Abstract
Background:
Prophylactic drainage following hepatectomy is frequently performed despite evidence that drainage is unnecessary. It is unknown to what extent drain use is influenced by hospital practice patterns. The objectives of this study were to identify factors associated with the use of prophylactic drains following hepatectomy and assess hospital variation in drain use.
Methods:
Retrospective cohort study of patients following hepatectomy without concomitant bowel resection or biliary reconstruction from the ACS NSQIP Hepatectomy Targeted Dataset. Factors associated with the use of prophylactic drains were identified using multivariable logistic regression and hospital-level variation in drain use was assessed.
Results:
Analysis included 10,530 patients at 130 hospitals. Overall, 42.3% of patients had a prophylactic drain placed following hepatectomy. Patients were more likely to receive prophylactic drains if they were ≥65 years old (adjusted odds ratio [aOR]: 1.34, 95%CI: 1.16–1.56), underwent major hepatectomy (aOR: 1.42, 95%CI 1.15–1.74), or had an open resection (aOR 1.94, 95%CI 1.49–2.53). There was notable hospital variability in drain use (range: 0% to 100% of patients), and 77.5% of measured variation in drain placement was at the hospital level.
Conclusion:
Prophylactic drains are commonly placed in both major and minor hepatectomy. While some patient factors are associated with drain use, hospital-specific patterns appear to be a major driver and represent a target for improvement.
Introduction
As surgical technique and perioperative care have improved, rates of hepatic resection have increased and the incidence of surgical complications has fallen, particularly mortality and post-hepatectomy liver failure.1–4 Still, rates of complications, including post-operative bile leakage and intra-abdominal abscess, occur after these procedures.5–7 These complications often necessitate percutaneous or operative drainage in order to prevent clinical deterioration.8, 9 Prophylactic abdominal drainage has been utilized following hepatic resection to reduce the occurrence of these events and decrease their associated morbidity.10, 11
In recent years, the practice of prophylactic drainage after hepatic resection has come under increasing scrutiny. Numerous studies, including retrospective analyses, prospective trials, and meta-analyses have suggested that prophylactic drainage after hepatic resection is unnecessary, and may be harmful.11–20 This trend has held true across numerous high risk subgroups of patients, a variety of surgical techniques, and multiple disease processes.21, 22 Despite randomized trial evidence suggesting that prophylactic abdominal drainage after hepatic resection is unnecessary, it is still frequently employed.23
Prior studies have identified patient and procedural characteristics that are associated with the decision to employ drain placement, however, these factors may not explain a large amount of the decision making.13 It is likely that institutional practices and biases are strong drivers of decisions regarding drainage after hepatic resection. The objectives of this study were to identify factors associated with the use of prophylactic surgical drains following hepatectomy and assess hospital variation in drain use.
Methods:
Study Design and Inclusion Criteria:
This retrospective cohort study was performed using the 2014–2017 American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) Hepatectomy Procedure Targeted Data File. Briefly, ACS NSQIP is a risk-adjusted multi-institutional quality improvement program that collects data on more than 150 variables including preoperative risk factors, intraoperative variables, and 30-day postoperative outcomes. ACS NSQIP has been described in detail elsewhere.24, 25 Hospitals participating in the hepatectomy targeted dataset collect additional variables following liver resection, including pathology, drain management, and bile leak. Missing data were imputed using maximum likelihood.26 The Northwestern IRB deemed this study exempt from review because it used pre-existing, de-identified data.
Hepatectomy procedures were identified by CPT© code. Right hepatectomy, left hepatectomy, and trisectionectomy were considered major hepatectomies. Other hepatic resections were considered minor hepatectomies, including multi-segment and multiple wedge resections. Patients with concomitant ablation were categorized with the highest level of hepatic resection as outlined above. Recorded pathologic indications for surgery included primary hepatic malignancies, hepatic metastatic disease, benign lesions, and other. Operations were classified as open or minimally invasive (including both laparoscopic and robotic), with surgical approach considered on an intention-to-treat basis (e.g., MIS converted to open is considered MIS for analytic purposes). Patients were excluded if the patient had a concomitant bowel resection, biliary reconstruction, ascites, cirrhosis, or if the patient was American Society of Anesthesiology (ASA) class 5. Patients were also excluded if the patient had unknown drain status, surgical approach, operative duration, or neoadjuvant therapy status.
Outcome Variables:
The primary outcome variable was utilization of prophylactic drainage following hepatectomy, defined by the presence of an intraabdominal drain at the conclusion of the index operation. Rates of utilization of prophylactic drainage were aggregated to the hospital-level to yield the rate of drain use for each facility. Secondary analyses were performed to assess infectious outcomes of patients with and without prophylactic drains, including surgical site infection (SSI; including superficial, deep, and organ space infections not present at the time of surgery) and bile leak. Bile leaks were defined as drain bile levels at least three times above the upper limit of normal serum bilirubin on or after POD3 or placement of a new drain.27 No data were available on intraoperative bile leaks or intraoperative leak tests.
Covariates:
Covariates included patient characteristics, documented comorbidities, preoperative laboratory values, and intraoperative variables. Variables were chosen for analysis based on clinical relevance in liver surgery. Variables of interest included patient age (grouped into age <55, 56–69, and ≥70), sex, race, and comorbidities (e.g. diabetes, hypertension, COPD, and hypoalbuminemia). Body mass index (BMI) was categorized using standard clinical cutoffs (<18.5 is underweight, 18.5–24.9 is normal, 25–29.9 is overweight, ≥30 is obese).28 ASA classification was categorized into two groups: classes 1–2 vs classes 3–4. Year of surgery was included in models to adjust for any temporal variation in utilization of prophylactic drains.
Statistical Analysis:
Bivariate chi-square tests were adjusted for patient clustering within hospitals, and presented odds ratios are the results of hierarchical multivariable logistic regression models. Reported confidence intervals are to the 95% level of significance, and tests of significance were 2-sided with p values considered significant at the 0.05 level. Because analysis was performed by internal ACS staff, hospital pseudo-identifiers were available for clustering, hierarchical modeling, and hospital-level frequency description. Serial hierarchical regression models were constructed to determine the relative contribution of patient-level characteristics in variation of prophylactic drain use following hepatectomy. Variance was ascribed to hospital-level random effects in both an empty model and in a model adjusted for patient characteristics. The residual hospital-level variance in the adjusted model is considered hospital variance not accounted for by differences in patient characteristics. Statistical analyses were performed using Stata v15.1 (StataCorp, College Station, TX).
Results
Patient Cohort Characteristics
Of 15,697 hepatectomies performed between 2014 and 2017, 1204 (7.6%) were excluded as biliary reconstructions, 2513 (16.0%) for concomitant bowel resection, 1273 (8.1%) for cirrhosis, 39 (0.2%) for ascites, 7 (<0.1%) for being ASA class 5, and 131 (0.8%) for missing outcome data leaving 10,530 patients to be included in subsequent analyses. Prophylactic drains were placed in 4455 patients (42.3%). Patient characteristics are shown in Table 1.
Table 1.
Characteristics of Patients Undergoing Hepatectomy
| Patient Characteristic | Overall N=10,530 n (%) |
|---|---|
| Age, y | |
| <55 | 3827 (36.3) |
| 55–64 | 2813 (26.7) |
| ≥65 | 3890 (36.9) |
|
| |
| Sex | |
| Female | 5707 (54.2) |
| Male | 4823 (45.8) |
|
| |
| Year | |
| 2014 | 2001 (19.0) |
| 2015 | 2620 (24.9) |
| 2016 | 2875 (27.3) |
| 2017 | 3034 (28.8) |
|
| |
| Race | |
| Non-Hispanic White | 6797 (64.6) |
| Non-Hispanic Black | 810 (7.7) |
| Hispanic | 485 (4.6) |
| Other/Unknown | 2438 (23.2) |
|
| |
| Diabetes | 1662 (15.8) |
|
| |
| Smoker | 1568 (14.9) |
|
| |
| COPD | 345 (3.3) |
|
| |
| Hypertension | 4654 (44.2) |
|
| |
| ASA Class | |
| 1–2 | 3124 (29.7) |
| 3–4 | 7406 (70.3) |
|
| |
| BMI (kg/m2) | |
| <18.5 | 178 (1.7) |
| 18.5–24.9 | 3180 (30.2) |
| 25–29.9 | 3550 (33.7) |
| ≥30 | 3622 (34.4) |
|
| |
| Histologic Indication | |
| Primary Hepatic Malignancy | 2383 (22.6) |
| Metastatic Disease | 4950 (47.0) |
| Benign | 2716 (25.8) |
| Unknown | 481 (4.6) |
|
| |
| Neoadjuvant Therapy Received | 3098 (29.4) |
|
| |
| Extent of Hepatectomy | |
| Minor | 7023 (66.7) |
| Major* | 3507 (33.3) |
|
| |
| Surgical Approach | |
| Open | 7608 (72.3) |
| Minimally Invasive** | 2922 (27.8) |
|
| |
| Length of Operation | |
| <3 hours | 4130 (39.2) |
| 3–5 hours | 4302 (40.9) |
| >5 hours | 2098 (19.9) |
|
| |
| Prophylactic Drain Placed | 4455 (42.3) |
Major hepatectomy includes left hepatectomy, right hepatectomy, and trisectionectomy
Includes laparoscopic/robotic converted to open
Factors Associated with Prophylactic Drain Use
Unadjusted bivariate analysis demonstrated associations between utilization of prophylactic drains and patient age, medical comorbidities, pathologic indication, technical approach, and operative time. There was also a significant difference in drain use by year without an obvious temporal trend (P=0.002). Full bivariate analysis are shown in Table 2.
Table 2.
Bivariate Associations Between Patient Characteristics and Drain Use (N=10,530)
| Patient Characteristic | Drain Use (%) | P Value |
|---|---|---|
| Overall Rate | 42.3 | |
|
| ||
| Age, y | <0.001 | |
| <55 | 38.7 | |
| 55–64 | 42.3 | |
| ≥65 | 45.9 | |
|
| ||
| Sex | 0.085 | |
| Female | 41.6 | |
| Male | 43.2 | |
|
| ||
| Year | 0.002 | |
| 2014 | 42.6 | |
| 2015 | 39.4 | |
| 2016 | 44.3 | |
| 2017 | 42.8 | |
|
| ||
| Race | <0.001 | |
| Non-Hispanic White | 44.6 | |
| Non-Hispanic Black | 44.2 | |
| Hispanic | 46.0 | |
| Other/Unknown | 34.5 | |
|
| ||
| Diabetes | 0.004 | |
| Yes | 45.6 | |
| No | 41.7 | |
|
| ||
| Smoker | <0.001 | |
| Yes | 47.9 | |
| No | 41.3 | |
|
| ||
| COPD | 0.035 | |
| Yes | 47.8 | |
| No | 42.1 | |
|
| ||
| Hypertension | <0.001 | |
| Yes | 45.0 | |
| No | 40.2 | |
|
| ||
| ASA Class | <0.001 | |
| 1–2 | 39.2 | |
| 3–4 | 43.6 | |
|
| ||
| BMI (kg/m2) | 0.023 | |
| <18.5 | 44.9 | |
| 18.5–24.9 | 40.8 | |
| 25–29.9 | 41.6 | |
| ≥30 | 44.2 | |
|
| ||
| Histologic Indication | <0.001 | |
| Primary Hepatic Malignancy | 53.3 | |
| Metastatic Disease | 37.9 | |
| Benign | 43.2 | |
| Unknown | 28.3 | |
|
| ||
| Neoadjuvant Therapy Received | 0.214 | |
| Yes | 41.4 | |
| No | 42.7 | |
|
| ||
| Extent of Hepatectomy | <0.001 | |
| Minor | 37.6 | |
| Major* | 51.8 | |
|
| ||
| Surgical Approach | <0.001 | |
| Open | 47.1 | |
| Minimally Invasive** | 30.0 | |
|
| ||
| Length of Operation | <0.001 | |
| <3 hours | 33.2 | |
| 3–5 hours | 46.0 | |
| >5 hours | 52.6 | |
Major hepatectomy includes left hepatectomy, right hepatectomy, and trisectionectomy
Includes laparoscopic/robotic converted to open
Hierarchical multivariable modeling results are shown in Table 3 and demonstrated associations between use of prophylactic drains and patient age, smoking status, pathologic indication, extent of hepatectomy, operative approach, and time of operation. Sensitivity analyses stratified by hepatectomy type yielded similar patient-level predictors.
Table 3.
Multivariable Model of Factors Associated with Use of Drains Following Hepatectomy
| All Patients (N=10,530) | Minor (n=7,023) | Major (n=3,507) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Patient Characteristic | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Age, y | ||||||
| <55 | 1.0 | REF | 1.0 | REF | 1.0 | REF |
| 55–64 | 1.17 (1.05–1.30) | 0.004 | 1.23 (1.08–1.40) | 0.002 | 1.02 (0.85–1.21) | 0.861 |
| ≥65 | 1.34 (1.16–1.56) | <0.001 | 1.42 (1.19–1.69) | <0.001 | 1.15 (0.96–1.37) | 0.143 |
|
| ||||||
| Year | ||||||
| 2014 | 1.0 | REF | 1.0 | REF | 1.0 | REF |
| 2015 | 0.90 (0.71–1.13) | 0.372 | 0.84 (0.64–1.11) | 0.221 | 0.98 (0.76–1.27) | 0.888 |
| 2016 | 1.10 (0.85–1.43) | 0.466 | 1.04 (0.79–1.38) | 0.768 | 1.23 (0.90–1.70) | 0.192 |
| 2017 | 1.08 (0.87–1.34) | 0.483 | 0.98 (0.78–1.23) | 0.863 | 1.27 (0.95–1.70) | 0.107 |
|
| ||||||
| Race | ||||||
| Non-Hispanic White | 1.0 | REF | 1.0 | REF | 1.0 | REF |
| Non-Hispanic Black | 0.93 (0.71–1.22) | 0.613 | 0.93 (0.70–1.24) | 0.626 | 0.91 (0.61–1.34) | 0.624 |
| Hispanic | 1.09 (0.80–1.47) | 0.583 | 1.04 (0.78–1.39) | 0.779 | 1.18 (0.70–1.99) | 0.534 |
| Other/Unknown | 0.63 (0.38–1.07) | 0.086 | 0.77 (0.50–1.21) | 0.264 | 0.50 (0.26–0.93) | 0.029 |
|
| ||||||
| Diabetes | ||||||
| Yes | 1.00 (0.91–1.11) | 0.962 | 0.99 (0.87–1.13) | 0.904 | 1.02 (0.82–1.26) | 0.874 |
| No | 1.0 | REF | 1.0 | REF | 1.0 | REF |
|
| ||||||
| Smoker | ||||||
| Yes | 1.26 (1.11–1.43) | <0.001 | 1.28 (1.08–1.51) | 0.005 | 1.26 (1.08–1.47) | 0.003 |
| No | 1.0 | REF | 1.0 | REF | 1.0 | REF |
|
| ||||||
| COPD | ||||||
| Yes | 0.99 (0.81–1.21) | 0.928 | 1.11 (0.89–1.39) | 0.359 | 0.73 (0.50–1.09) | 0.125 |
| No | 1.0 | REF | 1.0 | REF | 1.0 | REF |
|
| ||||||
| Hypertension | ||||||
| Yes | 1.06 (0.95–1.17) | 0.284 | 1.01 (0.89–1.14) | 0.924 | 1.18 (0.97–1.44) | 0.093 |
| No | 1.0 | REF | 1.0 | REF | 1.0 | REF |
|
| ||||||
| ASA Class | ||||||
| 1–2 | 1.0 | REF | 1.0 | REF | 1.0 | REF |
| 3–4 | 1.06 (0.91–1.25) | 0.450 | 1.03 (0.87–1.22) | 0.741 | 1.05 (0.84–1.32) | 0.645 |
|
| ||||||
| BMI (kg/m2) | ||||||
| <18.5 | 1.16 (0.83–1.63) | 0.391 | 1.65 (1.05–2.59) | 0.030 | 0.72 (0.44–1.21) | 0.216 |
| 18.5–24.9 | 1.0 | REF | 1.0 | REF | 1.0 | REF |
| 25–29.9 | 0.98 (0.88–1.10) | 0.775 | 1.12 (0.98–1.28) | 0.097 | 0.80 (0.66–0.97) | 0.026 |
| ≥30 | 1.06 (0.95–1.18) | 0.270 | 1.18 (1.04–1.35) | 0.011 | 0.90 (0.73–1.10) | 0.304 |
|
| ||||||
| Histologic Indication | ||||||
| Primary Hepatic Malignancy | 1.80 (1.53–2.12) | <0.001 | 1.87 (1.56–2.25) | <0.001 | 1.69 (1.32–2.16) | <0.001 |
| Metastatic Disease | 1.0 | REF | 1.0 | REF | 1.0 | REF |
| Benign | 1.77 (1.43–2.20) | <0.001 | 1.90 (1.52–2.39) | <0.001 | 1.52 (1.15–2.01) | 0.003 |
| Unknown | 0.69 (0.25–1.86) | 0.458 | 1.42 (0.78–2.60) | 0.250 | 0.22 (0.06–0.78) | 0.019 |
|
| ||||||
| Extent of Hepatectomy | ||||||
| Minor | 1.0 | REF | -- | -- | -- | -- |
| Major** | 1.42 (1.15–1.74) | 0.001 | -- | -- | -- | -- |
|
| ||||||
| Surgical Approach | ||||||
| Open | 1.94 (1.49–2.53) | <0.001 | 2.02 (1.55–2.63) | <0.001 | 1.68 (1.17–2.42) | 0.005 |
| Minimally Invasive*** | 1.0 | REF | 1.0 | REF | 1.0 | REF |
|
| ||||||
| Length of Operation | ||||||
| <3 hours | 1.0 | REF | 1.0 | REF | 1.0 | REF |
| 3–5 hours | 1.57 (1.28–1.94) | <0.001 | 1.52 (1.23–1.87) | <0.001 | 1.65 (1.16–2.34) | 0.006 |
| >5 hours | 2.13 (1.50–3.03) | <0.001 | 2.31 (1.64–3.26) | <0.001 | 2.10 (1.31–3.39) | 0.002 |
Major hepatectomy includes left hepatectomy, right hepatectomy, and trisectionectomy
Includes laparoscopic/robotic converted to open
Patient Outcomes
The overall rate of SSI and bile leak was 7.1% and 5.8%, respectively. Adjusted hierarchical multivariable models are shown in Table 4.
Table 4.
Multivariable Model of Factors Associated with Surgical Site Infection and Bile Leak Following Hepatectomy*
| Surgical Site Infection (n=748, 7.1%) | Bile Leak (n=611, 5.8%) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Patient Characteristic | Rate | OR (95% CI) | P Value | Rate | OR (95% CI) | P Value |
| Age, y | ||||||
| <55 | 6.5 | 1.0 | REF | 5.2 | 1.0 | REF |
| 55–64 | 7.3 | 1.00 (0.82–1.21) | 0.999 | 5.2 | 0.99 (0.78–1.25) | 0.931 |
| ≥65 | 7.5 | 1.02 (0.85–1.22) | 0.868 | 6.8 | 1.26 (1.01–1.57) | |
|
| ||||||
| Sex | ||||||
| Female | 5.8 | 1.0 | REF | 5.5 | 1.0 | REF |
| Male | 8.7 | 1.30 (1.09–1.55) | 0.004 | 6.2 | 1.01 (0.82–1.24) | 0.925 |
|
| ||||||
| Year | ||||||
| 2014 | 6.9 | 1.0 | REF | 6.0 | 1.0 | REF |
| 2015 | 7.3 | 1.11 (0.88–1.41) | 0.385 | 6.0 | 1.02 (0.76–1.37) | 0.875 |
| 2016 | 6.5 | 0.96 (0.77–1.21) | 0.761 | 6.6 | 1.13 (0.83–1.53) | 0.440 |
| 2017 | 7.7 | 1.17 (0.92–1.50) | 0.204 | 5.0 | 0.85 (0.59–1.21) | 0.368 |
|
| ||||||
| Race | ||||||
| Non-Hispanic White | 6.8 | 1.0 | REF | 5.7 | 1.0 | REF |
| Non-Hispanic Black | 7.2 | 1.08 (0.82–1.42) | 0.595 | 6.5 | 1.24 (0.91–1.69) | 0.182 |
| Hispanic | 7.0 | 1.08 (0.75–1.55) | 0.681 | 4.3 | 0.76 (0.49–1.18) | 0.220 |
| Other/Unknown | 7.8 | 1.20 (0.92–1.56) | 0.176 | 6.0 | 1.26 (0.86–1.84) | 0.231 |
|
| ||||||
| Diabetes | ||||||
| Yes | 8.2 | 1.02 (0.81–1.28) | 0.846 | 5.8 | 0.88 (0.68–1.12) | 0.300 |
| No | 6.9 | 1.0 | REF | 5.8 | 1.0 | REF |
|
| ||||||
| Smoker | ||||||
| Yes | 8.1 | 1.09 (0.91–1.30) | 0.367 | 5.8 | 0.88 (0.69–1.13) | 0.313 |
| No | 6.9 | 1.0 | REF | 5.8 | 1.0 | REF |
|
| ||||||
| COPD | ||||||
| Yes | 10.4 | 1.47 (1.03–2.11) | 0.034 | 6.4 | 1.05 (0.70–1.58) | 0.814 |
| No | 7.0 | 1.0 | REF | 5.8 | 1.0 | REF |
|
| ||||||
| Hypertension | ||||||
| Yes | 7.7 | 1.05 (0.87–1.27) | 0.600 | 6.0 | 0.93 (0.77–1.12) | 0.446 |
| No | 7.0 | 1.0 | REF | 5.6 | 1.0 | REF |
|
| ||||||
| ASA Class | ||||||
| 1–2 | 5.8 | 1.0 | REF | 6.1 | 1.0 | REF |
| 3–4 | 7.6 | 1.09 (0.86–1.38) | 0.481 | 5.0 | 1.07 (0.85–1.36) | 0.565 |
|
| ||||||
| BMI (kg/m2) | ||||||
| <18.5 | 3.9 | 0.54 (0.27–1.06) | 0.073 | 5.6 | 0.91 (0.47–1.75) | 0.783 |
| 18.5–24.9 | 7.0 | 1.0 | REF | 5.7 | 1.0 | REF |
| 25–29.9 | 6.9 | 0.93 (0.77–1.11) | 0.411 | 5.7 | 0.98 (0.82–1.18) | 0.851 |
| ≥30 | 7.5 | 1.04 (0.88–1.23) | 0.639 | 5.9 | 1.03 (0.83–1.27) | 0.817 |
|
| ||||||
| Histologic Indication | ||||||
| Primary Hepatic Malignancy | 8.9 | 1.12 (0.95–1.33) | 0.185 | 7.5 | 1.05 (0.82–1.33) | 0.714 |
| Metastatic Disease | 4.6 | 1.0 | REF | 5.6 | 1.0 | REF |
| Benign | 4.9 | 0.90 (0.73–1.12) | 0.363 | 4.9 | 1.01 (0.75–1.36) | 0.935 |
| Unknown | 5.6 | 0.72 (0.34–1.50) | 0.378 | 4.6 | 0.98 (0.55–1.76) | 0.954 |
|
| ||||||
| Neoadjuvant Therapy Received | ||||||
| Yes | 8.5 | 1.11 (0.89–1.38) | 0.346 | 6.3 | 1.01 (0.77–1.31) | 0.961 |
| No | 6.5 | 1.0 | REF | 5.5 | 1.0 | REF |
|
| ||||||
| Extent of Hepatectomy | ||||||
| Minor | 5.9 | 1.0 | REF | 4.3 | 1.0 | REF |
| Major** | 9.5 | 1.16 (0.98–1.37) | 0.078 | 8.7 | 1.38 (1.17–1.63) | <0.001 |
|
| ||||||
| Surgical Approach | ||||||
| Open | 8.4 | 1.92 (1.55–2.38) | <0.001 | 6.8 | 1.46 (1.11–1.92) | 0.007 |
| Minimally Invasive*** | 3.6 | 1.0 | REF | 3.1 | 1.0 | REF |
|
| ||||||
| Length of Operation | ||||||
| <3 hours | 4.3 | 1.0 | REF | 3.2 | 1.0 | REF |
| 3–5 hours | 7.9 | 1.54 (1.25–1.90) | <0.001 | 6.4 | 1.51 (1.19–1.93) | 0.001 |
| >5 hours | 10.9 | 2.04 (1.62–2.57) | <0.001 | 9.5 | 2.03 (1.52–2.72) | <0.001 |
|
| ||||||
| Prophylactic Drain Placed | ||||||
| Yes | 9.3 | 1.49 (1.24–1.78) | <0.001 | 11.2 | 6.08 (4.47–8.27) | <0.001 |
| No | 5.4 | 1.0 | REF | 1.8 | 1.0 | REF |
Surgical Site Infection (SSI) includes superficial, deep, and organ space infections not present at the time of surgery
Major hepatectomy includes left hepatectomy, right hepatectomy, and trisectionectomy
Includes laparoscopic/robotic converted to open
Hospital-Level Variation in Use of Prophylactic Drains Following Hepatectomy
Hospital-level analysis included 130 hospitals over the study period. The median number of observations at each hospital was 43 (IQR: 19–104; Range 1–550). The median rate of prophylactic drain use by hospital was 47% (IQR 21%−69%; range 0% to 100%). Similar results were obtained when limiting analysis to the 113 hospitals with at least 10 observations over the study period (median 47%, IQR 22%−67%; range 0% to 100%, Figure 1). A total of 47% (52/113) of hospitals with at least 10 observations used prophylactic drains in more than 50% of patients. For minor hepatectomy, 40% (45/113) left drains in more than half of patients, while 62% (70/113) left drains in more than half of patients following major hepatectomy. Hierarchical analysis demonstrated that 43% (95%CI 37% to 51%) of the overall variation in use of prophylactic drains was due to hospital-level practices. When adjusting for patient characteristics, 78% of measurable variation was at the hospital level, while 23% was due to patient selection factors.
Figure 1. Utilization of Prophylactic Drains at 113 hospitals.
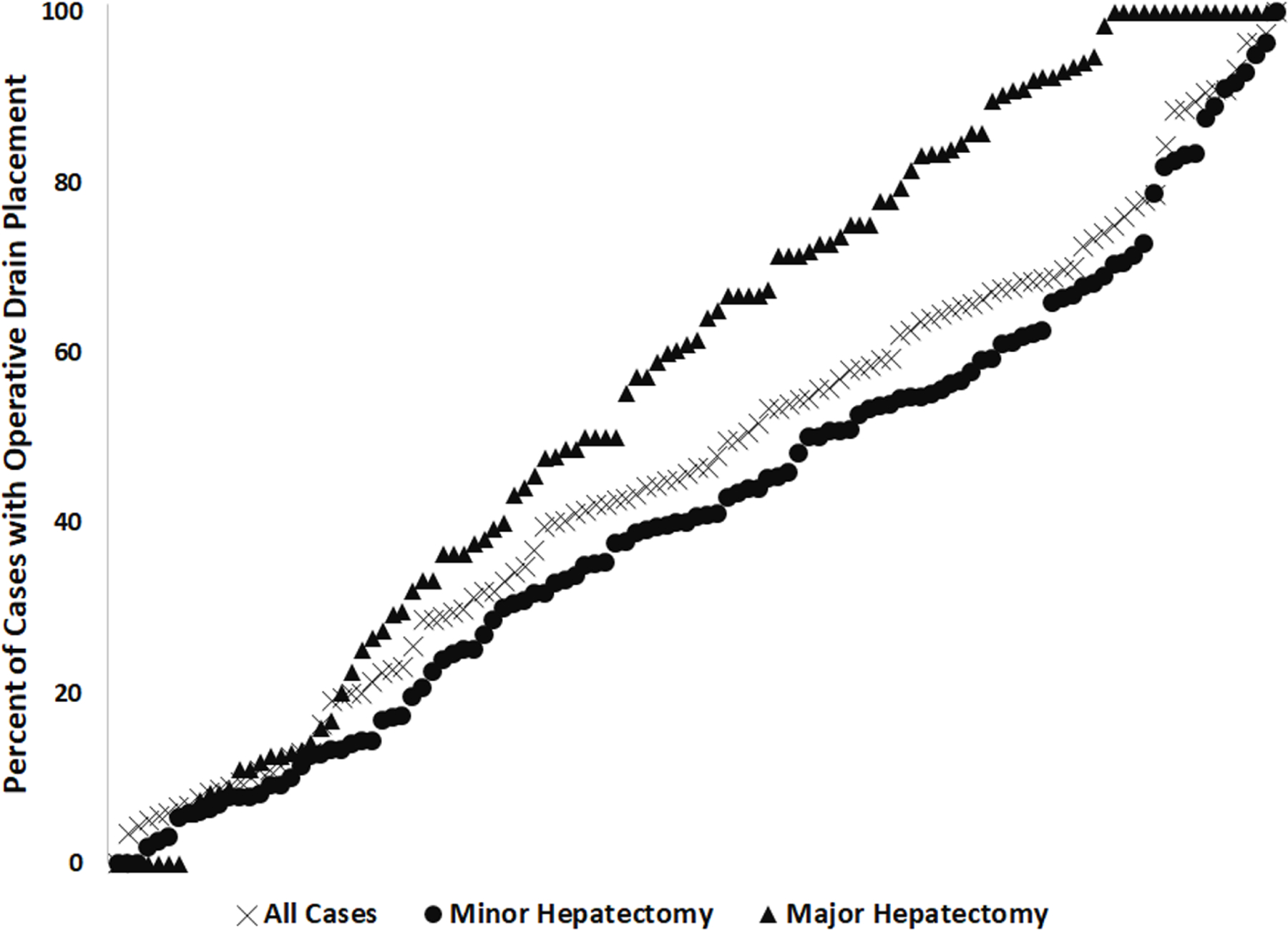
Hospital-level rates of prophylactic drain use. Each marker represents a hospital, with black representing overall use, light blue representing drain use in major hepatectomy, and purple representing drain use in minor hepatectomy. Graph depicts only those hospitals with at least 10 observations during the study period.
Discussion
In this study, a national cohort of patients undergoing hepatectomy without biliary reconstruction or concomitant bowel resection were analyzed to ascertain factors associated with the use of prophylactic operative drainage and hospital-level variation in the use of prophylactic drains. Several patient factors, including age, comorbidities, and pathology were associated with prophylactic drain use. Patients that received prophylactic drains were more likely to experience surgical site infection or bile leak even when adjusting for comorbidities and extent of resection. Variation in drain use appears to be heavily driven by hospital practice rather than patient characteristics.
One of the striking findings of this study is consistent use of prophylactic drains over time, despite high-level evidence indicating they do not improve outcomes, and may even contribute to postoperative infections.14–16 Final models demonstrated no change in drain use over time, with 42.6% of patients in 2014 and 42.8% of patients in 2017 receiving drains. While there is often a significant delay between publication of data and clinical implementation,29 the flat rates across the study period are somewhat surprising. Moreover, it was observed that drains were used in more than one third of minor hepatectomies. While some use of prophylactic drainage following major hepatectomy is expected due to surgical complexity or a high clinical concern for a postoperative bile leak (e.g., positive leak test), the persistently high rate of drain use following minor hepatectomy was less predictable and surprising.
The worse outcomes in patients with prophylactic drain placement is in line with prior extensive literature, including previous observational studies of ACS NSQIP, which have linked infectious complications including bile leak and SSI with use of prophylactic drains.13 Previous work by Brauer et al demonstrated higher rates of postoperative infections complications in patients with prophylactic drains in a propensity matched analysis.13 These results persist in this more modern analysis, despite a relatively restricted cohort (isolated hepatectomy without biliary reconstruction, bowel resection, cirrhosis, or ascites).
The most important and unique results of this study are the statistics on hospital variation in use of prophylactic drains. Inclusion criteria for this study were designed specifically to approximate those procedures that surgeons would be most comfortable leaving undrained, which is reflected by many facilities having very low rates of operative drainage. Importantly, analysis of variance in use of prophylactic drains indicated that more than only a minority of differences in hospital drain practices are explained by differences at the patient level, with significantly more variation being between facilities and independent of patient characteristics. Similar hospital variation was also observed in both major and minor hepatectomy, further indicating that drain use may be more dependent on local practice patterns than patient or technical considerations. In this cohort, made up on relatively straightforward resections to approximate those patients in which drain use is least supported, the substantial hospital variation provides a notable target for improvement.
There are caveats in interpretation of these findings. First, annual volume data were not available and it is possible that facilities with high drain use rates are also relatively low volume and thus more uncomfortable with the procedure or unfamiliar with the literature. The impact of this limitation is mitigated by using the ACS NSQIP hepatectomy targeted dataset, which is comprised mostly of relatively high-volume centers. Second, a lack of surgeon-level data makes it impossible to differentiate between surgeon and hospital practice, though it is likely that individual surgeon practice underlies the hospital-level findings. Third, ACS NSQIP does not have some data that might influence the use of prophylactic drains (e.g. anatomic variation, difficulty of the resection). However, the fact that many hospitals had very low or no drain use indicates that these missing data do not explain hospital variation.
Conclusion
Despite high-level evidence indicating that routine use of prophylactic drainage following hepatectomy is not required, drains are still commonly placed in both major and minor hepatectomy. While some patient factors are associated with drain use, hospital-specific patterns appear to be a major driver and could be significantly improved. Additional high-level data from multi-institutional prospective trials may be required to further change national practice patterns.
Funding:
RJE was supported by a postdoctoral research fellowship (Agency for Healthcare Research and Quality 5T32HS000078). RJE and BCB were supported by the American College of Surgeons Clinical Scholars in Residence Program. The American College of Surgeons as an organization had no role in the design and conduct of the study; analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Views expressed in this work represent those of the authors only. RPM is supported by the Agency for Healthcare Quality (K12HS026385) and an Institutional Research Grant from the American Cancer Society (IRG-18-163-24).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: the authors report no conflicts of interest, financial or otherwise, related to this work.
References
- 1.Tsao JI, Loftus JP, Nagorney DM, Adson MA, Ilstrup DM. Trends in morbidity and mortality of hepatic resection for malignancy. A matched comparative analysis. Annals of surgery 1994;220(2):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengmark S, Hafstrom L, Jeppsson B, Sundqvist K. Primary carcinoma of the liver: improvement in sight? World journal of surgery 1982;6(1):54–60. [DOI] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Annals of surgery 2002;236(4):397–406; discussion −7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanabe KK. The past 60 years in liver surgery. Cancer 2008;113(7 Suppl):1888–96. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita Y, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Shimada M, et al. Bile leakage after hepatic resection. Annals of surgery 2001;233(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capussotti L, Ferrero A, Vigano L, Sgotto E, Muratore A, Polastri R. Bile leakage and liver resection: Where is the risk? Arch Surg 2006;141(7):690–4; discussion 5. [DOI] [PubMed] [Google Scholar]
- 7.Guillaud A, Pery C, Campillo B, Lourdais A, Sulpice L, Boudjema K. Incidence and predictive factors of clinically relevant bile leakage in the modern era of liver resections. HPB : the official journal of the International Hepato Pancreato Biliary Association 2013;15(3):224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakamoto K, Tamesa T, Yukio T, Tokuhisa Y, Maeda Y, Oka M. Risk Factors and Managements of Bile Leakage After Hepatectomy. World journal of surgery 2016;40(1):182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donadon M, Costa G, Cimino M, Procopio F, Del Fabbro D, Palmisano A, et al. Diagnosis and Management of Bile Leaks After Hepatectomy: Results of a Prospective Analysis of 475 Hepatectomies. World journal of surgery 2016;40(1):172–81. [DOI] [PubMed] [Google Scholar]
- 10.Bona S, Gavelli A, Huguet C. The role of abdominal drainage after major hepatic resection. Am J Surg 1994;167(6):593–5. [DOI] [PubMed] [Google Scholar]
- 11.Messager M, Sabbagh C, Denost Q, Regimbeau JM, Laurent C, Rullier E, et al. Is there still a need for prophylactic intra-abdominal drainage in elective major gastro-intestinal surgery? Journal of visceral surgery 2015;152(5):305–13. [DOI] [PubMed] [Google Scholar]
- 12.Belghiti J, Kabbej M, Sauvanet A, Vilgrain V, Panis Y, Fekete F. Drainage after elective hepatic resection. A randomized trial. Annals of surgery 1993;218(6):748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brauer DG, Nywening TM, Jaques DP, Doyle MB, Chapman WC, Fields RC, et al. Operative Site Drainage after Hepatectomy: A Propensity Score Matched Analysis Using the American College of Surgeons NSQIP Targeted Hepatectomy Database. J Am Coll Surg 2016;223(6):774–83e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooke-Smith M, Figueras J, Ullah S, Rees M, Vauthey JN, Hugh TJ, et al. Prospective evaluation of the International Study Group for Liver Surgery definition of bile leak after a liver resection and the role of routine operative drainage: an international multicentre study. HPB : the official journal of the International Hepato Pancreato Biliary Association 2015;17(1):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fong Y, Brennan MF, Brown K, Heffernan N, Blumgart LH. Drainage is unnecessary after elective liver resection. American journal of surgery 1996;171(1):158–62. [DOI] [PubMed] [Google Scholar]
- 16.Sun HC, Qin LX, Lu L, Wang L, Ye QH, Ren N, et al. Randomized clinical trial of the effects of abdominal drainage after elective hepatectomy using the crushing clamp method. Br J Surg 2006;93(4):422–6. [DOI] [PubMed] [Google Scholar]
- 17.Squires MH 3rd, Lad NL, Fisher SB, Kooby DA, Weber SM, Brinkman A, et al. Value of primary operative drain placement after major hepatectomy: a multi-institutional analysis of 1,041 patients. Journal of the American College of Surgeons 2015;220(4):396–402. [DOI] [PubMed] [Google Scholar]
- 18.Petrowsky H, Demartines N, Rousson V, Clavien PA. Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Annals of surgery 2004;240(6):1074–84; discussion 84–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurusamy KS, Samraj K, Davidson BR. Routine abdominal drainage for uncomplicated liver resection. The Cochrane database of systematic reviews 2007(3):CD006232. [DOI] [PubMed] [Google Scholar]
- 20.Shwaartz C, Fields AC, Aalberg JJ, Divino CM. Role of Drain Placement in Major Hepatectomy: A NSQIP Analysis of Procedure-Targeted Hepatectomy Cases. World journal of surgery 2017;41(4):1110–8. [DOI] [PubMed] [Google Scholar]
- 21.Liu CL, Fan ST, Lo CM, Wong Y, Ng IO, Lam CM, et al. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Annals of surgery 2004;239(2):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YI, Fujita S, Hwang VJ, Nagase Y. Comparison of Abdominal Drainage and No-drainage after Elective Hepatectomy: A Randomized Study. Hepato-gastroenterology 2014;61(131):707–11. [PubMed] [Google Scholar]
- 23.Spolverato G, Ejaz A, Kim Y, Hall BL, Bilimoria K, Cohen M, et al. Patterns of care among patients undergoing hepatic resection: a query of the National Surgical Quality Improvement Program-targeted hepatectomy database. J Surg Res 2015;196(2):221–8. [DOI] [PubMed] [Google Scholar]
- 24.Cohen ME, Ko CY, Bilimoria KY, Zhou L, Huffman K, Wang X, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg 2013;217(2):336–46e1. [DOI] [PubMed] [Google Scholar]
- 25.User Guide for the 2016 ACS NSQIP Participant Use Data File (PUF) https://www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx: American College of Surgeons National Surgical Quality Improvement Program; October2017. [
- 26.Allison PD. Handling Missing Data by Maximum Likelihood. SAS Global Forum 2012: Statistics and Data Analysis 2012.
- 27.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149(5):680–8. [DOI] [PubMed] [Google Scholar]
- 28.Guidelines (2013) for managing overweight and obesity in adults. Preface to the Expert Panel Report (comprehensive version which includes systematic evidence review, evidence statements, and recommendations). Obesity (Silver Spring) 2014;22Suppl 2:S40. [DOI] [PubMed] [Google Scholar]
- 29.Balas EA, Boren SA. Managing Clinical Knowledge for Health Care Improvement. Yearb Med Inform 2000(1):65–70. [PubMed] [Google Scholar]


