Visual Abstract
Key Words: CLEC4E, inflammation, ischemia-reperfusion injury, magnetic resonance imaging, myocardial remodeling
Abbreviations and Acronyms: ACS, acute coronary syndrome; AMI, acute myocardial infarction; ANOVA, analysis of variance; CAD, coronary artery disease; Car3, carbonic anhydrase 3; CLEC4E, C-type lectin domain family 4 member E; CMC, cardiac myocyte; Cxcl2, CXC chemokine ligand 2; Cxcr2, CXC chemokine receptor 2; DAMP, damage-associated molecular pattern; ECM, extracellular matrix; Efna2, ephrin A2; ESV, end-systolic volume; Grk2, G protein–coupled receptor kinase 2; hs-TnI, high-sensitivity troponin I; I/R, ischemia-reperfusion; LAD, left anterior descending coronary artery; LV, left ventricular; MPO, myeloperoxidase; MRI, magnetic resonance imaging; NS, not significant; PRR, pattern recognition receptor; qRT-PCR, quantitative reverse transcription polymerase chain reaction; RNA, ribonucleic acid; SMC, smooth muscle cell; STEMI, ST-segment elevation myocardial infarction; TnT, troponin T; WT, wild-type
Highlights
-
•
The role of the CLEC4E during myocardial healing after ischemia-reperfusion injury is unknown.
-
•
CLEC4E deletion is associated with reduced cardiac injury, inflammation, and left ventricular structural and functional remodeling.
-
•
CLEC4E is a promising target to modulate myocardial inflammation and enhance repair after ischemia-reperfusion injury.
Summary
The bacterial C-type lectin domain family 4 member E (CLEC4E) has an important role in sterile inflammation, but its role in myocardial repair is unknown. Using complementary approaches in porcine, murine, and human samples, we show that CLEC4E expression levels in the myocardium and in blood correlate with the extent of myocardial injury and left ventricular (LV) functional impairment. CLEC4E expression is markedly increased in the vasculature, cardiac myocytes, and infiltrating leukocytes in the ischemic heart. Loss of Clec4e signaling is associated with reduced acute cardiac injury, neutrophil infiltration, and infarct size. Reduced myocardial injury in Clec4e–/– translates into significantly improved LV structural and functional remodeling at 4 weeks’ follow-up. The early transcriptome of LV tissue from Clec4e–/– mice versus wild-type mice reveals significant upregulation of transcripts involved in myocardial metabolism, radical scavenging, angiogenesis, and extracellular matrix organization. Therefore, targeting CLEC4E in the early phase of ischemia-reperfusion injury is a promising therapeutic strategy to modulate myocardial inflammation and enhance repair after ischemia-reperfusion injury.
Cardiac repair after myocardial infarction is a highly orchestrated and complex cascade of events characterized by an initial inflammatory phase with immune cell infiltration and release of danger-associated molecular patterns (DAMPs) that subsequently transits into a reparative and proliferative phase (1, 2, 3). The early inflammatory response with binding of DAMPS to cognate pattern recognition receptors (PRRs) needs to be properly and timely coordinated to allow subsequent tissue repair and prevent maladaptive left ventricular (LV) remodeling, defective scar formation, and impaired patient outcome (4,5). Excessive or prolonged inflammation would result in ventricular dilatation and systolic dysfunction, putting patients at increased risk for developing heart failure (6), whereas deficient early inflammation would fail to clear necrotic cardiac cells and matrix components and hamper subsequent tissue repair. This complex and tightly regulated innate immune activation response emphasizes the critical need for a better understanding of the cellular and molecular pathways governing this biphasic repair process after ischemia-reperfusion (I/R) injury.
Previously, we investigated the temporal changes in transcriptional profile of patients with an acute myocardial infarction (AMI) in circulating blood cells (7). We confirmed a robust activation of pro-inflammatory pattern recognition receptors, including the C-type lectin domain family 4 member E receptor (CLEC4E) that is usually expressed on leukocytes and activated in response to bacteria (8,9). This innate immune receptor senses necrotic material induced by ischemic injury in vitro (10) and adversely modulates the inflammatory response in experimental murine brain injury (11,12). However, the role of CLEC4E in myocardial I/R injury remains unknown.
The current study investigated the role of CLEC4e signaling on the early inflammatory response and the subsequent healing phase after acute myocardial ischemic injury, and explored its potential as a new target for intervention in myocardial I/R injury.
Methods
Ethics
All animal procedures were performed according to the Belgian law on care and use of experimental animals and were approved by the Ethics Committee for Animal Experimentation at KU Leuven (P244/2014 and P064/2017). The patient study protocol complied with the Declaration of Helsinki, was approved by the regional ethical committee, and all patients signed informed consent (Ethical Committee ML8525, Belgian trial no. B322201214942, S54129) (7).
Porcine model of I/R injury and cardiac magnetic resonance imaging
Ten domestic pigs (body weight: 20-30 kg) were sedated, anesthetized, and underwent I/R injury by transient balloon occlusion of the left anterior descending coronary artery (LAD) distal to the first diagonal branch for 50 minutes, followed by 4 hours’ reperfusion, as previously described (7). After 4 hours’ reperfusion, a subgroup of 6 pigs underwent 3-T cardiac magnetic resonance imaging (MRI) (Prisma-Tim, Siemens) imaging to evaluate infarct size (MI/left ventricle), end-systolic volume (ESV), end-diastolic volume, and ejection fraction, followed by euthanasia (13). Biopsy specimens from ischemic, border, and remote zones of the left ventricle were collected for differential gene expression analysis and histopathology. Details are provided in the Supplemental Materials and Methods, and Supplemental Table 1.
Mouse model of I/R injury and cardiac MRI
Twelve- to 14-week-old male C57Bl6/J wild-type (WT) mice were cross-bred with Clec4e–/– mice (031936-UCD), obtained at the Mutant Mouse Resource and Research Center. Mice underwent I/R injury by 60 minutes’ LAD ligation followed by reperfusion, as described previously (14). They were randomly allocated to evaluate arms with 24 hours’ (WT, n = 12; Clec4e–/–, n = 14), 72 hours’ (WT, n = 5; Clec4e–/–, n = 5), or 4 weeks’ (WT, n = 17; Clec4e–/–, n = 16) reperfusion. Cardiac MRI data were acquired at 4 weeks’ reperfusion on a Bruker BioSpec 70/30 7T MRI system (Bruker BioSpin) by investigators blinded to the genotype of the mice. After euthanasia, blood was collected from the inferior caval vein to measure high-sensitivity troponin I (hs-TnI) as a surrogate marker of cardiac injury and to isolate neutrophils to study their phenotype and migration capacity; LV tissue was collected for histological analysis and transcriptome studies at 24 hours after I/R injury. Organs were perfused with saline for 10 minutes before harvesting for histological and differential gene expression analysis using ribonucleic acid (RNA)-sequencing and quantitative real-time polymerase chain reaction (qRT-PCR). Additional details are provided in the Supplemental Materials and Methods.
In vitro analysis of Clec4e gene deletion on chemokine signaling
Peripheral blood neutrophils were isolated from WT and Clec4e–/– mice by using the Neutrophil Isolation Kit for mice (130-097-658, Miltenyi), according to the manufacturer’s instructions. To study the effect of Clec4e on neutrophil migration, a transwell migration assay toward a Cxcl2 gradient (10 ng/mL) was performed by using WT and Clec4e–/– neutrophils. To investigate whether Clec4e influences Cxcr2 protein expression, bone marrow cells were collected from the femurs of WT and Clec4e–/– mice as previously described (15). Proteins were then isolated by using radioimmunoprecipitation assay buffer supplemented with protease inhibitors, and Cxcr2 expression was analyzed according to immunoblot analysis. Additional details are provided in the Supplemental Materials and Methods.
Analysis of microarray CLEC4E expression and validation with qRT-PCR in patients with AMI
We identified CLEC4E as one of the top upregulated transcripts in a profiling study in 65 patients with acute coronary syndrome (ACS) (GEO accession number GSE123342) (7). We validated CLEC4E expression at the time of admission in an extended cohort of 138 patients with AMI via qRT-PCR and in 20 patients with stable coronary artery disease (CAD). CLEC4E whole blood transcript levels were then correlated with peak high-sensitivity troponin T (TnT) levels and LV ejection fraction before discharge.
Statistical analysis
Data are shown as mean ± SD or median (interquartile range) for the number of animals studied. Data analysis was performed blinded, and normal distributions were tested by using Shapiro-Wilk and Kolmogorov-Smirnov tests. CLEC4E expression in porcine tissue was analyzed by using one-way analysis of variance (ANOVA) with Tukey's correction for multiple pairwise testing. CLEC4E expression in human peripheral blood samples was analyzed by using a Kruskal-Wallis test with Dunn’s correction for multiple comparisons. Intergroup differences were analyzed by using a 2-tailed Student’s t-test for normally distributed data or nonparametric Mann-Whitney U test for non-normally distributed data. For experiments performed at different time points (24 and 72 hours), 2-way ANOVA with Šidák’s correction for multiple testing was performed. Pearson correlations were used for normally distributed data and Spearman correlations for non-normally distributed data to analyze linear or general associations between variables, respectively. Categorical variable differences were determined with the chi-square test or Fisher exact test and are presented with the odds ratio and 95% confidence intervals. P values < 0.05 were considered statistically significant. The statistical analyses were performed by using Prism version 8.0 software (GraphPad Software).
Details regarding the extended methods are provided in the Supplemental Materials and Methods section, and in Supplemental Figures 1, 2, 3, and 4.
Results
CLEC4E expression levels in the ischemic porcine myocardium and correlation with LV function
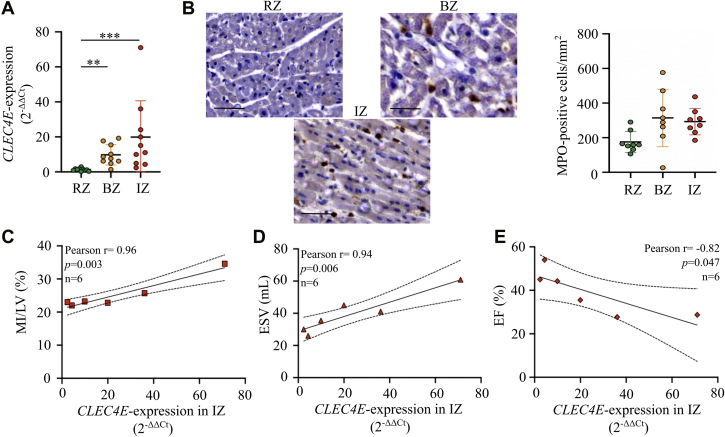
To examine whether CLEC4E expression is upregulated in the heart after I/R injury, we collected 3 different regions of porcine heart tissue 4 hours after I/R injury (remote zone, border zone, and ischemic core, n = 10) and measured CLEC4E expression levels. CLEC4E expression was significantly upregulated in both the ischemic core and border zone, with the highest levels in the ischemic core (P = 0.004 and P < 0.001 vs remote zone, respectively) (Figure 1A).
Figure 1.
CLEC4E Is Significantly Upregulated in the Ischemic Myocardium and Correlates With Infarct Size and Left Ventricular Function in the Acute Inflammatory Response After I/R Injury in Pigs
(A) Quantitative reverse transcription polymerase chain reaction of C-type lectin domain family 4 member E (CLEC4E) expression (mean with SD, 2-ΔΔCt) in porcine remote zone (RZ), border zone (BZ), and ischemic core zone (IZ) heart tissue, normalized to peptidylprolyl isomerase A (n = 10). (B) Quantification of myeloperoxidase (MPO)-positive cells/mm2 (mean ± SD) in RZ, BZ, and IZ heart tissue. (C to E) Pearson correlations of CLEC4E expression in IZ versus infarct size as percentage of left ventricular mass (myocardial infarction [MI]/left ventricle [LV], %), left ventricular end-systolic volume (ESV), and ejection fraction (EF) (n = 6). One-way analysis of variance test, with Tukey’s correction for multiple testing. Dotted lines indicate 90% CI. ∗∗P < 0.01, ∗∗∗P < 0.001. Scale bar = 20 μm. I/R = ischemia-reperfusion.
To investigate whether increased CLEC4E expression in the border zone and ischemic core can be accounted for by infiltrating inflammatory cells, which in the acute phase are predominantly neutrophils, we performed immunohistochemical stains for neutrophil-specific myeloperoxidase (MPO) and measured the number of MPO-positive cells (Figure 1B). However, neutrophil infiltration did not correlate with LV CLEC4E expression (Pearson r = 0.12; P = not significant [NS]; n = 8), suggesting that CLEC4E was also upregulated in resident or parenchymal cells within the ischemic myocardium. The latter is consistent with our findings that CLEC4E expression in the ischemic core positively correlates with MRI-based measurements of infarct size (MI/left ventricle) (r = 0.96; P = 0.003) (Figure 1C) and LV ESV (r = 0.94; P = 0.006) (Figure 1D), and inversely relates to LV ejection fraction (r = –0.82; P = 0.047) (Figure 1E).
Leukocyte recruitment and Clec4e-expression in the infarcted murine heart during the acute inflammatory phase of I/R injury
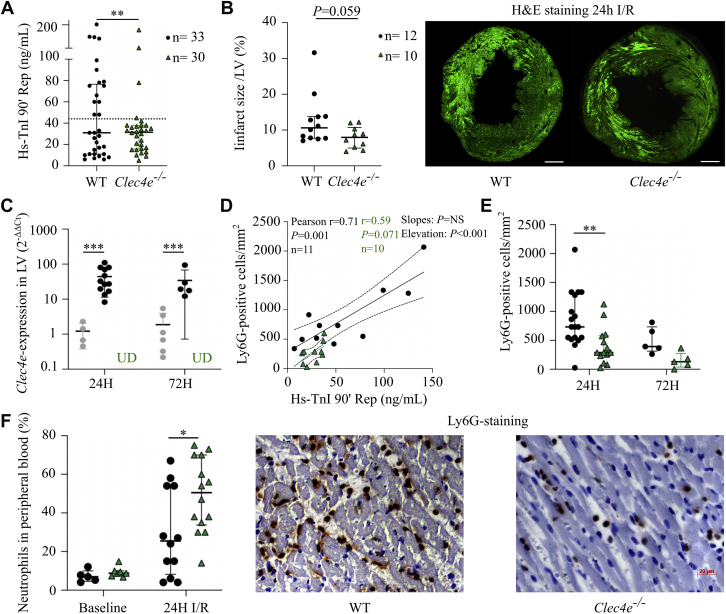
To investigate how Clec4e gene function modulates the myocardial response to acute ischemic injury, transient ligation of the LAD was performed in Clec4e–/– mice and WT control mice. We first examined whether Clec4e gene function affects the initial ischemic injury. Significantly greater ischemic injury 90 minutes after reperfusion was measured in WT mice (n = 33) versus Clec4e–/– mice (n = 30), as evidenced by the greater proportion of mice with plasma hs-TnI levels exceeding the average value of 44 ng/mL (P = 0.006) (Figure 2A). The data suggest that the odds of having TnI above the average of 44 ng/mL is 5.4 times higher in WT mice compared with Clec4e–/– mice (odds ratio: 5.4; 95% CI: 1.6-16.5), despite having comparable risk areas after occlusion of the LAD (Supplemental Figure 1A). The difference observed in cardiac necrosis markers at 90 minutes is consistent with a trend toward reduced infarct size (P = 0.059) in Clec4e–/– mice 24 hours after I/R injury (Figure 2B).
Figure 2.
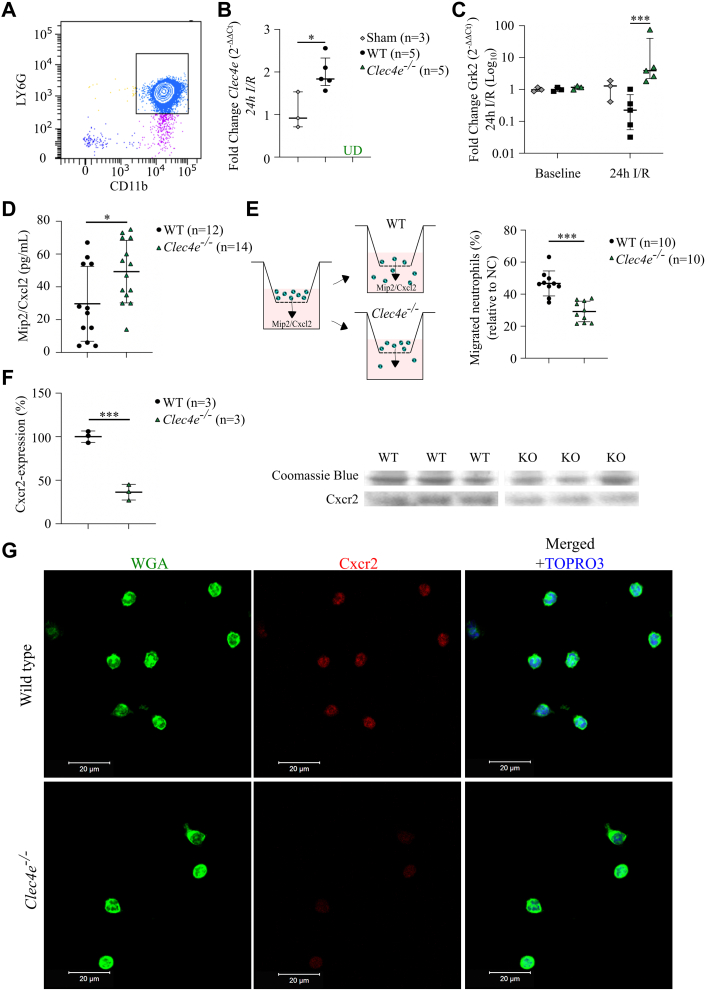
Clec4e Expression Is Upregulated in the Murine Heart, and Clec4e Signaling Influences the Recruitment of Leukocytes After I/R Injury
(A) High-sensitivity troponin I (hs-TnI) plasma levels at 90 minutes’ reperfusion (Rep) after 60 minutes’ ischemia (wild-type [WT], n = 33; Clec4e–/–, n = 30); Mann-Whitney U test. (B) Infarct size in the left ventricle (LV) based on hematoxylin and eosin (H&E) staining under 488 nm wavelength light to visualize autofluorescence of necrotic cells, with representative sections per genotype. Scale bar = 500 μm. (C) Quantitative reverse transcription polymerase chain reaction of Clec4e expression (log2-fold) in left ventricular tissue in sham-operated mice (gray), WT control mice (black), and Clec4e–/– mice (green) 24 hours (n = 4, n = 12, and n = 10, respectively) and 72 hours (n = 6, n = 5, and n = 5) after I/R injury. Two-way analysis of variance (ANOVA) with Šidák’s correction. (D) Pearson correlation between TnI at 90 minutes’ reperfusion versus lymphocyte antigen 6 complex locus G6D (Ly6G)-positive cells at 24 hours’ reperfusion in WT (n = 11) and Clec4e–/– (n = 10) mice. (E) Number of Ly6G-positive cells in the LV/mm2 in WT control mice (and Clec4e–/– mice (n = 14) at 24 hours (n = 18 and n = 14, respectively) and 72 hours (n = 5 and n = 5). Two-way ANOVA with Šidák’s correction. (F) Left: Percent neutrophils in peripheral blood at baseline and 24 hours (n = 12 and n = 14). Two-way ANOVA with Šidák’s correction. Right: Ly6G-staining of left ventricular tissue in WT and Clec4e–/– mice at 24 hours. Scale bar = 20 μm. Results are shown as mean with SD or as median with interquartile range. Dotted lines indicate 95% confidence interval. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. NS = not significant; UD = undetectable; other abbreviations as in Figure 1.
We next measured Clec4e expression in the murine heart at 24 and 72 hours after I/R injury using qRT-PCR and found significant upregulation of Clec4e transcript levels in WT mice compared with sham mice at 24 and 72 hours (both, P < 0.001) (Figure 2C). To investigate whether increased Clec4e signaling is associated with recruitment of different leukocyte subpopulations in the infarcted myocardium during the early post–I/R inflammatory phase, we measured neutrophil infiltration in the left ventricle at 24 hours’ reperfusion and monocyte/macrophage infiltration at 72 hours’ reperfusion. Early TnI plasma levels significantly correlated with the number of infiltrating neutrophils at 24 hours in a similar way in both genotypes (P = NS) (Figure 2D), but the slope in Clec4e–/– was significantly lower (P < 0.001). The number of infiltrating lymphocyte antigen 6 complex locus G6D–positive neutrophils in Clec4e–/– mice at 24 hours was significantly decreased compared with WT mice (P = 0.003) (Figure 2E), while neutrophil counts in peripheral blood were significantly higher (P = 0.025) (Figure 2F). At 72 hours, monocytes/macrophages (lysosome-associated membrane protein 2 [Mac3]) infiltration was significantly and similarly increased in the left ventricle of WT and Clec4e–/– mice compared with findings at the 24-hour time point (both, P < 0.001). Consistent with our earlier observations in pigs, no correlation between Clec4e expression in the ischemic murine myocardium and the number of infiltrating neutrophils was observed (Pearson r = –0.38; P = NS; n = 12).
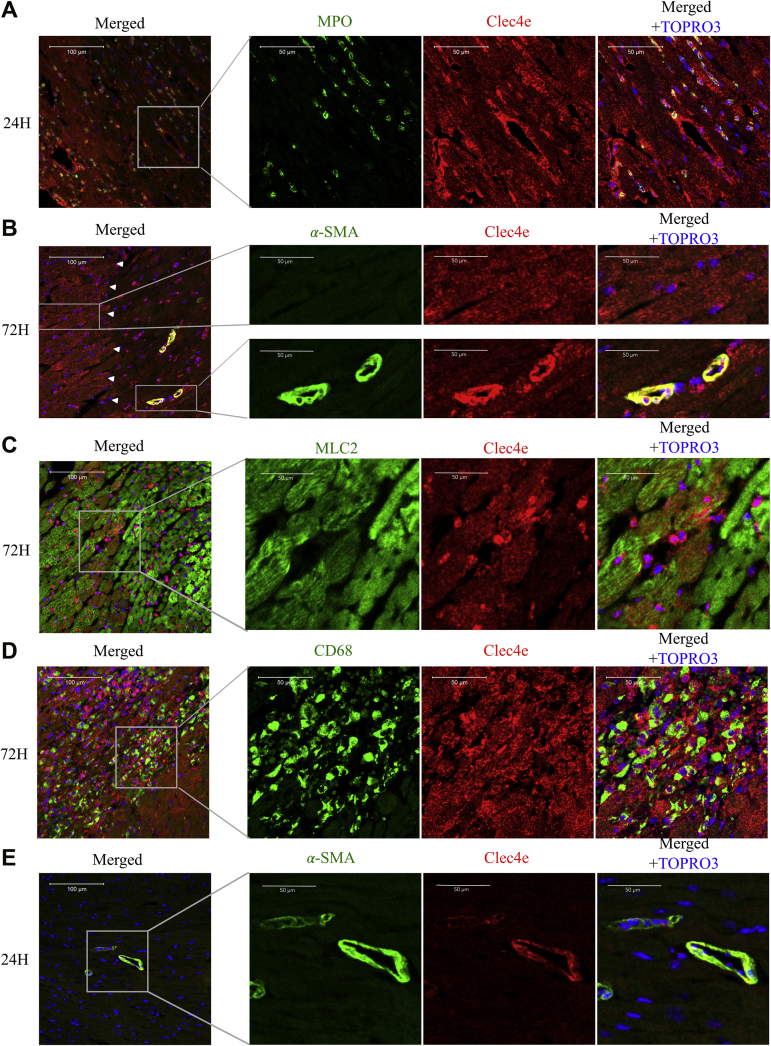
To determine whether Clec4e expression is increased in parenchymal cardiac cells upon I/R injury, we performed immunohistochemical analysis on midventricular heart sections of WT C57Bl6/J mice at 24 and 72 hours after I/R injury. Clec4e protein expression co-localizes with MPO-positive cells, with cardiac myocytes (CMCs), and in muscularized vasculature 24 hours after I/R injury (Figure 3A). At 72 hours after I/R injury, Clec4e expression is markedly increased in smooth muscle cells (SMCs), infiltrating macrophages, and CMCs (Figures 3B to 3D), more specifically in viable CMCs at the border zone (Figure 3B, left inset). Interestingly, Clec4e expression also co-localizes with CD68, a marker for monocytes and macrophages in the ischemic myocardium. In remote, nonischemic myocardium, Clec4e immunoreactivity was only detectable in SMCs, although much less than in ischemic myocardium (Figure 3E). Together, these results indicate that Clec4e is not only expressed by infiltrating inflammatory cells but also by resident myocardial parenchymal and vascular cells.
Figure 3.
Temporal and Spatial Clec4e Expression in the Ischemic Heart 24 and 72 Hours After I/R Injury
Heart tissue was stained for Clec4e (red) in combination with antibodies against MPO to identify neutrophils (green) at 24 h’ post–I/R injury (A) and α-smooth muscle actin (α-SMA) to highlight smooth muscle cells (green) 72 h’ post–I/R injury (B). Upper panels show viable BZ tissue, and lower panels show IZ tissue. MLC2 to visualize cardiomyocytes (green) (C) and CD68 identified infiltrating monocytes/macrophages (D) at 72 h’ post–I/R injury. (E) Sections from the RZ were stained with α-SMA antibody at 24 h’ post–I/R injury. Nuclei are counterstained with TO-PRO-3 (blue; Life Technologies). Scale bar is 100 μm in left panel (merged) and 50 μm in 3 right panels. Left panel inA, 20× magnification; 3 right panels, 40× magnifications. Abbreviations as in Figure 1.
Effects of Clec4e deletion on chemokine signaling
To explore the potential mechanism of reduced neutrophil infiltration in Clec4e–/– mice, we investigated the effect of Clec4e–/– on chemokine signaling. One of the most important chemokine receptors responsible for neutrophil recruitment is CXC chemokine receptor 2 (CXCR2), post-translationally regulated by the G protein–coupled receptor kinase 2 (Grk2) (16). Grk2 is responsible for the phosphorylation of chemokine receptors, leading to internalization, receptor desensitization, and reduced responsiveness of neutrophils to migrate toward chemokine gradients produced during inflammation (17,18). To investigate whether Clec4e signaling alters Grk2 expression in the setting of sterile inflammation induced by I/R injury, we isolated peripheral blood neutrophils from WT and Clec4e–/– mice 24 hours after I/R injury (Figure 4A) and performed qRT-PCR for Clec4e and Grk2. We measured significant upregulation of Clec4e in WT mice compared with sham mice (P = 0.016) (Figure 4B). There was no difference in Grk2 expression levels in neutrophils from WT (n = 3) and Clec4e–/– (n = 3) mice at baseline (Figure 4C). Interestingly, at 24 hours’ post–I/R injury, Grk2 expression levels were downregulated in WT mice but significantly increased in Clec4e–/– mice (P < 0.001). In addition, increased Cxcl2 levels were recorded in plasma at 24 hours’ post–I/R injury (P = 0.025) (Figure 4D).
Figure 4.
Potential Mechanism for Reduced Neutrophil Infiltration in Response to I/R Injury
(A) Flow cytometry confirms isolation of neutrophils, positive for both LY6G and cluster of differentiation 11 b (CD11b) antigens. (B) Quantitative reverse transcription polymerase chain reaction of isolated neutrophils from sham (n = 3), WT (n = 5), and Clec4e–/– mice 24 h’ post–I/R injury. Clec4e expression. (C) Baseline (n = 3 vs 3) and 24-h G protein–coupled receptor kinase 2 (Grk2) expression in WT and Clec4e–/– mice (n = 5 vs 5). Two-way ANOVA with Šidák’s correction. (D) Cxcl2 plasma levels in WT (n = 12) and Clec4e–/– (n = 14) mice 24 h’ post–I/R injury; Student’s t-test. (E) Neutrophil migration assay toward Mip2/Cxcl2-chemokine gradient in WT (n = 10) and Clec4e–/– (n = 10) mice, normalized to negative control (NC) (medium alone); Student’s t-test. (F) Right: Cxcr2 expression levels in isolated bone marrow–derived cells, normalized to average Cxcr2 expression in WT value in n = 3 for both genotypes. Left: Image of Coomassie blue stain (loading control) and Cxcr2 Western blot. Results are shown as mean ± SD or median with interquartile range. (G) Neutrophils isolated from health WT and Clec4e-/- mice were stained for the chemokine receptor Cxcr2 (Red) in combination with wheat-germ agglutinin (WGA; Green) to delineate cell membranes. Nuclei are counterstained with TO-PRO-3 (Blue; Life Technologies). Scale bar is 20 μm. ∗P < 0.05, ∗∗∗P < 0.001. Scale bar = 20 μm. KO = knockout; WGA = wheat germ agglutinin; other abbreviations as in Figures 1 and 2.
To determine whether Clec4e–/– neutrophils showed reduced migration toward the Cxcr2 receptor CXC chemokine ligand 2 (Cxcl2), we performed a Cxcl--induced migration assay, using freshly isolated neutrophils from WT and Clec4e–/– mice (Figure 4E). Of note, Clec4e–/– neutrophils exhibited significantly reduced migration compared with WT neutrophils (P < 0.001).
We next evaluated whether reduced neutrophil migration could be caused by lower Cxcr2 receptor expression in Clec4e–/– cells and performed immunoblot analysis on isolated bone marrow–derived cells from both genotypes. The results revealed significantly lower expression of Xxcr2 in Clec4e–/– cells compared with WT cells (P < 0.001) (Figure 4F). To validate these results in isolated peripheral blood neutrophils from WT and Clec4e–/– mice, we performed confocal microscopy using Cxcr2-specific antibody and confirmed markedly reduced total Cxcr2 expression in isolated neutrophils from Clec4e–/– mice compared with WT mice (Figure 4G).
LV remodeling and function of the murine heart 4 weeks after I/R injury
To investigate whether Clec4e signaling and the altered neutrophil infiltration in early post–I/R injury influences LV functional and structural remodeling, we performed cardiac MRI analysis and fibrosis staining of LV tissue at the 1-month follow-up in 17 WT mice and 16 Clec4e–/– mice. Cardiac MRI analysis revealed a significantly higher number of Clec4e–/– mice with ejection fraction >52% (odds ratio: 6.0; 95% CI: 1.1-24.6; P = 0.032) and thus better preserved LV function after I/R injury, consistent with the smaller initial ischemic injury. Linear regression analysis to quantify the relationship between cardiac MRI–based LV end-systolic and end-diastolic dimensions at 1-month follow-up with the initial TnI plasma levels exhibited significant genotype-dependent differences in slopes for the regression lines (P = 0.004 and P = 0.006, respectively) (Figures 5A and 5B). This was not observed for LV ejection fraction (Figure 5C). These data suggest significantly better LV functional and structural LV remodeling after I/R injury in Clec4e–/– mice.
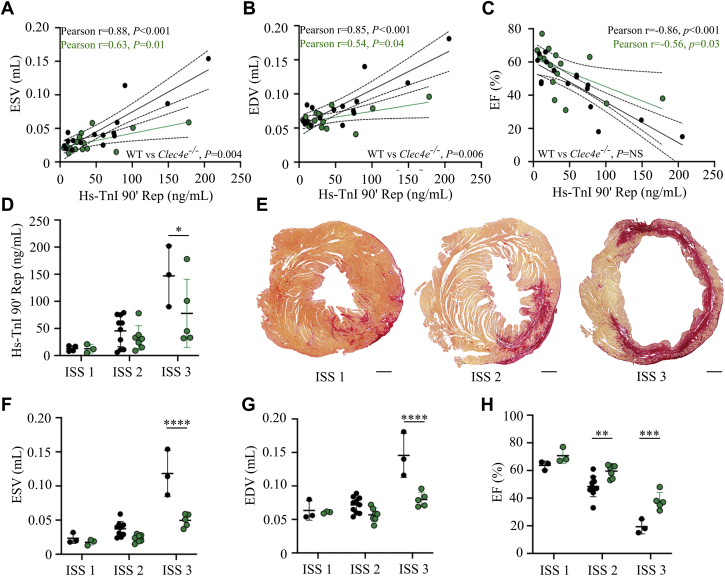
Figure 5.
Cardiac MRI in WT Control Mice and Clec4e–/– Mice 4 Weeks After I/R Injury
Pearson correlation between high-sensitivity troponin I (hs-TnI) plasma levels measured at 90 min’ reperfusion (Rep) and ESV (A), end-diastolic volume (EDV) (B), and EF (C) in WT control mice (n = 16) and Clec4e–/– mice (n = 15). (D) hs-TnI at 90 min’ reperfusion for WT and Clec4e–/– mice (mean ± SD). (E) Representative mosaic images of fibrosis staining for each infarct severity score (ISS). (F) ESV, (G) EDV, and (H) EF. To compare the 2 genotypes and the 3 levels of infarct severity, a 2-way ANOVA with Šidák’s correction was performed. Scale bar = 500 μm. Dotted lines indicate 95% CI. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations as in Figures 1 and 2.
The degree of replacement fibrosis at 4 weeks as a percentage of the LV surface area paralleled the severity of the initial ischemic injury, reflected by hs-TnI levels at 90 minutes in both genotypes (WT, Pearson r = 0.92, P < 0.001; Clec4e–/–, Pearson r = 0.39, P = NS). In an additional exploratory analysis, 3 independent and blinded researchers scored and stratified mice according to tertiles of replacement fibrosis in the left ventricle (post-mortem histopathological analysis) (Figure 5E). WT and Clec4e–/– mice with an infarct severity score of 1 (lowest tertile or replacement fibrosis <8% of LV surface area; WT, n = 4; Clec4e–/–, n = 3) had similar hs-TnI plasma levels at 90 minutes’ reperfusion (Figure 5D), MRI-based LV dimensions (Figures 5F and 5G), and LV global function parameters (Figure 5H). In contrast, Clec4e–/– mice with a severity score of 2 (middle tertile with fibrotic area between 8% and 25% of the LV surface area; WT, n = 10; Clec4e–/–, n = 8) and with a severity score of 3 (upper tertile with replacement fibrosis >25% of the LV surface area; WT, n = 3; Clec4e–/–, n = 5) exhibited better preservation of LV function and a lesser degree of adverse remodeling; these findings were evidenced by significantly smaller ESV and end-diastolic volume (both, P < 0.001) and significantly higher LV ejection fraction in Clec4e–/– mice with an infarct severity score of 2 to 3 (P = 0.003 and P = 0.001, respectively). Of note, hs-TnI plasma levels at 90 minutes’ reperfusion were again significantly lower in Clec4e–/– mice in those with an infarct severity score of 3 (P = 0.039).
Transcriptome of the left ventricle during the early inflammatory response after I/R injury
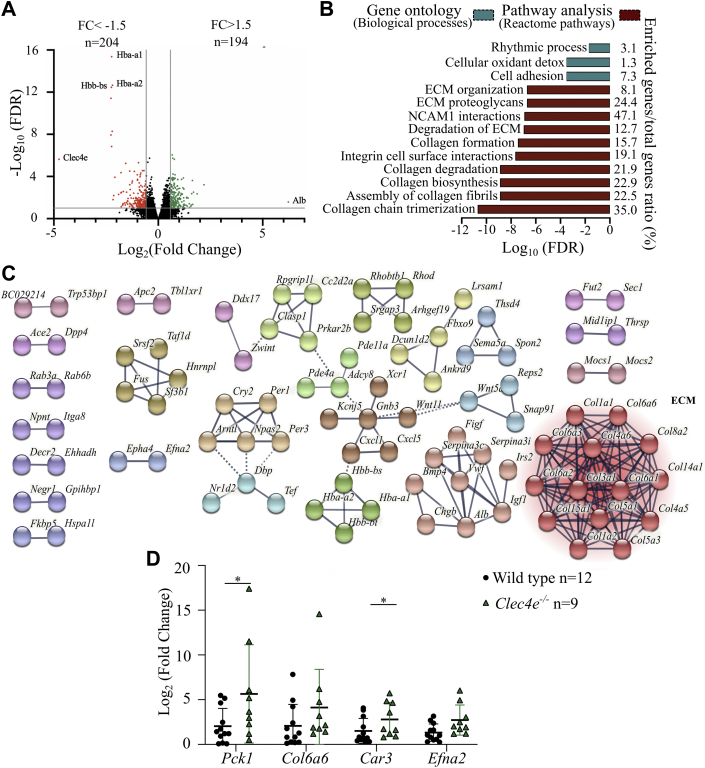
To explore potential pathogenic mechanisms associated with increased Clec4e expression, we performed RNA-sequencing of LV tissue (apex to mid–left ventricle) extracts from 5 WT mice and 5 Clec4e–/– mice at 24 and 72 hours after I/R injury. We detected 398 differentially expressed genes, of which 204 were downregulated and 194 were upregulated (P < 0.100) (Figure 6A). The top 20 significantly differentially expressed genes (fold-change) included genes involved in cardiac remodeling (Mir22 host gene [Mir22hg]), gluconeogenesis (phosphoenolpyruvate carboxykinase 1 [Pck1]), angiogenesis, endothelial barrier function (UBX domain protein 10 [Ubxn10], ephrin A2 [Efna2]), secondary messenger signaling (adenylate cyclase 8 [Adcy8], phosphodiesterase 11A [Pde11a]), scavenging of reactive oxygen species (carbonic anhydrase 3 [Car3]), and extracellular matrix (ECM) (collagen type VI alpha 6 chain [Col6a6]) (Supplemental Table 2).
Figure 6.
RNA Sequencing of WT Versus Clec4e–/– LV Tissue 24 Hours After I/R Injury Shows Differential Expression of Genes Involved in ECM Regulation
(A) Volcano plot of differentially expressed genes. Red dots reflect 204 significantly downregulated transcripts, and green dots reflect 194 significantly upregulated transcripts (adjusted P < 0.10). (B) Gene ontology and pathway analysis shows significant differential regulation of biological processes and pathways involved in extracellular matrix (ECM) regulation. (C) STRING network confirms clustering of genes involved in ECM regulation. (D) Polymerase chain reaction analysis of selected transcripts. Two-way ANOVA. Mean values ± SD in WT mice (n = 12) and Clec4e–/– mice (n = 10). ∗P < 0.05. Car3 = carbonic anhydrase 3; Col6a6 = collagen type IV alpha 6 chain; Efna2 = ephrin A2; FC = fold change; FDR = false discovery rate; NCAM = neural cell adhesion molecule; Pck1 = phosphoenolpyruvate carboxykinase 1; other abbreviations as in Figures 1 and 2.
Gene ontology analysis of these 398 transcripts with the DAVID (Database for Annotation, Visualization and Integrated Discovery) functional gene annotation tool revealed their involvement in cell adhesion and cellular oxidant detoxification (Figure 6B, blue). In addition, network analysis with the online STRING database confirmed clustering of these genes related to ECM organization (Figure 6C). Moreover, pathway analysis with the Reactome Pathway Database showed enrichment of genes involved in collagen chain trimerization pathways, assembly of collagen fibrils, and collagen biosynthesis (Figure 6B, red). We validated the profiling data by RT-qPCR for Pck1, Col6a6, Car3, and Efna2 in WT (n = 12) and Clec4e–/– (n = 10) mice and confirmed significant upregulation of Pck1 (P = 0.047) and Car3 (P = 0.047) and a trend for Efna2 (P = 0.056) (Figure 6D).
At 72 hours after I/R injury, only 8 genes were significantly differentially regulated (Supplemental Figure 5), suggesting that CLEC4E signaling plays a more prominent role in the early inflammatory response during the first 24 hours after I/R injury.
CLEC4E expression in whole blood of patients with AMI in the acute phase of AMI
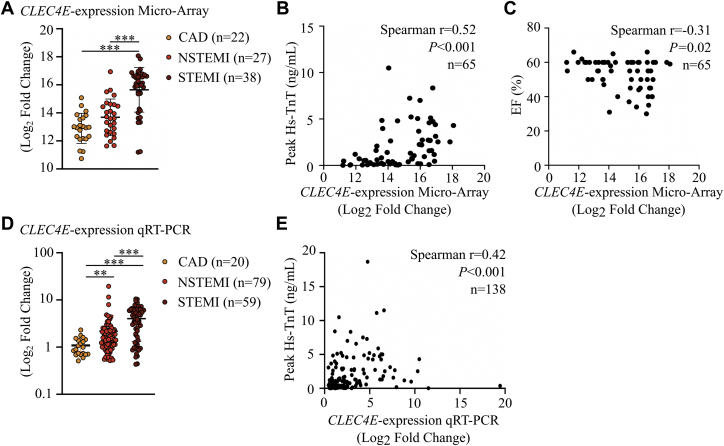
We detected significant upregulation of CLEC4E in patients with ST-segment elevation myocardial infarction (STEMI) compared with patients with chronic CAD (P < 0.001) (Figure 7A). CLEC4E expression significantly correlated with peak TnT levels (r = 0.52; P < 0.001) (Figure 7B) and ejection fraction (r = –0.31; P = 0.018) (Figure 7C) in these patients with ACS (n = 38 with STEMI and n = 27 with non-STEMI).
Figure 7.
CLEC4E Is Significantly Upregulated in the Acute Phase of AMI and Correlates With Cardiac Damage on Admission
(A) Microarray analysis of CLEC4E expression in 65 patients with acute coronary syndrome (ACS) on admission and 22 control subjects with chronic coronary artery disease (CAD). Spearmann correlation between CLEC4E expression determined by microarray and peak high-sensitivity troponin T (hs-TnT) levels (B) and EF (C) on admission. n = 65. (D) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) validation of CLEC4E expression in a cohort of 158 patients with ACS. (E) Spearmann correlation between CLEC4E expression determined by PCR and peak TnT levels in 138 patients. Results are shown as mean ± SD. To test the differences in CLEC4E expression, a Kruskal-Wallis test with Dunn’s correction for multiple comparisons was performed. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. NSTEMI = non–ST-segment elevation myocardial infarction; STEMI = ST-segment elevation myocardial infarction; other abbreviations as in Figure 1.
We then validated CLEC4E expression in an extended cohort of 138 patients with AMI using qRT-PCR. We confirmed the expression data from the microarray with a significant increase of CLEC4E in patients with non-STEMI (P = 0.004) and STEMI (P < 0.001) versus patients with chronic CAD (Figure 7D) and a significant correlation with peak TnT levels (r = 0.42; P < 0.001) (Figure 7E).
Discussion
In the current study, we report for the first time that the CLEC4E receptor of the innate immune system is upregulated during the acute inflammatory phase in porcine and murine I/R injury. Confocal analysis showed that the early induction at 24 hours after I/R injury is accounted for in part by infiltrating neutrophils and in part attributable to increased expression in resident CMCs and vascular SMCs, with a further increase at 72 hours in infiltrating monocytes/macrophages. Clec4e deletion in mice was associated with significantly less acute ischemic cardiac injury, reduced early neutrophil infiltration, and a strong trend toward smaller infarct size at 24 hours’ post–I/R injury. Isolated neutrophils from Clec4e–/– mice 24 hours’ post–I/R injury showed increased expression of Grk2 kinase, known to desensitize CXCR2-receptor signaling, and Clec4e–/– neutrophils exhibited reduced migration toward CXCL2 in vitro. Furthermore, isolated bone marrow cells from Clec4e–/– mice displayed a significant reduction in baseline Cxcr2 protein expression. Reduced early ischemic injury translated into favorable functional and structural LV remodeling 4 weeks after I/R injury. Differential gene expression and STRING network analysis of LV tissue samples identified a transcriptome in Clec4e–/– mice that was predominantly enriched in biological processes and pathways involved in ECM regulation. Finally, exploratory analysis of patients with AMI confirmed a highly significant correlation between CLEC4E expression levels in whole blood on admission, peak high-sensitivity TnT levels, and LV ejection fraction. Taken together, our data highlight for the first time an important role for CLEC4E signaling during acute myocardial ischemic injury, not only as a marker of infarct severity and adverse LV remodeling but also as a novel target for pharmacologic intervention.
The first key observation of our study relates to the temporal and spatial dynamics of myocardial CLEC4E expression in the setting of acute ischemic damage. We measured a gradient of increased Clec4e expression toward the most vulnerable ischemic area that was not only attributable to early leukocyte recruitment in the porcine or murine ischemic myocardium. Confocal analysis confirmed that Clec4e immunoreactivity is not restricted to infiltrating immune cells but is also widely present in CMCs and in vascular SMCs. Although endothelial cells express different types of PRRs that are partially responsible for endothelial cell activation (19) and subsequent inflammatory cell extravasation, we did not detect Clec4e expression in endothelial cells. These pathological observations are in line with bone marrow chimera experiments by Arumugam et al (12) in a murine brain injury model, which also revealed marked Clec4e expression in resident brain cells.
The second key observation is that Clec4e seems to play a major role in the induction and propagation of ischemic inflammation in response to the release of DAMPs after I/R injury. Our data support the widely held model of Clec4e as a necrotic cell receptor directing inflammatory responses to areas of necrotic cell damage. It was long thought that PRRs were predominantly expressed on leukocytes, but recent studies also showed an important role of PRRs on CMCs and SMCs. CMCs have been shown to express functional PRRs and increase inflammatory signaling in response to myocardial injury (20,21). Increased inflammatory signaling and downstream chemokine production reportedly exert cardioprotective effects in vitro. Tarzami et al (22) showed that 2 important early-phase chemokines (Mip2/Cxcl2 and Mcp1/Cxcl1) reduce hypoxia-induced cell death in cultured CMCs. In line with the protective effects of Clec4e–/– we showed on acute injury and leukocyte infiltration after I/R injury, our results suggest that Clec4e deletion increases viability of CMCs in response to hypoxic stress, which might be due to a change in chemokine response. These cardioprotective effects are consistent with previous data of neuroprotection in similar Clec4e transgenic models of ischemic stroke (11,12). In SMCs, PRRs are also functionally expressed and have been shown to induce a proinflammatory phenotype upon activation (23). After activation of PRRs, vascular SMCs contribute to the early inflammatory response by producing proinflammatory mediators that increase leukocyte infiltration and reactive oxygen species production. These proinflammatory effects have direct consequences on tissue perfusion and vascular permeability and could therefore influence acute injury and leukocyte infiltration.
The role of PRRs, and more specifically CLEC4E, on monocytes/macrophages has been investigated in more detail in the setting of atherosclerosis. Multiple studies showed that Clec4e favors a proinflammatory, proatherogenic phenotype of macrophages, contributing to plaque progression (24,25). In the setting of myocardial ischemia, CLEC4E is upregulated in the acute phase of AMI in peripheral blood monocytes and could promote the proinflammatory phenotype of infiltrating monocytes/macrophages, thereby increasing phagocytic activity and degradation properties. Although we did not observe a genotype-dependent difference in monocyte/macrophage infiltration 72 hours after I/R injury (data not shown), we cannot exclude transcriptional differences in the inflammasome or a shift toward a more reparative macrophage phenotype in Clec4e knockout mice.
Taken together, these data show an important role of Clec4e on cardioprotection, but elucidation of the underlying mechanisms requires additional single-cell multi-omics analysis.
Third, we measured a significant increase in Grk2 expression in circulating neutrophils from Clec4e–/– mice 24 hours after I/R injury compared with WT mice. In addition, we observed reduced migration in response to Cxcl2 of neutrophils isolated from Clec4e–/– mice and noted significantly reduced baseline Cxcr2 expression levels on isolated Clec4e–/– bone marrow cells, confirmed in isolated neutrophils using immunocytochemistry. We also recorded a significant increase in Mip2/Cxcl2 levels in plasma collected 24 hours after I/R injury. Together, these results suggest a significant effect of Clec4e deletion on the CXCR2/CXCL2 chemokine–signaling pathway. The effect of Clec4e genetic deletion has been previously reported in a mouse model of polymicrobial septic peritonitis, showing reduced neutrophil infiltration in the peritoneum of Clec4e–/– mice and Grk2-mediated desensitization of the CXCR2 receptor (17,18). We hypothesized that a similar mechanism would render neutrophils less responsive for chemokine gradients produced by the ischemic heart (26,27), and our results confirm a comparable effect of Clec4e on chemokine signaling in our setting of sterile inflammation. Neutrophils isolated from healthy WT or Clec4e–/– mice did not exhibit a significant difference in baseline Grk2 expression, suggesting that the effects of Clec4e signaling on Grk2 expression are only induced in response to I/R injury. Interestingly, protein analysis revealed that there is a reduction in baseline Cxcr2 receptor expression in Clec4e–/– mice, which could explain why Clec4e–/– cells showed reduced migration toward Cxcl2 gradients and reduced neutrophil infiltration in ischemic myocardium. The increase in plasma Mip2/Cxcl2 in Clec4e–/– mice could be a counterregulatory mechanism in response to reduced neutrophil infiltration, as proposed in the setting of polymicrobial septic peritonitis (26). It has been shown that Toll-like receptor-4 signaling, which is closely linked to CLEC4E signaling (27), cross-talks with chemokine signaling via downstream activation of MAP/ERK Kinase Kinase (MEKK) and results in transcriptional downregulation of Grk2, promoting leukocyte migration (16) (Figure 8). The exact downstream pathway and interactions between Clec4e gene function, Grk2, and chemokine signaling require further investigations in follow-up studies.
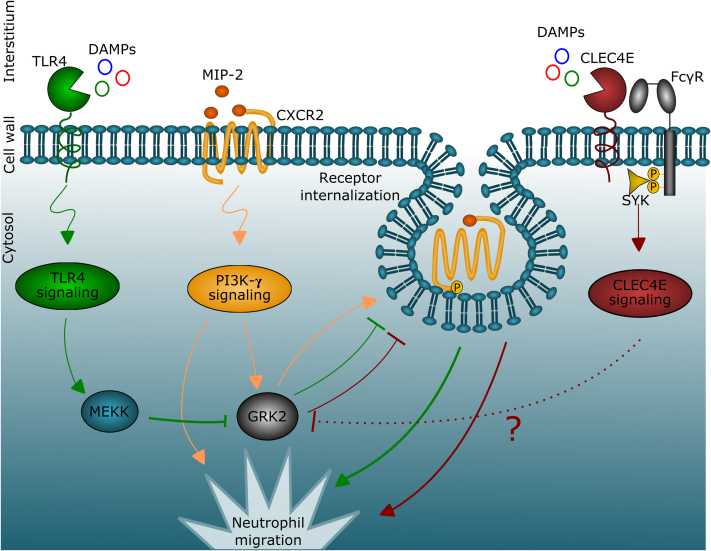
Figure 8.
Schematic Overview of the Interplay Between Chemokine-Signaling and PRR-Signaling Pathways
The release of the chemokine MIP-2 during I/R injury triggers the activation of the CXC chemokine receptor 2 (CXCR2) receptor on neutrophils, leading to downstream phosphatidylinositol 3-kinase-γ (PI3K-γ) signaling and subsequent neutrophil chemotaxis. The CXCR2 receptor also directly regulates its own expression via upregulation of GRK2, which leads to CXCR2 phosphorylation, receptor internalization, and recycling. During sterile inflammation, neutrophil recruitment is required, and the negative feedback induced by CXCR2 needs to be reduced. When Toll-like receptor 4 (TLR4) signaling is activated by damage-associated molecular patterns (DAMPs), it downregulates GRK2 in an MEKK-dependent manner, and promotes neutrophil chemotaxis. In addition to TLR4, the CLEC4E receptor also promotes neutrophil migration by downregulating GRK2. Together, the triggers for neutrophil chemotaxis outweigh the negative feedback of chemokine signaling, but when CLEC4E gene function is deleted, the balance will shift toward increased GRK2 expression, CXCR2 receptor desensitization, and reduced chemotaxis. MEKK = MAP/ERK Kinase Kinase; PRR = pattern recognition receptor; other abbreviations as in Figures 1, 2, and 4.
Fourth, comprehensive cardiac MRI analysis found that Clec4e–/– mice have improved LV remodeling after I/R injury. The benefit is most prominent in mice with the largest area at risk and a scar burden of >25% of LV surface area, which we classified as the highest infarct severity score, but it was also detectable in mice with a scar burden >8% and <25% of LV surface area. In contrast, Arumugam et al (12) did not report a statistical difference in LV volumes or LV function after permanent ligation of the LAD in heterozygous Clec4e+/– mice. Major differences in experimental model (permanent vs transient ligation), genetic background (partial Clec4e deletion vs homozygous deletion), and in cardiac phenotyping (transthoracic echocardiography versus 7-T MRI) likely account for the respective findings.
Fifth, we performed unbiased RNA-sequencing to better understand downstream pathways that mediate the differential remodeling processes in both genotypes and measured 398 significantly differentially expressed genes, predominantly involved in ECM regulation and cell adhesion. Although the ECM serves as an endogenous DAMP to PRR on innate immune cells (28,29), studies have found that PRR signaling also influences collagen production and ECM remodeling (30). Our bioinformatics analysis and validation by qRT-PCR revealed enrichment for genes involved in collagen assembly and biosynthesis but also in cellular oxidant detoxification and neoangiogenesis.
Finally, to explore the translational relevance of our preclinical injury models, we performed exploratory microarray analysis on peripheral blood collected from patients with AMI (7) and confirmed significant upregulation of CLEC4E, with the highest expression in patients with STEMI. Subsequent RT-PCR validation in a separate cohort of 138 patients with AMI and 20 patients with CAD revealed a highly significant correlation of CLEC4E transcript levels in peripheral blood with peak high-sensitivity TnT cardiac necrosis marker. These data are consistent with our experimental data in porcine and murine I/R injury and extend previous reports on significantly upregulated CLEC4E levels in circulating monocytes from patients with CAD and in atherosclerotic plaques (24,25). Others have investigated the potential role of peripheral blood CLEC4E expression levels, as a biomarker for CAD severity (31), thereby also validating the gene expression tests for determining CAD risk. In concert, these data highlight the increasing importance and complex interplay of CLEC4E signaling in CAD and ACS.
Study limitations
First, to reduce sex-related variability, we only considered male C57Bl6/J mice. It has been reported that the estrogen-receptor ß has potential cardioprotective effects (32). Therefore, future I/R experiments in female mice are required. Second, the relatively short follow-up period of 4 weeks in I/R mice limits the possibility of evaluating survival rates and the development of advanced heart failure. Third, a baseline cardiac MRI assessment was not performed for the murine experiments. Fourth, the RNA-sequencing data need further validation and mechanistic studies to prove causality. Finally, further studies are required to elucidate the molecular mechanisms between Clec4e signaling and chemokine signaling.
Conclusions
Our data in 2 different experimental models of acute myocardial ischemia and in patients with AMI emphasize an important role of the innate immune receptor CLEC4E as a direct activator in the local response to cardiac damage. Observations in Clec4e knockout mice also indicate that blocking CLEC4E improves cardiac repair after myocardial I/R injury and point toward a new modifiable target of sterile inflammation during acute myocardial ischemic damage.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The CLEC4E-receptor plays an important role in myocardial healing after ischemia-reperfusion injury by increasing cardiac damage, inflammation, and LV-remodeling.
TRANSLATIONAL OUTLOOK: Our combined data in mice, pigs, and patients with acute coronary syndromes suggest that targeting CLEC4E might hold a promising strategy in the treatment of ischemic injury.
Funding Support and Author Disclosures
This work was supported by a KU Leuven financing grant (C14/20/095). Ms Veltman holds a grant from the Royal Academy for Medicine in Belgium (KAGB, ZDK4478). Dr Janssens is holder of the AstraZeneca chair in Cardiology at KU Leuven. Dr Sinnaeve is co-holder of the Bayer Chair in Cardiology at KU Leuven. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors acknowledge the work of Genewiz RNA-sequencing on our murine I/R LV-samples, the work of Petra Vandervoort for performing flow cytometry on isolated neutrophils, and the work of Marleen Lox for performing additional murine I/R experiments during the revision process.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental Materials and Methods, tables, and figures, please see the online version of this paper.
Appendix
References
- 1.Eltzschig H.K., Bratton D.L., Colgan S.P. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13(11):852–869. doi: 10.1038/nrd4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong T., Liu L., Jiang W., Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 3.Arslan F., De Kleijn D.P., Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol. 2011;8(5):292–300. doi: 10.1038/nrcardio.2011.38. [DOI] [PubMed] [Google Scholar]
- 4.Frangogiannis N.G. The inflammatory response in myocardial injury, repair and remodeling. Nat Rev Cardiol. 2014;11(5):255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong S.B., Hernández-Reséndiz S., Crespo-Avilan G.E. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73–87. doi: 10.1016/j.pharmthera.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St. John Sutton M.G., Sharpe N. Left ventricular remodeling after myocardial infarction: Pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 7.Vanhaverbeke M., Vausort M., Veltman D. Peripheral blood RNA levels of QSOX1 and PLBD1 are new independent predictors of left ventricular dysfunction after acute myocardial infarction. Circ Genomic Precis Med. 2019;12(12):561–572. doi: 10.1161/CIRCGEN.119.002656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingeter L.M., Lin X. C-type lectin receptor-induced NF-κB activation in innate immune and inflammatory responses. Cell Mol Immunol. 2012;9(2):105–112. doi: 10.1038/cmi.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X., Nagata M., Yamasaki S. Mincle: 20 years of a versatile sensor of insults. Int Immunol. 2018;30(6):233–239. doi: 10.1093/intimm/dxy028. [DOI] [PubMed] [Google Scholar]
- 10.Yamasaki S., Ishikawa E., Sakuma M., Hara H., Ogata K., Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9(10):1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y., Nakano Y., Mishiro K. Involvement of mincle and syk in the changes to innate immunity after ischemic stroke. Sci Rep. 2013;3:1–7. doi: 10.1038/srep03177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arumugam T.V., Manzanero S., Furtado M. An atypical role for the myeloid receptor Mincle in central nervous system injury. J Cereb Blood Flow Metab. 2017;37(6):2098–2111. doi: 10.1177/0271678X16661201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu M., Dhooge J., Ganame J. Non-invasive characterization of the area-at-risk using magnetic resonance imaging in chronic ischaemia. Cardiovasc Res. 2011;89(1):166–174. doi: 10.1093/cvr/cvq257. [DOI] [PubMed] [Google Scholar]
- 14.Lux A., Pokreisz P., Swinnen M. Concomitant phosphodiesterase 5 inhibition enhances myocardial protection by inhaled nitric oxide in ischemia-reperfusion injury. J Pharmacol Exp Ther. 2016;356(2):284–292. doi: 10.1124/jpet.115.227850. [DOI] [PubMed] [Google Scholar]
- 15.Liu X., Yamashita T., Chen Q. Interleukin 1 type 1 receptor restore: a genetic mouse model for studying interleukin 1 receptor-mediated effects in specific cell types. J Neurosci. 2015;35(7):2860–2870. doi: 10.1523/JNEUROSCI.3199-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan J., Malik A.B. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9(3):315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 17.Raghuwanshi S.K., Su Y., Singh V., Haynes K., Richmond A., Richardson R.M. The chemokine receptors CXCR1 and CXCR2 couple to distinct G protein-coupled receptor kinases to mediate and regulate leukocyte functions. J Immunol. 2012;189(6):2824–2832. doi: 10.4049/jimmunol.1201114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penela P., Ribas C., Sánchez-Madrid F., Mayor F. G protein-coupled receptor kinase 2 (GRK2) as a multifunctional signaling hub. Cell Mol Life Sci. 2019;76(22):4423–4446. doi: 10.1007/s00018-019-03274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mai J., Virtue A., Shen J., Wang H., Yang X.F. An evolving new paradigm: endothelial cells—conditional innate immune cells. J Hematol Oncol. 2013;6(1):1–13. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell J.A., Ryffel B., Quesniaux V.F.J., Cartwright N., Paul-Clark M. Role of pattern-recognition receptors in cardiovascular health and disease. Biochem Soc Trans. 2007;35(pt 6):1449–1452. doi: 10.1042/BST0351449. [DOI] [PubMed] [Google Scholar]
- 21.Yu L., Feng Z. In: Mediators Inflamm. 2018. Agrawal A., editor. 2018. The role of Toll-like receptor signaling in the progression of heart failure; p. 9874109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarzami S.T., Cheng R., Miao W., Kitsis R.N., Berman J.W. Chemokine expression in myocardial ischemia: MIP-2 dependent MCP-1 expression protects cardiomyocytes from cell death. J Mol Cell Cardiol. 2002;34(2):209–221. doi: 10.1006/jmcc.2001.1503. [DOI] [PubMed] [Google Scholar]
- 23.Shea-Donohue T., Notari L., Sun R., Zhao A. Mechanisms of smooth muscle responses to inflammation. Neurogastroenterol Motil. 2012;24(9):802–811. doi: 10.1111/j.1365-2982.2012.01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clément M., Basatemur G., Masters L. Necrotic cell sensor Clec4e promotes a proatherogenic macrophage phenotype through activation of the unfolded protein response. Circulation. 2016;134(14):1039–1051. doi: 10.1161/CIRCULATIONAHA.116.022668. [DOI] [PubMed] [Google Scholar]
- 25.Pfeifer P., Steinmetz M., Habel S.T. CLEC4E plays an essential role in human atherogenesis. Clin Cardiol. 2018;107(April):2018. [Google Scholar]
- 26.Lee W Bin, Yan J.J., Kang J.S. Mincle activation enhances neutrophil migration and resistance to polymicrobial septic peritonitis. Sci Rep. 2017;7(January):1–13. doi: 10.1038/srep41106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoenen H., Huber A., Sonda N. Differential control of mincle-dependent cord factor recognition and macrophage responses by the transcription factors C/EBPβ and HIF1α. J Immunol. 2014;193(7):3664–3675. doi: 10.4049/jimmunol.1301593. [DOI] [PubMed] [Google Scholar]
- 28.Forte E., Furtado M.B., Rosenthal N. The interstitium in cardiac repair: role of the immune–stromal cell interplay. Nat Rev Cardiol. 2018;15(10):601–616. doi: 10.1038/s41569-018-0077-x. [DOI] [PubMed] [Google Scholar]
- 29.Frantz S., Bauersachs J., Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res. 2009;81(3):474–481. doi: 10.1093/cvr/cvn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhattacharyya S., Kelley K., Melichian D.S. Toll-like receptor 4 signaling augments transforming growth factor-β responses: a novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am J Pathol. 2013;182(1):192–205. doi: 10.1016/j.ajpath.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elashoff M.R., Wingrove J.A., Beineke P. Development of a blood-based gene expression algorithm for assessment of obstructive coronary artery disease in non-diabetic patients. BMC Med Genomics. 2011;4(1):26. doi: 10.1186/1755-8794-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deschamps A.M., Murphy E., Sun J. Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends Cardiovasc Med. 2010;20(3):73–78. doi: 10.1016/j.tcm.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.