Abstract
When stable and near-normoglycemic, patients with “A−β+” ketosis-prone diabetes (KPD) manifest accelerated leucine catabolism and blunted ketone oxidation, which may underlie their proclivity to develop diabetic ketoacidosis (DKA). To understand metabolic derangements in A−β+ KPD patients during DKA, we compared serum metabolomics profiles of adults during acute hyperglycemic crises, without (n = 21) or with (n = 74) DKA, and healthy control subjects (n = 17). Based on 65 kDa GAD islet autoantibody status, C-peptide, and clinical features, 53 DKA patients were categorized as having KPD and 21 type 1 diabetes (T1D); 21 nonketotic patients were categorized as having type 2 diabetes (T2D). Patients with KPD and patients with T1D had higher counterregulatory hormones and lower insulin-to-glucagon ratio than patients with T2D and control subjects. Compared with patients withT2D and control subjects, patients with KPD and patients with T1D had lower free carnitine and higher long-chain acylcarnitines and acetylcarnitine (C2) but lower palmitoylcarnitine (C16)-to-C2 ratio; a positive relationship between C16 and C2 but negative relationship between carnitine and β-hydroxybutyrate (BOHB); higher branched-chain amino acids (BCAAs) and their ketoacids but lower ketoisocaproate (KIC)-to-Leu, ketomethylvalerate (KMV)-to-Ile, ketoisovalerate (KIV)-to-Val, isovalerylcarnitine-to-KIC+KMV, propionylcarnitine-to-KIV+KMV, KIC+KMV-to-C2, and KIC-to-BOHB ratios; and lower glutamate and 3-methylhistidine. These data suggest that during DKA, patients with KPD resemble patients with T1D in having impaired BCAA catabolism and accelerated fatty acid flux to ketones—a reversal of their distinctive BCAA metabolic defect when stable. The natural history of A−β+ KPD is marked by chronic but varying dysregulation of BCAA metabolism.
Introduction
Ketosis-prone diabetes (KPD) is a heterogeneous form of “atypical” diabetes characterized by presentation with diabetic ketoacidosis (DKA) in patients lacking key features of autoimmune type 1 diabetes (T1D) (1–7). A validated “Aβ” classification scheme defines four subgroups, based on presence or absence of islet autoantibodies and presence or absence of β cell functional reserve, measured 2–4 months following the index episode of DKA, when the patients are clinically stable (1,3). The “β+” subgroups of KPD have substantial β cell functional reserve at that time, and the majority can discontinue insulin therapy while maintaining glycemic control (3,7).
We previously showed that unique defects in metabolic pathways involving BCAA underlie the proclivity of A−β+ KPD patients (who otherwise resemble patients with obesity-associated type 2 diabetes [T2D]), to develop DKA (8,9). A confluence of data from metabolomics and kinetic tracer studies in these patients when stable, near normoglycemic, and off insulin therapy delineated a pathogenic sequence of accelerated catabolism of the ketogenic amino acid leucine with increased ketone production, coupled with decreased lipolysis and fatty acid utilization for energy production. Tricarboxylic acid cycle (TCA) slowing due to altered anaplerosis at α-ketoglutarate (9) also impaired ketone oxidation, resulting in elevated plasma β-hydroxybutyrate (BOHB).
These investigations were performed months after the patients had completely recovered from the index DKA episode and were clinically stable. We hypothesized that if the defective pathways were immutable, A−β+ KPD patients might display their distinctive plasma metabolomics signatures even during acute presentation with DKA. Moreover, since the putative metabolic defects associated with ketosis in KPD appear unrelated to autoimmune β cell destruction or severely reduced insulin secretory capacity, they might manifest relatively preserved β cell function even at the time of DKA. To address these hypotheses, we investigated adult patients at the time of presentation with hyperglycemic crisis, both with and without DKA, in the emergency center of Ben Taub General Hospital.
Research Design and Methods
Study Participants
The study was approved by the Institutional Review Boards for Human Studies at Baylor College of Medicine and the Harris County Hospital District. Adult patients, sequentially admitted from 2015 to 2018 to the Ben Taub General Hospital emergency center, were recruited if they presented with acute hyperglycemic crisis, defined as an admission plasma glucose level ≥250 mg/dL with evidence of clinical decompensation. DKA was diagnosed if the patient also had serum BOHB >1.5 mmol/L, anion gap >12, venous pH ≤7.3, or serum total CO2 ≤15 mEq/L. A total of 102 patients were recruited and provided informed consent; 74 were diagnosed with DKA and 28 did not have DKA. Of the 74 DKA patients, 53 met criteria for A−β+ KPD based on absence of the 65 kDa GAD islet autoantibody (GAD65Ab), as well as significantly higher mean C-peptide, BMI, and age compared with those with DKA who were GAD65Ab positive. Twenty-one DKA patients were GAD65Ab positive and categorized as having T1D. Of the 28 hyperglycemic patients without DKA, 21 were GAD65Ab negative and categorized as having T2D. The remaining seven hyperglycemic patients without DKA were GAD65Ab positive and removed from the analysis because of small sample size.
Nondiabetic obese controls were recruited through advertisement. Selection criteria included age 18–65 years, fasting plasma glucose <100 mg/dL, HbA1c <5.6%, BMI >25 kg/m2, and good health, with no evidence of chronic or acute illness as assessed through medical history review, a physical exam, a complete blood count, a comprehensive metabolic panel, and liver function tests.
Sample Collection and Laboratory Analyses
Potential study participants were approached after diagnosis of acute hyperglycemic crisis in the emergency center, and blood samples were drawn immediately into prechilled tubes for those who provided informed consent. Blood samples for healthy control subjects were drawn into prechilled tubes containing heparin. Samples were immediately centrifuged at 4°C, and the serum/plasma was removed and stored at −70°C.
Serum insulin, C-peptide, and cortisol concentrations were measured by immunoassay (Cobas e411 analyzer; Roche Diagnostics, Indianapolis, IN), growth hormone and glucagon by ELISA (R&D, Minneapolis, MN), and epinephrine and norepinephrine by ELISA (Abnova, Taipei, Taiwan). Serum glucose concentration was measured by the glucose oxidase method (YSI 2300 STAT PLUS Glucose and Lactate Analyzer; YSI, Yellow Springs, OH), and BCAA and glutamate concentrations were measured by ultra-high-performance liquid chromatography (Waters Corporation, Milford, MA) using precolumn derivatization with 6-amino-quinolyl-N-hydroxysuccinimidyl carbamate.
Concentrations of all other metabolites were determined by the isotope dilution technique using stable isotope labeled compounds (Cambridge Isotope Laboratories, Inc., Andover, MA) as internal standards and mass spectrometric analysis. Serum 3-methylhistidine (3-MH) concentration was measured using [methyl-2H3]3-MH as the internal standard. 3-MH in the samples was converted into its 5-dimethylamino-1-napthalene sulfonamide derivative and then analyzed by liquid chromatography–tandem mass spectroscopy (LC-MS/MS) (Thermo Scientific TSQ Vantage; Thermo Fisher Scientific, San Jose, CA). Serum concentrations of the branched-chain ketoacids (BCKA) ketoisocaproate (KIC), ketoisovalerate (KIV), and ketomethylvalerate (KMV) were measured with use of [13C6]KIC, [13C5]KIV, and [13C6]KMV as internal standards. The samples were converted into their pentafluorobenzyl derivatives and analyzed by gas chromatography–mass spectrometry (6890N/5973B; Agilent Technologies, Wilmington, DE) in negative chemical ionization mode. Serum free carnitine and acylcarnitine concentrations were measured using internal standards (deuterated carnitine standards set B [NSK-B]). Isotopic analysis was performed by LC-MS/MS (TSQ Vantage).
Statistical Analysis
All values are reported as mean ± SE. For all normally distributed data, between-group comparisons were made with one-way ANOVA with Tukey post hoc test. Because insulin was administered to some participants before blood was drawn for the study, insulin concentrations were not normally distributed; for insulin and any other data not normally distributed, nonparametric (Kruskal-Wallis test) statistical analysis was performed with post hoc Dunn multiple comparisons. Two-factor multiple regression was performed to determine possible effects of insulin therapy on biochemical, hormonal, and metabolomics measures. Nonpaired t test was used to determine differences in demographic, anthropometric, and biochemical characteristics between patients with KPD and patients with T1D. Associations between and among metabolomics data were sought using Spearman correlation. P < 0.05 was considered significant for all outcome measures. All analyses were performed with GraphPad Prism, version 9 (GraphPad Software, San Diego, CA).
Data and Resource Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. No applicable resources were generated or analyzed during the current study.
Results
Demographic, anthropometric, and biochemical characteristics of all participants are presented in Table 1. As is characteristic of A−β+ KPD, the KPD patients had significantly higher mean C-peptide level and BMI and were older than the T1D patients. Fifteen of the patients with DKA (12 KPD, 3 T1D) also had new-onset diabetes. Overweight/obesity was present in 100% of control subjects and 91% of T2D, 55% of KPD, and 33% of T1D patients. T2D patients were significantly older than KPD and T1D patients and control subjects. There were more men than women, and more African American and Hispanic than White individuals, in all groups.
Table 1.
Demographic, anthropometric, and biochemical characteristics of groups
| Control subjects | T2D patients with hyperglycemia | KPD patients with DKA | T1D patients with DKA | |
|---|---|---|---|---|
| N | 17 | 21 | 53 | 21 |
| Age (years) | 34.7 ± 2.53 | 50.7 ± 2.48a | 41.7 ± 1.8b | 34.3 ± 3.4bc |
| Women/men | 4/13 | 12/9 | 19/34 | 9/12 |
| Ethnicity (AA/C/H) | 8/1/8 | 7/3/11 | 16/9/28 | 10/2/9 |
| BMI (kg/m2) | 33.5 ± 0.9 | 32.3 ± 1.8 | 27.2 ± 0.9ab | 23.6 ± 1.3abc |
| Overweight or obese (%) | 100 | 90.5 | 54.7 | 33.3 |
| Glucose (mg/dL) | 89 ± 2.2 | 540 ± 36a | 402 ± 30ab | 413 ± 37a |
| Lactate (mmol/L) | 1.0 ± 0.10 | 3.32 ± 0.34a | 2.20 ± 0.19ab | 2.53 ± 0.27a |
| Venous pH | n/a | 7.36 ± 0.01 | 7.23 ± 0.02b | 7.23 ± 0.03b |
| BOHB (mmol/L) | n/a | 0.57 ± 0.1 | 7.3 ± 0.5b | 7.5 ± 0.8b |
| Total carbon dioxide (mmol/L) | 22.4 ± 0.7 | 23.6 ± 1.2 | 15.4 ± 0.8ab | 14.1 ± 1.3ab |
| Anion gap (mEq/L) | n/a | 14.4 ± 1.1 | 16.9 ± 0.6 | 18.6 ± 1.4b |
| New-onset diabetes, n (%) | — | 0 | 12 (23) | 3 (14) |
| BUN (mg/dL) | 11.9 ± 0.6 | 21.5 ± 3.9 | 22.8 ± 2.9 | 22.9 ± 2.3 |
| Creatinine (mg/dL) | 0.9 ± 0.04 | 1.13 ± 0.15 | 1.08 ± 0.15 | 1.04 ± 0.1 |
| Hemoglobin (g/dL) | 14.5 ± 0.4 | 12.8 ± 0.6 | 14.4 ± 0.3b | 14.3 ± 0.4 |
| Albumin (g/dL) | 4.4 ± 0.06 | 3.5 ± 0.3a | 3.6 ± 0.11a | 3.8 ± 0.18 |
| AST (IU/L) | 22.9 ± 1.9 | 34.5 ± 5.6 | 27.1 ± 3.8 | 20.9 ± 2.9 |
| ALT (IU/L) | 28.8 ± 4.5 | 41.1 ± 6.6 | 37.3 ± 4.5 | 27.7 ± 3.6 |
| Total bilirubin (mg/dL) | 0.44 ± 0.06 | 0.56 ± 0.11 | 0.66 ± 0.05 | 0.74 ± 0.11 |
| Triglyceride (mg/dL) | 116 ± 19 | 257 ± 59a | 174 ± 16 | 146 ± 25 |
| HDL-C (mg/dL) | 48.3 ± 3.2 | 47.3 ± 5.9 | 52.0 ± 3.2 | 58.5 ± 5.7 |
Data are means ± SEM or absolute numbers unless otherwise indicated. AA, African American; BUN, blood urea nitrogen; C, Caucasian; HDL-C, HDL cholesterol; H, Hispanic; n/a, not available.
Value significantly different from that for control subjects (P < 0.05, one-way ANOVA with Tukey post hoc test).
Value significantly different from that for T2D group (P < 0.05, one-way ANOVA with Tukey post hoc test).
Value significantly different from that for KPD group (P < 0.05, nonpaired t test).
Due to the acuity of their presentation at the emergency center, 17 of the T2D patients, 38 of the KPD patients, and 13 of the T1D patients received a bolus injection of insulin with or without an insulin infusion before a blood sample was drawn for the study (median delay = 113 min). With the exception of serum lactate concentration, there was no statistically significant difference in any biochemical characteristic between patients who received or did not receive insulin before the blood sample was drawn. Glucose and lactate concentrations were significantly higher in all groups with diabetes compared with control subjects, and both were significantly lower in KPD compared with those in T2D patients. Venous pH and total CO2 were significantly lower while BOHB and anion gap were significantly higher in the KPD and T1D groups compared with those in T2D patients. There was no difference in serum creatinine or calculated estimated glomerular filtration rate (not shown) between the groups. Hemoglobin was significantly lower in T2D than in KPD patients, albumin was significantly lower in T2D and KPD patients than in control subjects, and triglycerides were significantly higher in T2D patients than in control subjects.
Hormonal profiles are presented in Table 2. Insulin concentration was significantly lower in KPD and T1D patients who did not receive insulin compared with the value in control subjects, but it was not different between T2D patients who did not receive insulin and control subjects. Insulin concentration of the T1D group who did not receive insulin was also significantly lower than the corresponding value of the T2D group. Mean serum C-peptide concentration of all KPD patients was significantly higher than that of all T1D patients. Only 13 of the 53 KPD patients (25%) had serum C-peptide concentrations below the normal range. There was no difference in C-peptide concentration between control subjects and T2D patients regardless of whether they received insulin therapy, and C-peptide levels in these two groups were higher than those of both KPD and T1D patients, regardless of whether the latter received insulin therapy.
Table 2.
Hormonal profile of groups
| T2D patients with hyperglycemia | KPD patients with DKA | T1D patients with DKA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control subjects | All | No insulin Rx* | Insulin Rx** | All | No insulin Rx* | Insulin Rx** | All | No insulin Rx* | Insulin Rx** | |
| N | 17 | 21 | 7 | 14 | 53 | 15 | 38 | 21 | 8 | 13 |
| Insulin (µU/mL) | 13.1 ± 1.7 | 135 ± 70a | 23.3 ± 8 | 190 ± 102a | 111 ± 38 | 10.3 ± 5.6a | 151 ± 52a | 54 ± 13 | 3.2 ± 1.6ab | 85 ± 16a |
| C-peptide (ng/mL) | 2.6 ± 0.2 | 3.8 ± 0.4 | 4.2 ± 0.7 | 3.5 ± 0.6 | 1.1 ± 0.2ab | 1.5 ± 0.5ab | 0.94 ± 0.1ab | 0.34 ± 0.1abc | 0.48 ± 0.3ab | 0.26 ± 0.1ab |
| Glucagon (pg/mL) | 52 ± 3.9 | 125 ± 19a | 105 ± 31 | 135 ± 24a | 117 ± 29 | 123 ± 33 | 114 ± 38 | 69 ± 9 | 78 ± 18 | 63 ± 10b |
| Cortisol (pg/mL) | 279 ± 32 | 386 ± 61 | 345 ± 99 | 406 ± 78 | 752 ± 72ab | 742 ± 128a | 755 ± 88a | 856 ± 114ab | 858 ± 166a | 855 ± 158ab |
| Epinephrine (nmol/L) | 0.23 ± 0.05 | 0.16 ± 0.04 | 0.24 ± 0.06 | 0.12 ± 0.04 | 0.71 ± 0.2ab | 0.6 ± 0.1 | 0.75 ± 0.2b | 0.82 ± 0.3ab | 0.56 ± 0.13 | 1.0 ± 0.5b |
| Norepinephrine (nmol/L) | 1.6 ± 0.3 | 4.5 ± 0.9 | 4.3 ± 0.9 | 4.6 ± 1.2 | 10.3 ± 1.5ab | 9.8 ± 2.3a | 10.5 ± 1.9a | 12.1 ± 1.8ab | 11.5 ± 3a | 12.4 ± 2ab |
| Growth hormone (ng/mL) | 0.09 ± 0.04 | 2.8 ± 0.9a | 1.2 ± 1.1 | 3.5.2a | 3.0 ± 0.6a | 3.7 ± 1.4a | 2.5 ± 0.6a | 8.3 ± 3.6ab | 3.9 ± 0.9ab | 11.2 ± 5.9a |
Data are means ± SEM. Data were analyzed by one-way ANOVA with Tukey post hoc test. When data were not normally distributed they were analyzed by nonparametric (Kruskal-Wallis test) with post hoc Dunn multiple comparisons. Rx, prescription.
Blood sampled before insulin administration.
Blood sampled after insulin administration.
Value significantly different from that for control subjects (P < 0.05).
Value significantly different from that for T2D group (P < 0.05).
Value significantly different from that for KPD group (P < 0.05).
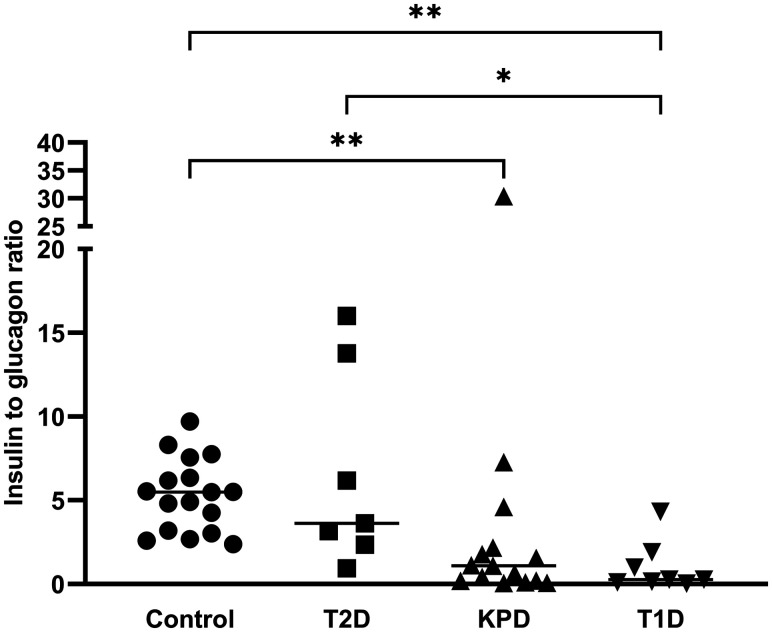
Serum glucagon concentration was significantly higher in all T2D patients and in those who received insulin than in control subjects. There was high variability in individual glucagon levels among KPD and T1D patients; hence, the difference from that of the control subjects was not statistically significant. The insulin-to-glucagon ratios (I/G ratios) of control subjects and all patients with diabetes who did not receive insulin therapy are presented in Fig. 1. There was no difference between the I/G ratios of T2D patients and control subjects. Similarly, there was no difference between the I/G ratios of KPD and T1D patients. However, both the KPD and T1D groups had significantly lower I/G ratios compared with those of control subjects, and T1D patients also had significantly lower I/G ratios than T2D patients.
Figure 1.
Individual serum I/G ratios of nondiabetic control subjects (Control), T2D patients in hyperglycemic crisis, KPD patients with DKA, and T1D patients with DKA. None of these patients received insulin therapy prior to blood collection. Median value is expressed as a line for each group. Data were analyzed by nonparametric one-way ANOVA (Kruskal-Wallis test) with post hoc Dunn multiple comparisons (*P < 0.05; **P < 0.01).
While there was no difference in serum cortisol, epinephrine, and norepinephrine concentrations between control subjects and T2D patients, the serum concentrations of these three hormones were significantly higher in both KPD and T1D patients than in control subjects and T2D patients. Serum growth hormone concentration was significantly higher in all three diabetes groups than in control subjects. It was also significantly higher in all T1D patients and in those who did not receive insulin compared with the corresponding values of the T2D group.
Metabolomics Data
Because there was no statistical difference in any metabolomics measurement based on whether patients with diabetes received insulin prior to blood sampling, their data were combined into a single group. Serum concentrations of BCAA and related metabolites are presented in Table 3. Serum leucine, isoleucine, and valine concentrations were significantly higher in KPD and T1D patients than in control subjects and T2D patients. Serum concentrations of the three BCKAs (KIC, KMV, and KIV) were also significantly higher in T1D patients compared with control subjects. Although all three BCKAs trended higher in the T2D and KPD groups, only KIV concentration was significantly higher than in the control subjects. However, the sum of the three BCKAs was significantly higher in both T1D and KPD patients than in control subjects.
Table 3.
Serum concentrations of BCAAs and related metabolites of groups
| Control subjects | T2D patients with hyperglycemia | KPD patients with DKA | T1D patients with DKA | |
|---|---|---|---|---|
| N | 17 | 21 | 53 | 21 |
| BCAA (µmol/L) | ||||
| Leucine | 119 ± 4.28 | 130 ± 10.6 | 201 ± 14.4ab | 236 ± 22.6ab |
| Isoleucine | 63.3 ± 2.26 | 75.6 ± 4.80 | 108 ± 6.73ab | 127 ± 12.4ab |
| Valine | 227 ± 9.07 | 231 ± 15.0 | 340 ± 20.0ab | 373 ± 30.5ab |
| Total | 410 ± 14.7 | 436 ± 28.9 | 648 ± 40.4ab | 736 ± 64.8ab |
| Ketoacids (µmol/L) | ||||
| KIC | 32.7 ± 1.58 | 39.9 ± 3.85 | 42.8 ± 1.83 | 56.9 ± 5.54a |
| KMV | 19.3 ± 0.677 | 24.6 ± 2.01 | 24.8 ± 1.02 | 33.6 ± 3.26a |
| KIV | 6.15 ± 0.285 | 7.83 ± 0.514a | 8.17 ± 0.279a | 9.24 ± 0.680a |
| Total | 58.2 ± 2.45 | 73.3 ± 6.14 | 75.8 ± 2.98a | 99.7 ± 9.34a |
| BCKA-to-BCCA ratios | ||||
| KIC-to-Leu ratio | 0.276 ± 0.010 | 0.306 ± 0.019 | 0.243 ± 0. 015b | 0.264 ± 0.022 |
| KMV-to-Ile ratio | 0.308 ± 0.011 | 0.328 ± 0.020 | 0.266 ± 0.014 ab | 0.286 ± 0.023 |
| KIV-to-Val | 0. 027 ± 0.001 | 0. 034 ± 0.001a | 0. 027 ± 0.002b | 0.027 ± 0.003b |
| Acylcarnitines (nmol/L) | ||||
| C5 | 108 ± 6.09 | 167 ± 25.4 | 167 ± 35.1 | 160 ± 25.8 |
| C3 | 461 ± 43.5 | 641 ± 114 | 486 ± 77.6 | 528 ± 68.1 |
| Acylcarnitine-to-BCKA ratios ×103 | ||||
| C5-to-KIC ratio | 3.35 ± 0.18 | 4.39 ± 0.50 | 3.78 ± 0.63b | 2.80 ± 0.42b |
| C5-to-KMV ratio | 5.65 ± 0.31 | 6.91 ± 0.81 | 6.57 ± 1.13 | 4.76 ± 0.67 |
| C5-to-KIC+KMV ratio | 2.10 ± 0.11 | 2.61 ± 0.26 | 2.39 ± 0.40b | 1.76 ± 0.26b |
| C3-to-KMV ratio | 24.4 ± 2.61 | 28.2 ± 5.36 | 19.7 ± 2.34 ab | 16.4 ± 1.78a |
| C3-to-KIV ratio | 77.7 ± 8.42 | 85.1 ± 13.9 | 58.5 ± 7.18ab | 57.0 ± 7.08 |
| C3-to-KMV+KIV ratio | 18.5 ± 1.98 | 21.0 ± 3.84 | 14.6 ± 1.75ab | 12.4 ± 1.33a |
| Other related ratios | ||||
| KIC+KMV-to-C2 ratio | 8.83 ± 0.68 | 6.19 ± 0.58 | 3.26 ± 0.28ab | 3.57 ± 0.31ab |
| KIC-to-BOHB ratio | n/a | 0.184 ± 0.068 | 0.007 ± 0.001b | 0.011 ± 0.002b |
Data are means ± SEM. Because data were not normally distributed, nonparametric one-way ANOVA (Kruskal-Wallis test) was performed with post hoc Dunn multiple comparisons.
Value significantly different from that of control subjects (P < 0.05);
Value significantly different from that of T2D group (P < 0.05). n/a, not applicable.
The ratio of each BCKA to its precursor BCAA serves as an index of its rate of formation by transamination. Compared with those of control subjects, KIC-to-Leu, KMV-to-Ile and KIV-to-Val ratios trended higher in T2D but lower in KPD and T1D patients. However, only KIV-to-Val ratio was significantly higher in T2D patients and KMV-to-Ile ratio was lower in KPD patients than in control subjects. KIC-to-Leu, KMV-to-Ile, and KIV-to-Val ratios were also lower in KPD than in T2D patients, and KIV-to-Val ratio was also lower in T1D than in T2D patients.
There were no group differences in concentrations of 2-methylbutyryl and isovalerylcarnitine (C5) and propionylcarnitine (C3) acylcarnitine esters derived from BCKAs. The ratio of each acylcarnitine to its precursor BCKA was used as an index of its rate of entry and metabolism within mitochondria. Compared with those of control subjects, all ratios trended higher in T2D and lower in KPD and T1D patients. In the KPD group, serum C3-to-KIV, C3-to-KMV, and C3-to-KIV+KMV ratios were lower than in both control subjects and T2D patients, while C5-to-KIC and C5-to-KIC+KMV ratios were lower than in T2D patients. In the T1D group, serum C3-to-KMV and C3-to-KIV+KMV were lower than in control subjects, while C5-to-KIC and C5-to-KIC+KMV were lower than in T2D patients. Since both KIC and KMV can be metabolized to acetyl CoA, and KIC also can be converted to acetoacetate (which is then quantitatively reduced to BOHB during DKA), the ratios of KIC+KMV to acetylcarnitine (C2) and KIC to BOHB were used as indicators of the relative conversions of the respective precursors to acetyl CoA and acetoacetate. KIC+KMV-to-C2 ratio was lower in KPD and T1D patients than in control subjects and T2D patients. Similarly, KIC-to-BOHB ratio was markedly lower in KPD and T1D than in T2D patients.
We used plasma free carnitine and acylcarninite concentrations and ratios as indices of β-oxidation of fatty acids based on their utility as biomarkers of fatty acid metabolism in conditions of either accelerated or impaired lipid metabolism (10), such as T2D, DKA, obesity, insulin resistance, HIV infection, and inborn errors of fatty acid oxidation (11). Though acylcarnitines are formed intracellularly in all organs and tissues except brain, there is increased efflux to the plasma compartment and excretion in the urine during conditions of increased lipolysis, possibly to prevent CoA trapping (thereby allowing maintenance of CoA-dependent metabolic processes [12,13]), and also possibly serving as a detoxification process (14). Most relevant to the current study, patients with DKA are reported to manifest a striking change in the distribution of plasma free carnitine and acylcarnitines, as free carnitine is markedly reduced and the plasma concentration of acylcarnitines, particularly short-chain acyl-carnitines, is elevated in comparison with healthy control subjects—and this, in parallel with plasma BOHB, promptly returns to normal on insulin treatment (15). Serum free carnitine concentrations were markedly lower in KPD and T1D patients than in both control subjects and T2D patients (Table 4). The opposite occurred for palmitoylcarnitine (C16) and myristoylcarnitine (C14) concentrations, which were higher in KPD and T1D patients than in control subjects and T2D patients. There were no group differences in octanoylcarnitine (C8) concentrations, but C2 was markedly higher in KPD and T1D patients than in both control subjects and T2D patients. With “longer” to “shorter” chain fatty acid–derived acylcarnitines, expressed as ratios, there were no group differences in C16-to-C14, C16-to-C8, or C14-to-C8 ratios, but C16-to-C2, C14-to-C2, and C8-to-C2 ratios were significantly lower in all groups with diabetes in comparisons with control subjects.
Table 4.
Serum carnitine, acylcarnitines, and acylcarnitine ratios of groups
| Control subjects | T2D patients with hyperglycemia | KPD patients with DKA | T1D patients with DKA | |
|---|---|---|---|---|
| N | 17 | 21 | 52 | 21 |
| Carnitine (µmol/L) | 27.0 ± 1.9 | 25.0 ± 2.4 | 14.9 ± 1.7ab | 15.1 ± 1.8ab |
| Acylcarnitine from fatty acids (µmol/L) | ||||
| C16 | 0.072 ± 0.005 | 0.075 ± 0.009 | 0.129 ± 0.009ab | 0.148 ± 0.018ab |
| C14 | 0.020 ± 0.003 | 0.019 ± 0.003 | 0.038 ± 0.005ab | 0.055 ± 0.011ab |
| C8 | 0.134 ± 0.019 | 0.147 ± 0.031 | 0.228 ± 0.032 | 0.236 ± 0.040 |
| C2 | 6.4 ± 0.46 | 13.4 ± 2.7 | 27.2 ± 2.7ab | 26.8 ± 2.3ab |
| Acylcarnitine ratios | ||||
| C16-to-C14 ratio | 3.9 ± 0.19 | 4.4 ± 0.32 | 3.5 ± 0.32 | 4.3 ± 0.26 |
| C16-to-C8 ratio | 0.67 ± 0.08 | 0.89 ± 0.16 | 0.84 ± 0.11 | 1.01 ± 0.11 |
| C16-to-C2 ratio | 0.012 ± 0.001 | 0.007 ± 0.001a | 0.006 ± 0.001a | 0.006 ± 0.001a |
| C14-to-C8 ratio | 0.182 ± 0.031 | 0.199 ± 0.028 | 0.254 ± 0.033 | 0.266 ± 0.047 |
| C14-to-C2 ratio | 0.003 ± 0.00 | 0.002 ± 0.000a | 0.002 ± 0.000a | 0.002 ± 0.000a |
| C8-to-C2 ratio | 0.022 ± 0.004 | 0.011 ± 0.001a | 0.009 ± 0.001a | 0.009 ± 0.001a |
Data are means ± SEM. Because data were not normally distributed, nonparametric one-way ANOVA (Kruskal-Wallis test) was performed with post hoc Dunn multiple comparisons.
Value significantly different from that of control subjects (P < 0.05).
Value significantly different from that of T2D group (P < 0.05).
Significant correlations between and among serum carnitine and BCAA-derived acylcarnitines are presented in Table 5. In all groups, there were significant relationships between serum carnitine and C5. There were also significant relationships between carnitine and C3 in all diabetes groups and between C3 and C5 in patients with T2D.
Table 5.
Significant correlations between and among serum concentrations of carnitine and BCAA-derived acylcarnitines by group
| Group | Variable 1 | Variable 2 | Spearman r | P (two tailed) |
|---|---|---|---|---|
| Control subjects | Carnitine | C5 | 0.532 | 0.030 |
| T2D patients with hyperglycemia | Carnitine | C5 | 0.675 | 0.001 |
| C3 | 0.826 | <0.001 | ||
| C5 | C3 | 0.804 | <0.001 | |
| KPD patients with DKA | Carnitine | C5 | 0.551 | <0.001 |
| C3 | 0.537 | <0.001 | ||
| T1D patients with DKA | Carnitine | C5 | 0.465 | 0.034 |
| C3 | 0.509 | 0.018 |
Correlations between and among serum concentrations of BOHB, carnitine, C16, and C14 are presented in Table 6. There were significant negative relationships between serum BOHB and carnitine in both KPD and T1D groups, and positive relationships between serum BOHB and serum C16 and C14 in the KPD group. There were also positive relationships between C16 and C2 in all groups.
Table 6.
Correlations between and among serum concentrations of ΒΟΗΒ, carnitine, C16, and C14 by group
| Group | Variable 1 | Variable 2 | Spearman r | P (two tailed) |
|---|---|---|---|---|
| Control subjects | C16 | C2 | 0.595 | 0.012 |
| T2D patients with hyperglycemia | BOHB | Carnitine | 0.06 | 0.82 |
| C16 | 0.21 | 0.45 | ||
| C14 | 0.36 | 0.19 | ||
| C16 | C2 | 0.489 | 0.025 | |
| KPD patients with DKA | BOHB | Carnitine | −0.279 | 0.05 |
| C16 | 0.464 | 0.001 | ||
| C14 | 0.348 | 0.017 | ||
| C16 | C2 | 0.489 | 0.025 | |
| T1D patients with DKA | BOHB | Carnitine | −0.551 | 0.026 |
| C16 | 0.398 | 0.09 | ||
| C14 | 0.315 | 0.18 | ||
| C16 | C2 | 0.533 | 0.013 |
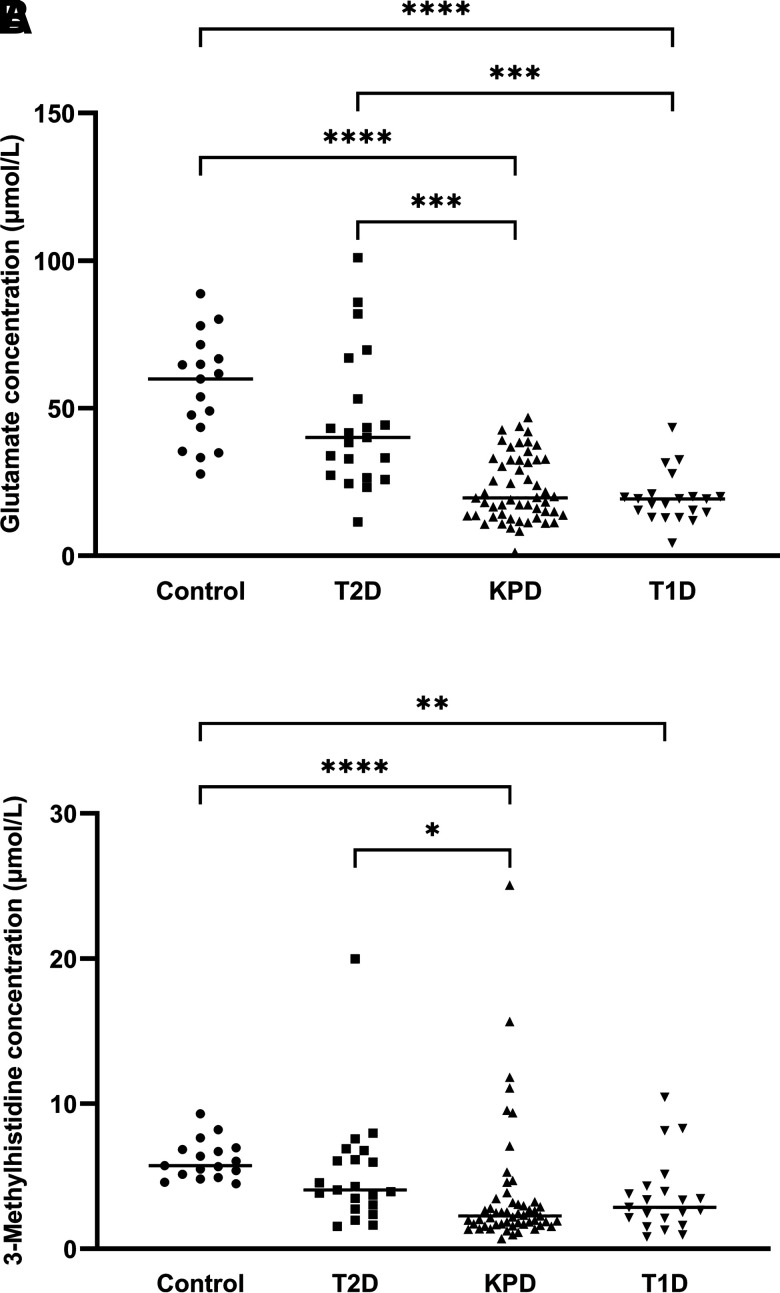
As shown in Fig. 2A, serum glutamate concentration was significantly lower in both KPD and T1D patients than in T2D patients and control subjects. Finally, serum 3-MH concentration, a marker of skeletal muscle protein breakdown rate (16), was lower in KPD and T1D patients than in both T2D patients and control subjects (Fig. 2B).
Figure 2.
Individual serum glutamate (A) and 3-MH (B) concentrations of healthy control subjects (Control), T2D patients in hyperglycemic crisis, KPD patients with DKA, and T1D patients with DKA. Median value is expressed as a line for each group. Data were analyzed by nonparametric one-way ANOVA (Kruskal-Wallis test) with post hoc Dunn multiple comparisons (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Discussion
A principal aim of this study was to determine whether the serum markers of aberrant BCAA/fatty acid metabolism previously described in stable, near-normoglycemic A−β+ KPD patients are also present when these patients decompensate and experience an acute episode of DKA. The current results suggest that during acute DKA, ketosis in KPD patients results primarily from excessive fatty acid–derived ketone production—just as in T1D patients. Higher concentrations of the long-chain acylcarnitines, C16 and C14, plus 3.3-fold higher C2 compared with the levels of control subjects suggest markedly increased rates of lipolysis and oxidation of long-chain fatty acids to acetyl CoA in the KPD patients. The serum concentration of carnitine, essential for shuttling long-chain fatty acids into mitochondria for β-oxidation, is markedly lower in the KPD group. There is a negative relationship between serum carnitine and BOHB in both KPD and T1D, suggesting accelerated flux of fatty acyl carbons into the β-oxidative pathway that exceeds the capacity of the TCA. Finally, the positive relationships of serum C16 and C14 with BOHB concentrations in the KPD group and between C16 and C2 in all groups suggest that the marked elevation of BOHB in KPD patients results from excessive acetyl CoA formation via β-oxidation.
We previously reported that clinically stable, fasting A−β+ KPD patients avidly catabolized BCAAs to acetyl CoA, with increased contribution to ketogenesis (9), suggesting preferential use of BCAAs as a source of energy and ketones. Our current finding of markedly higher BCAA and BCKA concentrations in A−β+ KPD patients suggests that this is not the case when they experience DKA—in fact, there seems to be a decrease in BCAA catabolism relative to their release from breakdown of body proteins in comparisons with T2D patients and control subjects. Thus, during an episode of DKA, KPD patients appear to switch to fatty acids as the preferred source for energy and ketone production, reverting to the classically defined mechanism of ketoacidosis in T1D patients (17). Notably, this switch occurs despite the fact that KPD patients maintain a substantial degree of β cell functional reserve even during the DKA episode, manifested by their significantly higher C-peptide levels compared with those of T1D patients.
The classic mechanism of DKA in a patient with typical autoimmune T1D requires a combination of severe insulin deficiency and increased concentrations of glucagon, catecholamines, and cortisol. This combination activates triglyceride hydrolysis in adipose tissues, with release of glycerol and nonesterified fatty acids. Glucagon facilitates the transport of nonesterified fatty acids into the mitochondrion for β-oxidation through inhibition of acetyl CoA carboxylase, thereby lowering the concentration of malonyl CoA, which normally inhibits carnitine palmitoyltransferase I, the rate-limiting enzyme for transesterification of fatty acyl-CoA to fatty acylcarnitine for transport into the mitochondrion (18–20). The higher concentrations of several counterregulatory hormones in the KPD and T1D groups confirm previous reports in the literature of these elevated hormonal levels in T1D patients during DKA (19,20). However, serum glucagon levels were highly variable and the mean values were not significantly higher in KPD or T1D patients compared with that of the control subjects. While the mean value for C-peptide in the KPD group was lower than that of T2D patients, it was significantly higher than that of the T1D patients during DKA. Hence, A−β+ KPD patients retain some β cell functional reserve even during acute DKA (1,3), and severe hypoinsulinemia does not seem to be the main factor contributing to the increased lipolysis in this situation. However, the combined lipolytic effects of elevated cortisol, growth hormone, norepinephrine, and, especially, epinephrine (21) may be sufficient to overwhelm the antilipolytic effect of endogenous insulin in the KPD patients. Furthermore, the insulin-to-glucagon ratio in KPD was lower than that of control subjects and trended lower than that of T2D patients (Fig. 1). The stimuli for the excessive release of the stress hormones remain to be understood, as a significant fraction of patients with A−β+ KPD, including half of the present cohort, develop DKA without an apparent precipitating stressful event (1,3).
Marked hyperglycemia due to decreased glucose uptake and metabolism with increased gluconeogenesis in all three diabetes groups would lead to depletion of TCA intermediates, hence, reduced TCA capacity. Also, increased flow of long-chain fatty acyl-CoA down the β-oxidative pathway consumes more NAD and FAD, leaving less available for expansion of redox reactions in the TCA. Further, the higher mitochondrial NADH-TO-NAD ratio (which permits conversion of acetoacetate to BOHB) suggests an inability of the electron transport chain to reoxidize the increased NADH being produced. Together, these events would result in intramitochondrial accumulation of long-chain-derived acylcarnitines and increased efflux to the plasma compartment resulting in elevated serum concentrations as observed in this study. The lower C16-to-C2, C14-to-C2, and C8-to-C2 ratios in all diabetes groups compared with those of control subjects (Table 4) suggest slowed entry of acetyl CoA into the TCA during hyperglycemic crisis.
The higher BCAA concentrations of the KPD and T1D patients are consistent with previous reports of a two to threefold elevation during DKA (22,23). Higher plasma BCAA in T1D patients during DKA has been thought to result from increased muscle protein breakdown due to insulin deficiency (22,24,25). The current findings suggest a different explanation—that BCAAs are elevated in intracellular and extracellular free pools because of decreased efflux into their catabolic pathways and not because of increased influx from breakdown of muscle proteins. The perception of increased muscle protein breakdown was based on higher plasma BCAA (22) with increased urinary excretion of 3-MH, a marker of skeletal muscle protein breakdown (16) during ketoacidosis (26,27). The marked depletion (∼41%) of 3-MH serum concentration in both the KPD and T1D groups (Fig. 2B) suggests that the serum 3-MH pool was not being replenished by a concomitant influx from increased muscle protein breakdown during DKA. Because essential amino acids can only be derived from protein breakdown in the fasting state, their plasma concentrations have been used as indices of protein breakdown rate. With the exception of the three BCAAs, studies of plasma amino acid concentrations in patients during DKA compared with healthy control subjects have reported no difference in lysine, methionine, phenylalanine, and histidine and lower threonine and tryptophan concentrations (22–25,28,29). Finally, no change in muscle protein balance has been demonstrated in experimental DKA in T1D patients (30). Together, these findings suggest that elevation of serum BCAA during DKA is due primarily to a marked decrease in BCAA catabolism rather than increased muscle protein breakdown.
The first two steps of BCAA catabolism are transamination by two BCAA transaminases (BCATs) in the cytosol and mitochondria to produce BCKAs, followed by irreversible oxidative decarboxylation by the BCKA dehydrogenase (BCKD) complex in the mitochondria to form CoA esters. These are subsequently converted to acetyl CoA, succinyl CoA, and acetoacetate and by-products such as C5 and C3 acylcarnitines (31). BCAT activity is highest in muscle and very low in liver, while BCKD activity is highest in liver and low in muscle (31). The higher serum concentrations of BCAAs and their respective BCKAs suggest that the initial transamination step in the cytosol of skeletal muscle cells is not impaired in KPD and T1D but the subsequent influx and metabolism in mitochondria is impaired, possibly in both muscle and liver. The result is elevation of BCKA concentrations. This interpretation is supported by the lower C5-to-KIC+KMV and C3-to-KIV+KMV ratios of KPD and T1D patients compared with those of T2D and control subjects, suggesting a relatively slower rate of entry and/or metabolism of BCKA into mitochondria. This is also supported by the lower KIC+KMV-to-C2 and KIC-to-BOHB ratios of the KPD and T1D patients, indicating that the relative contribution of leucine and isoleucine to acetyl CoA and acetoacetate production is markedly reduced during DKA.
Inability of muscle or liver cells to catabolize BCKAs may be secondary to the massive increase in β-oxidation of fatty acids during DKA. Reports in the literature suggest that lower pH, decreased carnitine availability, increased NADH-to-NAD and FADH-to-FAD ratios, and fatty acid–derived ketogenesis could all lead to impaired mitochondrial transport and metabolism of BCAA catabolites in KPD patients during acute DKA (32–37). In particular, the marked shortage of carnitine in the KPD and T1D patients would impair BCAA catabolism because accumulation of branched-chain acyl-CoA intermediates in mitochondria inhibits BCKD activity (31,36–38). Animal studies suggest that during DKA, BCKAs released into the circulation overwhelm hepatic uptake and catabolism (39–42), resulting in the higher serum BCKA concentrations as observed in the KPD and T1D patients.
A pathophysiologic question is, why do A−β+ KPD patients prefer BCAAs over fatty acids as a ketogenic source in the fasted state but behave like T1D patients in switching to excessive fatty acid–derived ketone production during acute DKA? A clue may reside in their regulation of glucagon. Some unique aspects of the metabolic profile of fasting, stable A−β+ KPD patients resemble those of individuals infused with glucagon (43,44). Persistent glucagon-like effects could contribute to the cascade of events that lead to elevated plasma ketones in KPD patients even when they are stable (9); any decrement in the I/G molar ratio in metabolic tissues might accelerate progression to ketoacidosis. Indeed, the I/G ratio of the KPD patients during DKA was closer to that of the T1D patients than that of the T2D patients (whose I/G ratio was similar to that of the control subjects) (Fig. 1).
Finally, the lower serum glutamate levels during DKA in KPD suggest reduced efflux into the circulation from hepatic and splanchnic tissues, the primary sites of net glutamate release (45–47). This could be due to either decreased synthesis or increased utilization and suggests profound disturbances in the nitrogen homeostasis of hepatic and/or splanchnic tissue cells. As glutamate plays a central role in facilitating the transfer of excess nitrogen (ammonia) either toward synthesis of several amino acids, nucleic acids, and nucleotides or to disposal via arginine and the urea cycle for renal excretion, its depletion would have inimical cellular effects (48,49). Glutamate is also a primary excitatory neurotransmitter, a precursor for the synthesis of the inhibitory neurotransmitter γ-aminobutyrate, and a substrate for the ubiquitous antioxidant glutathione. Hence, a shortage of glutamate in hepatic and splanchnic cells during DKA would accelerate oxidative stress and impair detoxification processes.
A caveat to understanding these data is that the interpretations and conclusions are based on static metabolomics measurements and, hence, should be viewed with circumspection. Ideally, the identified aberrant pathways in any metabolomics study should be further investigated using gold standard tracer studies to make kinetic measurements of metabolite fluxes. However, it would be virtually impossible to conduct such complex studies in the setting of the emergency room with such critically ill participants, especially prior to treating them with exogenous insulin. In our previous work identifying the BCAA and lipid metabolic defects in stable A−β+ KPD patients, the metabolomics data were extensively supported by kinetic (stable isotope/mass spectrometry) measurements of the relevant metabolic pathways (9).
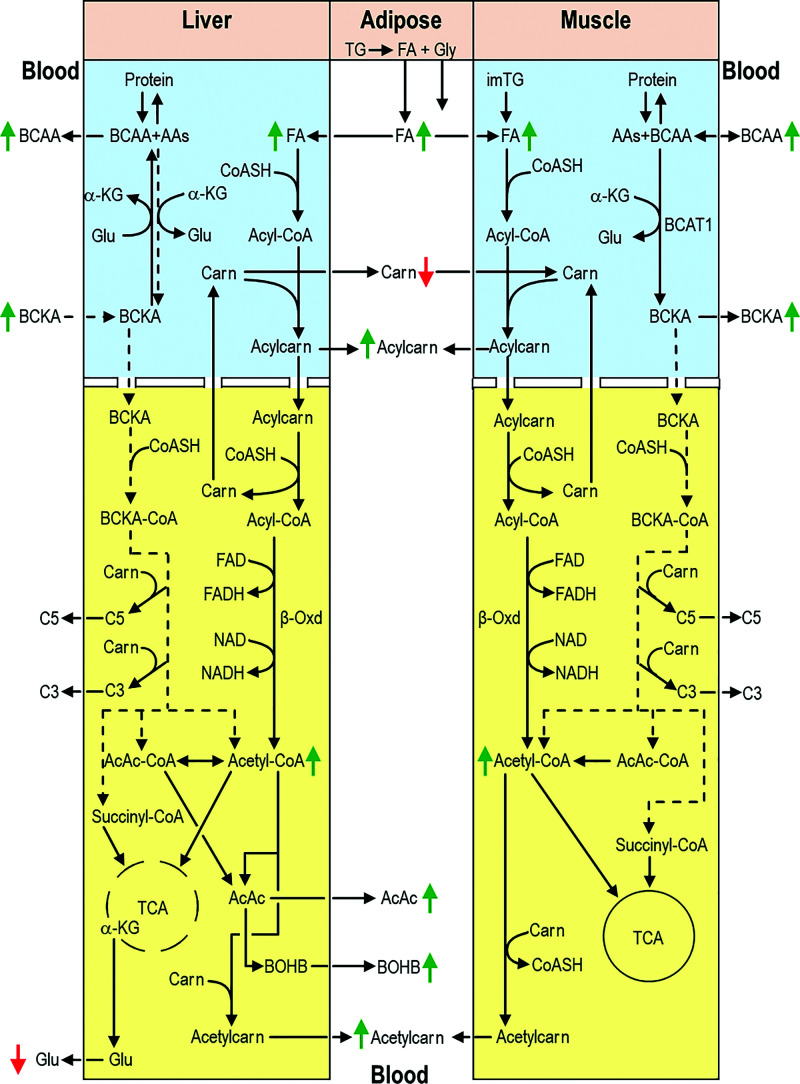
In summary (Fig. 3), A−β+ KPD patients experiencing DKA appear to have markedly increased lipolysis with reduced TCA capacity resulting in increased ketogenesis. Increased β-oxidation depletes whole-body carnitine availability, compromising mitochondrial BCKA (hence BCAA) catabolism especially in muscle, which cannot produce carnitine and has low BCKD activity. Consequently, net muscle uptake of BCAA is likely decreased and BCKA release into blood is greater than its uptake and catabolism by liver and other tissues, leading to higher blood levels of these metabolites. These defects are the opposite of those described in A−β+ KPD patients when they are clinically stable and near-normoglycemic and similar to those in T1D patients during DKA. Lower serum 3-MH concentrations suggest that increased skeletal muscle protein breakdown may not be the source of elevated BCAA in DKA. Thus, comparative serum metabolomics analysis reveals complex defects in pathways of energy and amino acid metabolism that vary with the clinical status of patients with A−β+ KPD.
Figure 3.
Schema of key findings in fatty acid and BCAA catabolic pathways in liver, skeletal muscle, adipose tissue, and blood of KPD and T1D patients during DKA. Blue and yellow backgrounds indicate cytosol and mitochondrion, respectively. Dashed arrows/lines indicate decreased substrate or metabolite flow through a pathway, and green and red arrows indicate increased and decreased concentrations, respectively. Markedly increased lipolysis in adipose tissues results in higher blood levels of fatty acids despite increased uptake and β-oxidation by liver and muscle. Increased β-oxidation depletes whole body carnitine availability, compromising mitochondrial BCKA (hence BCAA) catabolism, especially in muscle, which has lower BCKD activity and does not synthesize carnitine. Consequently, net muscle uptake of BCAA is decreased and BCKA release into blood is greater than its uptake and catabolism by liver and other tissues, leading to higher blood levels of these metabolites. See text for additional details. AAs, other amino acids; AcAc, acetoacetate; α-KG, α-ketoglutarate; Acetylcarn, acetylcarnitine; Acylcarn, acylcarnitine; β-Oxd, β-oxidation; Carn, carnitine; CoASH, coenzyme A; FA, fatty acids; Glu, glutamic acid; Gly, glycerol; im, intramyocellular; TG, triglyceride.
Article Information
Acknowledgments. The authors thank the clinical and research staff of the Ben Taub General Hospital emergency center and all the study participants.
Funding. This work was supported by Rutherford Chair, Baylor St. Luke’s Medical Center/Baylor College of Medicine (to A.B.), funds; National Institutes of Health grant RO1 DK101411 (F.J., A.B.); and funds from the Agricultural Research Service, U.S. Department of Agriculture, under Cooperative Agreement no. 58-6250-6001 (F.J.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. F.J. and A.B. designed the studies, analyzed data, and drafted the manuscript. J.W.H. performed mass spectrometric and biochemical analyses and analyzed data. K.R.K. and W.F.P. recruited the participants, implemented the clinical protocols, and developed the database. E.C. performed biochemical assays. R.B. and A.L. performed autoantibody analyses and reviewed the manuscript. P.B.M., R.G., and S.N.M. obtained, reviewed, and analyzed the clinical data and edited the manuscript. S.G.P. analyzed the metabolic data and edited the manuscript. A.B. and F.J. obtained funding for the study and supervised the protocols. A.B. and F.J. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in poster form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018, and at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
F.J. and J.W.H. are joint first authors.
P.B.M. is currently affiliated with Division of Endocrinology and Metabolism, University of California, San Francisco, San Francisco, CA.
References
- 1.Balasubramanyam A, Nalini R, Hampe CS, Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev 2008;29:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerji MA, Chaiken RL, Huey H, et al. GAD antibody negative NIDDM in adult black subjects with diabetic ketoacidosis and increased frequency of human leukocyte antigen DR3 and DR4. Flatbush diabetes. Diabetes 1994;43:741–745 [DOI] [PubMed] [Google Scholar]
- 3.Maldonado M, Hampe CS, Gaur LK, et al. Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and beta-cell functional classification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metab 2003;88:5090–5098 [DOI] [PubMed] [Google Scholar]
- 4.Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes 2004;53:645–653 [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Román MA, Piñero-Piloña A, Adams-Huet B, Raskin P. Comparison of type 1, type 2, and atypical ketosis-prone diabetes at 4 years of diabetes duration. J Diabetes Complications 2006;20:137–144 [DOI] [PubMed] [Google Scholar]
- 6.Sobngwi E, Mauvais-Jarvis F, Vexiau P, Mbanya JC, Gautier JF. Diabetes in Africans. Part 2: ketosis-prone atypical diabetes mellitus. Diabetes Metab 2002;28:5–12 [PubMed] [Google Scholar]
- 7.Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes 1995;44: 790–795 [DOI] [PubMed] [Google Scholar]
- 8.Mulukutla SN, Hsu JW, Gaba R, et al. Arginine metabolism is altered in adults with A-β + ketosis-prone diabetes. J Nutr 2018;148:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel SG, Hsu JW, Jahoor F, et al. Pathogenesis of A−β+ ketosis-prone diabetes. Diabetes 2013;62:912–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCann MR, George De la Rosa MV, Rosania GR, Stringer KA. L-carnitine and acylcarnitines: mitochondrial biomarkers for precision medicine. Metabolites 2021;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adeva-Andany MM, Calvo-Castro I, Fernández-Fernández C, Donapetry-García C, Pedre-Piñeiro AM. Significance of l-carnitine for human health. IUBMB Life 2017;69:578–594 [DOI] [PubMed] [Google Scholar]
- 12.Chalmers RA, Roe CR, Stacey TE, Hoppel CL. Urinary excretion of l-carnitine and acylcarnitines by patients with disorders of organic acid metabolism: evidence for secondary insufficiency of l-carnitine. Pediatr Res 1984;18:1325–1328 [DOI] [PubMed] [Google Scholar]
- 13.Ramsay RR, Gandour RD, van der Leij FR. Molecular enzymology of carnitine transfer and transport. Biochim Biophys Acta 2001;1546:21–43 [DOI] [PubMed] [Google Scholar]
- 14.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 2013;62:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genuth SM, Hoppel CL. Plasma and urine carnitine in diabetic ketosis. Diabetes 1979;28:1083–1087 [DOI] [PubMed] [Google Scholar]
- 16.McKeran RO, Halliday D, Purkiss P, Royston P. 3-methylhistidine excretion as an index of myofibrillar protein catabolism in neuromuscular disease. J Neurol Neurosurg Psychiatry 1979;42:536–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGarry JD, Foster DW. Hormonal control of ketogenesis. Biochemical considerations. Arch Intern Med 1977;137:495–501 [PubMed] [Google Scholar]
- 18.Foster DW. Malonyl-CoA: the regulator of fatty acid synthesis and oxidation. J Clin Invest 2012;122:1958–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerich JE, Lorenzi M, Bier DM, et al. Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism. Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin. J Clin Invest 1976;57: 875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGarry JD, Woeltje KF, Kuwajima M, Foster DW. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev 1989;5:271–284 [DOI] [PubMed] [Google Scholar]
- 21.Weiss M, Keller U, Stauffacher W. Effect of epinephrine and somatostatin-induced insulin deficiency on ketone body kinetics and lipolysis in man. Diabetes 1984;33:738–744 [DOI] [PubMed] [Google Scholar]
- 22.Felig P, Marliss E, Ohman JL, Cahill CF Jr. Plasma amino acid levels in diabetic ketoacidosis. Diabetes 1970;19:727–728 [DOI] [PubMed] [Google Scholar]
- 23.Szabó A, Kenesei E, Körner A, Miltényi M, Szücs L, Nagy I. Changes in plasma and urinary amino acid levels during diabetic ketoacidosis in children. Diabetes Res Clin Pract 1991;12:91–97 [DOI] [PubMed] [Google Scholar]
- 24.Aoki TT, Assal J-P, Manzano FM, Kozak GP, Cahill GF. Plasma and cerebrosponal fluid amino acid levels in diabetic ketoacidosis before and after corrective therapy. Diabetes 1975;24:463–467 [DOI] [PubMed] [Google Scholar]
- 25.Felig P, Wahren J, Sherwin R, Palaiologos G. Amino acid and protein metabolism in diabetes mellitus. Arch Intern Med 1977;137:507–513 [PubMed] [Google Scholar]
- 26.Marchesini G, Forlani G, Zoli M, Vannini P, Pisi E. Muscle protein breakdown in uncontrolled diabetes as assessed by urinary 3-methylhistidine excretion. Diabetologia 1982;23:456–458 [DOI] [PubMed] [Google Scholar]
- 27.Mårtensson J, Hermansson G. Sulfur amino acid metabolism in juvenile-onset nonketotic and ketotic diabetic patients. Metabolism 1984;33:425–428 [DOI] [PubMed] [Google Scholar]
- 28.Carl GF, Hoffman WH, Blankenship PR, Litaker MS, Hoffman MG, Mabe PA. Diabetic ketoacidosis depletes plasma tryptophan. Endocr Res 2002;28: 91–102 [DOI] [PubMed] [Google Scholar]
- 29.Lu J, Zhou J, Bao Y, et al. Serum metabolic signatures of fulminant type 1 diabetes. J Proteome Res 2012;11:4705–4711 [DOI] [PubMed] [Google Scholar]
- 30.Svart MV, Rittig N, Kampmann U, Voss TS, Møller N, Jessen N. Metabolic effects of insulin in a human model of ketoacidosis combining exposure to lipopolysaccharide and insulin deficiency: a randomised, controlled, crossover study in individuals with type 1 diabetes. Diabetologia 2017;60:1197–1206 [DOI] [PubMed] [Google Scholar]
- 31.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr 1984;4:409–454 [DOI] [PubMed] [Google Scholar]
- 32.Gibson R, Zhao Y, Jaskiewicz J, Fineberg SE, Harris RA. Effects of diabetes on the activity and content of the branched-chain alpha-ketoacid dehydrogenase complex in liver. Arch Biochem Biophys 1993;306:22–28 [DOI] [PubMed] [Google Scholar]
- 33.Hutson SM. Branched chain alpha-keto acid oxidative decarboxylation in skeletal muscle mitochondria. Effect of isolation procedure and mitochondrial delta pH. J Biol Chem 1986;261:4420–4425 [PubMed] [Google Scholar]
- 34.Hutson SM. pH regulation of mitochondrial branch chain alpha-keto acid transport and oxidation in rat heart mitochondria. J Biol Chem 1987;262: 9629–9635 [PubMed] [Google Scholar]
- 35.Landaas S. Inhibition of branched-chain amino acid degradation by ketone bodies. Scand J Clin Lab Invest 1977;37:411–418 [PubMed] [Google Scholar]
- 36.Owen OE, Trapp VE, Reichard GA Jr, Mozzoli MA, Smith R, Boden G. Effects of therapy on the nature and quantity of fuels oxidized during diabetic ketoacidosis. Diabetes 1980;29:365–372 [DOI] [PubMed] [Google Scholar]
- 37.Paul HS, Adibi SA. Effect of carnitine on branched-chain amino acid oxidation by liver and skeletal muscle. Am J Physiol 1978;234:E494–E499 [DOI] [PubMed] [Google Scholar]
- 38.Van Hinsbergh VM, Veerkamp JH, Zuurveld JG. Role of carnitine in leucine oxidation by mitochondria of rat muscle. FEBS Lett 1978;92:100–104 [DOI] [PubMed] [Google Scholar]
- 39.England BK, Greiber S, Mitch WE, et al. Rat muscle branched-chain ketoacid dehydrogenase activity and mRNAs increase with extracellular acidemia. Am J Physiol 1995;268:C1395–C1400 [DOI] [PubMed] [Google Scholar]
- 40.May RC, Hara Y, Kelly RA, Block KP, Buse MG, Mitch WE. Branched-chain amino acid metabolism in rat muscle: abnormal regulation in acidosis. Am J Physiol 1987;252:E712–E718 [DOI] [PubMed] [Google Scholar]
- 41.Price SR, Wang X, Bailey JL. Tissue-specific responses of branched-chain alpha-ketoacid dehydrogenase activity in metabolic acidosis. J Am Soc Nephrol 1998;9:1892–1898 [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Jurkovitz C, Price SR. Regulation of branched-chain ketoacid dehydrogenase flux by extracellular pH and glucocorticoids. Am J Physiol 1997;272:C2031–C2036 [DOI] [PubMed] [Google Scholar]
- 43.Battezzati A, Simonson DC, Luzi L, Matthews DE. Glucagon increases glutamine uptake without affecting glutamine release in humans. Metabolism 1998;47:713–723 [DOI] [PubMed] [Google Scholar]
- 44.Ramos-Roman MA, Burgess SC, Browning JD. Metabolomics, stable isotopes, and A−β+ ketosis-prone diabetes. Diabetes 2013;62:682–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aoki TT, Brennan MF, Müller WA, Moore FD, Cahill GF Jr. Effect of insulin on muscle glutamate uptake. Whole blood versus plasma glutamate analysis. J Clin Invest 1972;51:2889–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jang C, Hui S, Zeng X, et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab 2019;30:594–606.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marliss EB, Aoki TT, Pozefsky T, Most AS, Cahill GF Jr. Muscle and splanchnic glutmine and glutamate metabolism in postabsorptive andstarved man. J Clin Invest 1971;50:814–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brosnan JT, Brosnan ME. Glutamate: a truly functional amino acid. Amino Acids 2013;45:413–418 [DOI] [PubMed] [Google Scholar]
- 49.Brosnan ME, Brosnan JT. Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am J Clin Nutr 2009;90:857S–861S [DOI] [PubMed] [Google Scholar]