Abstract
Study Design:
Surface finish of titanium patient-specific craniofacial implants is known to affect their acceptability and durability and relevant literature still inconclusive on the best surface finishing protocol.
Objectives:
This study investigated surface topography of three-dimensionally (3D) printed and conventionally manufactured craniofacial titanium implants following non-contact 3D laser profile-meter analysis.
Methods:
Seven groups of titanium specimens (n = 10) were prepared and their surfaces were treated differently and included sole or combined treatment of mechanical polishing, gritting with 50 micron AL2O3, cold acid treatment using nitric acid for 20 hours (70% w/w), etching using acidic solution (69% nitric and 48% hydrofluoric acids) for 10 minutes and then electro-chemically anodized in another acidic solution (85% orthophosphoric and 98%sulphuric acid). Eighth group included specimens that were 3D printed. 3D micro-roughness parameters Sa, Sp, Sv, and Sz were determined (μm) for each specimen. Data was analyzed using one way ANOVA and Dunett T3 post-hoc tests (p < 0.05).
Results:
There were statistically significant effects of surface finishing protocols (p < 0.05). Sa values were 2.72-13.75 and specimens which were electroplated or mechanically polished and acid treated were the smoothest (p < 0.05). Sp was in the range 9.07-43.56 as sandblasting significantly roughened surfaces (p < 0.05). The same inferior effect was evident for the Sv (p < 0.05). The Sz values were 19.46-107.05 and was the highest for sandblasted surfaces (p < 0.05) and the lowest for surfaces of electro-chemical treatment (p < 0.05).
Conclusion:
Titanium surfaces are affected by the finishing procedure and electro-chemical treatment or mechanical polishing combined with acid treatment produced clinically-favorable smooth surfaces.
Keywords: skull reconstruction, titanium implant, surface topography, cranioplasty
Introduction
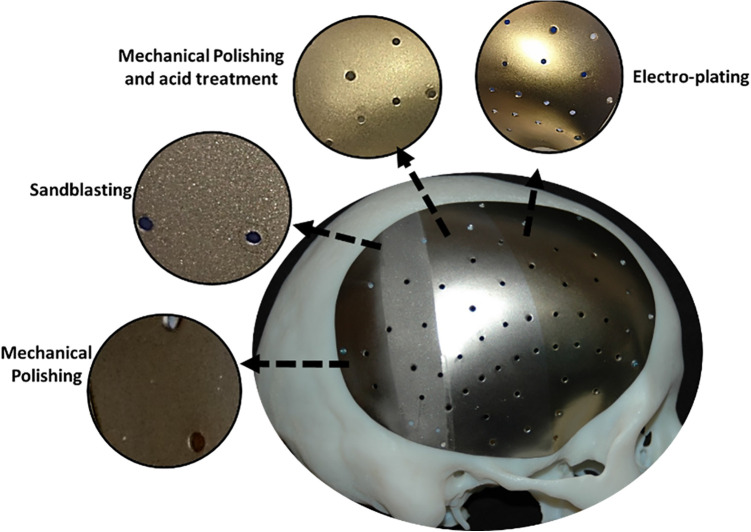
Titanium alloys are widely used in reconstructing missing bony structures in head and neck region due to their favorable biocompatibility, mechanical and electrochemical properties.1,2 Their use is successfully increasing3 reaching over 1 million titanium implantation every year, mostly intra-oral implants.4 The titanium craniofacial implants can be produced conventionally in which a titanium plate is pressed to the shape of missing bone.3,5–8 Alternatively, it is manufactured following computer-aided design/computer-aided manufacture (CAD/CAM) principle utilizing computerized novel additive manufacturing (AM) technologies such as Direct Metal Laser Sintering (DMLS) or Electron Beam Melting (EBM) technologies that use lasers to sinter the metal powder layer by layer.3,6,9 This aids to restore bony defects of skull (cranioplasty plate),8,10,11 orbital floor,12–14 and mandibular jaw.9,15–18 Other bodily uses may include orthopedics, fixing plates and hip replacements. The implant surface composition, hydrophilicity, and micro-topography are key factors to determine effective interaction between the implant and surrounding tissue, and subsequently its success.4,19–21 Success of titanium implant depends on effective biomaterial-tissue interaction, which is affected by the implant surface composition, hydrophilicity, and morphology including micro-geometry and roughness. Different surface treatments are followed,19,22–24 and include abrasive smoothening and polishing, gritting with micro-particles (i.e. Al2O3), cold treatment using hydrofluoric acid, or passivation and anodization.4,24–27 However, each treatment leaves distinctive surface characters and there is no ideal one treatment that can produce clinically favorable implant surface (Figure 1). Recent study showed that these treatments significantly change the surface topography and affect its micro-hardness and biocompatibility.28
Figure 1.
An example of skull implant showing 4 different surface treatments.
On the other hand, 3D printed implants (i.e. produced by DMLS or EBM technologies) are increasingly used but claimed to be rough and necessitate surface polishing for smoothness and removal of microstructural flaws, thus reducing chances of fatigue failures and compromised clinical performance.29 Although surface roughness of EBM processed titanium was reported to be higher than that of DMLS processed; still the surfaces were non-cytotoxic in processed and polished forms.30
The aim of the current work is to characterize surface topography of craniofacial titanium implants manufactured following conventional finishing protocols and DMLS technique. Our null hypothesis indicates that finishing protocol and DMLS will not affect the surface topography of titanium implant.
Materials and Methods
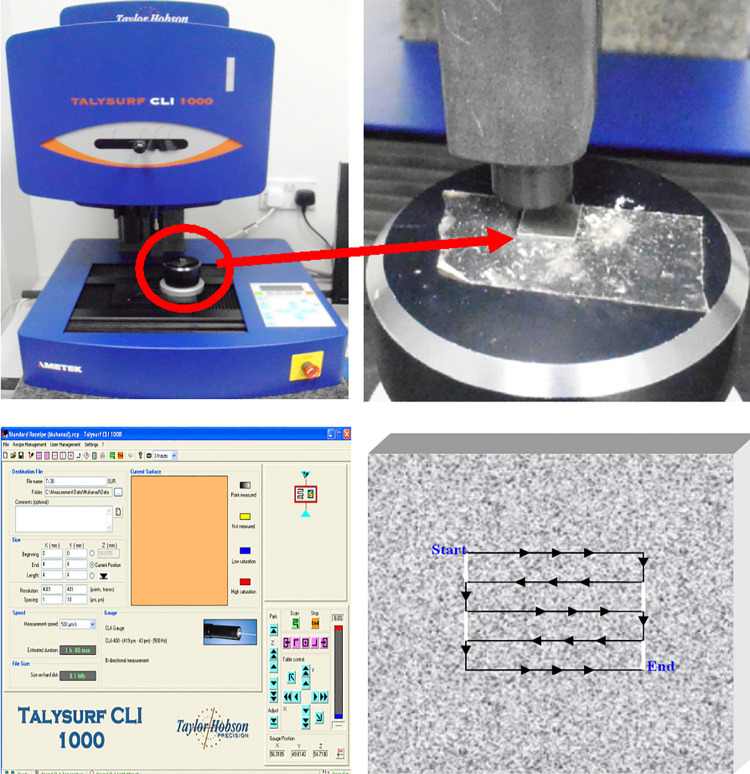
Readily available titanium sheets (300 mm × 300 mm × 0.7 mm thick) ASTM F136 (TiAL6V4; Titanium International; Birmingham, UK) were cut in small square shaped specimens (10 mm × 0.7 mm). A total of 70 specimen was produced and allocated to 7 groups (n = 10) of different surface treatment. Group 1 was control and received no treatment. Group 2 specimens were polished using abrasive powder (pumice and lathe) for 10 seconds. Group 3 specimens were submerged in nitric acid for 20 hours (70% w/w). Specimens of group 4 were held about 5 cm from the nozzle head and air abraded for 10 seconds with micro-particle Al2O3 (50 μm) at controlled air pressure (3-4 bar). Group 5 specimens received 2 different treatments of mechanical polishing and then immersed in acid as described earlier. Group 6 specimens received 2 different treatments of air abrasion then acid immersion as previously mentioned. Group 7 specimens were etched in an acidic solution (69% nitric and 48% hydrofluoric acids) for 10 minutes and then anodized electro-chemically in another acidic solution (85% orthophosphoric and 98% sulphuric acid) at 12 mV. An additional 10 specimens were manufactured using Direct Metal Laser Sintering Technology (DMLS) from a Ti64 ELI alloy (Electro Optical Systems-EOS GmbH, Krailling, Germany) and then polished and prepared per manufacturer instructions and these were assigned to group 8. Specimens of each group were cleaned in ultrasonic cleaning bath for 10 minutes before commencing three dimensional Micro-roughness (3D micro-Roughness) measurements. A non-contact surface profilometry (Talysurf CLI 1000, Taylor Hobson precision) was used. Bi-directional canning (X and Y axes) was performed at scanning speed of 500 µm/sec. The instrument consisted of 2 cross-slides on the X and Y axes. Scanning area was 4 × 4 mm and estimated scanning time for the full 16 mm2 area was 60 minutes. Scanning resolution was 1 and 10 (in μm) at X and Y axes respectively.
Statistical Analysis
Statistical Package for Social Sciences software (SPSS) was used (IL, USA). Data was analyzed using parametric test one way Analysis Of Variance (ANOVA) to check for statistically significant difference between the 8 groups. And Dunett T3 post-hoc was used to run paired comparisons between each 2 groups (p < 0.05).
Results
Results and statistical significances are presented in Table 1. 3D images of the groups were created using the non-contact surface profilometry (Figure 2).
Table 1.
Mean and SD Values of Micro-Roughness Results (µm).
| Group (n = 10) | Sa | Sp | Sv | Sz |
|---|---|---|---|---|
| 1 | 12.55a | 29.74a | 30.61a | 60.35a |
| 4.03 | 7.32 | 8.20 | 15.37 | |
| 2 | 6.00b | 14.26b | 14.90b | 29.16b |
| 1.69 | 3.23 | 3.71 | 6.82 | |
| 3 | 4.88b | 14.86b | 15.88b | 30.74b |
| 1.72 | 4.56 | 3.27 | 7.65 | |
| 4 | 11.98a | 34.91c | 56.64c | 107.05d |
| 4.95 | 9.54 | 13.24 | 51.82 | |
| 5 | 2.72c | 12.02b | 9.59b | 23.83b |
| 0.88 | 5.71 | 2.86 | 13.40 | |
| 6 | 13.95a | 43.56c | 62.67c | 106.23d |
| 4.33 | 15.42 | 8.03 | 18.59 | |
| 7 | 2.31c | 9.07b | 10.39b | 19.46b |
| 1.16 | 3.26 | 2.73 | 5.55 | |
| 8 | 4.29b | 23.23a | 19.55b | 42.75c |
| 2.09 | 6.18 | 2.72 | 7.78 |
* Sp (maximum value of peak height); Sv (maximum value of valley depth); Sz (sum of Sp and Sv); and Sa (Arithmetical mean height of the surface).
* Similar superscript letters within the same measurement indicate homogeneous subsets (P > 0.05).
Figure 2.
3D non-contact profilometer used and close up view of the scanning table (top row). Scanning parameters followed and schematic diagram of scanning topography (bottom row).
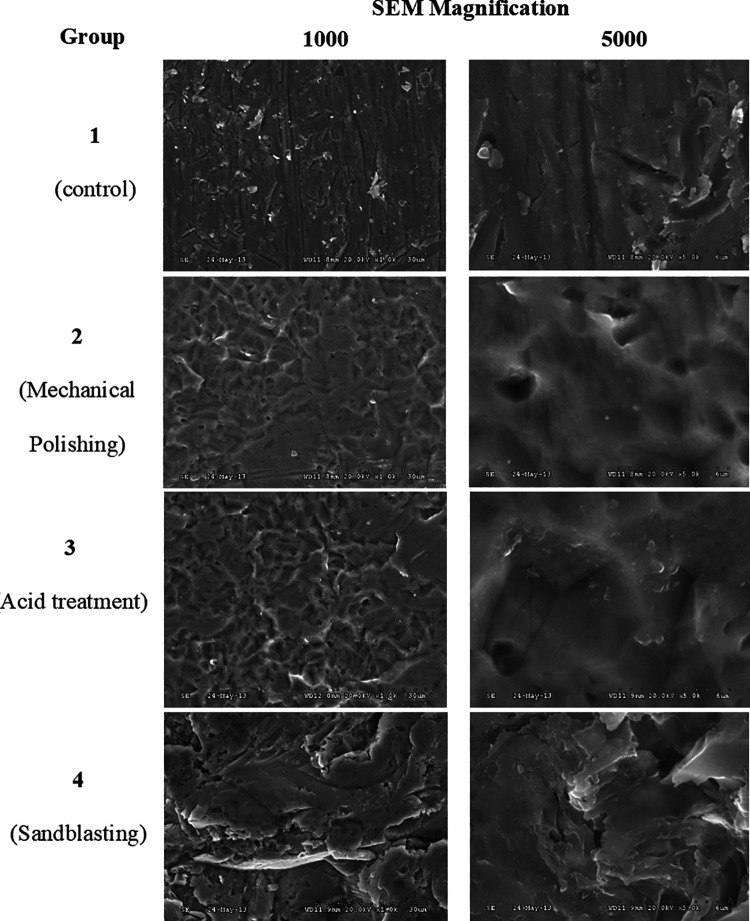
The 3D surface roughness parameters were (ISO 25178) Sp (Maximum height of peaks); Sv (Maximum height of valleys); Sz (Maximum height of the surface); and Sa (Arithmetical mean height of the surface). Surface images of the specimens under different treatments were captured using SEM at various magnifications (1000 and 5000) (Figure 3). Mean surface images of surface roughness (3D) and surface topography (2D) are shown in Figure 4.
Figure 3.
SEM images of the specimen surfaces after 4 major surface treatments and at 2 different magnifications (1000 and 5000).
Figure 4.
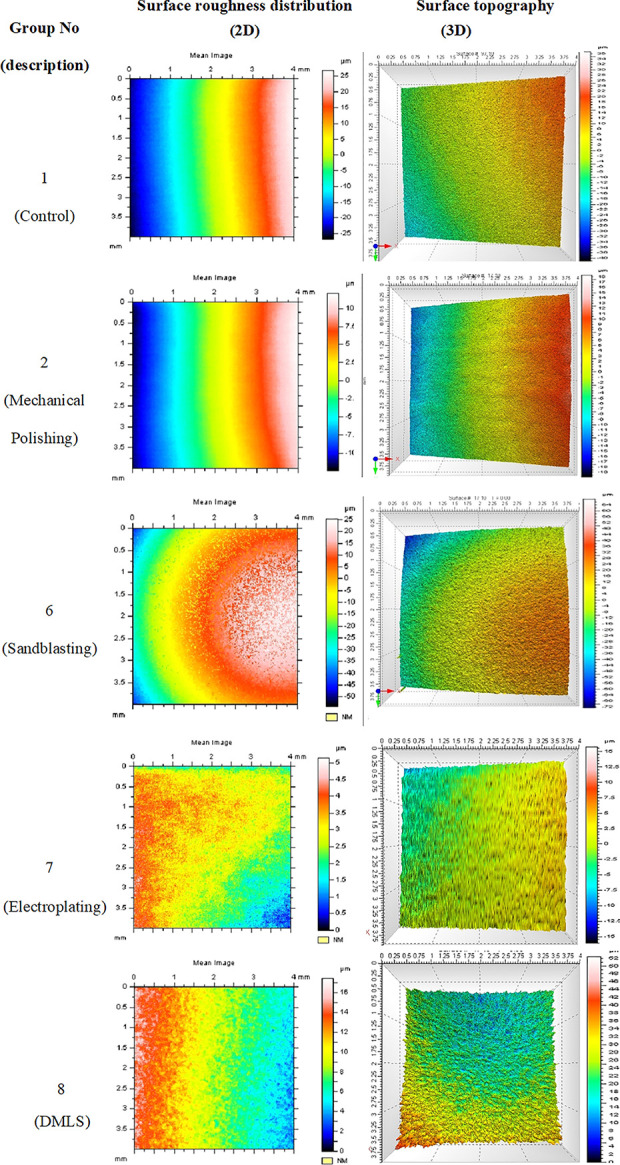
2D and 3D reconstruction of mean images of titanium surfaces of selected groups (1, 2, 6, 7, and 8). The 3D images show average surface with deep cavities colored in red and observed in the mechanical polishing group.
There were statistically significant effects of surface finishing protocols on micro-roughness parameters (p < 0.05). Statistically significantly smoothest surface was evident after following electro-chemical treatment as micro-roughness parameters were the lowest (p < 0.05).
Height parameter (amplitude mean in the height direction) Sa value (μm) was in the range 2.72-13.75. Specimens which were electroplated or mechanically polished and then acid treated were the lowest (P < 0.05) while sandblasting had no effect (p > 0.05). For the height parameters (peaks and valleys) Sp peak height values (μm) were in the range 9.07-43.56 and specimens that were sandblasted (with or without acid treatment) inferiorly affected this parameter (P < 0.05); while the remaining surface treatments had smoothening effect (p < 0.05) except for the 3D printed specimens which had no effect (P > 0.05). The same inferior effect was clear for the valleys depths (Sv) (P < 0.05) and values (μm) were in the range 9.59-62.67.
The Sz parameter which expresses the sum of the maximum value of peak height and the maximum value of valley depth was in the range 19.46-107-05. It was the highest for surfaces treated with sandblasting (with or without acid treatment) (P < 0.05) and was the least for surfaces treated with acid treatment with or without mechanical polishing, or electroplating (P < 0.05) and in the range (19.46-2916).
Discussion
Titanium implant surface topography plays an integral role toward its acceptance by the body, post-implantation healing time, and the implant/body interface. It determines implant’s surface quality and its chemical, physical, and mechanical properties. The different properties interact with each other, and a change in surface topography may change surface energy, thickness of oxide layer and surface chemical composition. The topography relates to the degree of roughness of the surface and the orientation of the surface irregularities. Smooth surfaces are advisable for out of load implants such as craniofacial implants and plates, and rough surfaces are required to promote osseointegration when they are under functional load such as dental implants and fixing screws. Current study showed that the roughness of the implant surfaces varied with the surface finish protocol, hence we rejected our null hypothesis.
The implant surface should warrant a good corrosion and wear resistance, anti-galling properties as well as an optimal biological response. Its treatment encompasses wide range of protocols that modify the surface topography by means of chemical deposition, grinding, polishing, or etching.24–26 Current study showed that micro-roughness parameters are affected by these protocols. Smooth implant surfaces are always desirable especially in craniofacial implant applications where implant surface plays an interactive role with overlying soft and hard tissue structures and significantly affects implant durability, functionality, stability and its overall success. Reconstructed 3D images obtained by the non-contact profilometry confirms the waviness of the surfaces but with different magnitudes as shown in Figure 4. At micro level, these surfaces exhibited significant variation in surface topography at 2 different magnifications 500 and 1000 as shown in Figure 3.
The 3D surface parameters calculated highlight a surface’s waviness, micro-roughness, wear ability and lubricant retention.31 These are defined through a set of standard “S Parameters” in 4 general categories: amplitude, spatial, hybrid and functional. The results for the titanium specimens included only the amplitude data of Sv, Sz, and Sa,31 that are clinically relevant. Sa represents amplitude mean in the height direction and the lesser the value the better the surface finish. Electroplating or mechanical polishing along with acid treatment exhibited the lowest value (P < 0.05). This is purely because polishing removes surface irregularities at micro level and anodization seals the surface be depositing titanium oxide layer of controlled thickness. Sp represents the maximum peak height value on the surface in the measured area and usually used for evaluation of frictional force and electrical contact resistance. The value decreased after mechanical polishing with or without acid treatment. The same was evident for the Sv parameter, which represents an area below the average level of the surface and it is often used for evaluation of surface strength and corrosion resistance. The Sz parameter expresses the sum of Sp and Sv on the surface within the measured area and lower value indicates a glossy and luster surface. The Sz values were highest after sandblasting (with or without acid treatment). The results showed that air abrasion produced matt finish and rough surfaces. Generally, sandblasting should be avoided in treating craniofacial implants surface though it is preferable in other applications like dental implants. Regardless, earlier work showed that cell attachment and cell proliferation are not affected by the surface finishing protocol employed.28
On the other hand, our results showed that DMLS produced titanium implants are comparable in surface topography to machined (custom made) titanium surfaces. This emphasizes the wide spread of Direct Metal Laser Sintering of titanium (DMLS) to manufacture customized mesh like bone screws, orbital implants, mesh for bone augmentation, and acetabular cup and femoral knee implants.2,3 However, microscopic view showed metal beads that are not affected by the laser from the DMLS process. This is in harmony with an earlier research30 and it might cause flaws within the structure and cause fatigue fracture if the implant is loaded under function.
For a careful topographical characterization it is necessary to use measuring methods that provide numerical and visual images. Specimens’ roughness was determined using non-contact 3D profilometry. It provides a reliable methodology in reporting surface topography in controlled reliable protocols as shown in Figure 2. The appropriate method must be chosen with respect to the desired measuring range, height range, resolution and material to be measured. No method is optimal for every purpose. A “true” surface roughness value does not exist as it varies among other things with the capability of the measuring equipment.31 This can be a limitation to our methodology.
Conclusion
Within the limitations of this study, it can be concluded that craniofacial titanium surfaces are affected by the finishing procedure. The surface roughness was comparable among conventionally and DMLS produced titanium craniofacial implants. Electro-chemical treatment or Mechanical polishing combined with acid treatment produce favorably smooth surface and should be followed in preparing surfaces of titanium craniofacial implants.
Footnotes
Ethical Approval: This research required no Institutional Review Board (IRB) or Ethical Committee approval.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Muhanad M. Hatamleh, PhD  https://orcid.org/0000-0003-3808-4695
https://orcid.org/0000-0003-3808-4695
References
- 1.Ninomi M. Metallic biomaterials. J Artif Organs. 2008;11(3):105–110. [DOI] [PubMed] [Google Scholar]
- 2.Rack HJ, Qazi JI. Titanium alloys for biomedical applications. Mater Sci Eng C. 2006;26(8):1269–1277. [Google Scholar]
- 3.Parthasarathy J.3D modelling, custom implants and its future perspectives in craniofacial surgery. Ann Maxillofac Surg. 2014;4(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Guehennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23(7):844–854. [DOI] [PubMed] [Google Scholar]
- 5.Eufinger H, Wehmoller M, Machtens E, et al. Reconstruction of craniofacial bone defects with individual alloplastic implants based on CAD/CAM-manipulated CT-data. J Craniomaxillofac Surg. 1995;23(3):175–181. [DOI] [PubMed] [Google Scholar]
- 6.Derand P, Rannar LE, Hirsch JM. Imaging, virtual planning, design, and production of patient-specific implants and clinical validation in craniomaxillofacial surgery. Craniomaxillofac Trauma Reconstr. 2012;5(3):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sing SL, An J, Yeong WY, Wiria FE. Laser and electron-beam powder-bed additive manufacturing of metallic implants: a review on processes, materials and designs. J Orthop Res. 2016;34(3):369–385. [DOI] [PubMed] [Google Scholar]
- 8.Day RE, Guy DT, Kop AM, Morrison DA. The Royal Perth Hospital method for the design and manufacture of titanium cranioplasty plates. Br J oral Maxfac Surg. 2012;50(4):376–377. [DOI] [PubMed] [Google Scholar]
- 9.Foley BD, Thayer WP, Honeybrook A, McKenna S, Press S. Mandibular reconstruction using computer-aided design and computer-aided manufacturing: an analysis of surgical results. J Oral Maxillofac Surg. 2013;71(2):e111–e119. [DOI] [PubMed] [Google Scholar]
- 10.Sharma SD, Lim B, Bentley RP. Preservation of the temporalis muscle during cranioplasty. Br J oral Maxfac Surg. 2012;50(3):e36–e37. [DOI] [PubMed] [Google Scholar]
- 11.Wiggins A, Austerberry R, Morrison D, Ho KM, Honeybul S. Cranioplasty with custom-made titanium plates—14 years experience. Neurosurgery. 2013;72(2):248–256; discussion 256. [DOI] [PubMed] [Google Scholar]
- 12.Gerbino G, Zavattero E, Zenga F, et al. Primary and secondary reconstruction of complex craniofacial defects using polyetheretherketone custom-made implants. J Craniomaxillofac Surg. 2015;43(8):1356–1363. [DOI] [PubMed] [Google Scholar]
- 13.Kozakiewicz M, Elgalal M, Walkowiak B, Stefanczyk L. Technical concept of patient-specific, ultrahigh molecular weight polyethylene orbital wall implant. J Craniomaxillofac Surg. 2013;41(4):282–290. [DOI] [PubMed] [Google Scholar]
- 14.Brie J, Chartier T, Chaput C, et al. A new custom made bioceramic implant for the repair of large and complex craniofacial bone defects. J Craniomaxillofac Surg. 2013;41(5):403–407. [DOI] [PubMed] [Google Scholar]
- 15.Ciocca L, Mazzoni S, Fantini M, et al. CAD/CAM guided secondary mandibular reconstruction of a discontinuity defect after ablative cancer surgery. J Craniomaxillofac Surg. 2012;40(8):e511–e515. [DOI] [PubMed] [Google Scholar]
- 16.Watson J, Hatamleh M, Alwahadni A, Srinivasan D. Correction of facial and mandibular asymmetry using a computer aided design/computer aided manufacturing prefabricated titanium implant. J Craniofac Surg. 2014;25(3):1099–1101. [DOI] [PubMed] [Google Scholar]
- 17.Hou JS, Chen M, Pan CB, et al. Immediate reconstruction of bilateral mandible defects: management based on computer-aided design/computer-aided manufacturing rapid prototyping technology in combination with vascularized fibular osteomyocutaneous flap. J Oral Maxillofac Surg. 2011;69(6):1792–1797. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch DL, Garfein ES, Christensen AM, et al. Use of computer-aided design and computer-aided manufacturing to produce orthognathically ideal surgical outcomes: a paradigm shift in head and neck reconstruction. J Oral Maxillofac Surg. 2009;67(10):2115–2122. [DOI] [PubMed] [Google Scholar]
- 19.Keller JC, Draughn RA, Wightman JP, Dougherty WJ, Meletiou SD. Characterization of sterilized CP titanium implant surfaces. Int J Oral Maxillofac Impl. 1990;5(4):360–367. [PubMed] [Google Scholar]
- 20.Marinucci L, Balloni S, Becchetti E, et al. Effect of titanium surface roughness on human osteoblast proliferation and gene expression in vitro. Int J Oral Maxillofac Impl. 2006;21(5):719–725. [PubMed] [Google Scholar]
- 21.Martin JY, Schwartz Z, Hummert TW, et al. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J Biomed Mater Res. 1995;29(3):389–401. [DOI] [PubMed] [Google Scholar]
- 22.Ku CH, Pioletti DP, Browne M, Gregson PJ. Effect of different Ti-6Al-4V surface treatments on osteoblasts behaviour. Biomaterials. 2002;23(6):1447–1454. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Bhat V, Balakrishnan M, et al. Bioactivity and surface characteristics of titanium implants following various surface treatments: an in vitro study. J Oral Implantol. 2015;41(5):e183–e188. [DOI] [PubMed] [Google Scholar]
- 24.Rosa AL, Beloti MM. Effect of cpTi surface roughness on human bone marrow cell attachment, proliferation, and differentiation. Braz Den J. 2003;14(1):16–21. [DOI] [PubMed] [Google Scholar]
- 25.Anselme K, Linez P, Bigerelle M, et al. The relative influence of the topography and chemistry of TiAl6V4 surfaces on osteoblastic cell behaviour. Biomaterials. 2000;21(15):1567–1577. [DOI] [PubMed] [Google Scholar]
- 26.Ask M, Lausmaa J, Kasemo B. Preparation and surface spectroscopic characterization of oxide films on Ti6Al4V. Appl Surf Sci. 1988;35:283–301. [Google Scholar]
- 27.Puippe JC. Surface treatment of titanium implants. Euro Cells Mater. 2003;5:32–33. [Google Scholar]
- 28.Hatamleh MM, Wu X, Alnazzawi A, Watson J, Watts D. Surface characteristics and biocompatibility of cranioplasty titanium implants following different surface treatments. Dent Mater. 2018;34(4):676–683. [DOI] [PubMed] [Google Scholar]
- 29.Sidambe AT. Biocompatibility of advanced manufactured titanium implants—a review. Materials. 2014;7(12):8168–8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuomi JT, Bjorkstrand RV, Pernu ML, et al. In vitro cytotoxicity and surface topography evaluation of additive manufacturing titanium implant materials. J Mater Sci Mater Med. 2017;28(3):53. [DOI] [PubMed] [Google Scholar]
- 31.Deleanu L, Cantaragiu A, Georgescu C, Botan M. Influence of measurements of 3D roughness parameters. Mech Test Diagn. 2011;1:42–53. [Google Scholar]