Abstract
Background:
Two-stage hepatectomy (TSH) is an important tool in the management of bilateral colorectal liver metastases (CRLM). This study sought to examine the presentation, management and outcomes of patients completing TSH in major hepato-biliary centers in the United States.
Methods:
A retrospective review from 5 liver centers in the United States identified patients who completed a TSH procedure for bilateral CRLM.
Results:
From December 2000 to March 2016, a total of 196 patients were identified. The majority of procedures were performed with an open technique (n = 194, 99.5%). The median number of tumors was 7 (range, 2–33). One-hundred twenty-eight (65.3%) patients underwent portal vein embolization. More patients received chemotherapy prior to the first stage than chemotherapy administration preceding the second stage (92% vs. 60%, p = 0.308). Median overall survival was 50 months with a median follow-up of 28 months (range, 2–143). Hepatic artery infusion chemotherapy was administered to 64 (32.7%) patients with similar overall survival (OS) to those managed without an infusion pump (p = 0.848). Postoperative morbidity following the second stage resection was 47.4%. Chemotherapy prior to the second stage did not demonstrate an increased complications rate (p = 0.202). Readmission following the second stage was 10.3% and was associated with a decrease in disease-free survival (p = 0.003). Overall survival was significantly decreased by positive resection margins and increased estimated blood loss (EBL) (p = 0.036 and p = 0.05, respectively).
Conclusion:
This is the largest TSH series in the U.S. and demonstrates evidence of safety and feasibility in the management of bilateral CRLM. Outcomes are influenced by margin status and operative EBL.
INTRODUCTION
Colorectal cancer is the third most common type of cancer and cancer-related deaths in the US. An estimated of 51,020 deaths from colorectal cancer are expected to occur in 20191. Of the patients who receive a diagnosis, about one third will have metastases confined to the liver and half will develop liver metastases during the course of their disease2. Further, out of the cancers with metastasis to the liver, colorectal cancer is the most common source, as the portal circulation acts as a conduit for metastasis3. Better understanding of tumor biology, improved techniques for liver resection, and multidisciplinary treatments have improved management of metastatic disease in the liver4,5. Despite these advances, only 10%–25% of patients with colorectal cancer metastasis confined to the liver will be surgical candidates based on the extent of their disease6. Many patients are not considered surgical candidates because of inadequate future liver remnant (FLR).
As 5- and 10-year survival rates after resection of colorectal liver metastases (CRLM) can be as high as 74% and 69% respectively7–11, new strategies to provide curative surgical treatment for patients previously considered only for palliative care, are needed. One important approach is two-stage hepatectomy (TSH). In 2000, Adam et al. published the first series of TSH in patients with unresectable bilateral CRLM who were not amenable to resection in a single operation, even with preoperative chemotherapy and portal vein embolization (PVE)12. Since this report, PVE and preoperative chemotherapy have become standard approaches for the management of select patients with CRLM.
TSH is a planned and potentially curative strategy that allows removal of all tumors with minimal risk of ensuing liver failure. TSH consists of the resection of tumors in one hemiliver during a 1st stage, followed by resection of remaining tumors in the contralateral hemiliver during a 2nd stage. A prudent time interval is valuable to ensure regeneration of the liver to decrease risk of liver failure and in order to evaluate tumor biology. This study sought to examine the presentation, management and outcomes of patients completing TSH with the collaboration of 5 major hepato-biliary surgery centers, creating the largest series in the United States.
METHODS
Study design
Patients who completed TSH with curative-intent between December 2000 and March 2016 for bilateral CRLM at 5 major hepato-biliary surgery centers in the United States were examined (Medical College of Wisconsin, Milwaukee, WI; MD Anderson Cancer Center, Houston, TX; Memorial Sloan Kettering Cancer Center, New York, NY; Johns Hopkins University, Baltimore, MD; and Emory University; Atlanta, GA). This study was approved by the Institutional Review Boards (IRB) from each institution. Curative-intent was defined as planned complete extirpation of all known liver tumors. Peri-operative therapies were administered at the discretion of the treatment team.
Definitions of outcome measures
Standard demographic and clinicopathologic data were collected for each patient, including gender, age, race, body mass index (BMI), information about the original cancer diagnosis, adjuvant therapy, preoperative serum tumor markers (carcinoembryonic antigen) and molecular markers (RAS, BRAF). Tumor characteristics collected on primary tumor included: location, American Joint Commission on Cancer (AJCC)/International Union against Cancer (UICC) stage (T, N, M), presence of perineural (PNI) and lymphovascular invasion (LVI) and presentation of liver metastasis (synchronous vs. metachronous). Synchronous disease was defined as development of metastatic disease within 6 months of primary tumor resection. The number of hepatic metastases at the time of diagnosis was assessed by preoperative imaging. Perioperative details included use of chemotherapy and other alternative therapies (e.g. ablation, hepatic artery infusion pump), laparoscopic vs. open procedure, extent of hepatic resection, blood loss, length of stay, readmission rate, morbidity and 90-day mortality. During collection of data, there was no distinction between the type of ablation preformed. The resected liver specimen was pathologically examined for number and size of lesions and resection margin status (R0 vs. R1/R2). For those patients in whom recurrent disease developed, disease-free intervals were calculated. Complications were reported based on the Clavien-Dindo criteria13. The data set was limited to those completing both stages and did not capture intent to treat or those who only completed the first stage. Overall Survival (OS) reflects death from any cause. Deaths were ascertained by clinic and hospital records. OS and disease-free survival (DFS) were calculated from the date of the second liver resection.
Statistical analysis
Unless otherwise specified, continuous variables were presented as median (IQR). Descriptive statistics were calculated for all variables and the normality of the data examined. A log-rank test was used to analyze OS and DFS. Survival curves were generated using the Kaplan-Meier method. Factors associated with recurrence and survival were examined using univariate Cox proportional hazards regression. A multivariant analysis was performed as it relates to DFS and OS. A p-value less than 0.05 was considered statistically significant.
RESULTS
Patient, tumor and perioperative characteristics
We identified 196 patients who underwent TSH for bilateral CRLM and their characteristics are summarized in Table 1. This represents a highly selective group and a small portion of the hepatectomies performed at these centers. Median follow-up for all patients was 28 (2–143) months. These patients were mostly male (n = 117, 59.7%) and Caucasian (n = 170, 86.7%). The median age was 52.4 years (20.5–77.5). The median BMI was 26.6 (11.7–44.8), with 52 (26.5%) patients considered obese (BMI ≥ 30).
Table 1.
Demographic and clinicopathologic characteristics of included patients (N=196)
| Variables | Total |
|---|---|
| Age, median (IQR) | 52.4 (20.5–77.5) |
| Gender | |
| Female | 79 (40.3%) |
| Male | 117 (59.7%) |
| BMI, median (IQR) | 26.6 (11.7–44.8) |
| BMI ≥ 30 | 52 (26.5%) |
| Race | |
| Caucasian | 170 (86.7%) |
| Hispanic | 10 (5.1%) |
| Asian | 6 (3.1%) |
| African American | 5 (2.6%) |
| Other | 5 (2.6%) |
| Primary Location | |
| Colon | 157 (80.1%) |
| Rectum | 36 (18.7%) |
| N/A | 3 (1%) |
| Primary T-stage | |
| T 1–2 | 21 (10.7%) |
| T 3–4 | 159 (81.1%) |
| T N/A | 16 (8.1%) |
| Primary N-stage | |
| N 0 | 37 (18.8%) |
| N 1–2 | 146 (74.4%) |
| N N/A | 13 (6.6%) |
| Lymphovascular invasion/N | 116/162 (71.6%) |
| Perineural invasion/N | 63/149 (42.2%) |
| Synchronous disease | |
| Yes | 172 (87.7%) |
| No | 24 (12.2%) |
| Number of Liver Mets at Diagnosis, median (IQR) | 7.0 (2.0–33.0) |
| Disease-free interval (months), median (IQR) | 12.5 (2–143) |
| Portal vein embolization | |
| Yes | 128 (65.3%) |
| No | 68 (34.6%) |
| Carcinoembryonic antigen level, median (IQR) | 5.8 (1.0–3566) |
| Molecular Markers | |
| KRAS mut/N | 48/137 (32.6%) |
| NRAS mut/N | 4/52 (7.7%) |
| BRAF mut/N | 1/106 (0.9%) |
BMI = Body Mass Index; N/A = Not Available
The location of the primary cancer was: 157 (80.1%) patients in colon and 36 (18.7%) in rectum. The distribution of the tumors in the colon was: 30 (19.1%) in ascending, 13 (8.2%) transverse, 17 (10.8%) descending and 97 (61.7%) in the sigmoid colon. Staging the primary tumor included: 21 (10.7%) patients were T1/2 and 159 (81.1%) were T3/4. Lymph node staging showed 37 (18.8%) patients with negative lymph nodes (N0) and 146 (74.4%) with positive lymph nodes (N1/2). Lymphovascular invasion and perineural invasion were present in 116 out of 162 (71.6%) and 63 (42.2%) out of 149 specimens, respectively.
Liver metastases were found to be synchronous in the majority of patients (n=172, 87.7%) and metachronous in 24 (12.2%) patients. The median number of lesions based on pre-operative imaging was 7 (2–33) with 87 patients (44.3%) having ≥ 6 lesions. PVE was used in 128 cases (65.3%) at the discretion of the surgeon from each institution based on adequacy of the future remnant liver volume. The remaining cases did not undergo a portal vein procedure.
The median carcinoembryonic antigen (CEA) level was 5.8 (1.0–3566). A total of 147 patients had molecular data available for analysis. KRAS mutation was present in 48 patients out of 147 (32.6%), NRAS mutation in 4 out of 52 patients (7.7%) and BRAF mutation in 1 patient out of 106 (0.9%).
Comparing surgical procedures for each stage (Table 2), the majority of operations were performed with an open technique. Only 8.2% of cases (n = 16) were performed laparoscopically at the 1st stage and 0.5% (n = 1) on 2nd stage. Most of 1st stage operations included minor hepatectomies, the majority being single segmental resection (42%), followed by non-anatomical wedge resections (29%), and two segment resections (26%). The majority of the 2nd stage included major liver resections with ≥4 segments (n = 148, 75.5%).
Table 2.
Perioperative characteristics of each surgical stage
| Variables | 1st Stage | 2nd Stage |
|---|---|---|
| Technique | ||
| Open | 180 (91.8%) | 194 (99.5%) |
| Laparoscopic | 16 (8.2%) | 1 (0.5%) |
| N/A | 1 | |
| Number of lesions resected, median (IQR) | 2.0 (1.0–8.0) | N/A |
| Size of lesions resected (cm), median (IQR) | 1.6 (0–24.0) | 3.0 (0–15.0) |
| R0 resection | 157/186 (84.4%) | 174/189 (92.1%) |
| Preoperative chemotherapy | ||
| Yes | 177 (92.2%) | 118 (60.5%) |
| No | 15 (7.8%) | 77 (39.5%) |
| N/A | 4 | 1 |
| Major liver resection (≥ 4 segments) | ||
| Yes | 5 (2.5%) | 148 (75.5%) |
| No | 188 (97.4%) | 47 (23.9%) |
| N/A | 3 | 1 |
| Morbidity | 47 (23.9%) | 93 (47.4%) |
| Biliary fistula/Liver abscess | 6 (3%) | 35 (17.8%) |
| Liver failure | 0 | 19 (9.7%) |
| Thromboembolic events | 0 | 6 (3%) |
| Wound-related events | 16 (8.1%) | 13 (6.6%) |
| Clavien-Dindo ≥ 3 | 10 (5.1%) | 46 (23.4%) |
| Length of stay (days), median (IQR) | 6.0 (1–17) | 7.0 (1–42) |
| Readmission within 30 days | 14 (7.2%) | 20 (10.3%) |
| 90-day mortality | 0 | 9 (4.5%) |
N/A = Not Available
R0 resection was achieved in 157 (84.4%) patients after 1st stage and 174 (92.1%) after 2nd stage. Data regarding a reresection if the first stage was R1 was not captured in the analysis. The mean time interval between the 1st and 2nd stage hepatectomy was 4 ± 3.1 months. This mean time is longer than the typical 6–8 weeks often used following PVE. The interval was at the discretion of the individual treatment team and allowed for maximal remnant growth, a biologic test prior to the second stage resection, and the opportunity to administer chemotherapy is 60% of the cases.
Chemotherapy was given to 177 out of 192 patients (92.2%) prior to 1st stage and 118 out of 195 patients (60.5%) prior to 2nd stage. Administration of systemic chemotherapy prior to 2nd stage was not associated with increased rates for any complication (HR 1.36, p = 0.202). A total of 64 (32.6%) patients received regional therapy via hepatic artery infusion pump (HAIP) in addition to systemic therapy. Patients who received HAIP had similar OS to those who received systemic therapy only (HR 0.956, p = 0.848).
Other therapies evaluated included intraoperative ablation of liver in 38 (19.3%) patients during 1st stage procedure and 18 (9.1%) of patients during 2nd stage. Ablative therapies were not significantly associated with OS (p = 0.661).
Surgical outcomes
Overall complications occurred in 47 (23.9%) patients and 93 (47.4%) patients related to 1st and 2nd stage, respectively. Out of these patients, 5.1% (n = 10) and 23.4% (n = 46) during 2nd were considered to have major complications (classified as Grade ≥ 3 Clavien-Dindo) during the 1st and 2nd stage, respectively. Postoperative adverse events are reported in Table 2. No intraoperative deaths occurred during any procedure. The 90-day mortality rate following the second stage was 4.5%.
The median length of stay was 6.0 (1–17) days for 1st stage and 7.0 (1–42) days for 2nd stage procedure. There were 14 (7.2%) and 20 (10.3%) readmissions after 1st and 2nd stage, respectively.
Prognostic factors on survival
Median OS for the entire cohort was 50 months (1–142). The OS at 1-, 3- and 5-years was 89%, 64% and 44% respectively. On univariate analysis, factors associated with worse survival were positive margins (R1/R2 resection) after second stage hepatectomy (HR 2.05, p = 0.036) (Figure 1) and increased EBL (HR 1.034, p = 0.005). Positive margins after 1st stage did not show an impact on OS (HR 1.36, p = 0.410). Age, gender, BMI, number of liver lesions, CEA level and colorectal cancer staging were not associated with prognosis (p > 0.05; Table 3). When total EBL was calculated for both procedures, each 100 mL increase was significantly associated with a higher risk of death (HR 1.034, p = 0.005). The use of blood transfusion after 2nd stage was not statistically significant for changes in survival rate (HR 1.024, p = 0.939). Multivariant analysis was performed as it related to DFS and OS. This analysis was inconclusive due to low power and missing data and added no additional predictors to the model beyond the bivariant and univariant findings.
Figure 1.
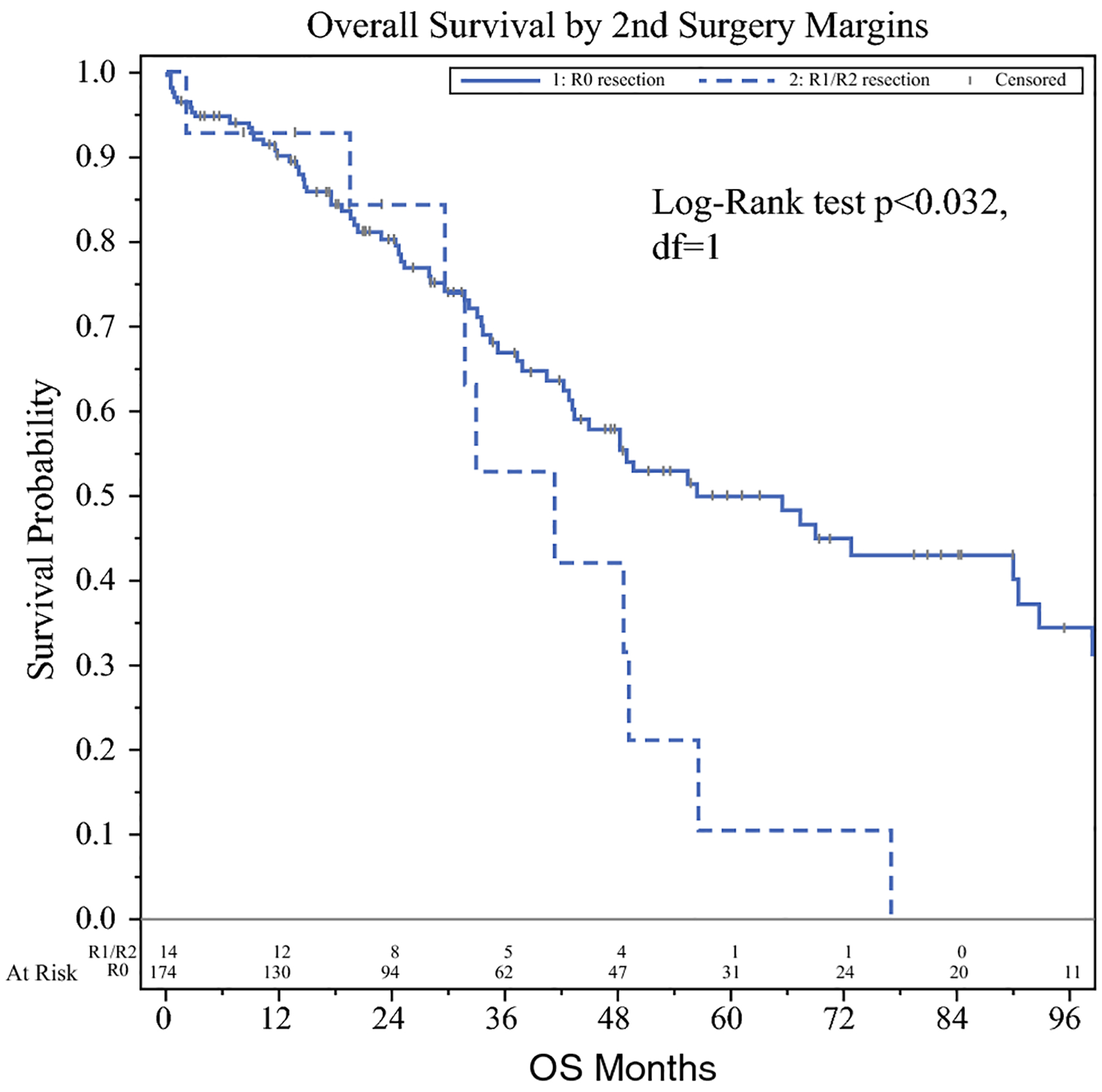
Resection margins predicts overall survival after 2nd stage. Negative margins (solid line) is associated with greater overall survival when compared to patients with positive margins (dotted line).
Table 3.
Bivariate overall survival analysis
| Variables | HR | 95% CI | p-value |
|---|---|---|---|
| Age | |||
| < 60 years | 0.996 | 0.957–1.017 | 0.862 |
| ≥ 60 years | 0.70 | 0.41–1.20 | 0.196 |
| Gender | |||
| Female | 1.51 | 0.971–2.36 | 0.068 |
| BMI ≥ 30 | 0.83 | 0.50–1.37 | 0.462 |
| Primary T-stage | |||
| T 3–4 | 0.62 | 0.15–2.54 | 0.507 |
| Primary N-stage | |||
| N 1–2 | 1.27 | 0.71–2.25 | 0.422 |
| Lymphovascular invasion | 0.61 | 0.37–1.014 | 0.057 |
| Perineural invasion | 0.63 | 0.37–1.099 | 0.105 |
| Synchronous disease | |||
| Metachronous | 1.31 | 0.67–2.54 | 0.426 |
| Number of liver mets at diagnosis | 1.004 | 0.957–1.054 | 0.862 |
| Portal vein embolization | 0.955 | 0.61–1.49 | 0.839 |
| Carcinoembryonic antigen level | 1.000 | 0.999–1.001 | 0.690 |
| Tumor genetic status | |||
| KRAS mut | 1.55 | 0.89–2.70 | 0.118 |
| NRAS mut | 4.63 | 1.26–17.03 | 0.021 |
| Chemotherapy prior to 1st stage | 1.17 | 0.51–2.70 | 0.706 |
| Chemotherapy prior to 2nd stage | 1.36 | 0.85–2.17 | 0.202 |
| R1/R2 resection after 1st stage | 1.36 | 0.65–2.86 | 0.410 |
| R1/R2 resection after 2nd stage | 2.05 | 1.049–4.01 | 0.036 |
| Total estimated blood loss (per 100 mL) | 1.034 | 1.011–1.059 | 0.005 |
BMI = Body Mass Index
DFS at 1, 3 and 5-years was 43%, 19% and 18% respectively. A total of 118 (60%) patients had a recurrence after completion of TSH. The distribution of recurrence was 44.6% intrahepatic, 38.7% extrahepatic and 16.8% both intra and extrahepatic. Median disease-free interval was 12.5 (2–143) months. On univariate analysis, similarly to OS, each 100 mL increase of total EBL calculated for both procedures were significantly associated with decreased DFS (HR 1.026, p = 0.010) (See Table 4). Readmission following 2nd stage hepatectomy was also associated with a decrease in DFS (HR 2.22, p = 0.003).
Table 4.
Univariate disease-free survival analysis
| Variables | HR | 95% CI | p-value |
|---|---|---|---|
| Age | |||
| ≥ 60 years | 0.87 | 0.60–1.27 | 0.473 |
| Gender | |||
| Female | 1.17 | 0.84–1.63 | 0.357 |
| BMI ≥ 30 | 0.939 | 0.64–1.38 | 0.749 |
| Primary T-stage | |||
| T 3–4 | 0.60 | 0.22–1.63 | 0.316 |
| Primary N-stage | |||
| N 1–2 | 1.048 | 0.68–1.62 | 0.833 |
| Lymphovascular invasion | 0.89 | 0.60–1.32 | 0.549 |
| Perineural invasion | 0.985 | 0.68–1.44 | 0.938 |
| Synchronous disease | |||
| Metachronous | 0.79 | 0.47–1.34 | 0.386 |
| Number of liver mets at diagnosis | 1.017 | 0.986–1.050 | 0.286 |
| Portal vein embolization | 0.85 | 0.61–1.18 | 0.331 |
| Carcinoembryonic antigen level | 1.000 | 0.999–1.001 | 0.618 |
| Tumor genetic status | |||
| KRAS mut | 1.085 | 0.72–1.63 | 0.691 |
| NRAS mut | 1.94 | 0.59–6.41 | 0.278 |
| Chemotherapy prior to 1st stage | 1.10 | 0.58–2.10 | 0.765 |
| Chemotherapy prior to 2nd stage | 1.19 | 0.85–1.67 | 0.308 |
| R1/R2 resection after 1st stage | 3.13 | 0.77–12.76 | 0.112 |
| R1/R2 resection after 2nd stage | 1.37 | 0.77–2.44 | 0.279 |
| Total estimated blood loss (per 100 mL) | 1.026 | 0.999–1.048 | 0.010 |
BMI = Body Mass Index
DISCUSSION
Just a few decades ago, patients with unresectable disease were relegated to palliative chemotherapy only14. Newer chemotherapeutic agents and surgical techniques have expanded our armamentarium for treating patients with advanced multifocal colorectal metastases to the liver. As such, PVE initially was intended for patients requiring extended resections in order to induce ipsilateral atrophy and contralateral hypertrophy of the remnant liver, thereby decreasing the risk of severe postoperative liver failure15. Likewise, ablative techniques such as radiofrequency ablation (RFA) and microwave ablation (MWA) when combined with hepatectomy, have been shown to be safe, feasible and a reasonable approach to patients with bilateral CRLM in need of parenchymal sparing surgery16,17. However, these techniques are at times insufficient to remove all the tumors in patients with extensive bilateral disease. Adam et al. reported TSH for the management of these patients, where two sequential procedures removed tumors not amenable to a single operation12. TSH is a strategy that takes advantage of the liver’s regenerative capabilities to allow for radical extirpation of extensive disease. Many specialized centers worldwide have reported promising short- and long-term outcomes. However, most of these reports are single institution series with small number of patients, which limit the power of the observations and conclusions drawn from them18–20. Therefore, the objective of the present study was to perform a large, multi-institutional analysis of patients who completed TSH for bilateral CRLM in the United States.
The present study shows that TSH strategy can be performed safely with acceptable morbidity and mortality rates following completion of the 2nd stage. The overall morbidity rate after completion of 2nd stage TSH was 47.4%, which is similar to that reported by Wicherts et al. (59%) but differs from that reported by Regimbeau et al. (25.1%) and Tsai et al. (25.7%)18, 19,21. It is important to highlight the therapeutic approach by Tsai et al., where the resection of the major disease was initially performed during the 1st stage with a shift of strategy later in the series to a more aggressive approach during the 2nd stage19. In 2017, Regimbeau et al. published the largest data reported to date for patients who completed TSH, obtained from the international registry LiverMetSurvey21. This group evaluated retrospectively 5,786 patients who underwent resection of multiple and/or bilateral CRLM in one surgical procedure (hepatectomy group) vs. a group of 869 patients with intended TSH. Among the intended TSH group, 625 patients completed TSH and 244 (28.1%) completed only the 1st stage. Differences were also found when comparing 90-day mortality rate, where the present study found a rate of 4.5%, similar to Tsai et al. (5%), while Regimbeau et al. reported almost double (9.3%). This difference in morbidity and mortality rates may be attributed to sample size. The current study only focuses on patients completing both stages and does not capture data regarding patients who failed to reach the second stage.
The present study confirms the importance of obtaining negative margins for curative resection. R1 or R2 resections after the 2nd stage procedure were associated with significantly decreased 5-year OS (55% vs. 10%, p < 0.032). Similarly, positive surgical margins have been described as an independent predictor of worse OS in multiple studies22–25. Recently, Wang et al. published their experience of 633 patients undergoing curative-intent resection for CRLM22. This group found improved overall survival associated with R0 resection when compared to either definition of R1 resection (involved vs. sub-mm margins). No significant survival differences were detected among patients who had involved vs sub-mm margins (p = 0.31), suggesting surgeons should strive for at least a 1-mm margin.
Recent literature shows the relationship of blood transfusion with peri-operative and long-term outcomes after major hepatectomy for metastatic colorectal cancer. A multi-institutional study of 456 patients found blood transfusions to be independently associated with increased major complications (OR 2.61, p < 0.001)26. In their report, transfusion rate was associated with extended hepatectomy, larger tumor size and increased operative blood loss. The present study showed increase in EBL was associated with decreased OS and DFS, however did not show blood transfusion correlated with survival. The findings could be attributed due to the small sample size of our study.
This study only presented patients who completed both stages of the planned approach, in some cases with the aid of ablative techniques. According to recent reports, approximately 22–28% of patients intended to undergo TSH, fail to complete the 2nd stage hepatectomy due to disease progression, poor performance status or death19,21. Other alternatives such as associating liver partition and portal vein ligation (ALPPS) or single-stage resection with ablation have recently been introduced.
The ALPPS procedure induces rapid hypertrophy of the FLR and shortens the time interval between the two stages of surgery. In ALPPS, the 2nd stage surgery can be performed 1 to 2 weeks after the 1st stage27. Although completion rates after ALPPS are very high, initially there was some debate in the literature regarding morbidity, mortality and the oncologic outcomes. Two systematic reviews and comparative meta-analyses of the ALPPS and TSH procedures discuss this subject28,29. These meta-analyses identified 9 studies comparing ALPPS with TSH including a total of 650 patients. The researchers found that ALPPS procedure did not lead to higher overall FLR, but final liver volume was reached in a shorter time (faster kinetic growth rate). Also, ALPPS was associated with greater mortality and morbidity rates than TSH, with comparable oncological outcomes. Ratti et al. have reported that after the 2nd stage, an overall and major morbidity for ALPPS of 83.3% and 41.7% respectively, which was significantly higher than the morbidity for their TSH cases (38.2% and 17.6%, respectively; p = 0.011)30. Their results from the TSH group are comparable to our study, with an overall and major morbidity after 2nd stage of TSH of 47.4% and 23.4%, respectively. More recently, a multicenter randomized controlled trial (RCT - LIGRO Trial) including 97 patients with diagnosis of CRLM and a standardized FLR (sFLR) < 30%, compared ALPPS to TSH31. This trial confirmed the higher resection rates after ALPPS (92%) compared to TSH (57%) (p < 0.0001) with no differences in severe complications, 90-day mortality or R0 resection rates. The results of the LIGRO Trial are of major significance as this is the first RCT comparing these two surgical procedures for the management of advanced CRLM. However, long-term oncological outcomes remain uncertain. Adam et al. reported a median OS of 20 months for patients undergoing ALPPS vs. a median OS of 37 months for TSH (p = 0.006)32. Our study reports a median OS of 50 months. This is partially due to the improved patient selection with TSH, as only patients with favorable biology will undergo 2nd stage.
The current study has several limitations, most of which are inherent to its retrospective design. Given the long duration of the study, changes in systemic therapy may have influenced the results. Selection bias could be due to the degree of multi-center participation and the fact that cases were performed in highly specialized centers. Furthermore, we were unable to collect details regarding chemotherapeutic drugs and regimens utilized however the series is confined to the modern era. Also, with this population, we were not able to establish a comparison group that could not complete TSH or compare this technique with other surgical/interventional approaches. Nonetheless, this is the largest series of TSH in the U.S. with the collaboration of internationally recognized liver centers.
CONCLUSION
This is the largest TSH series in the U.S. and demonstrates safety and acceptable perioperative and survival outcomes in select patients with initially unresectable bilateral CRLM. Optimal outcomes are associated to margin status and operative EBL. Further prospective controlled studies are required to better define criteria for the selection of patients to TSH and to compare it to other approaches.
SYNPOSIS.
A multicenter study of bilateral colorectal liver metastases (CRLM) patients who completed two-stage hepatectomy (TSH). The study includes 196 patients over a 16-year period, being the largest TSH series in the U.S. We conclude feasibility of TSH for bilateral CRLM.
ACKNOWLEDGEMENTS
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Disclosure: The authors declare that they have no conflict of interest or financial disclosures. This study was presented at 13th IHPBA World Congress 2018 - Geneva, Switzerland.
REFERENCES
- 1.Cancer Facts & Figures 2019 [database online]. Atlanta, GA; 2019. [Google Scholar]
- 2.Clavien P Malignant liver tumors: current and emerging therapies. Chichester, UK.: Wiley-Blackwell, 2010. [Google Scholar]
- 3.Groeschl RT, Nachmany I, Steel JL, et al. Hepatectomy for noncolorectal non-neuroendocrine metastatic cancer: a multi-institutional analysis. J Am Coll Surg 2012; 214(5):769–77. [DOI] [PubMed] [Google Scholar]
- 4.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000; 191(1):38–46. [DOI] [PubMed] [Google Scholar]
- 5.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002; 236(4):397–406; discussion 406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemeny N Presurgical chemotherapy in patients being considered for liver resection. Oncologist 2007; 12(7):825–39. [DOI] [PubMed] [Google Scholar]
- 7.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004; 239(6):818–25; discussion 825–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006; 141(5):460–6; discussion 466–7. [DOI] [PubMed] [Google Scholar]
- 9.Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol 2012; 4:283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomizawa N, Ohwada S, Ogawa T, et al. Factors affecting the prognosis of anatomical liver resection for liver metastases from colorectal cancer. Hepatogastroenterology 2006; 53(67):89–93. [PubMed] [Google Scholar]
- 11.Wei AC, Greig PD, Grant D, et al. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 2006; 13(5):668–76. [DOI] [PubMed] [Google Scholar]
- 12.Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg 2000; 232(6):777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adam R, De Gramont A, Figueras J, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist 2012; 17(10):1225–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wicherts DA, de Haas RJ, Andreani P, et al. Impact of portal vein embolization on long-term survival of patients with primarily unresectable colorectal liver metastases. Br J Surg 2010; 97(2):240–50. [DOI] [PubMed] [Google Scholar]
- 16.Imai K, Allard MA, Castro Benitez C, et al. Long-term outcomes of radiofrequency ablation combined with hepatectomy compared with hepatectomy alone for colorectal liver metastases. Br J Surg 2017; 104(5):570–579. [DOI] [PubMed] [Google Scholar]
- 17.Philips P, Groeschl RT, Hanna EM, et al. Single-stage resection and microwave ablation for bilobar colorectal liver metastases. Br J Surg 2016; 103(8):1048–54. [DOI] [PubMed] [Google Scholar]
- 18.Wicherts DA, Miller R, de Haas RJ, et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg 2008; 248(6):994–1005. [DOI] [PubMed] [Google Scholar]
- 19.Tsai S, Marques HP, de Jong MC, et al. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB (Oxford) 2010; 12(4):262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam VW, Laurence JM, Johnston E, et al. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford) 2013; 15(7):483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regimbeau JM, Cosse C, Kaiser G, et al. Feasibility, safety and efficacy of two-stage hepatectomy for bilobar liver metastases of colorectal cancer: a LiverMetSurvey analysis. HPB (Oxford) 2017; 19(5):396–405. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Margonis GA, Amini N, et al. The Prognostic Value of Varying Definitions of Positive Resection Margin in Patients with Colorectal Cancer Liver Metastases. J Gastrointest Surg 2018; 22(8):1350–1357. [DOI] [PubMed] [Google Scholar]
- 23.Miller CL, Taylor MS, Qadan M, et al. Prognostic Significance of Surgical Margin Size After Neoadjuvant FOLFOX and/or FOLFIRI for Colorectal Liver Metastases. J Gastrointest Surg 2017; 21(11):1831–1840. [DOI] [PubMed] [Google Scholar]
- 24.Laurent C, Adam JP, Denost Q, et al. Significance of R1 Resection for Advanced Colorectal Liver Metastases in the Era of Modern Effective Chemotherapy. World J Surg 2016; 40(5):1191–9. [DOI] [PubMed] [Google Scholar]
- 25.Andreou A, Aloia TA, Brouquet A, et al. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg 2013; 257(6):1079–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postlewait LM, Squires MH 3rd, Kooby DA, et al. The relationship of blood transfusion with peri-operative and long-term outcomes after major hepatectomy for metastatic colorectal cancer: a multi-institutional study of 456 patients. HPB (Oxford) 2016; 18(2):192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linecker M, Kuemmerli C, Clavien PA, et al. Dealing with insufficient liver remnant: Associating liver partition and portal vein ligation for staged hepatectomy. J Surg Oncol 2019; 119(5):604–612. [DOI] [PubMed] [Google Scholar]
- 28.Moris D, Ronnekleiv-Kelly S, Kostakis ID, et al. Operative Results and Oncologic Outcomes of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) Versus Two-Stage Hepatectomy (TSH) in Patients with Unresectable Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. World J Surg 2018; 42(3):806–815. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Z, Xu M, Lin N, et al. Associating liver partition and portal vein ligation for staged hepatectomy versus conventional two-stage hepatectomy: a systematic review and meta-analysis. World J Surg Oncol 2017; 15(1):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratti F, Schadde E, Masetti M, et al. Strategies to Increase the Resectability of Patients with Colorectal Liver Metastases: A Multi-center Case-Match Analysis of ALPPS and Conventional Two-Stage Hepatectomy. Ann Surg Oncol 2015; 22(6):1933–42. [DOI] [PubMed] [Google Scholar]
- 31.Sandström P, Røsok BI, Sparrelid E, et al. ALPPS Improves Resectability Compared With Conventional Two-stage Hepatectomy in Patients With Advanced Colorectal Liver Metastasis: Results From a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann Surg 2018; 267(5):833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adam R, Imai K, Castro Benitez C, et al. Outcome after associating liver partition and portal vein ligation for staged hepatectomy and conventional two-stage hepatectomy for colorectal liver metastases. Br J Surg 2016; 103(11):1521–9. [DOI] [PubMed] [Google Scholar]


