Abstract
The defining feature of pancreatic islet β-cell function is the precise coordination of changes in blood glucose levels with insulin secretion to regulate systemic glucose homeostasis. While ATP has long been heralded as a critical metabolic coupling factor to trigger insulin release, glucose-derived metabolites have been suggested to further amplify fuel-stimulated insulin secretion. The mitochondrial export of citrate and isocitrate through the citrate-isocitrate carrier (CIC) has been suggested to initiate a key pathway that amplifies glucose-stimulated insulin secretion, though the physiological significance of β-cell CIC-to-glucose homeostasis has not been established. Here, we generated constitutive and adult CIC β-cell knockout (KO) mice and demonstrate that these animals have normal glucose tolerance, similar responses to diet-induced obesity, and identical insulin secretion responses to various fuel secretagogues. Glucose-stimulated NADPH production was impaired in β-cell CIC KO islets, whereas glutathione reduction was retained. Furthermore, suppression of the downstream enzyme cytosolic isocitrate dehydrogenase (Idh1) inhibited insulin secretion in wild-type islets but failed to impact β-cell function in β-cell CIC KO islets. Our data demonstrate that the mitochondrial CIC is not required for glucose-stimulated insulin secretion and that additional complexities exist for the role of Idh1 and NADPH in the regulation of β-cell function.
Introduction
Pancreatic islet β-cells regulate glucose homeostasis by coupling glucose metabolism with insulin secretion. Signals generated from glucose metabolism are thought to trigger and amplify insulin secretion in two distinct phases (1,2). The triggering phase is activated by a metabolically driven action potential that begins with an increase in the ATP:ADP ratio, closure of KATP channels, plasma membrane depolarization, and opening of voltage-gated Ca2+ channels (3–7). The influx of Ca2+ allows for fusion of plasma membrane–docked insulin granules and subsequent exocytotic release of granule contents (8). In addition, glucose provides signals beyond simple membrane depolarization that further amplify insulin release (1,2,9,10). These signals, or coupling factors, are presumably derived from glucose metabolites that are exchanged between the mitochondria and cytosol and used to coordinate rates of tricarboxylic acid cycle (TCA) flux with the amplification of fuel-stimulated insulin secretion (11,12).
Cytosolic NADPH has garnered much attention as a potential metabolic signal that positively correlates with glucose-stimulated insulin secretion (GSIS) and can directly enhance β-cell exocytosis in some contexts (11,13–15); however, the coordination of TCA flux with NADPH production and insulin secretion is not well understood. Cytosolic NADPH can be produced following mitochondrial export of TCA intermediates citrate or isocitrate and subsequent oxidation by the cytosolic enzyme isocitrate dehydrogenase (Idh1) as part of the pyruvate-isocitrate cycle (11,12). Support for this pathway comes from studies in rat islets and insulinoma cells showing that suppression of Idh1 impairs insulin secretion along with reductions in NADPH levels (16). In addition, decarboxylation of mitochondrially derived malate by the cytosolic isoform of malic enzyme ME1 in the pyruvate-citrate cycle or the pyruvate-malate shuttle can similarly generate cytosolic NAPDH; however, genetic knockout (KO) of ME1 has a limited impact on stimulus-coupled insulin secretion (17). Further advances have now linked the generation of NADPH by Idh1 to the activation of the deSUMOylating enzyme SENP1 via glutaredoxin, which in turn amplifies insulin secretion through an unknown mechanism (15,18). More recent studies have also added a role for the reductive, “counterclockwise” TCA, utilizing mitochondrial Idh2 to regenerate isocitrate from α-ketoglutarate to further propagate the generation of mitochondrially derived isocitrate as a key substrate in the generation of cytosolic NADPH (19).
Mitochondrial export of citrate and isocitrate is regulated by a single protein, the citrate-isocitrate carrier (CIC), which is encoded by the SLC25A1 nuclear gene. CIC assembles in the inner mitochondrial membrane as a homodimer and catalyzes the exchange of citrate or isocitrate with cytosolic malate and/or phosphoenolpyruvate (PEP) (20,21). In insulinoma and isolated rat islet models, functional studies using siRNA suppression or pharmacological inhibitors have supported a role for CIC in the regulation of insulin secretion, which is postulated to provide a key substrate for Idh1 (22). While informative, the lack of genetic mouse models manipulating expression of Idh1 and CIC in the β-cell have hindered our understanding of the physiological significance of these pathways to glucose homeostasis. Specific loss-of-function mutations in human CIC are associated with combined D-2 and L-2 hydroxyglutaric aciduria, but currently, it is not known whether individuals with these conditions are predisposed to the development of β-cell dysfunction and diabetes (23,24). In mouse models of nonalcoholic fatty liver disease and steatohepatitis, pharmacological inhibition of CIC reduces and/or reverses pathologies associated with fatty liver disease, attenuates diet-induced obesity, and normalizes hyperglycemia and glucose intolerance (25). This is proposed to occur via reprogramming lipid metabolism in the liver and is supported by liver-specific deletion of CIC; however, the physiological impact of pharmacological inactivation of CIC in the β-cell was not investigated. In sum, inactivation of CIC has been shown to be both beneficial and detrimental to glucose homeostasis depending on the context. These unresolved issues encourage continued investigation into the physiological significance of CIC and mitochondrial citrate-isocitrate export in the regulation of glucose sensing in the islet β-cell.
In the current study, we generated two conditional CIC β-cell KO mouse lines using Ins1-Cre to produce constitutive β-cell KO and the tamoxifen-inducible MIP-CreERT to create adult β-cell KO. Using these approaches, we demonstrate that β-cell CIC is fully dispensable for glucose homeostasis. Both constitutive and adult β-cell CIC KO mice exhibit normal glucose tolerance in both sexes, no overt phenotype in response to diet-induced obesity, and similar insulin secretory responses in vivo to glucose challenge and ex vivo in response to various fuel secretagogues. Furthermore, the glucose-dependent rise in NADPH/NADP+ was impaired in CIC KO β-cells, yet glutathione (GSH) reduction was maintained. Using shRNA suppression, we demonstrate that the dicarboxylate carrier (DIC) does not compensate for β-cell CIC loss in the regulation of insulin secretion. In addition, while loss of Idh1 strongly inhibited insulin secretion in wild-type islets, as previously shown (16), Idh1 knockdown in β-cell CIC KO islets failed to impair GSIS. This apparent contradiction suggests that additional complexities exist for the contribution of Idh1 to the regulation of β-cell function. Together, our data demonstrate that mechanisms independent of citrate and isocitrate efflux can be used to amplify fuel-stimulated insulin secretion in the islet β-cell.
Research Design and Methods
Cell Culture and Reagents
Cell culture reagents were from Thermo Life Technologies unless specified otherwise. Chemical reagents were from Sigma-Aldrich unless specified otherwise. Mouse islets were isolated via collagenase V digestion and purified using Histopaque 1077 and 1119. Islets were cultured in RPMI medium supplemented with 10% FBS and 1% penicillin and streptomycin and maintained at 37°C in 5% CO2. Pools of islets were transduced with ∼2 × 107 infectious units/mL adenovirus (multiplicity of infection ∼100–200) for 18 h and assayed 72–96 h after treatment. Fatty acid solutions (10 mmol/L) containing palmitate or an oleate/palmitate mixture (2:1) were dissolved in water at 95°C and cooled to 50°C, and BSA (fatty acid free) was added to a final concentration of 10%. Solutions were maintained at 37°C for an additional 1 h.
Animal Studies
Mice containing a conditional, floxed Slc25a1 allele (CICfl) were generated by in vitro fertilization of C57BL6/N embryos with cryopreserved sperm obtained from KOMP harboring the Slc25a1tm1a allele. The Slc25a1tm1a allele mice were passed through mice expressing optimized Flp recombinase expressed ubiquitously by the ROSA26 locus to remove the neomycin-selectable marker producing Slc25a1tm1c (floxed allele) mice. Subsequently, CICfl mice were crossed into the C57BL6/J background. B6.Cg-Tg(Ins1-cre/ERT)1Lphi/J (MIP-CreERT) mice were provided by Dr. Louis Phillipson (University of Chicago, Chicago, IL) (26). B6(Cg)-Ins1 tm1.1(cre)Thor/J (Ins1-Cre) mice were purchased from The Jackson Laboratory. CIC floxed mice were bred with either MIP-CreERT or Ins1-Cre mice to generate heterozygous animals and then backcrossed for at least eight generations to the CIC floxed parental line selecting for the C57BL6/J NNT mutant background. All animals were verified by genotyping. Cre alleles (MIP-CreERT and Ins1-Cre) were maintained as hemizygous in all studies. CICfl/fl; MIP-CreERT and CICfl/fl mice at 8–12 weeks of age were injected with 2–3 mg i.p. tamoxifen dissolved in corn oil containing 5–10% ethanol on 5 consecutive days. Four to six weeks after tamoxifen treatment, glucose tolerance was measured in 4–6-h fasted mice given a 1–2 mg/g body wt i.p. glucose challenge. In some studies, mice (8 weeks of age) were placed on a Western diet (D12079B; Research Diets) for up to 12 weeks. Blood glucose was determined using a OneTouch Ultra 2 glucometer. Plasma insulin was determined by ultrasensitive rat/mouse ELISA (ALPCO). All animal protocols were approved by the University of Iowa institutional animal use and care committee.
Plasmids and Viruses
A shuttle plasmid containing a U6 promoter, multiple cloning sites, and a phosphoglycerate kinase (PGK) promoter–expressing mCherry reporter was generated by Gibson assembly (Integrated DNA Technologies) and subcloned into pENTR2b. Primers containing Slc25a10 shRNA target sequences were inserted into pU6-MCS-PGK-mCherry by restriction digest and subcloned into a modified pAd-PL/DEST (27) using LR Clonase II. Plasmids containing a U6 promoter–driven Idh1 shRNA targeting sequences in an adenoviral backbone were obtained from VectorBuilder. Nontargeting control (shSAFE) sequence was provided by the University of Iowa Viral Vector Core Facility. Recombinant adenoviruses were generated in HEK293 cells and purified by cesium chloride gradient. Adenovirus-containing rat insulin promoter (RIP) expressing Grx1-GFP2 was a gift from A. Linneman (Indiana University School of Medicine, Indianapolis, IN) (28). All sequences were verified by the Iowa Institute of Human Genetics, University of Iowa.
GSIS
Insulin secretion using ∼40 islets was performed by perifusion in secretion assay buffer using a Biorep Perifusion System with a flow rate of 100 µL/min at 37°C. Perifusate was collected at 1–2-min intervals. Following stabilization of insulin release under basal (2.5 mmol/L) glucose conditions (up to 32 min), islets were stimulated with various secretagogues, including glucose alone (11.2 mmol/L or 16.7 mmol/L), KCl (35 mmol/L), glucose-diazoxide-KCl cocktail (11.2 mmol/L glucose + 100 μmol/L diazoxide + 35 mmol/L KCl), amino acid stimulation (10 mmol/L glutamine + 1 mmol/L leucine), glucose plus fatty acid (11.2 mmol/L glucose + 0.5 mmol/L oleate/palmitate 2:1), pyruvate kinase M2 activator (TEPP46) (10 μmol/L), or 1,2,3-benzene tricarboxylate (BTC) (2 mmol/L), where indicated. Islets were collected from perifusion chambers and lysed in radioimmunoprecipitation assay buffer for content determination. Static incubations for insulin secretion were performed on pools of 10 islets as previously described using a BSA solution containing free fatty acid (29). Insulin (secreted and content) was measured by ELISA, rodent 80-INSMR-CH10 (ALPCO), or AlphaLISA AL204C (Perkin-Elmer).
Immunoblot Analysis
Clarified cell lysates were resolved on 4–12% NuPAGE gels and transferred to supported nitrocellulose membranes. Membranes were probed with diluted antibody raised against Slc25a1 (Proteintech) or γ-tubulin. Donkey anti-mouse and anti-rabbit antibodies coupled to IRDye 680 or 800 (LI-COR) were used to detect primary antibodies. Blots were developed using an Odyssey CLx instrument (LI-COR).
Quantitative RT-PCR
RNA from mouse islets was harvested using the RNeasy Microkit (QIAGEN) and cDNA synthesized in an iScript Reaction Mix (Bio-Rad). RNA from human islets was isolated using TRIzol reagent, and cDNA was synthesized in a SuperScript IV VILO Master Mix. Real-time PCRs were performed using the ABI 7700 sequence detection system and software (Applied Biosystems). All primer sequences are available upon request.
Live-Cell Microscopy
For GSH disulfide (GSSG)/GSH redox measurements, isolated islets treated with AdRIP-Grx1-GFP2 were partially dispersed into monolayers using Accutase and plated on HTB9-coated 6-cm glass-bottom dishes (MatTek) 24 h postinfection, as previously described (29,30). Islets were cultured in secretion assay buffer containing 2.5 mmol/L glucose for 20 min and imaged by an SP8 confocal microscope with a 20× objective (HC PL APO CS2, 0.75 NA; Leica) using sequential excitations at 405 and 488 nm detected at 530 ± 30 nm. Islets were subsequently treated with 20 mmol/L glucose for 12 min and reimaged followed by dithiothreitol (DTT) (5 mmol/L) and diamide (500 μmol/L) for 12 min each. Fluorescence intensities of each channel were calculated from masked images (to remove background) of whole cells using Fiji software (National Institutes of Health). Ratiometric intensities (405 nm/488 nm) were normalized by comparing with DTT (0%)– and diamide (100%)–treated samples using GraphPad Prism software.
For NADPH/NADP+ measurements, dispersed islets were cotransduced with recombinant adenovirus expressing the Venus-tagged Apollo-NADP+ sensor and adenovirus-expressing mCherry under control of the insulin promoter to facilitate identification of β-cells. β-Cells were incubated at 1 mmol/L glucose for 1 h and sequentially treated with 5 mmol/L glucose and 15 mmol/L glucose followed by oxidation with diamide. Anisotropy values were collected 5 min after each treatment was administered as previously described (31) and normalized to diamide treatment.
Human Islets
Human islets from donors without diabetes and donors with type 2 diabetes (T2D) (Supplementary Table 1) were cultured in CMRL1066 containing 1% human serum albumin, 1% penicillin and streptomycin, and 1% L-glutamate overnight at 37°C and 5% CO2 before analyses. The institutional review board of the University of Iowa determined this to be a nonhuman study.
Statistical Analysis
Data are presented as the mean ± SEM. For statistical significance determinations, unpaired Student t test or two-way ANOVA (regular or repeated measures for time courses) with Šidák post hoc analysis for multiple group comparisons were used (GraphPad Prism).
Data and Resource Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Results
Constitutive β-Cell Loss of CIC Does Not Impair Glucose Homeostasis
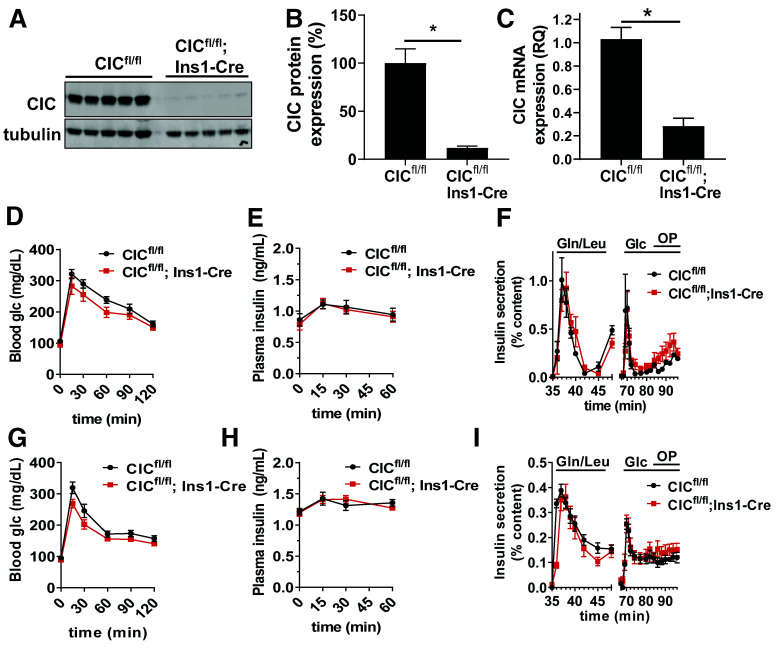
Previous studies have highlighted a critical role for mitochondrial export of citrate/isocitrate to generate coupling factors necessary to support GSIS in the pancreatic islet β-cell (11,16,22). Mitochondrial citrate/isocitrate export is thought to be solely performed by the CIC, which has been shown through pharmacological and knockdown studies to regulate insulin secretion (22); however, the physiological effect of CIC loss on β-cell function is not known. To explore this, we generated a β-cell specific KO of CIC using homozygous CIC floxed mice expressing constitutively active Ins1-Cre (CICfl/fl; Ins1-Cre). Using this system, we achieve ∼90% loss of CIC protein expression by immunoblot analysis (Fig. 1A and B and Supplementary Fig. 1A and B) and >80% reduction of mRNA by quantitative RT-PCR (qRT-PCR) in islets isolated from CICfl/fl; Ins1-Cre mice compared with CICfl/fl controls (Fig. 1C). In both male and female β-cell CIC KO mice (CICfl/fl; Ins1-Cre), we observed no change in glucose tolerance compared with CICfl/fl control mice (Fig. 1D and G) accompanied by normal plasma insulin responses to a glucose challenge (Fig. 1E and H). Next, we isolated islets from both male and female CICfl/fl control and β-cell CIC KO (CICfl/fl; Ins1-Cre) mice and examined insulin secretion in response to various secretagogues by perifusion. While we were able to detect robust increases in insulin release upon stimulation by amino acids, glucose, and glucose and fatty acids, there was no difference between control and β-cell CIC KO mice in either sex (Fig. 1F and I).
Figure 1.
Constitutive β-cell–specific deletion of CIC does not impair β-cell function. Islets isolated from CICfl/fl; Ins1-Cre vs. CICfl/fl mice were analyzed for protein expression by immunoblot (A), quantified (B) (n = 5), and analyzed for mRNA expression by qRT-PCR (C) (n = 6). CIC expression was first normalized to tubulin (immunoblot) or peptidylpropyl isomerase B (qRT-PCR) and expressed as percent relative to CICfl/fl control. Four- to 6-h fasted CICfl/fl; Ins1-Cre vs. CICfl/fl male (D and E) (n = 8–12) and female (G and H) (n = 10–12) mice (10–14 weeks of age) were injected with glucose 1.5 mg/g body wt i.p. Blood glucose (D and G) and plasma insulin (E and H) were recorded at the indicated times. Isolated islets from male (F) (n = 6) and female (I) (n = 3) CICfl/fl; Ins1-Cre vs. CICfl/fl mice (10–14 weeks of age) were perifused for 32 min at basal (2.5 mmol/L) glucose stimulated with amino acids glutamine (Gln) (10 mmol/L) and leucine (Leu) (1 mmol/L) for 16 min, returned to basal (2.5 mmol/L) glucose for 16 min, and stimulated with glucose alone (11.2 mmol/L) for 16 min followed by glucose (11.2 mmol/L) and oleate/palmitate (OP) (0.5 mmol/L, 2:1) for 16 min. Data are mean ± SEM. *P < 0.05 by two-tailed unpaired t test (B and C) or repeated-measures two-way ANOVA (D–I). Glc, glucose; RQ, relative quantification.
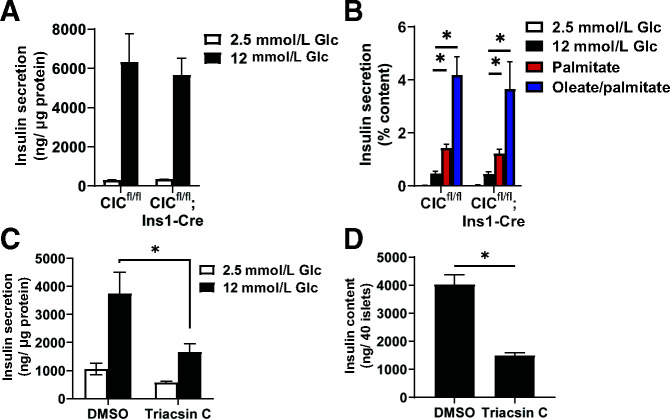
We next examined insulin secretion using static incubations to consider minor effects of β-cell CIC loss that may not be evident by perifusion. As shown in Fig. 2A, islets from CICfl/fl control and CIC β-cell KO (CICfl/fl; Ins1-Cre) mice had identical levels of GSIS. Additional stimulation via palmitate or oleate/palmitate achieved similar levels of insulin release (Fig. 2B). This lack of effect in the β-cell CIC KO is in contrast to direct inhibition of fatty acid production via triacsin C, which strongly reduces GSIS (Fig. 2C), albeit the effect may be driven, in part, by a reduction in insulin content (Fig. 2D).
Figure 2.
Static GSIS in β-cell CIC KO islets is indistinguishable from control islets. A and B: Islets were isolated from CICfl/fl; Ins1-Cre vs. CICfl/fl mice (10–20 weeks of age, n = 3–5 per genotype). Islets were incubated at basal (2.5 mmol/L) glucose followed by stimulation with glucose (12 mmol/L) alone, glucose and palmitate (0.5 mmol/L), or glucose and oleate/palmitate (1 mmol/L, 2:1) for 1 h each as indicated. C and D: Islets isolated from C57BL6/J mice (10–20 weeks of age, n = 3) were treated with triacsin C (10 μmol/L) for 72 h (C). Islets were sequentially incubated at basal (2.5 mmol/L) glucose followed by stimulatory (12 mmol/L) glucose for 1 h each as indicated (D). Insulin content was derived from whole-cell lysates. Data are mean ± SEM. *P < 0.05 by two-way ANOVA (A and B) or two-tailed unpaired t test (C and D). Glc, glucose.
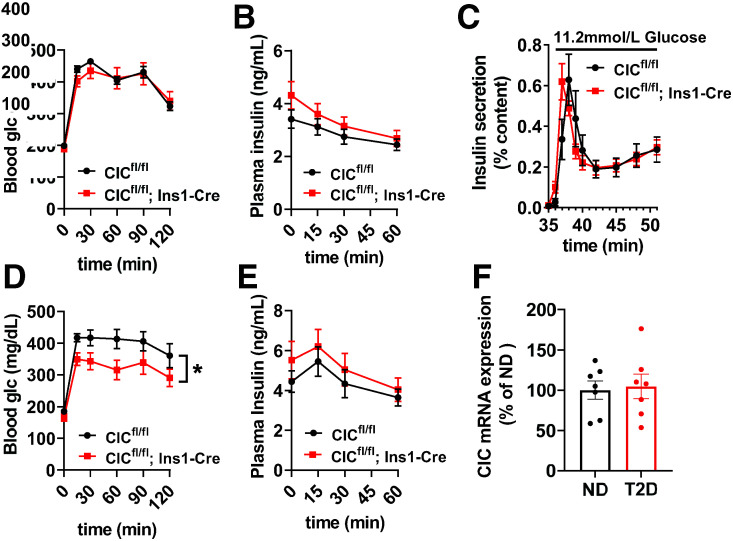
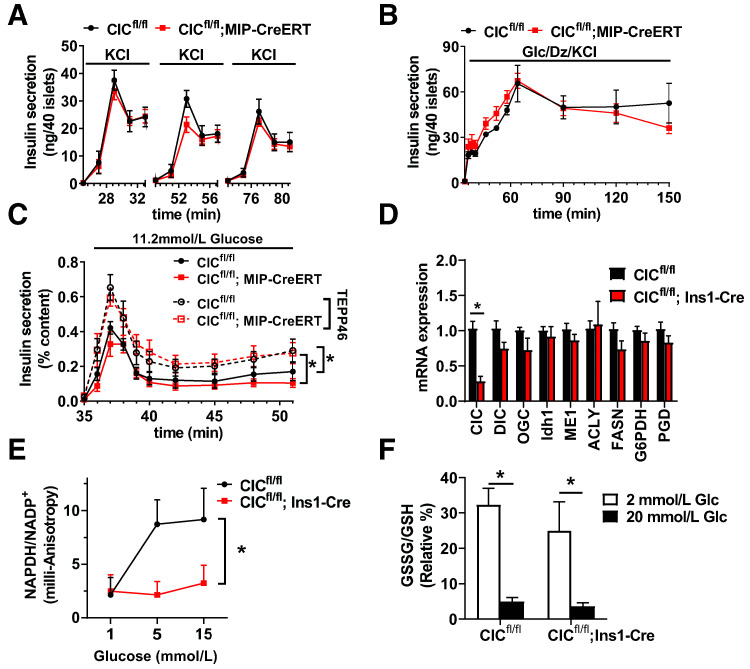
We next examined whether β-cell CIC KO mice would have an altered β-cell response to diet-induced obesity. To test this, β-cell CIC KO mice were placed on a Western diet (40% fat/kcal, 43% carbohydrate/kcal) for 8 weeks. Similar weight gain (data not shown) and glucose intolerance (Fig. 3A) compared with control (CICfl/fl) mice was observed. In addition, fasting hyperinsulinemia and plasma insulin response to glucose challenge were similar between β-cell CIC KO and control mice (Fig. 3B). We isolated islets following 8 weeks of diet and observed no difference between genotypes in GSIS by perifusion (Fig. 3C). We further extended the dietary intervention to 12 weeks and assessed glucose homeostasis. We observed a modest improvement in glucose tolerance in β-cell CIC KO mice compared with controls (Fig. 3D) but no difference in plasma insulin (Fig. 3E). Furthermore, no change in CIC mRNA expression was observed in islets isolated from human donors with T2D compared with control subjects without diabetes (Fig. 3F).
Figure 3.
Constitutive β-cell–specific deletion of CIC does not alter β-cell response to diet-induced obesity. Male CICfl/fl; Ins1-Cre vs. CICfl/fl mice were maintained on a Western diet (40% fat/kcal, 43% carbohydrate) for up to 12 weeks. A and B: Following 8 weeks of diet, 4–6-h fasted mice were injected with glucose 1 mg/g body wt i.p. (n = 11–16). Blood glucose (A) and plasma insulin (B) were recorded at the indicated times. C: Following 8 weeks of diet, isolated islets were perifused for 32 min at basal (2.5 mmol/L) glucose and then stimulatory glucose (11.2 mmol/L) for the indicated times (n = 6). D and E: Following 12 weeks of Western diet, 4–6-h fasted mice were injected with glucose 1 mg/g body wt i.p. (n = 5–7). Blood glucose (D) and plasma insulin (E) were recorded at the indicated times. F: CIC mRNA expression was determined by qRT-PCR in human islets derived from cadaveric donors (n = 7) classified as nondiabetic (ND) or diabetic (T2D). Data are mean ± SEM. *P < 0.05 by repeated-measures two-way ANOVA. glc, glucose.
Inducible β-Cell Loss of CIC Does Not Impact β-Cell Function
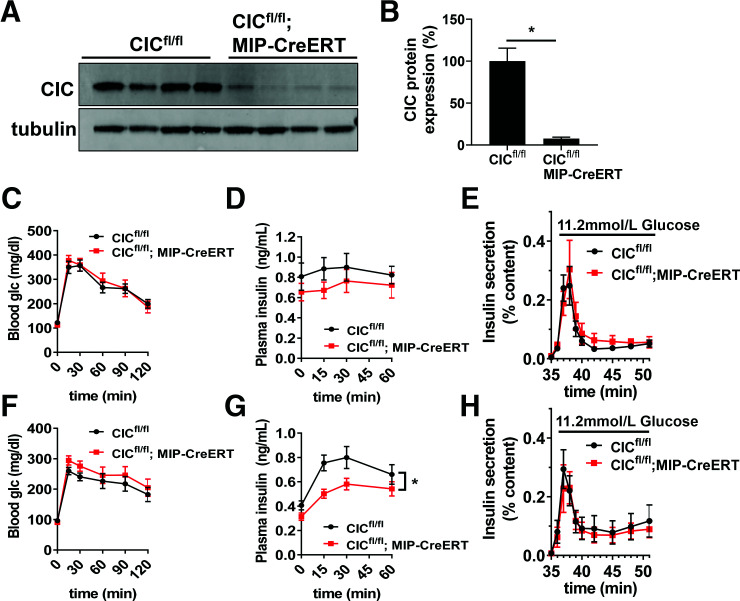
Potentially, early β-cell loss of CIC using the constitutive Ins1-Cre driver may lead to compensatory changes that maintain normal β-cell function in the absence of CIC. To test this, we generated an additional mouse line using homozygous CIC floxed mice expressing the tamoxifen-inducible MIP-CreERT transgene (CICfl/fl; MIP-CreERT). Using this approach, islets isolated from tamoxifen-treated CICfl/fl; MIP-CreERT mice had an ∼90% loss of CIC protein expression (Fig. 4A and B and Supplementary Fig. 1C and D) compared with tamoxifen-treated CICfl/fl control mice, which is similar to knockdown levels previously used to examine CIC function in rat islets (22). Similar to our findings with the constitutive β-cell KO of CIC (Fig. 1D and G), tamoxifen-induced β-cell KO of CIC had no impact on glucose tolerance in either male or female mice compared with control mice (Fig. 4C and F). While no difference in plasma insulin levels in response to glucose challenge was seen in male β-cell CIC KO mice using the inducible Cre model (Fig. 4D), female β-cell CIC KO mice showed a modest reduction in circulating insulin (Fig. 4G) following glucose injection. Note that we did not observe any effects of the MIP-CreERT transgene compared with wild-type mice with respect to glucose tolerance and plasma insulin levels in our lines (Supplementary Fig. 2) in accordance with previous observations (32). We next examined GSIS ex vivo in isolated islets by perifusion from tamoxifen-treated CICfl/fl; MIP-CreERT and CICfl/fl control mice. Consistent with our data using the constitutive Ins1-Cre KO model (Fig. 1), adult (tamoxifen-induced) β-cell KO of CIC had no direct effect on GSIS in either male or female mice (Fig. 4E and H). Finally, we also assessed the specificity of the CIC inhibitor BTC previously used to examine the contribution of CIC to β-cell function (22). We demonstrated that both control (CICfl/fl) and β-cell CIC KO (CICfl/fl; MIP-CreERT) mice were equally sensitive to BTC-mediated inhibition of GSIS (Supplementary Fig. 3), suggesting that the actions of BTC on β-cell function operate independently of CIC, as previously suggested (33). Overall, our data, using two different Cre lines, demonstrate that CIC is not required for islet β-cell function.
Figure 4.
Adult-inducible β-cell–specific deletion of CIC does not impair β-cell function. CICfl/fl; MIP-CreERT vs. CICfl/fl mice were analyzed 4–6 weeks after tamoxifen (i.p. injection). A: Isolated islets were analyzed for protein expression by immunoblot. B: CIC protein expression was normalized to tubulin and expressed as percent relative to CICfl/fl controls (n = 4). Four- to 6-h fasted male (C and D) (n = 16) and female (F and G) (n = 16) mice (12–16 weeks of age) were injected with glucose 1.5 mg/g body wt i.p. Blood glucose (C and F) and plasma insulin (D and G) were recorded at the indicated times. Isolated islets from male (E) (n = 8) and female (H) (n = 9) mice were perifused for 32 min at basal (2.5 mmol/L) glucose followed by stimulatory glucose (11.2 mmol/L). Data are mean ± SEM. *P < 0.05 by two-tailed unpaired t test (B) or repeated-measures two-way ANOVA (C–H). glc, glucose.
Glucose Amplification of Insulin Secretion Is Maintained in β-Cell CIC KO Islets
Insulin secretion can be divided between the KATP-dependent triggering phase and the KATP-independent amplifying phase (1,2,10). Mitochondrial citrate/isocitrate export is largely thought to amplify insulin secretion during the KATP-independent phase of glucose stimulation (11,12). To first test the contribution of CIC to the KATP-dependent phase of insulin secretion, we simulated ATP-mediated suppression of KATP channels via direct membrane depolarization using a train of KCl stimulations at basal glucose followed by rest periods (12 min) of basal glucose alone. As shown in Fig. 5A, no difference was observed in response to three consecutive KCl challenges in islets isolated from adult β-cell CIC KO animals (CICfl/fl; MIP-CreERT) compared with controls (CICfl/fl). To examine insulin release independent of the KATP channel, we used diazoxide to prevent KATP channel closure in the presence of stimulatory glucose and elevated KCl. In these studies, we also observed no difference in stimulated insulin release between adult β-cell CIC KO (CICfl/fl; MIP-CreERT) and control (CICfl/fl) islets (Fig. 5B). We next examined whether augmenting metabolic activity via activation of pyruvate kinase M2 using TEPP46 (34) could unveil differences in insulin secretion in CIC KO animals. As shown in Fig. 5C, TEPP46 enhanced GSIS to similar levels in islets from inducible β-cell CIC KO and control mice.
Figure 5.
Stimulated insulin secretion is maintained in CIC KO mice. A–D: Islets were isolated from CICfl/fl; MIP-CreERT vs. CICfl/fl mice 4–6 weeks after tamoxifen (14–20 weeks of age) or CICfl/fl; Ins1-Cre vs. CICfl/fl mice (10–20 weeks of age), as indicated. A–C: Insulin secretion profiles of perifused islets following stabilization at basal (2.5 mmol/L) glucose are shown. Islets were stimulated for 12 min with KCl (35 mmol/L) at basal (2.5 mmol/L) glucose followed by 12 min at basal glucose in the absence of KCl and repeated twice (A) (n = 3–4); a cocktail containing 11.2 mmol/L glucose, 100 μmol/L diazoxide (Dz), and 35 mmol/L KCl (B) (n = 5–7); and 11.2 mmol/L glucose with or without TEPP46 (C) (n = 7–8). D: mRNA expression levels were determined by qRT-PCR (n = 4–6). E: Isolated islets expressing the Apollo-NADP+ sensor were cultured at basal glucose (1 mmol/L) for 1 h and sequentially treated with the indicated glucose concentration for 5 min each followed by diamide. Milli-anisotropy values were collected by two-photon microscopy and normalized to diamide treatment (12–18 cells per mouse, n = 3 mice per genotype). F: Isolated islets expressing Grx1-roGFP2 were cultured at basal glucose (2.5 mmol/L) for 30 min followed by stimulatory glucose (20 mmol/L), DTT (5 mmol/L), and diamide (5 μmol/L) for 12 min each. Ratiometric data were collected by confocal imaging and GSSG/GSH ratio normalized to DTT (0%) and diamide (100%) (20–40 cells imaged per condition, n = 5 mice per genotype). Data are mean ± SEM. *P < 0.05 by two-way ANOVA. ACLY, ATP-citrate lyase; Glc, glucose.
Distinct Effects of β-Cell CIC KO on NADPH Production and GSH Redox
Previous studies have demonstrated a correlation between GSIS and increased levels of cytosolic NADPH/NADP+ (13–16). Mitochondrial export of citrate/isocitrate is thought to be necessary for this process via serving as a substrate for the NADPH-generating cytosolic enzyme Idh1 (11,16,22). We first examined whether β-cell loss of CIC was accompanied by changes in expression of genes that control NADPH production and consumption but observed no differences in islets isolated from β-cell CIC KO (CICfl/fl; Ins1-Cre) versus control (CICfl/fl) mice (Fig. 5D). Next, we examined changes in NADPH/NADP+ levels in response to glucose stimulation by fluorescent anisotropy using the Venus-tagged Apollo sensor previously demonstrated to measure glucose-dependent NADPH flux in situ (31). In our studies, isolated islets were cotreated with recombinant adenoviruses expressing cytomegalovirus-driven Apollo sensor and insulin promoter–driven mCherry to specifically identify β-cells. β-Cells were sequentially treated with increasing glucose concentrations followed by oxidation with diamide for normalization. As shown in Fig. 5E, islet β-cells from control (CICfl/fl) mice demonstrated a strong glucose-dependent increase in NADPH/NADP+, whereas β-cells from the constitutive β-cell CIC KO (CICfl/fl; Ins1-Cre) mice failed to elevate NADPH in response to glucose treatment, consistent with a previous report (22).
The glucose-dependent increase in NADPH/NADP+ is thought to be necessary for GSH reduction via the NADPH-dependent enzyme glutathione reductase (GR) (13–15). On the basis of this, we examined the redox levels of GSSG/GSH in islet β-cells using a recombinant adenovirus expressing the ratiometric Grx1-roGFP2 biosensor under control of a β-cell specific promoter, RIP (15,28,35). In these studies, islet β-cells were sequentially treated with increasing glucose concentrations as indicated followed by reduction with DTT and oxidation with diamide to establish minimum and maximum GSSG/GSH levels, respectively. As shown in Fig. 5F, we observed a rapid glucose-dependent fall in the GSSG/GSH ratio, consistent with a rise in the level of reduced GSH (13,15,28), to similar levels in both control and β-cell CIC KO β-cells. These data suggest that citrate/isocitrate export is not required for the change in GSH redox status in response to glucose stimulation but may be necessary for the observed increase in NADPH/NADP+ levels.
Idh1 Suppression of Insulin Secretion Requires β-Cell CIC
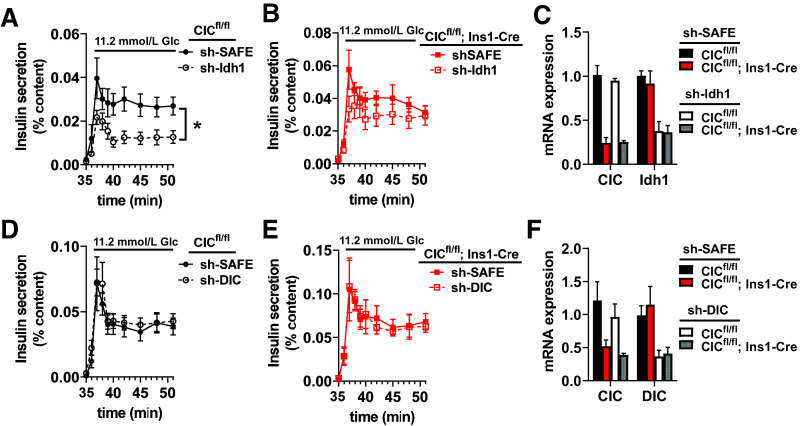
Loss of Idh1 has been shown to impair GSIS and is accompanied by decreased NADPH production and diminished effects on GSH redox status (15,16). Our data indicate that mitochondrial export of the Idh1 substrate isocitrate is not required for GSIS or glucose-dependent changes in GSH redox status. We therefore reasoned that in the absence of CIC, Idh1 may not be required for GSIS. To test this, we generated a recombinant adenovirus expressing an shRNA targeting Idh1 and treated islets isolated from control (CICfl/fl) and β-cell CIC KO (CICfl/fl; Ins1-Cre) mice. Consistent with previous reports (16), a 65% suppression of Idh1 in control islets (expressing CIC) impaired GSIS compared with islets treated with virus expressing a nontargeting shRNA control, shSAFE (Fig. 6A and C). In contrast, islet Idh1 knockdown in the β-cell CIC KO background had no discernible effect on GSIS, with secretion levels comparable to shSAFE controls (Fig. 6B and C). We further explored whether DIC could compensate for CIC loss as an alternative pathway for the efflux of TCA intermediates. As shown in Fig. 6D–F, a 60% shRNA suppression of DIC in either control or β-cell CIC KO islets had no effect on GSIS. Together, these data demonstrate that CIC is not required for insulin secretion and highlight additional complexity in the role of Idh1 in the regulation of β-cell function.
Figure 6.
Idh1 suppression of insulin secretion requires β-cell CIC. Islets were isolated from CICfl/fl; Ins1-Cre vs. CICfl/fl mice (10–20 weeks of age, n = 3–6) and treated with recombinant adenovirus expressing a nontargeting control shRNA (shSAFE), Idh1 shRNA, or DIC shRNA as indicated. A, B, D, and E: Insulin secretion profiles of perifused islets stimulated with 16.7 mmol/L glucose are shown. C and F: qRT-PCR analysis of gene expression is shown. Data are mean ± SEM. from n = 4 mice per group. *P < 0.05 by two-way ANOVA. Glc, glucose.
Discussion
Glucose-derived coupling signals in the form of TCA metabolite exchange between the mitochondria and cytosol and are thought to directly translate rates of TCA flux into amplifying signals for insulin secretion (1,2,9,10). Mitochondrial export of citrate and isocitrate has become a prominent mechanism proposed to provide substrate for either Idh1, which generates cytosolic NADPH, or ATP-citrate lyase, which funnels carbons into de novo lipogenesis, both of which are suggested to support β-cell function (11,12). To date, a single carrier, CIC, regulates mitochondrial citrate and isocitrate export in mammalian tissues. Previous studies using shRNA-mediated suppression and pharmacological inhibition in isolated rat islets and insulinoma cells suggested that CIC played a supporting role in insulin secretion (22). In contrast, administration of a newly developed pharmacological inhibitor of CIC reduces hyperglycemia and improves glucose tolerance in models of diet-induced obesity and fatty liver disease (25). These conflicting observations highlight the need for genetic animal models to better examine the physiological contribution of CIC to islet β-cell function. Our studies using two different β-cell CIC KO mouse lines, constitutive and inducible, demonstrate that β-cell CIC loss fails to negatively impact glucose homeostasis, response to diet-induced obesity, or GSIS in vivo or ex vivo. In sum, our data strongly argue against a significant role for CIC in the regulation of islet β-cell function; however, it should be noted that due to incompleteness of the KO model, remaining CIC expression, albeit low, could be sufficient for the maintenance of β-cell function. Regarding the discrepancies between previously published results using shRNA suppression of CIC (22) and our data presented here using genetic KO models, it is possible that adenoviral effects and/or species-dependent differences (rat vs. mouse models) are contributing factors; however, we note that the inhibitor BTC, which was used in prior studies (22), suppresses insulin secretion independent of β-cell CIC KO, potentially acting on other citrate-regulated metabolic enzymes as previously suggested (33). Taken together, our studies underscore the need for additional physiological examination of potential β-cell amplification pathways emanating from mitochondrial citrate and isocitrate export.
Mouse β-cells typically have significantly lower second phase insulin secretion compared with rat or human β-cells (36–38) and thus may not have a sufficient second phase response to facilitate detection of a CIC-dependent effect on GSIS. To address this, we directly examined KATP-independent insulin secretion and show that the full amplification of glucose-driven insulin secretion can occur in the absence of mitochondrial citrate/isocitrate export. Moreover, additional activation of glucose metabolism via the pyruvate kinase M2 activator, which led to increased insulin secretion, was unable to dissociate control and β-cell CIC KO islets. As a further test, we used diet-induced obesity, which is a well-established model to induce a hyperinsulinemic response from β-cells due to hyperglycemia and underlying insulin resistance. Here again, control and β-cell CIC KO mice had similar phenotypes with respect to glucose tolerance, plasma insulin levels, and ex vivo β-cell function. These data are consistent with the lack of change in CIC expression in human T2D islets; however, the role of CIC in human islets was not examined in our study. Moreover, increased expression of CIC has been observed in INS-1 cells in response to glucolipotoxic stress, indicating that CIC may be negatively associated with β-cell function (39). In support of this, longer duration of diet exposure was less severe in β-cell CIC KO mice, which maintained better glycemic control and trended toward higher plasma insulin levels compared with control animals. Potentially, β-cell CIC KO may be partially protected from the deleterious effects of diet-induced obesity by limiting NADPH production and, thereby, limiting reactive oxygen species generation by NOX2, which is negatively correlated with β-cell function (40).
Increased NADPH production has been correlated with insulin secretion by multiple studies (11,13,15,16) and directly augments β-cell exocytosis under some circumstances (14,15). Cytosolic Idh1 has been suggested to convey rates of TCA flux via generation of cytosolic NADPH using mitochondrially derived isocitrate to amplify insulin secretion (16) (Fig. 6). While a causal relationship between NADPH and insulin secretion is not well established, the generation of GSH reducing equivalents by the NADPH-dependent enzyme GR has been proposed as a key downstream effect (14,15). In support of this, loss of Idh1 negatively impacts insulin secretion, NADPH production, and GSH redox status in response to glucose (16). In the current study, we show that CIC KO β-cells failed to mount a glucose-dependent increase in NADPH/NADP+, confirming that the incremental rise in NADPH upon glucose stimulation likely depends on citrate/isocitrate export and Idh1 activity as previously suggested (11,16,22); however, our data also dissociate a direct role for glucose-stimulated NADPH production as a stimulus for the generation of GSH reducing equivalents and in the amplification of insulin secretion, which were both normal in β-cell CIC KO islets. Consistent with our data, previous observations examining loss of the α-ketoglutarate carrier (2-oxoglutarate carrier [OGC]) showed similar discordance between lack of NADPH production and normal insulin secretion in response to amino acid stimulation (41); however, OGC expression was unaltered in our CIC β-cell KO lines. Potentially, NADPH production may be limited in our β-cell CIC KO islets, but above a threshold level, such that rapid consumption by GR precludes our ability to detect a surplus of NADPH (15,16,22).
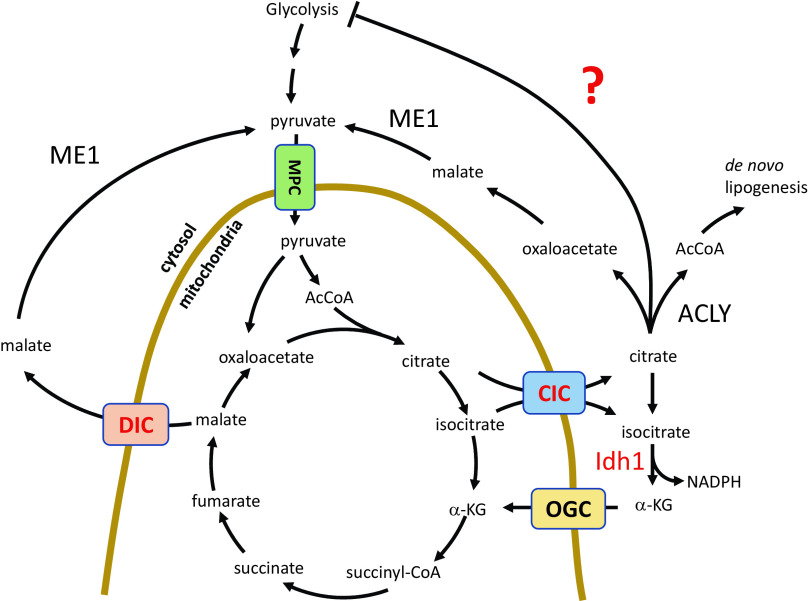
Upon examination of events downstream of CIC, we confirm that Idh1 loss negatively impacts β-cell function as previously described (16); however, our data demonstrate that this phenotype is context dependent (Fig. 7). In the absence of CIC, and presumably the mitochondrially derived Idh1 substrate isocitrate, Idh1 suppression no longer impacts GSIS. On the basis of these data, we propose that Idh1 functions to relieve citrate/isocitrate-mediated inhibition of a glycolytic enzyme to maintain steady rates of glucose metabolic flux rather than Idh1 acting as a direct positive regulator of GSIS as previously suggested (16). Citrate is well established to allosterically inhibit phosphofructokinase-1 (42,43), the rate-limiting step in glycolysis (particularly in the context of increased ATP levels) as well as pyruvate kinase (44,45), which are critical to carbon flow into the mitochondria. Considering that the essential function of the islet β-cell is to rapidly coordinate dose-dependent changes in blood glucose levels with insulin secretion, limiting substrate-dependent allosteric inhibition of glycolysis at two key regulatory nodes would seem to be a critical evolutionary advantage to ensure β-cell responsivity within the full range of physiological glucose levels.
Figure 7.
β-Cell model of mitochondrial metabolism. This model shows the export of TCA-derived citrate and isocitrate via CIC, which has been proposed to regulate insulin secretion following generation of NADPH by Idh1. Our data indicate that CIC is required for NADPH production but not insulin secretion. Furthermore, we suggest that Idh1 conversion of isocitrate to α-ketoglutarate (α-KG) is necessary to prevent a negative feedback of glycolytic flux by (iso)citrate that suppresses insulin secretion. AcCoA, acetyl CoA; ACLY, ATP-citrate lyase; MPC, mitochondrial pyruvate carrier.
The larger question remaining in the field is the identification of glucose-derived amplification signals in the β-cell that sustain insulin secretion. Our data using shRNA suppression and previous studies from ME1 KO mice (17) demonstrate that DIC and decarboxylation by ME1 are unlikely to contribute to GSIS either directly or as a compensatory pathway in the absence of CIC. Furthermore, our data support the notion that de novo lipogenesis is necessary for β-cell function and is clearly distinct from CIC loss. The precise details of how de novo lipogenic pathways contribute to β-cell function remain controversial (46), but on the basis of our data and published work using a pharmacological inhibitor (25), these pathways can operate in the absence, or with very limited, CIC function. The glutamate carrier OGC has also been suggested to regulate GSIS (41,47). While the precise mechanism remains elusive, mitochondrial glutamate export may represent an additional or compensatory pathway operating independently of CIC to supply TCA intermediates for GSIS. Recent studies have highlighted a novel amplifying pathway that is independent of citrate export and NADPH production, termed ADP privation, involving pyruvate kinase and mitochondrial PEP cycling (34,48,49). In this emerging pathway, mitochondria oscillate between an anaplerotic state to generate PEP from malate via mitochondrial PEP carboxykinase and an oxidative phosphorylation state to produce ATP. Key to this pathway is the mitochondrial export of PEP to sustain pyruvate kinase activity. Our data indicate that if the PEP cycle is central to the operative amplification pathway, mitochondrial PEP export into the cytosol does not require either CIC or DIC. These emerging questions highlight the need for continued dissection of key molecular players coordinating glucose sensing and insulin secretion.
Article Information
Acknowledgments. The authors acknowledge the use of the University of Iowa Central Microscopy Research Facility and technical support provided by Thomas Moninger (University of Iowa, Iowa City, IA).
Funding. This work was supported by a Canadian Institutes of Health Research project grant to J.V.R.; National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01-DK-090490 and Department of Veterans Affairs grant I01 BX005107 to Y.I.; NIDDK grant R01-DK-104998 and University of Iowa Pappajohn Biomedical Institute Convergence Pilot Grant to E.B.T.; and startup funds provided by the Fraternal Order of Eagles Diabetes Research Center, University of Iowa, to S.B.S. The authors used human pancreatic islets provided by the NIDDK-funded Integrated Islet Distribution Program at the Beckman Research Institute, City of Hope (2UC4-DK-098085).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.J.B, K.E.R., V.P., S.L., and S.B.S. performed the experiments. C.J.B., K.E.R., J.V.R., Y.I., and S.B.S conceived and designed the studies. C.J.B, C.K.B, and S.B.S. designed and generated constructs. E.B.T. generated the Slc25a1 floxed mouse line. S.B.S. wrote the manuscript. S.B.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14666160.
References
- 1.Henquin JC, Ishiyama N, Nenquin M, Ravier MA, Jonas JC. Signals and pools underlying biphasic insulin secretion. Diabetes 2002;51:S60–S67 [DOI] [PubMed] [Google Scholar]
- 2.Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia 2009;52:739–751 [DOI] [PubMed] [Google Scholar]
- 3.Rorsman P, Renström E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003;46:1029–1045 [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft FM, Harrison DE, Ashcroft SJH. Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells. Nature 1984;312:446–448 [DOI] [PubMed] [Google Scholar]
- 5.Rorsman P, Trube G. Glucose dependent K+-channels in pancreatic beta-cells are regulated by intracellular ATP. Pflugers Arch 1985;405:305–309 [DOI] [PubMed] [Google Scholar]
- 6.Dean PM, Matthews EK. Electrical activity in pancreatic islet cells. Nature 1968;219:389–390 [DOI] [PubMed] [Google Scholar]
- 7.Satin LS, Cook DL. Voltage-gated Ca2+ current in pancreatic B-cells. Pflugers Arch 1985;404:385–387 [DOI] [PubMed] [Google Scholar]
- 8.Pace CS, Tarvin JT, Neighbors AS, Pirkle JA, Greider MH. Use of a high voltage technique to determine the molecular requirements for exocytosis in islet cells. Diabetes 1980;29:911–918 [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Aizawa T, Komatsu M, Okada N, Yamada T. Dual functional role of membrane depolarization/Ca2+ influx in rat pancreatic B-cell. Diabetes 1992;41:438–443 [DOI] [PubMed] [Google Scholar]
- 10.Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest 1992;89:1288–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 2008;295:E1287–E1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prentki M, Matschinsky FM, Madiraju SRM. Metabolic signaling in fuel-induced insulin secretion. Cell Metab 2013;18:162–185 [DOI] [PubMed] [Google Scholar]
- 13.Ivarsson R, Quintens R, Dejonghe S, et al. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 2005;54:2132–2142 [DOI] [PubMed] [Google Scholar]
- 14.Reinbothe TM, Ivarsson R, Li D-Q, et al. Glutaredoxin-1 mediates NADPH-dependent stimulation of calcium-dependent insulin secretion. Mol Endocrinol 2009;23:893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferdaoussi M, Dai X, Jensen MV, et al. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional β cells. J Clin Invest 2015;125:3847–3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronnebaum SM, Ilkayeva O, Burgess SC, et al. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem 2006;281:30593–30602 [DOI] [PubMed] [Google Scholar]
- 17.Ronnebaum SM, Jensen MV, Hohmeier HE, et al. Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucose-stimulated insulin secretion from rodent islets. J Biol Chem 2008;283:28909–28917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai XQ, Plummer G, Casimir M, et al. SUMOylation regulates insulin exocytosis downstream of secretory granule docking in rodents and humans. Diabetes 2011;60:838–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang G-F, Jensen MV, Gray SM, et al. Reductive TCA cycle metabolism fuels glutamine- and glucose-stimulated insulin secretion. Cell Metab 2021; 33:804–817.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klingenberg M. Kinetic study of the tricarboxylate carrier in rat liver mitochondria. Eur J Biochem 1972;26:587–594 [DOI] [PubMed] [Google Scholar]
- 21.Kotaria R, Mayor JA, Walters DE, Kaplan RS. Oligomeric state of wild-type and cysteine-less yeast mitochondrial citrate transport proteins. J Bioenerg Biomembr 1999;31:543–549 [DOI] [PubMed] [Google Scholar]
- 22.Joseph JW, Jensen MV, Ilkayeva O, et al. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem 2006;281:35624–35632 [DOI] [PubMed] [Google Scholar]
- 23.Majd H, King MS, Smith AC, Kunji ERS. Pathogenic mutations of the human mitochondrial citrate carrier SLC25A1 lead to impaired citrate export required for lipid, dolichol, ubiquinone and sterol synthesis. Biochim Biophys Acta Bioenerg 2018;1859:1–7 [DOI] [PubMed] [Google Scholar]
- 24.Nota B, Struys EA, Pop A, et al. Deficiency in SLC25A1, encoding the mitochondrial citrate carrier, causes combined D-2- and L-2-hydroxyglutaric aciduria. Am J Hum Genet 2013;92:627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan M, Mosaoa R, Graham GT, et al. Inhibition of the mitochondrial citrate carrier, Slc25a1, reverts steatosis, glucose intolerance, and inflammation in preclinical models of NAFLD/NASH. Cell Death Differ 2020;27:2143–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wicksteed B, Brissova M, Yan W, et al. Conditional gene targeting in mouse pancreatic β-cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 2010;59:3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haldeman JM, Conway AE, Arlotto ME, et al. Creation of versatile cloning platforms for transgene expression and dCas9-based epigenome editing. Nucleic Acids Res 2019;47:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reissaus CA, Piñeros AR, Twigg AN, et al. A versatile, portable intravital microscopy platform for studying beta-cell biology in vivo. Sci Rep 2019;9:8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephens SB, Edwards RJ, Sadahiro M, et al. The prohormone VGF regulates β cell function via insulin secretory granule biogenesis. Cell Rep 2017;20:2480–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayes HL, Peterson BS, Haldeman JM, Newgard CB, Hohmeier HE, Stephens SB. Delayed apoptosis allows islet β-cells to implement an autophagic mechanism to promote cell survival. PLoS One 2017;12:e0172567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cameron WD, Bui CV, Hutchinson A, Loppnau P, Gräslund S, Rocheleau JV. Apollo-NADP(+): a spectrally tunable family of genetically encoded sensors for NADP(+). Nat Methods 2016;13:352–358 [DOI] [PubMed] [Google Scholar]
- 32.Smith EP, An Z, Wagner C, et al. The role of β cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab 2014;19:1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stucki JW. Influence of 1,2,3-benzene-tricarboxylate on pyruvate metabolism in rat-liver mitochondria. Eur J Biochem 1977;78:183–187 [DOI] [PubMed] [Google Scholar]
- 34.Lewandowski SL, Cardone RL, Foster HR, et al. Pyruvate kinase controls signal strength in the insulin secretory pathway. Cell Metab 2020;32:736–750.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutscher M, Pauleau A-L, Marty L, et al. Real-time imaging of the intracellular glutathione redox potential. Nat Methods 2008;5:553–559 [DOI] [PubMed] [Google Scholar]
- 36.Berglund O. Different dynamics of insulin secretion in the perfused pancreas of mouse and rat. Acta Endocrinol (Copenh) 1980;93:54–60 [DOI] [PubMed] [Google Scholar]
- 37.Blackard WG, Nelson NC. Portal and peripheral vein immunoreactive insulin concentrations before and after glucose infusion. Diabetes 1970;19:302–306 [DOI] [PubMed] [Google Scholar]
- 38.Nunemaker CS, Wasserman DH, McGuinness OP, Sweet IR, Teague JC, Satin LS. Insulin secretion in the conscious mouse is biphasic and pulsatile. Am J Physiol Endocrinol Metab 2006;290:E523–E529 [DOI] [PubMed] [Google Scholar]
- 39.Brun T, Scarcia P, Li N, et al. Changes in mitochondrial carriers exhibit stress-specific signatures in INS-1Eβ-cells exposed to glucose versus fatty acids. PLoS One 2013;8:e82364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li N, Li B, Brun T, et al. NADPH oxidase NOX2 defines a new antagonistic role for reactive oxygen species and cAMP/PKA in the regulation of insulin secretion. Diabetes 2012;61:2842–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Odegaard ML, Joseph JW, Jensen MV, et al. The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion. J Biol Chem 2010;285:16530–16537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kemp RG. Rabbit liver phosphofructokinase. Comparison of some properties with those of muscle phosphofructokinase. J Biol Chem 1971;246:245–252 [PubMed] [Google Scholar]
- 43.Kemp RG, Foe LG. Allosteric regulatory properties of muscle phosphofructokinase. Mol Cell Biochem 1983;57:147–154 [DOI] [PubMed] [Google Scholar]
- 44.Evans CT, Ratledge C. Partial purification and properties of pyruvate kinase and its regulatory role during lipid accumulation by the oleaginous yeast Rhodosporidium toruloides CBS 14. Can J Microbiol 1985;31: 479–484 [Google Scholar]
- 45.Duggleby RG, Dennis DT. Pyruvate kinase, a possible regulatory enzyme in higher plants. Plant Physiol 1973;52:312–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell JE, Newgard CB. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat Rev Mol Cell Biol 2021;22:142–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casimir M, Lasorsa FM, Rubi B, et al. Mitochondrial glutamate carrier GC1 as a newly identified player in the control of glucose-stimulated insulin secretion. J Biol Chem 2009;284:25004–25014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abulizi A, Cardone RL, Stark R, et al. Multi-tissue acceleration of the mitochondrial phosphoenolpyruvate cycle improves whole-body metabolic health. Cell Metab 2020;32:751–766.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stark R, Pasquel F, Turcu A, et al. Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. J Biol Chem 2009;284:26578–26590 [DOI] [PMC free article] [PubMed] [Google Scholar]