Abstract
Background:
Hepatic artery infusion (HAI) chemotherapy is associated with overall survival (OS) in patients with resected colon cancer liver metastases (CLM). The prognostic impact of primary tumor location in CLM following hepatic resection in patients receiving regional HAI is unknown. This study sought to investigate the prognostic impact of HAI in relation to laterality in this patient population.
Methods:
Consecutive patients with resected CLM, known primary tumor site treated with and without HAI were reviewed from a prospective institutional database. Correlations between HAI, laterality, other clinicopathological factors and survival were analyzed; Cox proportional hazard regression was used to determine whether laterality was an independent prognostic factor.
Results:
From 1993–2012, 487 patients (182 right colon cancer (RCC), 305 left colon cancer (LCC)) were evaluated with a median follow up of 6.5 years. Fifty-seven percent (n = 275) received adjuvant HAI. Patients with RCC had inferior 5-year OS compared with LCC (56% vs. 67%, P = 0.01). HAI was associated with improved 5-year OS in both RCC (68% vs. 45%; P < 0.01) and LCC (73% vs. 55%; P < 0.01). In multivariable analysis, HAI remained associated with improved OS (HR, 0.52; 95% CI, 0.39 to 0.70; P < 0.01) but primary tumor site did not (HR, 0.83; 95% CI, 0.63 to 1.11; P = 0.21). Additional significant prognostic factors on multivariable analysis included age, number of tumors, node-positive primary, positive margins, RAS mutation, 2-stage hepatectomy and extrahepatic disease. Cox proportional hazard regression determined no significant interaction between HAI and laterality on OS (parameter estimate [SEM], 0.12 [0.28]; P = 0.67)
Conclusions:
Our data show an association of adjuvant HAI and increased OS in patients who underwent curative hepatectomy, irrespective of primary tumor location. Laterality should therefore not impact decision making when offering adjuvant HAI.
INTRODUCTION
Colorectal cancer (CRC) is no longer regarded as a single disease entity. For the past decades, there has been a persistent trend in the increasing percentage of right-sided colon cancers (RCC) [1, 2]. Furthermore, a large body of literature since the 1990s has demonstrated differences in biology with regards to epidemiology, clinical behavior, and somatic genetic expression profiles in colon cancers originating in the right and left colon [3, 4]. RCC usually present at more advanced stage compared with left-sided colon cancer (LCC) [5]. Mucinous features, microsatellite instability, and BRAF mutations [6–8] have been found to be more common in RCC. Prognostic implications of primary tumor location have also been reported. Multiple retrospective series and post-hoc analyses of clinical trials have demonstrated worse survival outcomes in patients with unresectable, metastatic RCC compared to LCC [9–12]. Although most studies similarly show inferior outcomes for RCC compared with LCC in patients with CLM after curative hepatectomy[13, 14], long-term cure rates after hepatic resection have been reported to be similar [15].
More than 50% of colon cancers present with or develop liver metastasis and complete resection remains the only chance of cure. Despite that, relapse is a common clinical challenge and occurs in up to 80% of patients after resection of CLM. Multiple randomized controlled trials have demonstrated that adjuvant regional hepatic artery infusion (HAI) chemotherapy after hepatic resection decreases hepatic recurrence and overall recurrence, and improves overall survival [16–18]. In our largest study evaluating 2,368 consecutive patients after complete [19] resection of CLM, the median OS for patients treated with HAI (n = 785) was 67 months versus 44 months treated without HAI (n = 1,583; P < 0.001), despite more advanced disease in the HAI group. More recently, we showed that adjuvant HAI was associated with improved OS in patients with colorectal liver metastases after hepatic resection, irrespective of RAS-mutational status [20]. While numerous prognostic factors were evaluated in both studies, location of primary tumor was not. Whether adjuvant HAI is a prognostic factor for survival based on the anatomic location of the primary site for CLM after hepatic resection remains unknown. Therefore, the objective of this study was to study the differential impact of adjuvant HAI and systemic therapy on outcomes by primary tumor location in patients with resected CLM from a large institutional database.
METHODS
Patients
From 1993 to 2012, consecutive patients with liver metastases from colonic origin who underwent complete resection and treated with and without HAI chemotherapy were assessed from a prospectively maintained institutional database. Patients who presented with upfront resectable liver-only metastases, those who initially were downstaged with chemotherapy (systemic and/or HAI therapy) and then underwent complete resection, and those who had completely resected extrahepatic disease at the time of prior to liver resection were included in the final analysis. Patients who underwent ablations exclusively or had R2 resections were excluded. Patients with rectal cancer, multiple primaries, BRAF mutations, or unknown location of primary tumor or unknown RAS status were excluded. Patients with rectal cancer were excluded due to its unique therapeutic approach [3, 21, 22]. Finally, patients who received regional HAI for recurrent CLM were also excluded from this analysis. The study was approved by the Institutional Review Board of MSKCC.
Treatment
All patients completed hepatic resection, including 2-stage hepatectomy and additional intraoperative ablative therapies in some cases. Standard placement of the hepatic artery infusion pump was performed as previously described [17]. Adjuvant HAI chemotherapy consisted of infusion of FUDR mixed with dexamethasone plus heparinized saline administered for a two-week infusion alternating with 2 weeks of heparinized saline alone. With tolerable toxicity, this treatment is generally administered for a total of six cycles. HAI chemotherapy was routinely administered concomitantly with systemic chemotherapy as previously reported [17]. The treatment groups therefore were defined as adjuvant HAI + systemic therapy (HAI) versus systemic therapy alone (non-HAI). Patients who initially received HAI prior to completion of hepatic resection, were continued on adjuvant HAI therapy with systemic chemotherapy. Selection of post-operative systemic chemotherapy and details of the treatment plan were determined by the treating medical oncologist and based on prior chemotherapy exposure [17].
Variables
Laterality was defined as location of primary tumor derived from the pathology report. RCC was defined as tumors arising from the cecum through the transverse colon; LCC originated from the splenic flexure to rectosigmoid junction [23, 24]. Liver metastases diagnosed prior (within 6 months of diagnosis of the primary lesion) to or at the time of resection of the primary tumor were defined as synchronous disease. Node-positive primary tumors were staged according to 8th AJCC guidelines [25]. Total number and tumor size were derived from pathology reports. Positive surgical margins were defined as tumor cells at the resection margin. Serum CEA level was recorded closest to and within 3 months prior to liver resection. RAS mutational status (NRAS and KRAS) were obtained from institutional genomic platforms. Disease-free interval (DFI) was defined as time from resection of primary tumor to diagnosis of liver metastasis. Clinical risk score was calculated using DFI, CEA, node positivity and size of primary tumor, and number of metastases as previously described [26]. Surveillance scans were performed every 3 to 6 months unless otherwise indicated. Recurrent disease was confirmed by imaging (including CT, MRI, or PET) and/or biopsy. Only initial recurrence was recorded for each patient. Overall survival (OS) was calculated from the time of hepatic resection (2nd resection in cases with 2 stage hepatectomies) to date of death, with censoring at date of last follow-up. Recurrence-free survival (RFS) was calculated from date of hepatic resection to date of first recurrence, with censoring at date of last follow-up or death prior to recurrence.
Statistical Analysis
Clinical and pathological factors were summarized by laterality and HAI using frequencies and percentages or medians and ranges, as appropriate. Chi-square tests and Wilcoxon rank sum tests were used to assess the association of each factor with laterality and HAI. Three- and five-year OS and RFS were estimated using the Kaplan-Meier method, along with their 95% confidence intervals; comparisons of these survival probabilities by laterality and HAI were performed using z-tests. The log rank test was used to compare OS and RFS with respect to laterality and HAI separately and in combination; the stepwise log-rank tests was used to assess the interaction between laterality and HAI. Multivariable Cox regression models of OS and RFS were created to estimate hazard ratios and 95% confidence intervals adjusted for clinically important variables that have been shown to be significant in previous studies [20]. A separate Cox regression model of RFS and OS including interaction between HAI and laterality was performed to determine whether the effect of HAI on RFS differed between right- and left-sided tumors. All analyses were performed in SAS version 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
Patient Characteristics
Between 1993 and 2012, a total cohort of 2690 patients underwent complete resection of CLM at our institution. Seven-hundred seventy-five patients had verified location of the primary tumor based on pathology with available RAS mutational status. Patients with rectal tumors (n = 215) and multiple primary tumors (n = 11) were excluded from the analysis. Lastly, patients who received HAI for recurrent liver metastases (n = 54) and those who did not complete 2-stage hepatectomy and/or resection of their extrahepatic disease (n = 8) were excluded from the final analysis (Figure 1).
Figure 1.
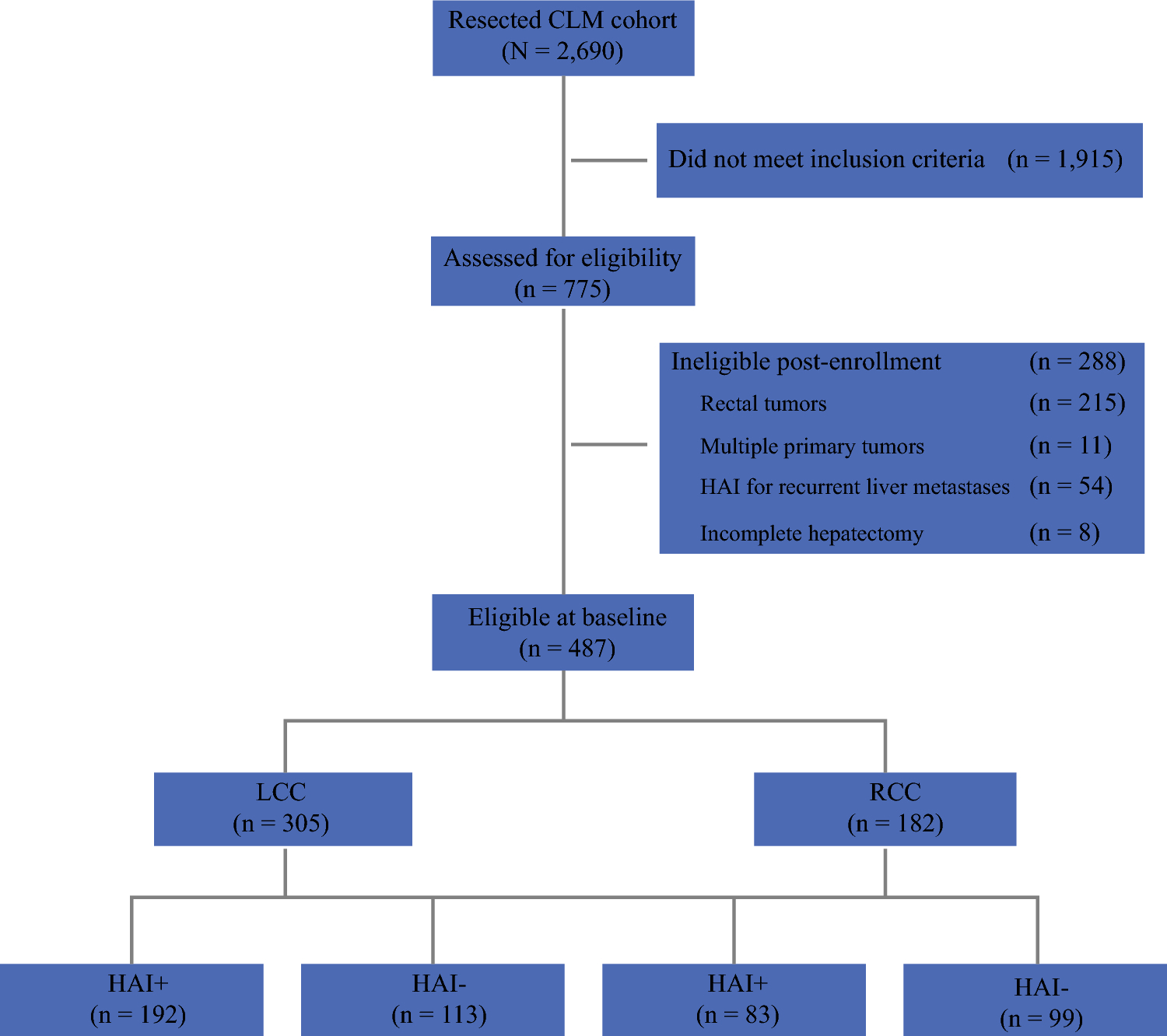
Flowchart of study cohort. CLM, colon cancer liver metastases; RCC, right colon cancer; LCC, left colon cancer; HAI, hepatic artery infusion.
The final analysis included 305 LCC (63%) and 182 RCC (37%) cases. Patients with RCC tumors were of older median age (62.5 years (27.2–90.7) vs. 56.5 years (25.3–87.0); P < 0.001). Right sided tumors more commonly harbored RAS mutation (53%) compared to patients with LCC (30%; P < 0.001). There was also a trend towards more extensive disease (P = 0.07). and more extra-hepatic disease in patients with RCC (P = 0.07). The demographics and clinical characteristics of the patients in these 2 groups are summarized in Table 1.
Table 1.
Clinical and pathologic characteristics of all consecutive patients who underwent curative-intent resection (n = 487)
| Variable | All n = 487 | RCC n = 182 | LCC n = 305 | P-value† | HAI n = 275 | Non-HAI n = 212 | P-valuea |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age | 58.8 (25.3–90.7) | 62.5 (27.2–90.7) | 56.5 (25.3–87.0) | < 0.01 | 55.5 (25.3–84.2) | 64.6 (25.7–90.7) | < 0.01 |
| Gender | |||||||
| Male | 265 (54) | 98 (54) | 167 (55) | 0.85 | 148 (54) | 117 (55) | 0.76 |
| Female | 222 (46) | 84 (46) | 138 (45) | 127 (46) | 95 (45) | ||
| Laterality | |||||||
| Right | 182 (37) | N/A | N/A | N/A | 83 (30) | 99 (47) | < 0.01 |
| Left | 305 (63) | 192 (70) | 113 (53) | ||||
| Adjuvant HAI | |||||||
| Yes | 275 (56) | 83 (46) | 192 (63) | < 0.01 | N/A | N/A | N/A |
| No | 212 (44) | 99 (54) | 113 (37) | ||||
| RAS status | |||||||
| Wild-type | 299 (61) | 85 (47) | 214 (70) | < 0.01 | 178 (65) | 121 (57) | 0.09 |
| Mutant | 188 (39) | 97 (53) | 91 (30) | 97 (35) | 91 (43) | ||
| Number of metastases | 2 (1–15) | 2 (1–10) | 2 (1–15) | 0.09 | 3 (1–15) | 2 (1–12) | < 0.01 |
| Solitary metastasis | |||||||
| Yes | 158 (32) | 60 (33) | 98 (32) | 0.85 | 62 (23) | 96 (45) | < 0.01 |
| No | 329 (68) | 122 (67) | 207 (68) | 213 (77) | 116 (55) | ||
| 3+ CLM | |||||||
| Yes | 169 (35) | 54 (30) | 115 (38) | 0.07 | 123 (45) | 46 (22) | < 0.01 |
| No | 318 (65) | 128 (70) | 190 (62) | 152 (55) | 166 (78) | ||
| Synchronous | 310 (64) | 115 (63) | 195 (64) | 0.87 | 185 (67) | 125 (59) | 0.06 |
| Metachronous | 177 (36) | 67 (37) | 110 (36) | 90 (33) | 87 (41) | ||
| DFI < 12 months | 389 (80) | 149 (82) | 240 (79) | 0.34 | 228 (83) | 161 (76) | 0.06 |
| Size of largest lesion (cm) | 2.9 (0.2–20.0) | 2.8 (0.6–20.0) | 3.0 (0.2–20.0) | 0.42 | 2.5 (0.2–20.0) | 3.0 (0.6–15.6) | 0.04 |
| Nodal status of primary tumor | |||||||
| Node positive | 317 (65) | 121 (66) | 196 (64) | 0.62 | 178 (65) | 139 (66) | 0.85 |
| Node negative | 170 (35) | 61 (34) | 109 (36) | 97 (35) | 73 (34) | ||
| Margin Status | |||||||
| Positive | 44 (9) | 13 (7) | 31 (10) | 0.26 | 24 (9) | 20 (9) | 0.79 |
| Negative | 443 (91) | 169 (93) | 274 (90) | 251 (91) | 192 (91) | ||
| CRS 3–5 | 248 (53) | 94 (54) | 154 (52) | 0.68 | 154 (62) | 94 (38) | 0.03 |
| Lesions ablated | |||||||
| Yes | 105 (22) | 39 (21) | 66 (22) | 0.96 | 78 (28) | 27 (13) | < 0.01 |
| No | 382 (78) | 143 (79) | 239 (78) | 197 (72) | 185 (87) | ||
| Extra-hepatic disease | 35 (7) | 18 (10) | 17 (6) | 0.07 | 15 (5) | 20 (9) | 0.09 |
| 2-stage hepatectomy | 40 (8) | 14 (8) | 26 (9) | 0.75 | 35 (13) | 5 (2) | < 0.01 |
Abbreviations: RCC = right colon cancer; LCC = left colon cancer; HAI = hepatic artery infusion; CLM = colorectal liver metastases; DFI = disease-free interval; CRS = clinical risk score
P-value from chi-square test for categorical variables or Wilcoxon rank sum test for numeric variables
HAI Chemotherapy
Adjuvant HAI was administered to a total of 275 patients (56%). Patients with LCC were more likely to receive HAI therapy (63%) compared to patients with RCC (46%; P < 0.01). Patients who received HAI were younger and were more likely to have higher risk disease demonstrated by higher CRS (3–5), synchronous disease, higher number of tumors, and larger tumor size. Furthermore, rates of ablations (28% vs. 13%) and 2 stage-hepatectomy (13% vs. 2%) were higher for the HAI group. Table 1 demonstrates the baseline characteristics of the patients in the 2 treatment groups included in the final analysis.
Predictors of Recurrence-Free Survival
In the entire cohort, 3- and 5-year overall RFS were 36% and 31%, respectively. There was no difference in 5-year RFS observed in patients between RCC (31%) and LCC (30%). Three- and five-year RFS rates were similar in the adjuvant HAI and the non-HAI groups (38% vs. 33%; P = 0.47 and 34% vs. 27%; P = 0.2, respectively). Among patients with LCC adjuvant HAI was associated with improved 5-year RFS (34% vs. 22%; P = 0.038). For RCC, 5-year RFS for patients treated with and without HAI were 33% and 29%, respectively (P = 0.65). In a Cox model of time to first recurrence, there was no significant interaction between HAI and laterality (P = 0.12).
In multivariable analysis, RFS was longer for patients who received HAI compared to patients who received systemic therapy only (HR, 0.68; 95% CI, 0.53–0.87; P = 0.002). The multivariable model confirmed additional significant prognostic factors of earlier recurrence including DFI < 12 months, number of tumors, node-positive primary, positive margins, size of largest tumor, lesions ablated, RAS mutation, extrahepatic disease (Table 2).
Table 2:
Multivariate Analysis of Predictors of recurrence-free survival (n = 480)
| Variable | Hazard Ratio | 95% Hazard Ratio CI | P-value |
|---|---|---|---|
|
| |||
| HAI therapy | 0.68 | 0.53 – 0.87 | < 0.01 |
| Age | 0.10 | 0.99 – 1.01 | 0.89 |
| Number of lesions | 1.13 | 1.07 –1.20 | < 0.01 |
| Size of largest lesion | 1.08 | 1.04 – 1.13 | < 0.01 |
| Laterality (left) | 1.13 | 0.89 – 1.44 | 0.32 |
| Lesions ablated | 1.36 | 1.03 – 1.79 | 0.03 |
| Sex (male) | 1.12 | 0.89 – 1.40 | 0.35 |
| RAS (mutant) | 1.49 | 1.17 – 1.88 | < 0.01 |
| Extra-hepatic disease | 2.01 | 1.33 – 3.03 | < 0.01 |
| 2-stage hepatectomy | 1.39 | 0.93 – 2.08 | 0.11 |
| DFI < 12 months | 1.74 | 1.26 – 2.42 | < 0.01 |
| Node positive tumor | 1.58 | 1.23 – 2.03 | < 0.01 |
| Positive margins | 1.79 | 1.23 – 2.60 | < 0.01 |
Abbreviations: HAI = hepatic artery infusion; DFI = disease-free interval
Predictors of Overall Survival
The median 3- and 5-year OS for the entire cohort was 79% and 63%, respectively. Considering all patients, the 5-year OS rates after hepatic resection in the RCC (56%) group was lower than that in the LCC (67%; P = 0.01; Figure 2) group. Adjuvant HAI therapy was associated with significantly prolonged 5-year OS (71%) compared to systemic therapy only (50%; P < 0.01; Figure 3). HAI was associated with prolonged 5-year OS for RCC (68% vs. 45%; P < 0.01) and LCC (73% vs. 55%; P < 0.01) as demonstrated in Figure 4. In a separate Cox model of OS, no significant interaction between HAI and laterality was observed (parameter estimate [SEM], 0.12 [0.28]; P = 0.67).
Figure 2.
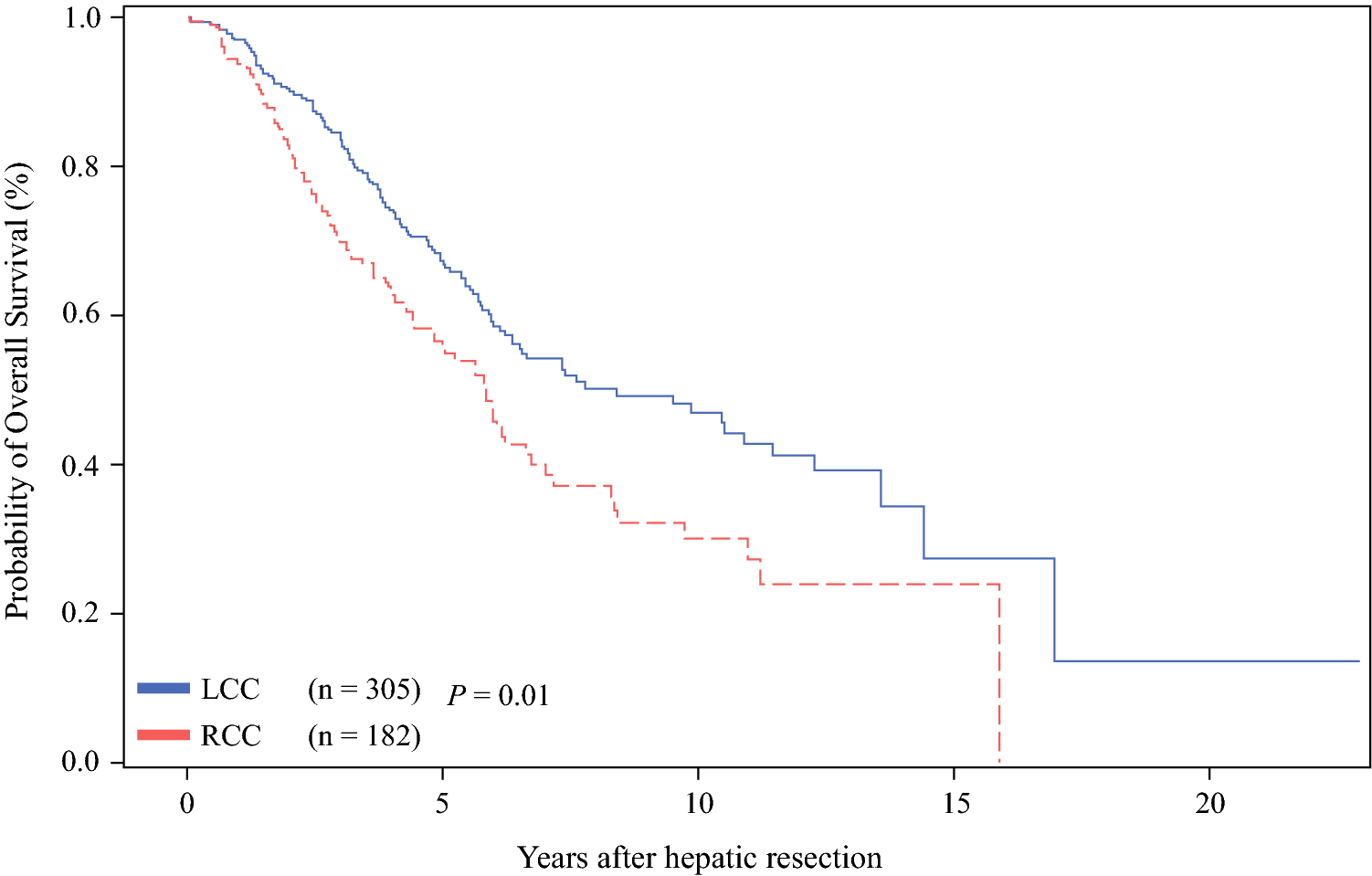
Overall survival (OS) rates after curative hepatectomy in RCC (right colon cancer) and in LCC (left colon cancer).
Figure 3.
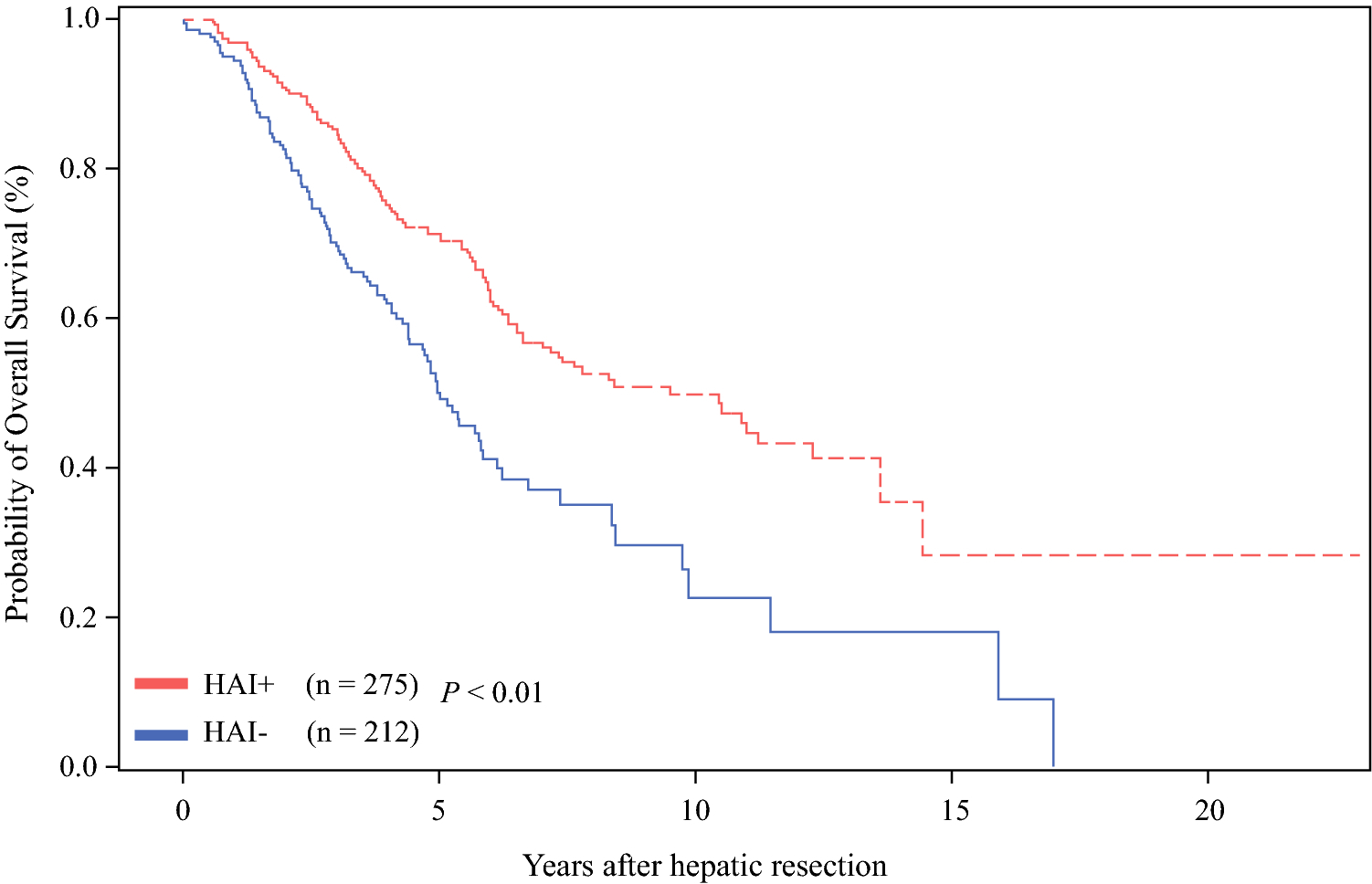
Overall survival (OS) rates with adjuvant HAI (hepatic artery infusion) therapy with systemic therapy (HAI +) and systemic therapy alone (HAI −).
Figure 4.
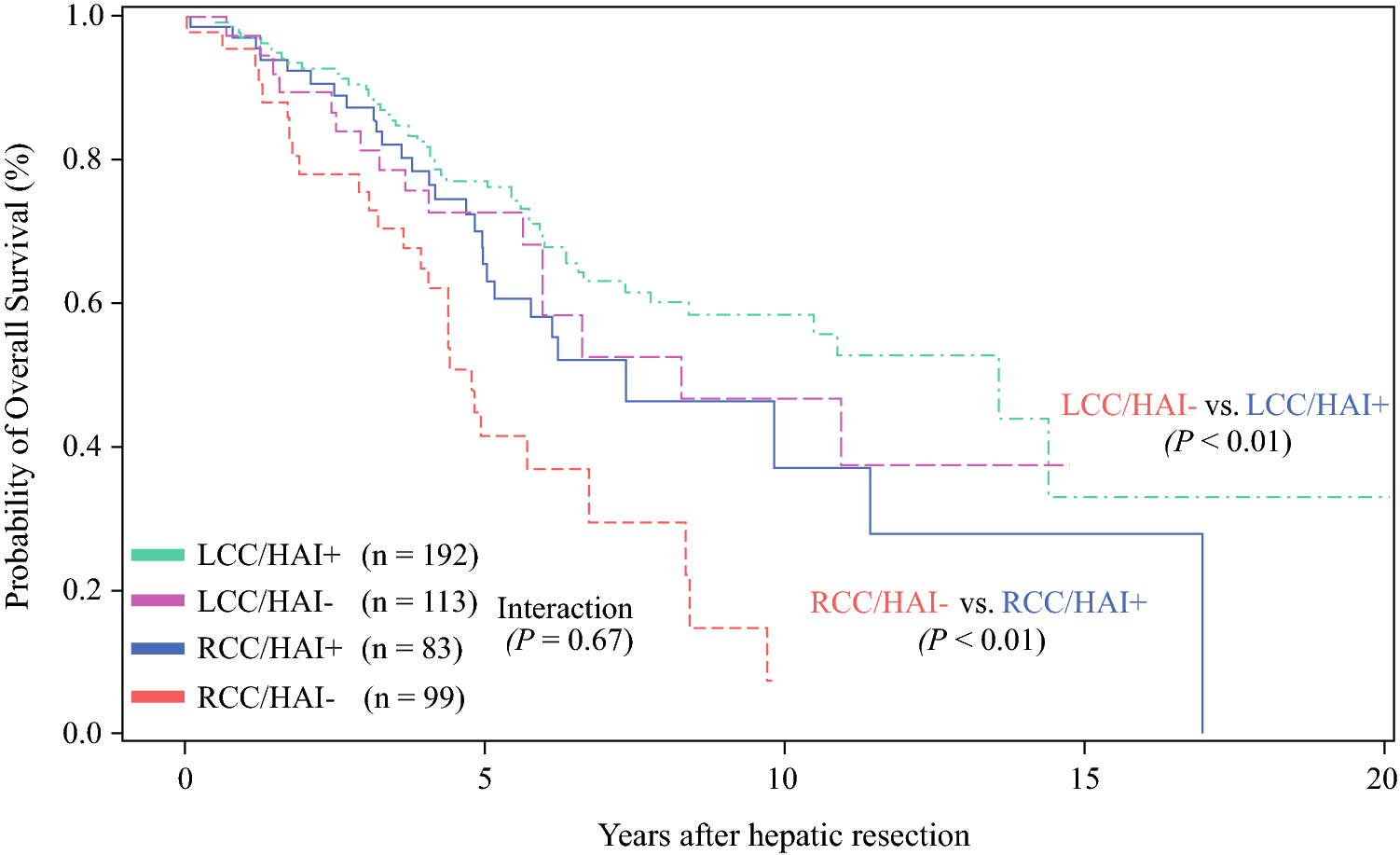
Overall survival (OS) rates for RCC (right colon cancer) and LCC (left colon cancer) stratified by treatment with (HAI +) and without (HAI −) therapy.
In multivariable analysis, adjuvant HAI was independently associated with improved OS with a HR of 0.52 (95% CI, 0.38–0.69; P < 0.01). Location of primary tumor was not a statistically significant independent predictor of OS (HR, 0.83; 95% CI, 0.63–1.11; P = 0.21). The multivariable model confirmed additional significant prognostic factors of worse survival including younger age, number of tumors, node-positive primary, positive margins, extrahepatic disease, 2-stage hepatectomy, and RAS mutational status as shown in Table 3.
Table 3.
Multivariate Analysis of Predictors of Overall Survival after Curative-Intent Resection of CLM (n = 487)
| Variable | Hazard Ratio | 95% Hazard Ratio CI | P-value |
|---|---|---|---|
|
| |||
| HAI therapy | 0.52 | 0.39 – 0.70 | < 0.01 |
| Age | 1.01 | 1.00 – 1.03 | 0.03 |
| Number of lesions | 1.13 | 1.05 – 1.22 | < 0.01 |
| Size of largest lesion | 1.04 | 1.00 – 1.09 | 0.08 |
| Laterality (left) | 0.83 | 0.63 – 1.11 | 0.21 |
| Lesions ablated | 1.29 | 0.92 – 1.81 | 0.15 |
| Sex (male) | 1.26 | 0.95 – 1.67 | 0.11 |
| RAS (mutant) | 1.60 | 1.20 – 2.13 | < 0.01 |
| Extra-hepatic disease | 2.56 | 1.62 – 4.06 | < 0.01 |
| 2-stage hepatectomy | 1.90 | 1.21 – 2.97 | < 0.01 |
| DFI < 12 months | 1.25 | 0.86 – 1.81 | 0.24 |
| Node positive tumor | 1.80 | 1.32 – 2.44 | < 0.01 |
| Positive margins | 2.55 | 1.70 – 3.81 | < 0.01 |
Abbreviations: HAI = hepatic artery infusion; DFI = disease-free interval
DISCUSSION
Differences in patient demographics, clinical presentation, tumor biology, and molecular carcinogenic pathways between right- and left-sided colon cancers have been long reported in the literature [27–30]. The prognostic impact of laterality has been well established in patients with stage IV unresectable metastatic CRC [11, 31]. However, it is unknown whether laterality determines the treatment effect of HAI and should guide the decision to offer HAI chemotherapy. We sought to investigate the impact of adjuvant HAI on outcome by location of the primary tumor for patients with resected CLM.
We reviewed 487 patients who completed with curative-intent hepatic resection with known primary location. Rectal tumors were excluded as they constitute a distinct clinical and prognostic subgroup [3, 21, 22]. For all patients, no difference in RFS was observed between RCC and LCC, however, OS was significantly shorter for RCC compared to LCC tumors (P = 0.001). Furthermore, adjuvant HAI was associated with prolonged RFS and OS regardless of primary tumor location. Although RFS rates were similar in patients who received HAI and those who did not, in multivariable analysis, HAI was a significant prognostic factor for RFS with a HR of 0.68 after accounting for other confounding factors. These findings are likely explained by the fact that patients who received HAI had overall worse prognostic indicators and more extensive disease. In multivariable analysis, laterality was not an independent prognostic factor associated with RFS (HR, 1.13; P = 0.32) or OS (HR, 0.83; P = 0.21), while adjuvant HAI therapy was (RFS: HR,0.68; P < 0.01 and OS: HR, 0.52; P < 0.01).
While the prognostic value of laterality on OS in patients with metastatic CRC has been well established [3], it is controversial if the same holds true for patients with resectable CLM. Several studies report positive prognostic value of sidedness of primary tumor in resected CLM [11, 32, 33]. A recent retrospective analysis of 475 patients demonstrated that patients with RCC undergoing hepatic resection had an associated improved RFS but worse OS (P = 0.03) and survival after recurrence (P = 0.01). The authors attributed these findings to higher number of recurrent lesions in patients with RCC, concluding that higher tumor burden in RCC translated into worse survival. Our recent analysis of 907 CLM patients undergoing hepatectomy showed no difference in RFS stratified by primary tumor location. In that report, left-sided primary tumors had a significantly improved median OS, but the observed cure rates were not different (RCC: 20% vs. LCC: 22%). Both studies have limitations. Sasaki’s group had a shorter follow up, and molecular data was not considered in Creasy’s analysis [15]. Similar results have been demonstrated by others showing shorter OS for RCC compared to LCC metastatic CRC undergoing curative hepatectomy. However, these studies included rectal tumors [34, 35] and therefore can’t be directly compared to other reports.
Similar to the results of the current analysis, other groups have published no prognostic value of laterality on survival outcomes in resected CLM. Wang et al. showed similar OS and RFS rates for RCC and LCC (5-year OS: 46.5% vs. 38.3%; P = 0.69 and 5-year RFS: 29.1% vs. 22.4%; P = 0.54) in 420 patients with CLM after hepatectomy[14]. Similarly, Marques et al. showed no difference in long term survival comparing RCC and LCC in 151 patients after hepatic resection [36]. It should be emphasized that in all these studies, RCC harbored higher rates of RAS mutation which could influence study findings. Moreover, variability in study design and selection bias may contribute to inconsistent results across all studies. For the entire cohort, current study shows lower overall survival for RCC compared to LCC, consistent with those seen in other studies [13, 15, 34]. However, laterality was not an independent predictor of survival in multivariable analysis for patients with resected CLM treated with adjuvant HAI and systemic therapy. We therefore do not think that laterality should not be a clinical factor to be included in the decision-making for consideration of HAI therapy.
In the metastatic setting, the anatomic location of the tumor appears to make a difference in OS and response to biologic treatments. Retrospective analysis from the CALGB/SWOG 80405 trial comparing bevacizumab and cetuximab in combination with chemotherapy as initial therapy for metastatic CRC suggests that the relative effectiveness of cetuximab and bevacizumab may differ depending on primary tumor location [36]. In the primary analysis, among patients with RCC, treatment with bevacizumab was associated with longer survival than that seen with cetuximab (24.2 vs. 16.7 months). Conversely, among patients with LCC, treatment with cetuximab was associated with longer OS than bevacizumab (36 vs. 31.4 months). In the setting of metastatic liver-only disease, studies have suggested that peri-operative chemotherapy may be more effective in high-risk tumors undergoing resection [36, 37]. Imai et al. looked at the impact of primary tumor location on the effectiveness of pre-operative chemotherapy and demonstrated that pre-operative chemotherapy is more effective in patients with RCC compared to those who received upfront hepatectomy. However, this study had a small size and did not account for RAS mutational status. To the contrary, no significant interaction of primary tumor location and HAI treatment was observed in the current study. Adjuvant HAI was associated with improved overall RFS and OS in all groups, irrespective of sidedness of the primary tumor. Finally, there was no difference in the effect of HAI on RFS or OS between RCC and LCC. These findings further demonstrate that when considering HAI therapy, tumor sidedness should not be a determining factor for patient selection.
The current work is a retrospective analysis and subject to biases associated with retrospective data. This study was conducted at a single center with significant experience on HAI treatment and consequently may not be generalizable. It needs to be recognized that at our center and most others, systemic therapy is in general given with adjuvant HAI. Furthermore, specific timing and details of chemotherapy regimens were not collected for this analysis. In this study, systemic chemotherapy was given according to standard of care and based on modern systemic chemotherapy in the later time periods as published before by our group. One would therefore not expect any significant differences in the treatment groups comparing HAI vs. non-HAI treated patients with regards to the systemic treatment received. Lastly, while this is the largest cohort addressing the effect of HAI on location of primary tumor, a large number of patients treated were excluded due to missing molecular marker data or unknown primary tumor location. Even though we considered RAS mutations and excluded patients with BRAF mutations, worse outcomes of patients with RCC may have been partially driven by other mutational factors not accounted for in this analysis. Further work is needed to more clearly elucidate genetic differences between RCC and LCC and mechanistic relationship between these mutations and primary tumor location and their prognostic significance. Moving forward, the implementation of broader Next Generation Sequencing approaches and tumor mutational burden may help to identify potential underlying reasons related to the survival differences noted in this and other studies.
CONCLUSIONS
In summary, this is the first study to evaluate the impact of adjuvant HAI and chemotherapy on outcomes by primary tumor location for resected CLM. Our data show an association of adjuvant HAI chemotherapy and increased OS in all patients who underwent curative hepatic resection, irrespective of location of primary tumor. Laterality was not a significant prognostic factor for OS. Therefore, we believe that primary tumor location should not be a determining factor for decision-making in selecting patients for adjuvant HAI treatment
SYNPOSIS.
A retrospective review of patients with resected colon cancer liver metastases. Our data show an association of adjuvant HAI and increased OS in patients who underwent curative hepatectomy, irrespective of primary tumor location. We conclude feasibility of HAI irrespective of tumor laterality.
ACKNOWLEDGMENTS
This work was supported in part by the NIH/NCI P30 CA008748 Cancer Center Support Grant.
Conflicts of Interest and Source of Funding: This work was supported in part by the NIH/NCI P30 CA008748 Cancer Center Support Grant
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version
REFERENCES
- 1.Jass JR: Subsite distribution and incidence of colorectal cancer in New Zealand, 1974–1983. Dis Colon Rectum 34(1):56–9, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Obrand DI, Gordon PH: Continued change in the distribution of colorectal carcinoma. Br J Surg 85(2):246–8, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Benedix F, Kube R, Meyer F, et al. : Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum 53(1):57–64, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Bufill JA: Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med 113(10):779–88, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Weiss JM, Pfau PR, O’Connor ES, et al. : Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol 29(33):4401–9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breivik J, Lothe RA, Meling GI, et al. : Different genetic pathways to proximal and distal colorectal cancer influenced by sex-related factors. Int J Cancer 74(6):664–9, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Hsu YL, Lin CC, Jiang JK, et al. : Clinicopathological and molecular differences in colorectal cancer according to location. Int J Biol Markers 34(1):47–53, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Shen H, Yang J, Huang Q, et al. : Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J Gastroenterol 21(21):6470–6478, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loupakis F, Yang D, Yau L, et al. : Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst 107(3), 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meguid RA, Slidell MB, Wolfgang CL, et al. : Is there a difference in survival between right-versus left-sided colon cancers? Ann Surg Oncol 15(9):2388–94, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tejpar S, Stintzing S, Ciardiello F, et al. : Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol 3(2):194–201, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yahagi M, Okabayashi K, Hasegawa H, et al. : The Worse Prognosis of Right-Sided Compared with Left-Sided Colon Cancers: a Systematic Review and Meta-analysis. J Gastrointest Surg 20(3):648–55, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Sasaki K, Andreatos N, Margonis GA, et al. : The prognostic implications of primary colorectal tumor location on recurrence and overall survival in patients undergoing resection for colorectal liver metastasis. J Surg Oncol 114(7):803–809, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Wang K, Xu D, Yan XL, et al. : The impact of primary tumour location in patients undergoing hepatic resection for colorectal liver metastasis. Eur J Surg Oncol 44(6):771–777, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Creasy JM, Sadot E, Koerkamp BG, et al. : The Impact of Primary Tumor Location on Long-Term Survival in Patients Undergoing Hepatic Resection for Metastatic Colon Cancer. Ann Surg Oncol 25(2):431–438, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemeny MM, Adak S, Gray B, et al. : Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy--an intergroup study. J Clin Oncol 20(6):1499–505, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Kemeny N, Huang Y, Cohen AM, et al. : Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 341(27):2039–48, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Kemeny NE, Gonen M: Hepatic arterial infusion after liver resection. N Engl J Med 352(7):34–5, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Groot Koerkamp B, Sadot E, Kemeny NE, et al. : Perioperative Hepatic Arterial Infusion Pump Chemotherapy Is Associated With Longer Survival After Resection of Colorectal Liver Metastases: A Propensity Score Analysis. J Clin Oncol 35(17):1938–1944, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gholami S, Kemeny NE, Boucher TM, et al. : Adjuvant Hepatic Artery Infusion Chemotherapy is Associated With Improved Survival Regardless of KRAS Mutation Status in Patients With Resected Colorectal Liver Metastases. Ann Surg, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Missiaglia E, Jacobs B, D’Ario G, et al. : Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 25(10):1995–2001, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Nitsche U, Stögbauer F, Späth C, et al. : Right Sided Colon Cancer as a Distinct Histopathological Subtype with Reduced Prognosis. Dig Surg 33(2):157–63, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Brulé SY, Jonker DJ, Karapetis CS, et al. : Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer 51(11):1405–14, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Charlton ME, Kahl AR, Greenbaum AA, et al. : KRAS Testing, Tumor Location, and Survival in Patients With Stage IV Colorectal Cancer: SEER 2010–2013. J Natl Compr Canc Netw 15(12):1484–1493, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin MB, Greene FL, Edge SB, et al. : The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67(2):93–99, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Fong Y, Fortner J, Sun RL, et al. : Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230(3):309–18, 1999. (dis 318–21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cucino C, Buchner AM, Sonnenberg A: Continued rightward shift of colorectal cancer. Dis Colon Rectum 45(8):1035–40, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Innocenti F, Ou FS, Qu X, et al. : Mutational Analysis of Patients With Colorectal Cancer in CALGB/SWOG 80405 Identifies New Roles of Microsatellite Instability and Tumor Mutational Burden for Patient Outcome. J Clin Oncol 37(14):1217–1227, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanza G Jr., Maestri I, Ballotta MR, et al. : Relationship of nuclear DNA content to clinicopathologic features in colorectal cancer. Mod Pathol 7(2):161–5, 1994 [PubMed] [Google Scholar]
- 30.Saltzstein SL, Behling CA: Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: a study of 213,383 cases from the California Cancer Registry. J Clin Gastroenterol 41(2):173–7, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Venook AP, Niedzwiecki D, Lenz HJ, et al. : CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol 32:LBA3–LBA3, 2014. (suppl 15) [Google Scholar]
- 32.Venook AP, Ou FS, Lenz HJ, et al. : Primary (1°) tumor location as an independent prognostic marker from molecular features for overall survival (OS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB / SWOG 80405 (Alliance). J Clin Oncol 35:3503–3503, 2017. (suppl 15) [Google Scholar]
- 33.Holch JW, Ricard I, Stintzing S, et al. : The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur J Cancer 70:87–98, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Dupré A, Malik HZ, Jones RP, et al. : Influence of the primary tumour location in patients undergoing surgery for colorectal liver metastases. Eur J Surg Oncol 44(1):80–86, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Price TJ, Beeke C, Ullah S, et al. : Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer 121(6):830–5, 201 [DOI] [PubMed] [Google Scholar]
- 36.Imai K, Yamashita YI, Miyamoto Y, et al. : Implication of primary tumor location for the indication of preoperative chemotherapy in patients with colorectal liver metastases. HPB (Oxford) 21(4):405–412, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Rahbari NN, Reissfelder C, Schulze-Bergkamen H, et al. : Adjuvant therapy after resection of colorectal liver metastases: the predictive value of the MSKCC clinical risk score in the era of modern chemotherapy. BMC Cancer 14:174, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]


