Abstract
This study examined the role of dopamine within the amygdala (AMY) in flavor preference learning induced by post-oral glucose. In Experiment 1, rats were trained with a flavor (CS+) paired with intragastric (IG) infusions of 8% glucose and a different flavor (CS−) paired with IG water infusions. The CS+ preference was evaluated in two-bottle tests following bilateral injection of the dopamine D1-like receptor antagonist, SCH23390, into the AMY at total doses of 0, 12, 24 and 48 nmol. SCH23390 produced dose-dependent reductions in CS+ intake but did not block the CS+ preference except at the two highest doses, which also greatly suppressed the CS intakes. In Experiment 2, new rats were injected daily in the AMY with either saline or SCH23390 (12 nmol), prior to training sessions with CS+/IG glucose and CS−/IG water. In the two-bottle tests, SCH rats, unlike the Control rats, failed to prefer the CS+ (55 vs. 81%). In Experiments 3 and 4, new rats were trained as in Experiment 2, except that brain injections were in the basolateral (BLA) and central (CeA) nuclei of AMY, respectively. SCH rats learned to prefer the CS+ to the CS−, although their preference was weaker than that displayed by the Control rats (Experiment 3: 59 vs. 80%; Experiment 4: 73 vs. 88%). These results show an essential role for D1-like receptor activation in the amygdala in the acquisition of flavor preference learning induced by the post-oral reinforcing properties of glucose. A distributed network mediating flavor-nutrient incentive learning is discussed.
Keywords: Carbohydrate, Conditioning, Forebrain, Learning, SCH23390, dopamine receptors, amygdala, behavioral pharmacology, Animal learning, reward
Introduction
Learning plays an important role in the development of flavor preferences and food selection in omnivores. There is extensive evidence from laboratory research that animals learn to prefer the flavor of foods and fluids that provide positive nutritional consequences. This is documented by studies showing that animals acquire strong and long-lasting preferences for flavored foods and fluids that either contain a nutrient or are paired with intragastric (IG) infusions of nutrients (Capaldi, 1996; Sclafani, 1999).
Flavor preference conditioning, like flavor aversion conditioning, is a form of classical conditioning in which a cue flavor (conditioned stimulus, CS) is associated with the oral and/or post-oral properties of a nutrient (unconditioned stimulus, US). There are two types of preference conditioning. In flavor-flavor conditioning, a preference develops for a cue flavor that is paired with the preferred flavor of a nutrient (e.g., sweet taste of sugar). In flavor-nutrient (or post-oral consequence learning), a preference develops for a cue flavor paired with the post-oral effects of a nutrient. The most common paradigm used to study conditioned flavor preferences is to pair one flavor (the CS+) with the nutrient US and a different flavor (the CS−) with water on alternate days and then assess preference learning by presenting the CS+ and CS− flavors in a two-choice test.
Flavor-nutrient learning, the subject of the present study, requires the neural integration of orosensory and viscerosensory information and the formation of long-term flavor memories. The brain mechanisms underlying these processes are incompletely understood. The results of lesion studies indicate that the pontine parabrachial nucleus, the lateral hypothalamus and the amygdala (AMY) play a crucial role (Touzani & Sclafani, 2001; Sclafani et al., 2001; Touzani & Sclafani, 2002; Touzani & Sclafani, 2005). Brain dopamine (DA) signaling is also implicated in flavor-nutrient conditioning. Using the same conditioning paradigm used in our lesion studies mentioned above, Mark et al. (1994) demonstrated an increase in dopamine efflux in the nucleus accumbens elicited by the consumption of the CS flavor that was paired with IG carbohydrate infusions but not with the CS flavor paired with IG water. A subsequent study by Azzara et al. (2001) provided further evidence of dopamine involvement in flavor-nutrient conditioning using systemic administration of D1- and D2-like receptor antagonists. Rats were trained to drink a CS+ flavored solution paired with IG infusions of 16% sucrose and a CS− flavor paired with IG water infusions. Unlike saline-treated control animals, rats treated with a D1-like receptor antagonist (SCH23390, 200 nmol/kg) during training failed to prefer the CS+ to the CS− in two-bottle choice tests. In contrast, the same dose of SCH23390 did not block the expression of a previously learned CS+ preference when the drug was administered at the time of two-bottle testing. Treatment with a D2-like receptor antagonist (raclopride; 200 nmol/kg), on the other hand, did not prevent the acquisition or expression of a CS+ preference. These finding indicate that flavor-nutrient learning is critically dependent upon D1-like but not D-2 like receptor transmission.
There is an extensive literature on the critical role of the mesocorticolimbic DA system in reward processes and reward-related learning (Wise, 2004; Berridge, 2007). In this system, DA neurons located in the ventral tegmental area (VTA) of the mesencephalon project to cortical and limbic structures including the nucleus accumbens (NAc), amygdala (AMY) and the prefrontal cortex (Swanson, 1982). In a recent study, we observed that injections of the D1-like receptor antagonist, SCH23390, into the NAc blocked the acquisition but not the expression of a flavor preference conditioned by IG glucose infusions (Touzani et al., 2008). This finding extends an earlier report that systemic administration of SCH23390, but not raclopride, a D2-like receptor antagonist, blocked flavor preference conditioning by IG sugar infusions (Azzara et al., 2001). Thus, D1-like receptor signaling in the NAc is critical for the formation of flavor-nutrient associations but this does not preclude the involvement of other brain targets of the mesocorticolimbic system.
The AMY has long been implicated in motivation and learning related to rewards (Cardinal et al., 2002; Baxter & Murray, 2002). In particular, recent studies have shown that AMY lesions impair flavor preference conditioning induced by both the orosensory and viscerosensory reinforcing properties of sugar (Gilbert et al., 2003; Touzani & Sclafani, 2005). Several lines of evidence suggest the possibility that activation of dopamine D1-like receptors in AMY is involved in flavor preference learning. The AMY receives DA innervation from VTA neurons and contains moderate to high density of D1 receptors (Dawson et al., 1986; Mansour et al., 1990; Huang et al., 1992; Asan, 1997; Leonard et al., 2003). Activation of dopamine D1-like receptors in the AMY is required for learning a sucrose-reinforced bar pressing response (Andrzejewski et al., 2005). Finally, neurochemical studies report an increase of DA efflux in the AMY by feeding and gastric load of nutrients (Heffner et al., 1980; Hajnal & Lenard, 1997), as well as during appetitive Pavlovian conditioning by predictive stimuli (Harmer & Phillips, 1999).
In the present study, we investigated the role of D1-like receptor signaling in AMY in flavor preference conditioning by IG glucose infusions. To this end, SCH23390 was injected into the AMY prior either to training or testing sessions. Central D2-like receptor signaling was not studied because systemic raclopride treatment failed to alter flavor conditioning by IG sugar infusions (Azzara et al., 2001). Based on our previous findings with systemic and NAc injections of SCH23390 (Azzara et al., 2001; Touzani et al., 2008), we predicted that SCH23390 injections in the AMY, including the basolateral complex (BLA) and the central amygdaloid nucleus (CeA) subdivisions, would impair the acquisition of a glucose-conditioned flavor preference but would have only a marginal effect on the expression of a previously learned flavor preference. Our recent findings with amygdala lesions (Touzani & Sclafani, 2005) also suggest that relatively large volume SCH23390 injections in the AMY will more completely block preference conditioning than smaller injections targeting the BLA or CeA regions.
Materials and methods
Subjects.
The subjects were 91 adult male Sprague-Dawley rats obtained from Charles River Laboratories (Wilmington, MA) or bred in our laboratory; they weighed 408-522 g at the time of brain surgeries. The rats were individually housed in plastic cages with stainless steel wire lids (Ancare, Bellmore, NY) in a vivarium maintained at 21°C and under a 12:12 h light:dark cycle (lights on at 0800h). They were maintained on chow (Laboratory Rodent Diet 5001, PMI Nutrition International, Brentwood, MO) and tap water. Experimental protocols were approved by Brooklyn College Animal Care and Use Committee and were performed in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
Surgery.
The rats were anesthetized with intraperitoneal injection of a ketamine hydrochloride (63 mg/Kg) and xylazine (9.4 mg/Kg) mixture and held in a Kopf stereotaxic apparatus with the incisor bar set 3.3 mm below the interaural line. Stainless steel guide cannulas (26-gauge, i.d. = 0.24 mm; o.d. = 0.46 mm, Plastics One Inc. Roanoke, VA) were aimed at bilateral placements in the AMY using the following coordinates: 2.0 mm posterior to Bregma, 4.2 mm lateral to the sagittal suture and 8.0 mm ventral from the surface of the skull for the whole AMY; 2.8 mm posterior to Bregma, 5.0 mm lateral to the sagittal suture and 8.2 mm ventral from the surface of the skull for the BLA; 2.2 mm posterior to Bregma, 4.2 mm lateral to the sagittal suture and 7.7 mm ventral from the surface of the skull for the CeA. The guide cannulae were secured on the skull with stainless steel screws and dental cement. During the same brain surgery session, the rats were fitted with a gastric catheter (silastic tubing, i.d. = 1.02 mm; o.d. = 2.16 mm) that was inserted in the fundus of the stomach and secured with sutures and polypropylene mesh. The tubing was routed under the skin and connected to a neck-mount connector pedestal that was mounted and secured on the animal’s neck with polypropylene mesh and sutures. Intramuscular penicillin (30,000 U) was given following the surgeries. One rat died after the surgery.
Apparatus.
As detailed in Touzani and Sclafani (2001), training and testing occurred in plastic cages that gave the rats access to one or two stainless steel drinking spouts. The spouts were attached to drinking bottles mounted on motorized holders that positioned the spouts at the front of the cage at the start of the sessions and retracted them at the end of the sessions. Licking behavior was monitored by an electronic lickometer interfaced to a microcomputer that activated a syringe pump as the rat drank. Plastic tubing connected the pump to the rat’s gastric catheter via the neck-mount connector pedestal. The infusion rate was 1.3 ml/min and the ratio of oral intake and infusion volume was maintained at approximately 1:1 by the computer unless indicated.
Test solutions.
The conditioned stimuli were cherry- and grape-flavored (0.05% Kool-Aid, General Foods, White Plains, NY) saccharin (0.2% sodium saccharin, Sigma, St. Louis, MO) solutions. The CS+ flavor was paired with IG infusions of 8% glucose (Bio-Serv, Frenchtown, NJ) and the CS− flavor was paired with IG water infusions. The specific flavor-infusion pairs were counterbalanced across subjects. All solutions were prepared with tap water.
Drugs and Infusion Procedures.
The dopamine D1-like receptor antagonist, SCH23390 (Sigma Chemical Company, St. Louis, MO) was dissolved in sterile isotonic saline (vehicle) and administered at a volume of either 0.5 μl/side (Experiments 1 and 2) or 0.25 μl/side (Experiments 3 and 4). Infusions of the drug or the vehicle into the amygdala were performed bilaterally using an infusion pump (Razel Scientific Instruments, Inc., Stamford, CT) and a 33-gauge (i.d. = 0.10 mm; o.d. = 0.20 mm) stainless steel internal cannula (Plastics one, Roanoke, VA) connected to a 2-μl Hamilton microsyringe (Hamilton Company, Reno, Nevada) by polyethylene tubing. At the moment of intracerebral injections, the rats were held gently, the stylus was removed and the cannulae were inserted. The tip of the injection cannulae protruded 1.0 mm beyond that of the guide. The injections were made at the rate of 0.5 μl/min and the cannulae were left in place one more minute before their removal.
Procedures.
Prior to the surgery, the rats were familiarized with unflavored 0.2% saccharin solution by giving them ad libitum access to the saccharin solution along with water and chow in their home cages for three days. Then the rats were housed in the test cages overnight with ad lib access to 0.2% saccharin solution, water and food to adapt them to the test cages. The saccharin and water bottles were automatically positioned to the front of the cages for 30 min every hour. Two to three weeks after the surgery, the rats were placed on a food restriction schedule and maintained at 85% of their ad libitum body weights. They were adapted to drink the saccharin solution in the test cages during 8-10 daily 30-min sessions. During the last four of these sessions, the rats were connected to the infusion system and were given IG water infusions as they drank the saccharin solution.
In Experiment 1, the rats (n=15) were given eight one-bottle training sessions (30 min/day). In sessions 1, 3, 5 and 7, intake of the CS+ solution was paired with concurrent IG infusions of 8% glucose; in sessions 2, 4, 6, and 8, intake of the CS− solution was paired with concurrent IG infusion of water. The right-left positions of the CS solutions were varied using an ABBA sequence. Following training, the rats were given a series of two-bottle tests with the CS+ vs. CS− solutions with no IG infusions. The rats received bilateral injections of 0 (saline), 12, 24 and 48 nmol of SCH23390 (0, 6, 12 and 24 nmol/0.5 μl/side) in the AMY, 10 min prior to the two-bottle tests with the CS+ vs. CS− solutions (eight 30 min/day sessions). Half of the rats received drug injections in an ascending order, and the other half in a descending order. Thus the rats received a total of six drug injections and two saline injections during testing. The left-right position of the CS solutions alternated daily, and the rats were injected twice with each drug dose to control for side preferences. Following each 2-day block of two-bottle tests, there was a 1-day break.
In Experiment 2, the rats (n=20) were divided into two groups equated for their pretraining intakes of saccharin. The Control group received bilateral injections of saline while the SCH group received injections of 12 nmol SCH23390 (6 nmol/0.5 μl/side) in the AMY 10 min prior to each of the CS+ and CS− training sessions (for a total of 8 injections). In sessions 1, 3, 5 and 7, intake of the CS+ solution was paired with 8 ml IG infusions of 8% glucose; in sessions 2, 4, 6, and 8, intake of the CS− solution was paired with 8 ml IG infusion of water. The IG infusions started after the rat emitted 20 licks, and continuous infusions were triggered once the rat reached 300 licks to deliver the fixed volume of 8 ml. Following each pair of training sessions with CS+ and CS− solutions, there was a 1-day break. In addition, the CS+ and CS− intakes of the Control rats were limited each day to the mean intakes of the SCH rats, which had unrestricted access to the solutions. Following training, two-bottle preference tests (four 30 min/day sessions) were conducted during which there were no brain injections or IG infusions, and CS intakes were unlimited.
In Experiments 3 and 4, the rats were trained with the same procedure used in Experiment 2 except that the injections of saline and 12 nmol SCH23390 (6 nmol/0.25 μl/side) were in the BLA (n=28) and the CeA (n=28) subdivisions of AMY, respectively.
Statistical analysis.
CS intakes were measured to the nearest 0.1 g and the data were analyzed using standard analyses of variance (ANOVA) procedures. Oral intakes during training and preference testing were averaged over 2- or 4-day blocks. Individual comparisons were evaluated using simple main effects tests or t-test when appropriate. Two-bottle preference data were also expressed as percent CS+ intake [(CS+ intake / total intake) x100]. The data were analyzed with ANOVA or t-test after an arcsine transformation as recommended by Kirk (1995).
Histological analysis.
At the completion of the experiments, the rats were deeply anesthetized and perfused transcardially with physiological saline followed by a 10% formalin solution. The brains were removed and soaked in a 10% formalin solution containing 20% sucrose for 3-6 days. The brains were coronally sectioned with a freezing microtome at 40 μm, and the sections were mounted on gelatin-coated glass slides and stained with thionin. The positions of the cannula tracks were examined under a light microscope and reconstructed on the appropriate frontal planes of the atlas of Paxinos and Watson (1998).
Results
Experiments 1 and 2: Effects of D1-like receptor antagonism in AMY on the acquisition and expression of glucose-conditioned flavor preference
Histology.
Cannula tip placements for all rats used in Experiments 1 and 2 are shown in Fig. 1. Placements were deemed appropriate for twelve rats in Experiment 1 (Fig. 1A) and 14 rats (seven Control rats and seven SCH rats) in Experiment 2 (Fig. 1B), and were primarily restricted to the rostral portion between the Frontal Planes −2.12 and −2.56 mm of the Paxinos and Watson (1998) atlas. The remaining nine rats had either a large unilateral lesion (one case), very rostral cannula placements between Frontal Planes −1.6 and −1.8 mm (six cases) or very dorsal between the central amygdala and the internal capsule (2 cases), and consequently their data were discarded. A photomicrograph of a representative bilateral microinjection site is shown in Fig. 1C.
Figure 1.
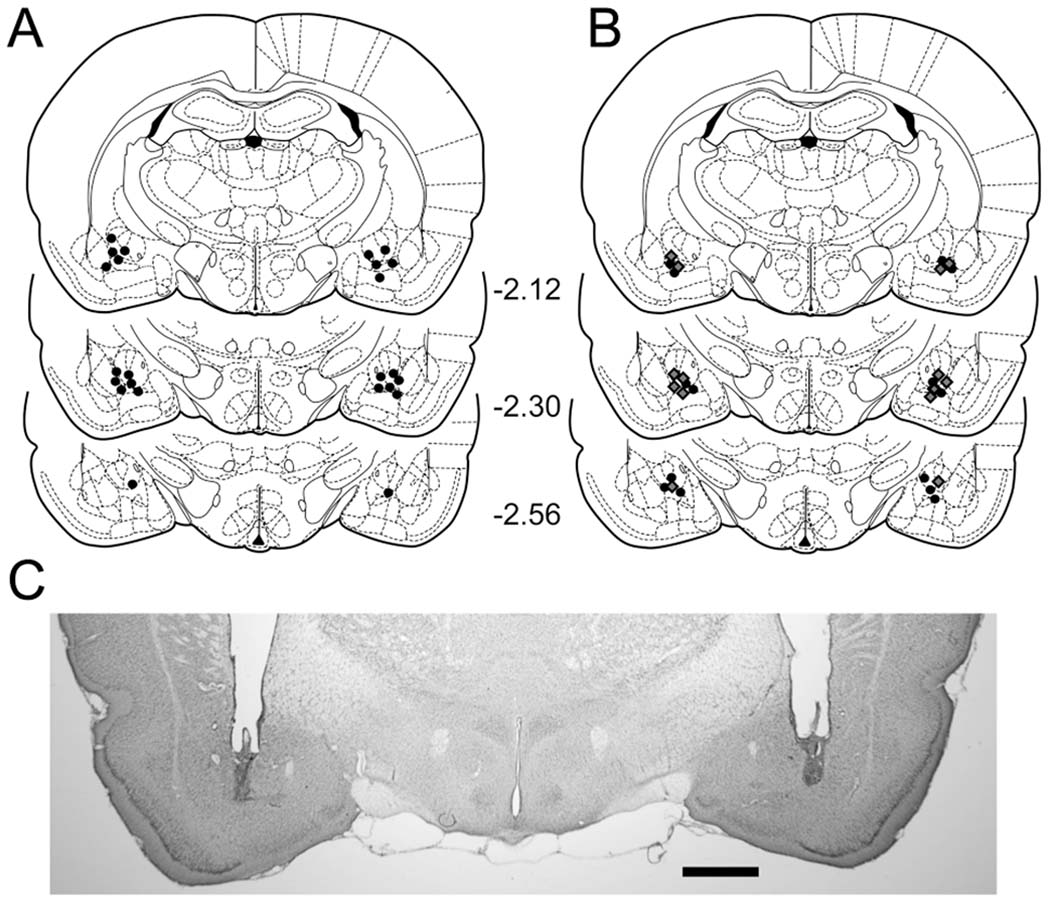
Schematic representations of cannula tip placements in the amygdala in Experiments 1 (1A) and 2 (1B). Coronal sections were adapted from Paxinos and Watson (1998) with permission. In Figure 1B, cannula tips are indicated by black circles in the SCH group and grey diamonds in the Control group. Numbers denote distance (in mm) posterior to bregma. Representative photomicrograph of a coronal section indicating bilateral cannula tracts terminating in the amygdala is shown in 1C. Scale bar: 1 mm.
Behavior.
Experiment 1.
During one-bottle training, the intakes of the CS+ and CS− failed to differ significantly (11.5 and 12.2 g/30 min, respectively). In the two-bottle preference tests (Figure 2), overall, the rats consumed more CS+ than CS− [F(1,11) = 78.2, p < 0.001] and their CS intakes decreased with increasing doses of SCH23390 [F(3,33) =142.4, p < 0.001]. Compared to intake at the 0 nmol dose, the two highest doses (24 and 48 nmol) greatly suppressed total CS intakes (from 18.8 to 3.6 and 2.0 g / 30 min, respectively). There was a CS x Dose interaction, F(3,33) = 69.8, p < 0.001 and the simple main effects analysis revealed that the rats consumed significantly (p <0.01) more CS+ than CS− at the 0 and 12 nmol dose. The CS+ vs. CS− difference approached significance at the 24 nmol dose (p = 0.087), but was not significant at the 48 nmol dose at which point intakes were minimal (1.3 vs. 0.8 g/30 min). Analysis of the percent CS+ intakes revealed that overall CS preferences declined as dose increased [F(3,33) = 6.88, p<0.001]. Individual tests indicated that CS preference did not differ at the 0 and 12 nmol dose (89 and 81%), and that both exceeded (p < 0.05) the percent intake at 48 nmol (62%). The preference at the 0 but not the 12 nmol dose also exceeded (p < 0.05) that at the 24 nmol dose (89 vs. 75%). Thus, SCH23390 treatment reduced CS intakes but did not significantly reduce CS+ preference except at the doses that greatly suppressed overall CS intakes.
Figure 2.
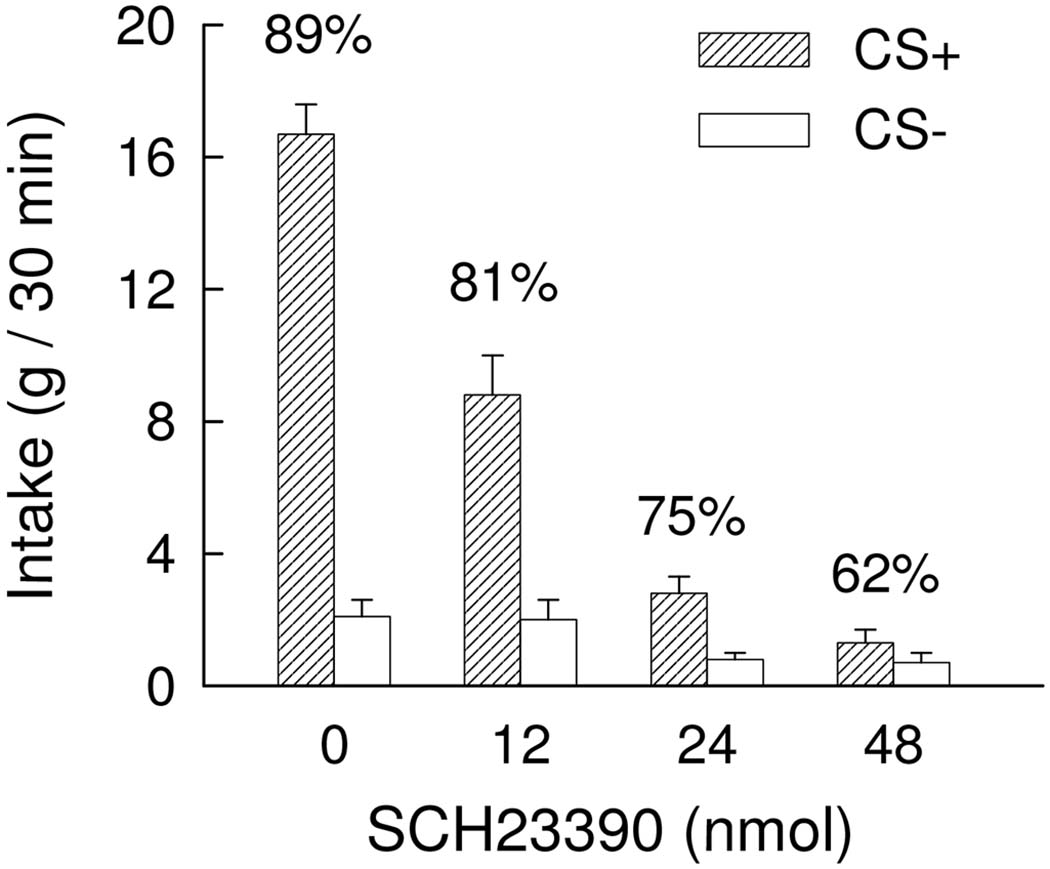
Experiment 1. Intakes (+S.E.M.) of the CS+ and CS− during two-bottle choice tests; data represent the mean of two 30-min sessions. Ten minutes prior to testing, the rats were injected with 0 (vehicle), 12, 24, 48 nmol of SCH23390 into the amygdala (6, 12, 24 nmol/side). The CS+ was paired with concurrent intragastric infusions of glucose and the CS− was paired with intragastric water infusions during training. No gastric infusions were given during testing. The asterisk denotes a significant (p < 0.05) difference between CS+ and CS− intakes. The numbers atop the bars represent the mean of the individual rat’s percent CS+ intakes.
Experiment 2.
The rats treated with 12 nmol SCH23390 during one-bottle training consumed only about 5 g of the CS+ or CS− during the 30-min sessions and the intakes of the Control group were limited to this amount. The results of the two-bottle preference tests are summarized in Figure 3. The SCH and Control groups did not significantly differ in their total CS intakes but there was a significant Group x CS interaction [F(1,12) = 7.93, p < 0.05]. Individual comparisons revealed that the Control group consumed more CS+ than CS− (p < 0.001) whereas the SCH group did not differ in its intake of the CS+ and CS− solutions. The groups did not differ in their intakes of the CS+ but the SCH rats consumed (p < 0.05) more CS− than did the Control rats. Consequently, the percent CS+ intake of the Control group exceeded that of the SCH group (81% vs. 55%, t (12) = 3.33, p < 0.01). Thus, the 12 nmol dose of SCH23390 that did not impair the expression of a previously acquired glucose-conditioned flavor preference in Experiment 1 totally blocked the acquisition of this preference when administered during training in the present experiment.
Figure 3.
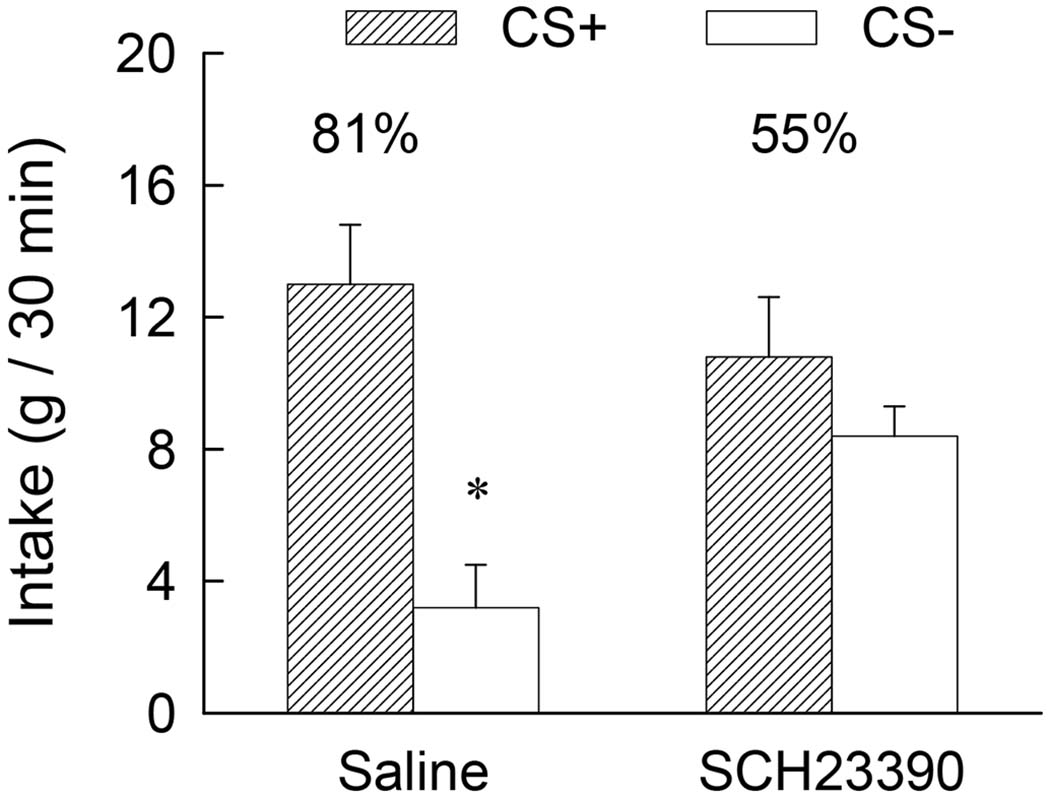
Experiment 2. Intakes (+S.E.M.) of the CS+ and CS− during two-bottle choice tests; data represent the mean of four 30-min sessions. The CS+ and CS− were paired with intragastric infusions of 8 ml glucose and water, respectively, during training. No gastric infusions were given during testing. The SCH group was given injections of 12 nmol of SCH23390 into the amygdala (6 nmol/side) ten minutes prior to the daily training sessions while the Control group was given vehicle injections. No injections were given prior to the two-bottle choice tests. The asterisk denotes a significant (p < 0.05) difference between CS+ and CS− intakes. The numbers atop the bars represent the mean of the individual rat’s percent CS+ intakes.
Experiment 3. Effects of D1-like receptor antagonism in BLA on the acquisition of glucose-conditioned flavor preference
Histology.
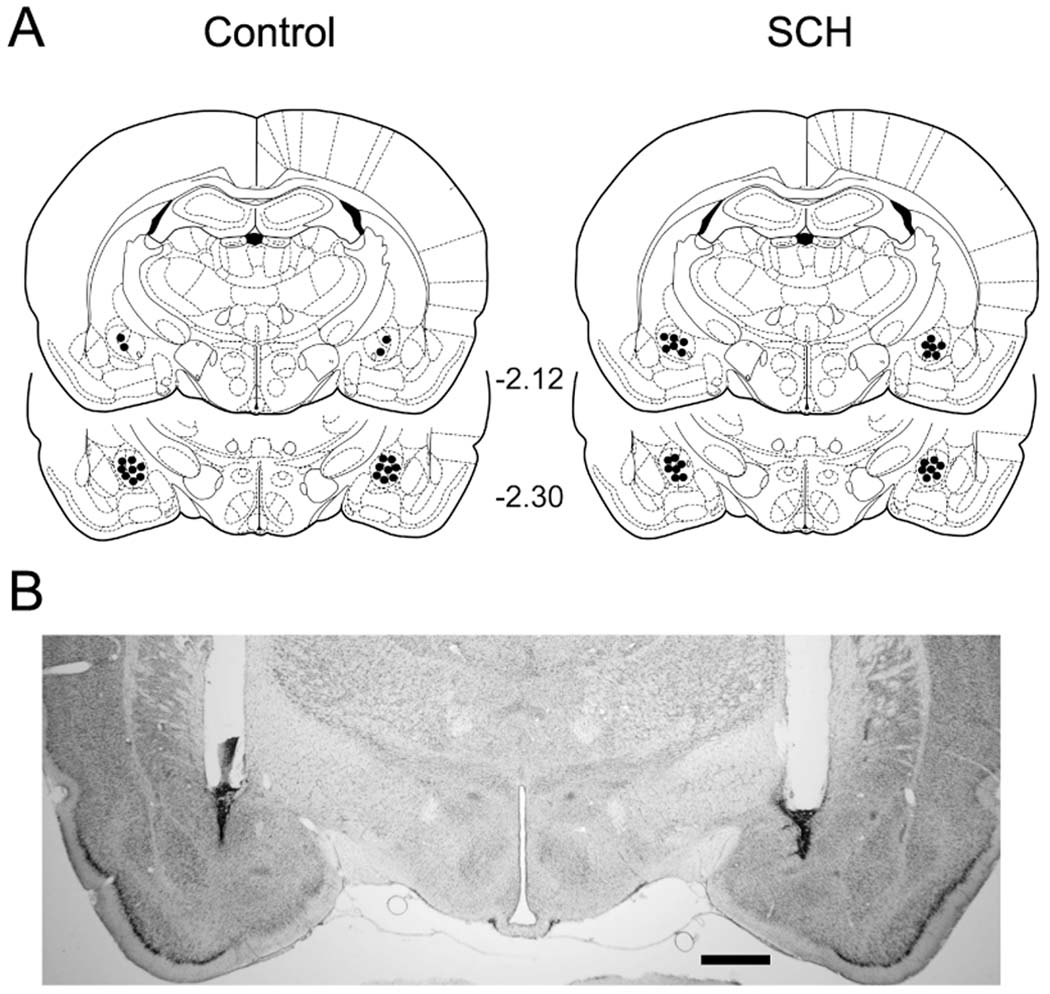
Cannula tips were localized in the BLA in eight Control rats and fourteen SCH rats (Fig. 4A) between frontal planes −2.56 and −3.30 mm of the Paxinos and Watson (1998) atlas. The remaining five rats had either necrosis around the cannula tips or had misplaced cannulae and, therefore, were not included in the analysis. A photomicrograph of a representative bilateral microinjection site is shown in Fig. 4B.
Figure 4.
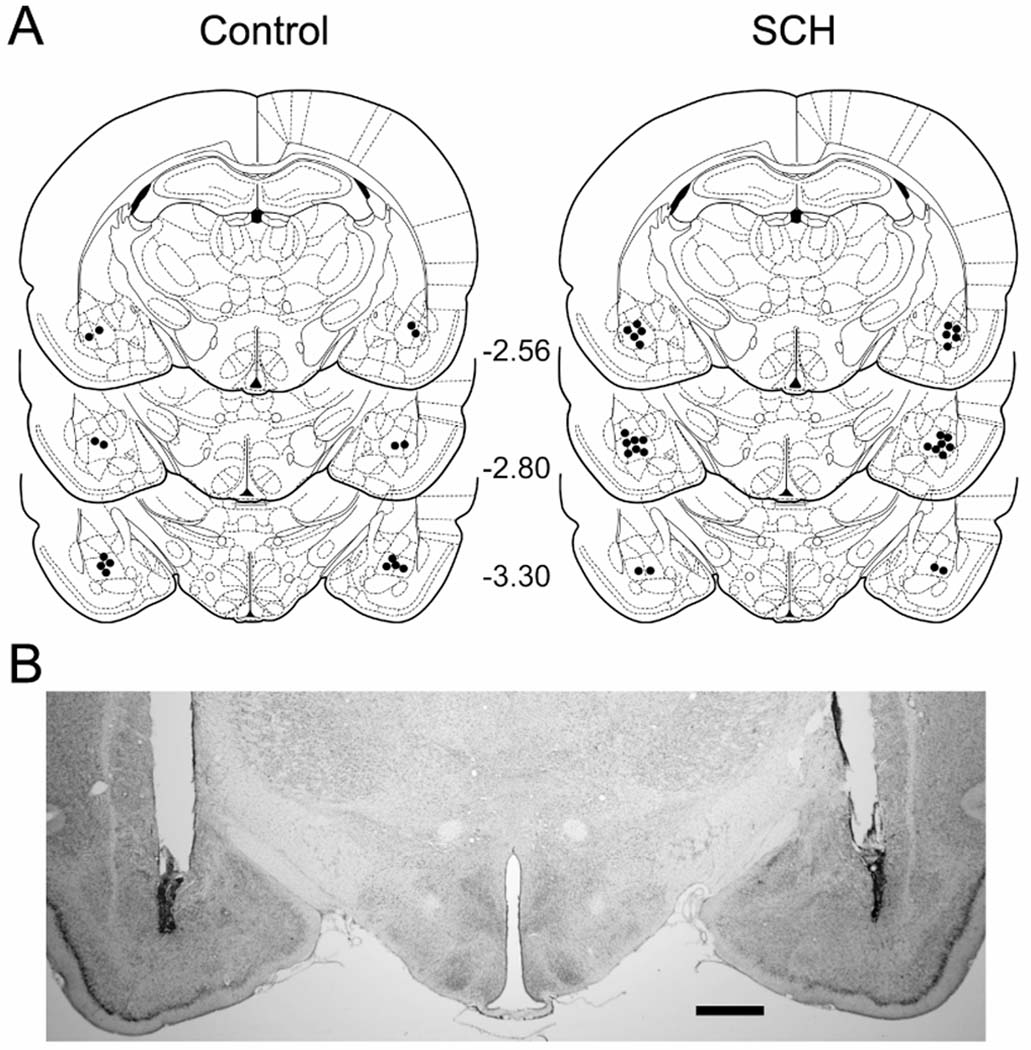
Experiment 3. 4A: Schematic representation of cannula tip placements (black circles) in the basolateral amygdala complex. Coronal sections were adapted from Paxinos and Watson (1998) with permission. Numbers denote distance (in mm) posterior to bregma. 4B: Representative photomicrograph of a coronal section indicating bilateral cannula tracts terminating in the basolateral amygdala complex. Scale bar: 1 mm.
Behavior.
The rats treated with 12 nmol SCH23390 consumed about 8 g of the CS+ and CS− during one-bottle training and the intakes of the Control group were limited to this amount. The results of the two-bottle preference tests are summarized in Figure 5. Overall, the rats consumed more CS+ than CS− [F(1,19) = 36.69; p< 0.001] and the two groups did not significantly differ in their total CS intakes. There was a significant Group x CS interaction [F(1,19) = 8.94, p < 0.01] although both groups consumed more (p < 0.05) CS+ than CS−. However, the SCH group consumed more (p < 0.05) CS− and tended to consume less (p = 0.086) CS+ compared to the Control group. Consequently, the percent CS+ intake of the Control group exceeded that of the SCH group (80% vs. 59%, t(19) = 3.85, p < 0.01). Thus, SCH23390 administration in the BLA during training attenuated but did not block the acquisition of glucose-conditioned flavor preference.
Figure 5.
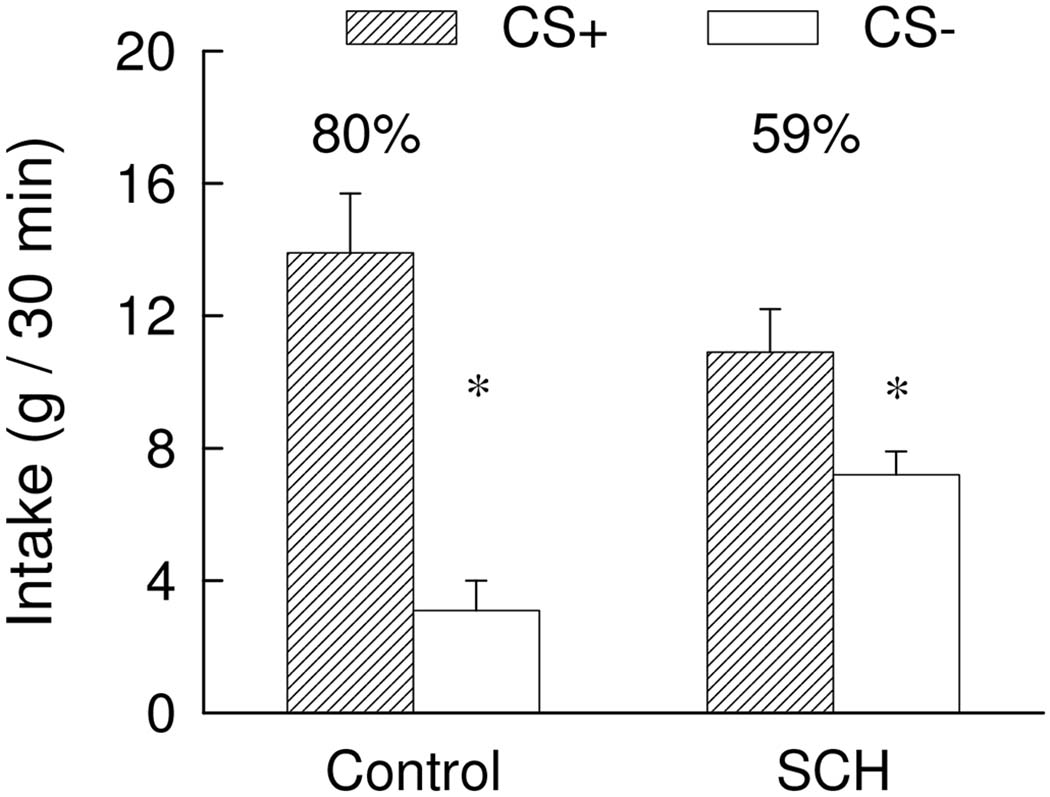
Experiment 3. Intakes (+S.E.M.) of the CS+ and CS− during two-bottle choice tests; data represent the mean of four 30-min sessions. The CS+ and CS− were paired with intragastric infusions of 8 ml glucose and water, respectively, during training. No gastric infusions were given during testing. The SCH group was given injections of 12 nmol of SCH23390 into the basolateral amygdala complex (6 nmol/side) ten minutes prior to the daily training sessions while the Control group was given vehicle injections. No injections were given prior to the two-bottle choice tests. The asterisk denotes a significant (p < 0.05) difference between CS+ and CS− intakes. The numbers atop the bars represent the mean of the individual rat’s percent CS+ intakes.
Experiment 4. Effects of D1-like receptor antagonism in CeA on the acquisition of glucose-conditioned flavor preference
Histology.
Cannula tip placements in the CeA for all rats used are shown in Fig. 6A. Placements were deemed appropriate for ten Control rats and thirteen SCH rats and were found between frontal planes −2.12 and −2.30 mm of the Paxinos and Watson (1998) atlas. The remaining five rats had misplaced cannula tips and, therefore, were not included in the analysis. A photomicrograph of a representative bilateral microinjection site is shown in Fig. 6B.
Figure 6.
Experiment 4. 6A: Schematic representation of cannula tip placements (black circles) in the central amygdaloid nucleus. Coronal sections were adapted from Paxinos and Watson (1998) with permission. Numbers denote distance (in mm) posterior to bregma. 6B: Representative photomicrograph of a coronal section indicating bilateral cannula tracts terminating in the central amygdaloid nucleus. Scale bar: 1 mm.
Behavior.
During training, the rats treated with 12 nmol SCH23390 consumed about 7 g of the CS+ and CS− during one-bottle training and the intakes of the Control group were limited to this amount. The results of the two-bottle preference tests are summarized in Figure 7. Overall, the two groups did not significantly differ in their CS intakes and the rats consumed more CS+ than CS− [F(1,21) = 91.02; p<0.001]. There was a significant Group x CS interaction [F(1,21) = 7.70, p < 0.05] although both groups consumed more (p < 0.01) CS+ than CS−. The SCH group consumed less (p < 0.05) CS+ than did the Control group but the groups did not differ in their CS− intakes. Consequently, the percent CS+ intake of the Control group exceeded that of the SCH group (88% vs. 73%, t(21) = 3.04, p < 0.01).
Figure 7.
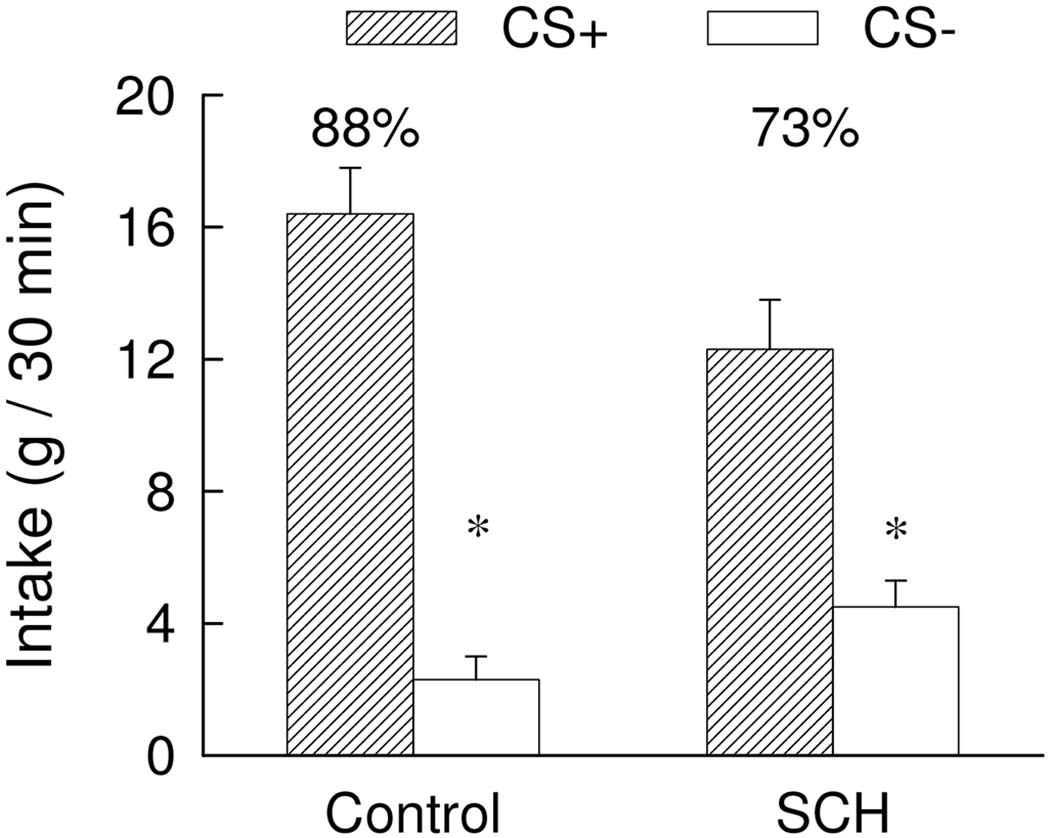
Experiment 4. Intakes (+S.E.M.) of the CS+ and CS− during two-bottle choice tests; data represent the mean of four 30-min sessions. The CS+ and CS− were paired with intragastric infusions of 8 ml glucose and water, respectively, during training. No gastric infusions were given during testing. The SCH group was given injections of 12 nmol of SCH23390 into the central amygdaloid nucleus (6 nmol/side) ten minutes prior to the daily training sessions while the Control group was given vehicle injections. No injections were given prior to the two-bottle choice tests. The asterisk denotes a significant (p < 0.05) difference between CS+ and CS− intakes. The numbers atop the bars represent the mean of the individual rat’s percent CS+ intakes.
DISCUSSION
This study explored the role of AMY dopamine D1-like receptors in flavor preference learning induced by the postoral actions of glucose. The role of D2-like receptors transmission within the AMY was not investigated because systemic treatment with the D2-like receptor antagonist, raclopride, failed to attenuate flavor-nutrient learning (Azzara et al., 2001). The results revealed that antagonism of the dopamine D1-like receptors with SCH23390 (12 nmol) during training totally blocked the acquisition of the glucose-conditioned flavor preference although the same drug dose during testing did not block the expression of a previously acquired flavor preference. Antagonism of the D1-like receptors in either the BLA or the CeA during training attenuated but did not completely block the acquisition of the glucose-conditioned flavor preference. These findings demonstrate that activation of dopamine D1-like receptors in the AMY is crucial for flavor-nutrient preference conditioning and add to the extensive literature on the importance of AMY dopamine D1-like receptors to incentive learning.
Amygdala D1-like receptors and flavor-nutrient preference learning
In Experiment 1, the rats were trained to consume a CS+ flavor paired with IG infusions of an 8% glucose solution and a CS− flavor paired with IG water infusions. In subsequent two-bottle choice tests, the rats exhibited a strong CS+ preference following vehicle (89%) and 12 nmol SCH23390 (81%) treatment although the 12 nmol dose reduced CS+ but not CS− intakes. We have previously observed reductions in absolute but not percent CS+ intakes following systemic and NAc injections of low SCH23390 doses (Azzara et al., 2001; Touzani et al., 2008). This drug-induced suppression of CS+ intake (acceptance) may be due to a reduction in food motivation. Reducing food motivation in rats by giving them caloric preloads or free access to food (prefeeding procedure) also reduces CS+ intake (acceptance) but not CS+ preference (Yiin et al., 2005). The higher SCH23390 doses (24 and 48 nmol) suppressed the intake of both CS+ and CS− solutions, as well as CS+ preference, which may represent a more substantial motivational deficit and/or general motor impairment.
The 12 nmol dose of SCH23390 that failed to impair the expression of a previously learned flavor preference totally blocked the acquisition of a new flavor preference when it was administered in the AMY throughout one-bottle training (Experiment 2). Indeed, the SCH rats consumed significantly less of the CS+ and more of the CS− than the Control rats during the two-bottle preference tests. During training, the CS intakes of the Control rats were matched to those of the SCH rats and all rats were infused with the same amount of glucose. Therefore, the failure of the SCH group to develop a significant CS+ preference cannot be attributed to reduced exposure to the CS+ or US (IG glucose). Altogether, these findings are consistent with our earlier finding that systemic administration of SCH23390 prevents carbohydrate-based flavor preference conditioning (Azzara et al., 2001), and show that activation of D1 dopamine receptors within the AMY is critical for the acquisition, but not the expression, of this type of learned flavor preference. Similar differential effects of D1 antagonism within the AMY on the acquisition and performance of instrumental learning have been reported (Andrzejewski et al., 2005) indicating that well learned behaviors become resistant to the effects of DA receptor antagonism, and are thereby less dependent on the mesolimbic dopamine system (Ikemoto & Panksepp, 1999).
Prior work indicates that pairing a saccharin-sweetened CS+ flavor, like that used in the present study, with IG carbohydrate infusions enhances the hedonic value of the flavor, as measured by a taste reactivity test (Myers & Sclafani, 2001) and the incentive value of the flavor, as measured by a progressive ratio operant task (Sclafani & Ackroff, 2006). The present findings indicate that D1-like receptor antagonism in the AMY prevents the CS+ flavor from acquiring enhanced reward value. This outcome may occur because SCH23390 treatment blocks the reinforcing properties of IG glucose. Arguing against this interpretation, total lesions of AMY did not block the conditioning by IG carbohydrate infusions of a preference for a compound taste CS+ (e.g., bitter-sweet) although it did block conditioning of a preference for a flavor CS+ (odor - taste compound, e.g., grape-sweet) (Touzani & Sclafani, 2005). These findings indicate that the perception and processing of the nutrient US produced by IG glucose infusions is at least partially preserved after AMY lesions. The findings that both AMY lesions and AMY D1-like receptor antagonism completely blocked flavor-nutrient preference learning point to the importance of D1-like receptors in amygdala cellular and molecular processes underlying this type of incentive learning. Whether AMY D1-like receptor antagonism, like AMY lesions, spared taste-nutrient learning remains to be determined. There is some evidence that Pavlovian incentive learning such as auditory fear conditioning induces associative long-term potentiation (LTP) in the AMY (Rogan et al., 1997), a form of neuronal plasticity believed to be involved in mechanisms underling learning and memory formation (Bliss & Collingridge, 1993), and that dopamine modulates both this associative LTP and auditory fear conditioning by activating D1-like receptors (Bissiere et al., 2003; Loretan et al., 2004). Taken together, the available data indicate that D1-like receptor activation within the AMY is critical for both appetitive and aversive associative learning.
Further evidence for the involvement of AMY dopamine receptors in flavor learning is provided by the results of a study investigating flavor conditioning by orally consumed fructose (Bernal et al., 2007). This is thought to be a form of flavor-taste learning because, unlike glucose, the fructose infusions do not support flavor conditioning using the procedures of the present study (Sclafani et al., 1999). In contrast to the present findings, AMY infusions of SCH23390 (12 nmol) during training did not block the acquisition of a fructose-conditioned flavor preference although it facilitated the extinction of the preference over repeated testing sessions. As in the present study, the same dose of SCH23390 administered during testing had little effect on the expression of a previously learned CS+ preference. Thus, while less effective in blocking flavor-taste learning than flavor-nutrient learning, D1 antagonism in the AMY did reduce the persistence of the flavor preference conditioned by the sweet taste of fructose. Other data indicate that, unlike flavor-nutrient learning, flavor-taste learning is modulated by D2- as well as D1-like receptors, suggesting that different processes mediate the two forms of learning (Baker et al., 2003; Bernal et al., 2008).
In contrast to flavor-nutrient learning, flavor-taste learning is modulated by D2- as well as D1-like receptors (Azzara et al., 2001; Baker et al., 2003; Bernal et al., 2008). On the other hand, flavor-aversion conditioning produced by LiCl treatment (Caulliez et al., 1996; Fenu et al., 2001), like flavor-nutrient preference conditioning (Azzara et al., 2001), is selectively disrupted by D1-like antagonism. Together these findings suggest that flavor conditioning by viscerosensory stimulation (LiCl or IG sugar) are processed differently in the brain than flavor conditioning by orosensory stimulation (sweet taste).
Potential contribution of amygdala subnuclei
Whereas administration of a relatively large volume (0.5 μl) of SCH23390 in the AMY completely blocked the acquisition of the glucose-conditioned flavor preference, microinjection of a smaller volume (0.25 μl) in either the BLA (Experiment 3) or CeA (Experiment 4) only attenuated the acquisition of this preference. This outcome is similar to our recent findings with total and subtotal AMY lesions (Touzani & Sclafani, 2005) and suggests that flavor-nutrient learning is a distributive function of the AMY. However, SCH23390 injections in the BLA attenuated flavor conditioning more than did drug injections in the CeA (CS+ preference 59% vs. 73%, p < 0.01). Conceivably, the CeA may not be directly involved in flavor-nutrient conditioning and the effect of SCH23390 infusion in the CeA results from drug diffusion into BLA. Although we did not control for the spread of the drug, such diffusion was certainly minimized. We used low injection volumes (0.25 μl) in Experiments 3 and 4 and the coordinates for the BLA and CeA used were differed in all directions. The CeA infusion sites were more anterior (−2.2 vs. −2.8), more medial (±4.2 vs. ±5.0) and more dorsal (−8.7 vs. −9.2). Moreover, other data indicate that a larger volume (0.5 μl) of drugs injected into either the CeA or the BLA produced differential effects on lever-pressing for food (Baldwin et al., 2000; Andrzejewski et al., 2004), indicating a regional specificity. Since the small BLA microinfusions of SCH23390 or lesions restricted to the BLA were less effective than larger AMY drug infusions or lesions (present study; Touzani & Sclafani, 2005), we reasoned that the BLA is not the only region of the amygdala involved in flavor-nutrient learning. Consequently, the effect of SCH23390 in the CeA was not due solely to drug diffusion to the BLA.
The differential effect on CS+ conditioning produced by the D1-like antagonism in the CeA and BLA is not readily explained by the density of D1 receptors which are reported to be similar (Boyson et al., 1986). It may be that dopamine transmission within the CeA and BLA is involved in different processes underlying flavor preference learning. Indeed, several lines of evidence have shown that lesions of the CeA and the BLA disrupt distinct aspects of Pavlovian and instrumental learning (Gallagher & Holland, 1994; Hall et al., 2001; Balleine et al., 2003; Holland & Gallagher, 2003; Everitt et al., 2003). Based on these findings, Balleine and Killcross (2006) proposed a parallel model of AMY function in which the BLA and CeA operate simultaneously and in parallel to mediate different aspects of incentive learning: the BLA mediates associations between predictive stimuli and specific reinforcing effects of rewards, where as the CeA mediates the establishment of general motivational/affective responses that underlie the nonspecific reinforcing features of those rewards. Our results, showing that D1-like receptors antagonism in either the CeA or the BLA impaired flavor-nutrient preference learning and that this antagonism in the CeA produced a relatively weaker effect, fit well this model. These data further contribute to the knowledge on the role of dopamine transmission within the limbic system in incentive learning. Further work is needed to elucidate the distinct incentive processes mediated by dopamine transmission within these two amygdaloid subdivisions.
Distributed network mediating flavor-nutrient preference learning
Our findings that dopamine D1-like receptor antagonism in the AMY prevented the formation of flavor-nutrient preference learning but not the expression of previously learned flavor preferences mirror those we recently reported with antagonism of the same receptors in the NAc (Touzani et al., 2008). Together, these findings suggest that dopamine transmission within different components of a distributed network is involved in flavor-nutrient preference learning. In this network, dopamine efferents from the VTA densely innervate cortical and forebrain structures such as the mPFC, AMY and NAc, (Swanson, 1982) and these structures are interconnected. This distributed network has also been proposed in reward-based instrumental learning (Baldwin et al., 2002; Andrzejewski et al., 2005). The NAc receives major glutamatergic inputs from both the mPFC and AMY (McGeorge & Faull, 1989; Brog et al., 1993; Zahm, 2000), and dopamine released in NAc facilitates firing of neurons elicited by these glutamatergic inputs (Nicola, 2007; Ishikawa et al., 2008). Interestingly, LTP induced by repeated tetanizations of either the NAc, mPFC or AMY neurons is modulated by dopamine D1-like receptors as well (Bissiere et al., 2003; Otani et al., 2003; Huang et al., 2004; Loretan et al., 2004; Schotanus & Chergui, 2008). Thus, as proposed by Wickens (1993) and Benninger (1993), it is possible that dopamine released in the AMY and NAc by nutrients or nutrient-associated cues and acting on D1-like receptors promote flavor-nutrient preference learning by altering the effectiveness of activated glutamatergic synapses in these structures. This does not imply that DA transmission within these two structures via the D1-like receptors underlies similar incentive processes. The meso-accumbens dopamine system may be involved in flavor-nutrient preference learning based on association between the predictive flavor cue (CS+) and the outcome of its consumption (stimulus-outcome association) as well as on the execution of actions upon the presentation of the CS+ (stimulus-action association) (Ikemoto, 2007), whereas the AMY and its dopamine receptors may be involved in flavor-nutrient preference learning based on association between the CS+ and the affective significance of the reinforcing properties of nutrients (Balleine & Killcross, 2006). Each of these incentive processes is a sine qua non for incentive learning and may develop according to a functional hierarchy within this distributed network. Indeed, more recent evidence indicates that AMY to NAc excitatory projection is required for NAc neuronal responses to reward-predictive cues, and that AMY neuronal activation evoked by predictive cues precedes that of NAc neurons (Ambroggi et al., 2008). Whether dopamine release in the AMY triggered by incentive cues also precedes that in the NAc is an interesting question.
As mentioned above, the mPFC is another component of the mesocorticolimbic dopaminergic network and plays a crucial role in reward-related learning (Kelley, 2004). It has intimate connections with the NAc and AMY and presents a moderate density of D1-like receptors. Interestingly, neurochemical studies have shown an increase of DA efflux in the mPFC by feeding and food-related cues in both Pavlovian and instrumental learning (D’Angio & Scatton, 1989; Hernandez & Hoebel, 1990; Izaki et al., 1999; Bassareo et al., 2002) suggesting a potential role of dopamine D1-like receptor signaling within this cortical region in flavor-nutrient preference learning. This possibility is currently under investigation.
Learning plays an important role in the establishment and strengthening of food preferences particularly for high-fat and high-sugar foods. Such foods may promote overeating and contribute to the current obesity epidemic. The appetite for high-fat and high-sugar foods and the learning processes through which these foods become more attractive and preferred are presumably mediated at least in part by the brain dopamine systems that are also linked to drug addiction. A better understanding of the basic cellular and molecular mechanisms involved in appetite and learned food preferences may provide insights into the clinical treatment of overeating and obesity.
Acknowledgements
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant DK071761.
Abbreviations
- AMY
amygdala
- BLA
basolateral amygdala complex
- CeA
central amygdaloid nucleus
- CS
conditioned stimulus
- DA
dopamine
- IG
intragastric
- LTP
long-term potentiation
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- US
unconditioned stimulus
- VTA
ventral tegmental area
References
- Ambroggi F, Ishikawa A, Fields HL & Nicola SM (2008) Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron, 59, 648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski ME, Sadeghian K & Kelley AE (2004) Central amygdalar and dorsal striatal NMDA receptor involvement in instrumental learning and spontaneous behavior. Behav. Neurosci, 118, 715–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski ME, Spencer RC & Kelley AE (2005) Instrumental learning, but not performance, requires dopamine D1-receptor activation in the amygdala. Neuroscience, 135, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan E (1997) Ultrastructural features of tyrosine hydroxylase-immunoreactive afferents and their targets in the rat amygdala. Cell Tissue Res, 288, 449–469. [DOI] [PubMed] [Google Scholar]
- Azzara AV, Bodnar RJ, Delamater AR & Sclafani A (2001) D1 but not D2 dopamine receptor antagonism blocks the acquisition of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol. Biochem. Behav, 68, 709–720. [DOI] [PubMed] [Google Scholar]
- Baker RM, Shah MJ, Sclafani A & Bodnar RJ (2003) Dopamine D1 and D2 antagonists reduce the acquisition and expression of flavor-preferences conditioned by fructose in rats. Pharmacol. Biochem. Behav, 75, 55–65. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Holahan MR, Sadeghian K & Kelley AE (2000) N-methyl-D-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behav. Neurosci, 114, 84–98. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Sadeghian K & Kelley AE (2002) Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J. Neurosci, 22, 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Killcross AS & Dickinson A (2003) The effect of lesions of the basolateral amygdala on instrumental conditioning. J. Neurosci, 23, 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW & Killcross S (2006) Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci, 29, 272–279. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA & Di Chiara G (2002) Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J. Neurosci, 22, 4709–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG & Murray EA (2002) The amygdala and reward. Nat. Rev. Neurosci, 3, 563–573. [DOI] [PubMed] [Google Scholar]
- Beninger RJ (1993) Role of D1 and D2 receptors in learning. In Waddington J (ed), D1, D2 Dopamine receptor interactions: Neuroscience and pharmacology. Academic Press, London, pp. 115–157. [Google Scholar]
- Bernal SY, Dostova I, Kest A, Abayev Y, Kandova E, Touzani K, Sclafani A & Bodnar RJ (2008) Role of dopamine D1 and D2 receptors in the nucleus accumbens shell on the acquisition and expression of fructose-conditioned flavor-flavor preferences in rats. Behav. Brain Res, 190, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal SY, Miner P, Abayev Y, Kandova E, Pulatov P, Touzani K, Sclafani A, and Bodnar RJ Aquisition of fructose-conditioned flavor preferences in rats: role of D1 and D2 dopamine receptors in the nucleus accumbens and amygdala. Program No. 629.2. 2007 Abstract Viewer/Itinerary Planner. San Diego, CA: Society for Neuroscience, 2007. Online. [Google Scholar]
- Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl), 191, 391–431. [DOI] [PubMed] [Google Scholar]
- Bissiere S, Humeau W & Luthi A (2003) Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat. Neurosci, 6, 587–592. [DOI] [PubMed] [Google Scholar]
- Bliss TV & Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature, 361, 31–39. [DOI] [PubMed] [Google Scholar]
- Boyson S, McGonigle P & Molinoff P (1986) Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J. Neurosci, 6, 3177–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY & Zahm DS (1993) The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol, 338, 255–278. [DOI] [PubMed] [Google Scholar]
- Capaldi ED (1996) Conditioned food preferences. In Capaldi ED (ed), Why We Eat What We Eat: The Psychology of Eating. American Psychological Association, Washington, DC, pp. 53–80. [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J & Everitt BJ (2002) Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biohehav. Rev, 26, 321–352. [DOI] [PubMed] [Google Scholar]
- Caulliez R, Meile M-J & Nicolaidis S (1996) A lateral hypothalamic D1 dopaminergic mechanism in conditioned taste aversion. Brain Res, 729, 234–245. [PubMed] [Google Scholar]
- D’Angio M & Scatton B (1989) Feeding or exposure to food odors increases extracellular DOPAC levels (as measured by in vivo voltammetry) in the prefrontal cortex of food-deprived rats. Neurosci. Lett, 96, 223–228. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Gehlert DR, McCabe RT, Barnett A & Wamsley JK (1986) D-1 dopamine receptors in the rat brain: a quantitative autoradiographic analysis. J. Neurosci, 6, 2352–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA & Robbins TW (2003) Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning. Ann. N. Y. Acad. Sci, 985, 233–250. [PubMed] [Google Scholar]
- Fenu S, Bassareo V & Di Chiara G (2001) A role for dopamine D1 receptors of the nucleus accumbens shell in conditioned taste aversion learning. J.Neurosci, 21, 6897–6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M & Holland PC (1994) The amygdala complex: multiple roles in associative learning and attention. Proc. Natl. Acad. Sci. U. S. A, 91, 11771–11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Campbell A & Kesner RP (2003) The role of the amygdala in conditioned flavor preference. Neurobiol. Learn. Mem, 79, 118–121. [DOI] [PubMed] [Google Scholar]
- Hajnal A & Lenard L (1997) Feeding-related dopamine in the amygdala of freely moving rats. Neuroreport, 8, 2817–2820. [DOI] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A & Everitt BJ (2001) Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur. J. Neurosci, 13, 1984–1992. [DOI] [PubMed] [Google Scholar]
- Harmer CJ & Phillips GD (1999) Enhanced dopamine efflux in the amygdala by a predictive, but not a non-predictive, stimulus: facilitation by prior repeated D-amphetamine. Neuroscience, 90, 119–130. [DOI] [PubMed] [Google Scholar]
- Heffner TG, Hartman JA & Seiden LS (1980) Feeding increases dopamine metabolism in the rat brain. Science, 208, 1168–1170. [DOI] [PubMed] [Google Scholar]
- Hernandez L & Hoebel BG (1990) Feeding can enhance dopamine turnover in the prefrontal cortex. Brain Res. Bull, 25, 975–979. [DOI] [PubMed] [Google Scholar]
- Holland PC & Gallagher M (2003) Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur. J. Neurosci, 17, 1680–1694. [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhou D, Chase K, Gusella JF, Aronin N & DiFiglia M (1992) Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proc. Natl. Acad. Sci. U. S. A, 89, 11988–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Simpson E, Kellendonk C & Kandel ER (2004) Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc. Natl. Acad. Sci. U. S. A, 101, 3236–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S (2007) Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Research Reviews, 56, 27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S & Panksepp J (1999) The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain. Res. Rev, 31, 6–41. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM & Fields HL (2008) Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J. Neurosci, 28, 5088–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki Y, Hori K & Nomura M (1999) Dopamine and acetylcholine elevation on lever-press acquisition in rat prefrontal cortex. Neurosci. Lett, 258, 33–36. [DOI] [PubMed] [Google Scholar]
- Kelley AE (2004) Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci. Biobehav. Rev, 27, 765–776. [DOI] [PubMed] [Google Scholar]
- Kirk RE (1995) Experimental Design: Procedures for the Behavioral Sciences Brooks/Cole, Pacific Grove, CA. [Google Scholar]
- Leonard SK, Petitto JM, Anderson CM, Mooney DH, Lachowicz JE, Schulz DW, Kilts CD & Mailman RB (2003) D1 dopamine receptors in the amygdala exhibit unique properties. Ann. N. Y. Acad. Sci, 985, 536–539. [Google Scholar]
- Loretan K, Bissiere S & Luthi A (2004) Dopaminergic modulation of spontaneous inhibitory network activity in the lateral amygdala. Neuropharmacology, 47, 631–639. [DOI] [PubMed] [Google Scholar]
- Mansour A, Meador-Wooruff JH, Bunzow JR, Civelli O, Akil H & Watson SJ (1990) Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J. Neurosci, 10, 2587–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark GP, Smith SE, Rada PV & Hoebel BG (1994) An appetitively conditioned taste elicits a preferential increase in mesolimbic dopamine release. Pharmacol. Biochem. Behav, 48, 651–660. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ & Faull RL (1989) The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience, 29, 503–537. [DOI] [PubMed] [Google Scholar]
- Myers KP & Sclafani A (2001) Conditioned enhancement of flavor evaluation reinforced by intragastric glucose: II. Taste reactivity analysis. Physiol. Behav, 74, 495–505. [DOI] [PubMed] [Google Scholar]
- Nicola SM (2007) The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology, 191, 521–550. [DOI] [PubMed] [Google Scholar]
- Otani S, Daniel H, Roisin MP & Crepel F (2003) Dopaminergic modulation of long-term synaptic plasticity in rat prefrontal neurons. Cereb. Cortex, 13, 1251–1256. [DOI] [PubMed] [Google Scholar]
- Paxinos G & Watson C (1998) The rat brain in stereotaxic coordinates Academic Press, San Diego, CA. [Google Scholar]
- Rogan MT, Staübli UV & LeDoux JE (1997) Fear conditioning induces associative long-term potentiation in the amygdala. Nature, 390, 604–607. [DOI] [PubMed] [Google Scholar]
- Schotanus SM & Chergui K (2008) Dopamine D1 receptors and group I metabotropic glutamate receptors contribute to the induction of long-term potentiation in the nucleus accumbens. Neuropharmacology, 54, 837–844. [DOI] [PubMed] [Google Scholar]
- Sclafani A (1999) Macronutrient-conditioned flavor preferences. In Berthoud H-R & Seeley R (eds), Neural and metabolic control of macronutrient intake. CRC Press, Boca Raton, FL, pp. 93–107. [Google Scholar]
- Sclafani A & Ackroff K (2006) Nutrient-conditioned flavor preference and incentive value measured by progressive ratio licking in rats. Physiol. Behav, 88, 88–94. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Azzara AV, Touzani K, Grigson PS & Norgren R (2001) Parabrachial nucleus lesions block taste and attenuate flavor preference and aversion conditioning in rats. Behav. Neurosci, 115, 920–933. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Fanizza LJ & Azzara AV (1999) Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiol. Behav, 67, 227–234. [DOI] [PubMed] [Google Scholar]
- Swanson LW (1982) The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull, 9, 321–353. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ & Sclafani A (2008) Activation of dopamine D1 receptors in the nucleus accumbens is critical for the acquisition, but not the expression, of flavor preference conditioned by intragastric glucose in rats. Eur. J. Neurosci, 27, 1525–1533. [DOI] [PubMed] [Google Scholar]
- Touzani K & Sclafani A (2001) Conditioned flavor preference and aversion: Role of the lateral hypothalamus. Behav. Neurosci, 115, 84–93. [DOI] [PubMed] [Google Scholar]
- Touzani K & Sclafani A (2002) Lateral hypothalamic lesions impair flavor-nutrient and flavor-toxin trace learning in rats. Eur. J. Neurosci, 16, 2425–2433. [DOI] [PubMed] [Google Scholar]
- Touzani K & Sclafani A (2005) Critical role of amygdala in flavor but not taste preference learning in rats. Eur. J. Neurosci, 22, 1767–1774. [DOI] [PubMed] [Google Scholar]
- Wickens J (1993) A theory of the striatum. Pergamon Press, Oxford. [Google Scholar]
- Wise RA (2004) Dopamine, learning and motivation. Nat. Rev. Neurosci, 5, 483–494. [DOI] [PubMed] [Google Scholar]
- Yiin Y-M, Ackroff K & Sclafani A (2005) Flavor preferences conditioned by intragastric nutrient infusions in food restricted and unrestricted rats. Physiol.Behav, 84, 217–231. [DOI] [PubMed] [Google Scholar]
- Zahm DS (2000) An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci. Biobehav. Rev, 24, 85–105. [DOI] [PubMed] [Google Scholar]


