Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype. Biotechnol Bioeng. 2014;111:1210–1221. doi: 10.1002/bit.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hachim D., LoPresti S.T., Yates C.C., Brown B.N. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials. 2017;112:95–107. doi: 10.1016/j.biomaterials.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamaly N., Fredman G., Fojas J.J.R. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano. 2016;10:5280–5292. doi: 10.1021/acsnano.6b01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan R.P., Chan A.H.P., Wei S. Bioactive materials facilitating targeted local modulation of inflammation. J Am Coll Cardiol Basic Trans Science. 2019;4:56–71. doi: 10.1016/j.jacbts.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honold L., Nahrendorf M. Resident and monocyte-derived macrophages in cardiovascular disease. Circ Res. 2018;122:113–127. doi: 10.1161/CIRCRESAHA.117.311071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sander J., Schmidt S.V., Cirovic B. Cellular differentiation of human monocytes is regulated by time-dependent interleukin-4 signaling and the transcriptional regulator NCOR2. Immunity. 2017;47:1051–1066.e12. doi: 10.1016/j.immuni.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin J.-D., Nishi H., Poles J. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4 doi: 10.1172/jci.insight.124574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cochain C., Vafadarnejad E., Arampatzi P. Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res. 2018;122:1661–1674. doi: 10.1161/CIRCRESAHA.117.312509. [DOI] [PubMed] [Google Scholar]
- 47.Barrett T.J. Macrophages in atherosclerosis regression. Arterioscler Thromb Vasc Biol. 2020;40:20–33. doi: 10.1161/ATVBAHA.119.312802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duda S.H., Bosiers M., Lammer J., Scheinert D., Zeller T., Oliva V. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. J Endovasc Ther. 2006;13:701–710. doi: 10.1583/05-1704.1. [DOI] [PubMed] [Google Scholar]
- 49.Müller-Hülsbeck S., Keirse K., Zeller T. Twelve-month results from the MAJESTIC trial of the eluvia paclitaxel-eluting stent for treatment of obstructive femoropopliteal disease. J Endovasc Ther. 2016;23:701–707. doi: 10.1177/1526602816650206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 51.Ikonomidis I., Lekakis J.P., Nikolaou M. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation. 2008;117:2662–2669. doi: 10.1161/CIRCULATIONAHA.107.731877. [DOI] [PubMed] [Google Scholar]
- 52.Giles J.T., Sattar N., Gabriel S. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol. 2020;72:31–40. doi: 10.1002/art.41095. [DOI] [PubMed] [Google Scholar]
- 53.Klein A.L., Imazio M., Brucato A. RHAPSODY: rationale for and design of a pivotal phase 3 trial to assess efficacy and safety of rilonacept, an interleukin-1α and interleukin-1β trap, in patients with recurrent pericarditis. Am Heart J. 2020;228:81–90. doi: 10.1016/j.ahj.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Chung E.S., Packer M., Lo K.H. Anti-TNF therapy against congestive heart failure investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 55.Bissonnette R., Tardif J.C., Harel F. Effects of the tumor necrosis factor-α antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circ Cardiovasc Imaging. 2013;6:83–90. doi: 10.1161/CIRCIMAGING.112.975730. [DOI] [PubMed] [Google Scholar]