Abstract
Background:
The utility of adjuvant chemotherapy after resection of colorectal liver metastasis (CLM) in patients with rapid recurrence after adjuvant chemotherapy for their primary tumor is unclear. The aim of this study was to evaluate the oncologic benefit of adjuvant hepatic arterial plus systemic chemotherapy (HAIC+Sys) in patients with early CLM.
Study design:
A retrospective analysis of patients with early CLM (≤12 months of adjuvant chemotherapy for primary tumor) who received either HAIC+Sys, adjuvant systemic chemotherapy alone (Sys), or active surveillance (Surgery alone) following resection of CLM was performed. Recurrence and survival were compared between treatment groups using Kaplan-Meier methods and Cox proportional hazards models.
Results:
Of 239 patients undergoing resection of early CLM, 79 (33.1%) received HAIC+Sys, 77 (32.2%) received Sys, and 83 (34.7%) had Surgery alone. HAIC+Sys was independently associated with reduced risk of RFS events (adjusted hazard ratio [HRadj]: 0.64, 95%CI:0.44–0.94, p=0.022) and all-cause mortality (HRadj: 0.54, 95%CI:0.36–0.81, p=0.003) compared to Surgery alone patients. Largest tumor >5cm (HRadj: 2.03, 95%CI: 1.41–2.93, p<0.001) and right-sided colon tumors (HRadj: 1.93, 95%CI: 1.29–2.89, p=0.002) were independently associated with worse OS.
Conclusion:
Adjuvant HAIC+Sys after resection of early CLM that occur after chemotherapy for node-positive primary is associated with improved outcomes.
Keywords: colorectal cancer, colorectal liver metastasis, hepatic arterial infusion chemotherapy, systemic chemotherapy, adjuvant therapy
Graphical Abstract
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related death in the United States, with approximately 140,000 new patients diagnosed annually 1. The risk of death is directly related to the development of distant metastatic disease, the prevention of which is the rationale for adjuvant therapy. The benefit of adjuvant chemotherapy for selected stage II and stage III colon cancer has been clearly established. Standard adjuvant chemotherapy regimens include fluorouracil (5-FU) with or without oxaliplatin 2,3. The relative risk of recurrence and death is reduced by approximately 20–30% with fluoropyrimidine monotherapy 3–5. When oxaliplatin is added to this treatment regimen, additional improvements in overall survival (OS) have been reported in three individual landmark trials 6–8 and a pooled analysis 9. Although colon and rectal cancers are frequently grouped as a single entity, there are important differences in treatment approach and pattern of recurrence between these two malignancies. Due to the lack of data on the adjuvant management of rectal cancer, extrapolation from colon cancer studies has been used to evaluate the optimal systemic therapy regimen in rectal cancer, but recent data suggest a benefit of adjuvant modern chemotherapy in stage II/III rectal cancer previously treated with neoadjuvant chemoradiation and surgery 10.
The utility of adjuvant systemic chemotherapy after resection of colorectal liver metastasis (CLM), especially in patients who have recently received chemotherapy for their node-positive primary tumor, is controversial. Several prospective randomized-controlled trials (RCT) have evaluated the role of perioperative or adjuvant systemic chemotherapy after resection of CLM 11–14. Recurrence-free survival (RFS) was improved in one of these trials; however, there was no difference in OS among patients receiving perioperative or adjuvant chemotherapy as compared to those who received surgery alone 11–15. Adjuvant hepatic arterial infusion chemotherapy (HAIC) combined with systemic 5-FU was associated with improved RFS and OS as compared to adjuvant systemic 5-FU alone in patients with resected CLM 16,17. Additionally, HAIC has proven efficacy in patients with advanced disease who have “failed” one or more lines of systemic treatment.
Patients who receive adjuvant systemic chemotherapy after resection of CRC and recur with resectable CLM soon afterward present a particularly difficult clinical situation. These patients are typically considered to be systemic chemotherapy failures and have limited options for adjuvant systemic chemotherapy at the time of resection of CLM. In the palliative setting, it is well known that response rates are very low after failure of first-line systemic therapy, an observation that calls into question the utility of further systemic treatment after resection of early liver metastases. This study investigated whether adjuvant therapy with HAIC and systemic chemotherapy (HAIC+Sys) or systemic chemotherapy alone (Sys) is associated with improved oncological outcome after resection of CLM in patients who had rapidly developed CLM after adjuvant chemotherapy for their node-positive primary tumor.
METHODS
Study Design
This retrospective analysis included patients with resected “early” CLM identified from a prospectively maintained institutional database at Memorial Sloan Kettering Cancer Center. Early CLM was defined as metachronous occurrence of CLM within 12 months of finishing adjuvant chemotherapy for primary CRC. Patients who developed CLM within the first 3 months after primary resection were excluded as 1) these tumors most likely constitute missed synchronous metastases and 2) patients had not received more than 1–2 cycles of chemotherapy in this time period and therefore did not meet the criteria of “failed” chemotherapy. Only patients with stage III CRC tumors (node-positive disease) who received adjuvant chemotherapy after resection of their primary tumor were included. Patients with completely resected extrahepatic disease diagnosed before or at the time of liver resection were included. Patients who were treated with ablative techniques exclusively or who suffered postoperative mortality in the first 90 days after liver surgery were excluded. The study was performed after approval by the Institutional Review Board at Memorial Sloan Kettering Cancer Center.
Therapeutic approach, demographic and clinical data, and clinical outcomes were extracted from the database. Preoperative staging at the time of liver surgery consisted of cross-sectional imaging, primarily contrast-enhanced computed tomography of the chest, abdomen, and pelvis; magnetic resonance imaging and fluorodeoxyglucose positron emission tomography were utilized selectively at the discretion of the treating clinician. Systemic chemotherapy regimens varied over time and were determined by the treating medical oncologist based on guidelines, chemotherapy history, and ongoing clinical trials. Modern chemotherapy was defined as regimens containing oxaliplatin or irinotecan. Adjuvant systemic chemotherapy for CLM was defined as systemic chemotherapy starting within 3 months after resection. Neoadjuvant chemotherapy was defined as systemic chemotherapy given after detection of CLM but prior to liver resection. All patients receiving adjuvant HAIC were scheduled to also receive systemic 5-FU with or without additional systemic chemotherapy, such as irinotecan or oxaliplatin 17. Patients received HAIC in the setting of a clinical trial or outside a trial at the discretion of their treating physician. For those receiving HAIC, an implantable pump was placed at the time of hepatic resection and positioned subcutaneously in the abdominal wall, with the catheter tip typically positioned in the gastroduodenal artery, as previously reported 17. Bilobar hepatic perfusion and lack of extrahepatic perfusion were confirmed by both intraoperative dye testing and postoperative technetium-99–labeled macroaggregated albumin nuclear medicine scanning. These patients received intra-arterial floxuridine (FUDR, Roche Pharmaceuticals), with six cycles scheduled. Patients who did not complete all six cycles due to toxicity continued on systemic chemotherapy as tolerated and were included in the HAIC group for all analyses. Patients who never received HAIC because of technical failure of the pump were included in the systemic chemotherapy or surgery alone group, depending on the treatment received. HAIC was routinely offered to patients at MSKCC during the study period.
Clinical risk scores (CRS) were calculated using a previously reported scoring system based on 5 factors: node-positive primary, disease-free interval (DFI) of CLM <12 months (synchronous), >1 CLM (multiple), size of the largest CLM >5 cm, and carcinoembryonic antigen (CEA) >200 µg/L, each scoring 1 point 18. Patients with a score of 0–3 were considered low-risk for recurrence, whereas patients with a score of 4–5 were deemed high-risk.
Consistent with previous publications, right-sided colon primary tumors were defined as tumors in the cecum, ascending colon, hepatic flexure, or transverse colon 19,20. Left-sided primary tumors were defined as those in the splenic flexure, descending colon, or sigmoid. Tumors within 16 cm of the anal verge were classified as rectal cancer. Patients with multiple primaries or unknown location of primary were excluded. TNM staging was performed based on the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 8th Edition.
Statistical Analysis
Disease and treatment characteristics were summarized using frequency and percentages for categorical variables and median and range for continuous variables; comparisons were made using the Chi-square test and Wilcoxon rank-sum test. The Fisher’s exact test was used for subgroups with numbers <5. All patients were followed every 3 to 6 months. A physical examination, CEA level determination, and cross-sectional imaging were performed at each visit. Time of recurrence was defined as the time of the first imaging that reported definitive or suspicious new tumors. For patients with biopsy-proven recurrence, the date of positive cytological or histological results was defined as the time of recurrence. The DFI was defined as time elapsed from primary resection to hepatic recurrence. RFS and OS were calculated from the date of liver resection until the first relapse or death, whichever came first (for RFS) or until the time of death (for OS). Patients who did not experience the event of interest by the end of the study were censored at the time of the last follow-up. RFS and OS were estimated using Kaplan-Meier methods and were compared between treatment groups using a log-rank test. A Cox proportional hazards model was used to evaluate independent associations between treatment groups and outcomes.
Covariates for inclusion in the multivariable OS and RFS survival analyses were chosen a priori. These covariates included nodal status of primary disease (N1 vs N2), largest CLM tumor size (>5 vs ≤5 cm), number of CLM tumor (1 vs >1), CEA prior hepatectomy (>200 vs ≤200 µg/L), DFI from surgical resection of primary cancer to CLM diagnosis (<12 vs ≥12 months) and tumor location (right colon vs left colon vs rectum) and they have been previously established as important known confounders in this disease group 18,19. MSKCC implemented modern chemotherapy into clinical practice in early 2002. To further control the heterogeneity of the study cohort due to different option of systemic therapies, and improved quality imaging over time, surgical era was dichotomized into patients resected between 1992–2001 vs 2002–2014 and included in the multivariable OS and RFS models. The estimates from the multivariable models were reported as adjusted hazard ratios (HRadj) along with 95% confidence interval (CI). All p values were based on 2-tailed statistical analysis, and p<0.05 was considered statistically significant. All analyses were performed with SAS version 9.4 (SAS Institute, Cary, North Carolina) or R version 3.6.0 (R Foundation for Statistical Computing Vienna, Austria).
RESULTS
Patient and Clinicopathological Characteristics
Of 2,623 patients who underwent resection for CLM between January 1992 and December 2014, 239 patients (9.1%) had complete resection of early CLM after receiving adjuvant chemotherapy for node-positive stage III CRC. Of the 239 patients, 79 (33.1%) received adjuvant HAIC+Sys, 77 (32.2%) received only adjuvant Sys, and 83 (34.7%) had no adjuvant therapy (Surgery alone). The demographic and clinicopathological characteristics for all 239 patients are summarized in Table 1. Extrahepatic disease at the time of liver resection was present in 6 patients in the Sys group, 3 in HAIC+Sys, and 1 in Surgery alone. All of these patients had incidental portal lymph node metastasis on surgical pathology and no distant metastatic disease.
Table 1.
Patient and clinicopathological variables.
| Variable | Overall (n = 239) |
HAIC+Sys (n = 79) |
Sys (n = 77) |
Surgery alone (n = 83) |
p value |
|---|---|---|---|---|---|
| Age at liver resection (years) | 60.8 (18.9 – 86.8) | 57.5 (37.2 – 81.3) | 59.2 (18.9 – 82.7) | 67.9 (34.3 – 86.8) | <0.001 |
| Body mass index | 26.5 (17.2 – 50.5) | 27.4 (17.9 – 41) | 26.3 (19.5 – 50.5) | 25.8 (17.2 – 37.6) | 0.075 |
| Number of resected liver segments | 3 (0.2 – 6) | 4 (1 – 5.2) | 4 (0.2 – 6) | 2 (0.2 – 6) | 0.003 |
| Size of largest CLM (cm) | 3.4 (0.7 – 16.5) | 3 (0.7 – 13) | 3.5 (1.1 – 16.5) | 3.5 (1.2 – 11) | 0.523 |
| Estimated blood loss at hepatectomy (mL) | 445 (10 – 5500) | 450 (50 – 5500) | 500 (50 – 2600) | 350 (10 – 2200) | 0.107 |
| CEA prior to liver resection (µg/L) | 14.4 (0.5 – 2375) | 13.2 (0.5 – 2375) | 18 (0.9 – 387) | 13.3 (1.3 – 823) | 0.298 |
| Sex | 0.150 | ||||
| Male | 131 | 44 (33.6) | 48 (36.6) | 39 (29.8) | |
| Female | 108 | 35 (32.4) | 29 (26.9) | 44 (40.7) | |
| Location of primary | 0.338 | ||||
| Right colon | 49 | 22 (44.9) | 15 (30.6) | 12 (24.5) | |
| Left colon | 136 | 41 (30.1) | 44 (32.4) | 51 (37.5) | |
| Rectum | 54 | 16 (29.6) | 18 (33.3) | 20 (37) | |
| Type of chemotherapy after primary resection | 0.043 | ||||
| 5-FU alone | 178 | 51 (28.7) | 60 (33.7) | 67 (37.6) | |
| 5-FU + oxaliplatin/irinotecan | 61 | 28 (45.9) | 17 (27.9) | 16 (26.2) | |
| CRS | 0.149 | ||||
| Low risk (0–3) | 218 | 76 (34.9) | 69 (31.7) | 73 (33.5) | |
| High risk (4–5) | 21 | 3 (14.3) | 8 (38.1) | 10 (47.6) | |
| Disease-free interval (DFI) | 0.385 | ||||
| ≥12 months | 136 | 40 (29.4) | 46 (33.8) | 50 (36.8) | |
| <12 months | 103 | 39 (37.9) | 31 (30.1) | 33 (32) | |
| Number of liver tumors | 0.078 | ||||
| Single lesion | 117 | 34 (29.1) | 34 (29.1) | 49 (41.9) | |
| Multiple lesions | 122 | 45 (36.9) | 43 (35.2) | 34 (27.9) | |
| Largest CLM tumor size | 0.979 | ||||
| ≤5cm | 187 | 62 (33.2) | 60 (32.1) | 65 (34.8) | |
| >5cm | 51 | 17 (33.3) | 17 (33.3) | 17 (33.3) | |
| N/A | 1 | 0 (0) | 0 (0) | 1 (100) | |
| Neoadjuvant chemotherapy prior hepatectomy | 0.836 | ||||
| No | 206 | 67 (32.5) | 66 (32) | 73 (35.4) | |
| Yes | 33 | 12 (36.4) | 11 (33.3) | 10 (30.3) | |
| Surgical era | 0.012 | ||||
| 1992–2001 | 144 | 38 (26%) | 47 (34%) | 59 (41%) | |
| 2002–2014 | 95 | 41 (43%) | 30 (32%) | 24 (25%) |
HAIC=hepatic arterial infusion chemotherapy; Sys=systemic chemotherapy; 5-FU=5-fluorouracil; CLM=colorectal liver metastasis;
CEA=carcinoembryonic antigen; CRS=clinical risk score; N/A=not available
For continuous variables, median (range) is shown. For categorical variables, n (row percentage) is shown
Recurrence-Free and Overall Survival
The median follow-up for survivors was 96 (range: 0.4–287) months, and 165 (range: 19–297), 150 (range: 45–246), and 55 (range: 0.4–205) months for patients treated with HAIC+Sys, Sys only, and Surgery alone, respectively. At the time of analysis, a total of 190 events (recurrence or death) for RFS (58 in HAIC+Sys, 68 in Sys and 64 in Surgery alone) were observed. The median RFS was 17.4 months (95%CI: 14.5–22.4) for the entire cohort, with a 5-year RFS of 26% (95%CI: 21–32%). In univariate analysis, patients treated with adjuvant HAIC+Sys after liver resection had a prolonged median RFS (HAIC+Sys: 22.5 months [95%CI: 14.7–47.3] vs. Sys: 14.4 months [95%CI: 11.3–22.4] vs. Surgery alone: 17.3 months [95%CI: 13.1–27.1]; p=0.046) (Figure 1A). Additional factors that were significantly associated with improved RFS from univariate analyses are shown in Supplemental Table 1.
Figure 1.
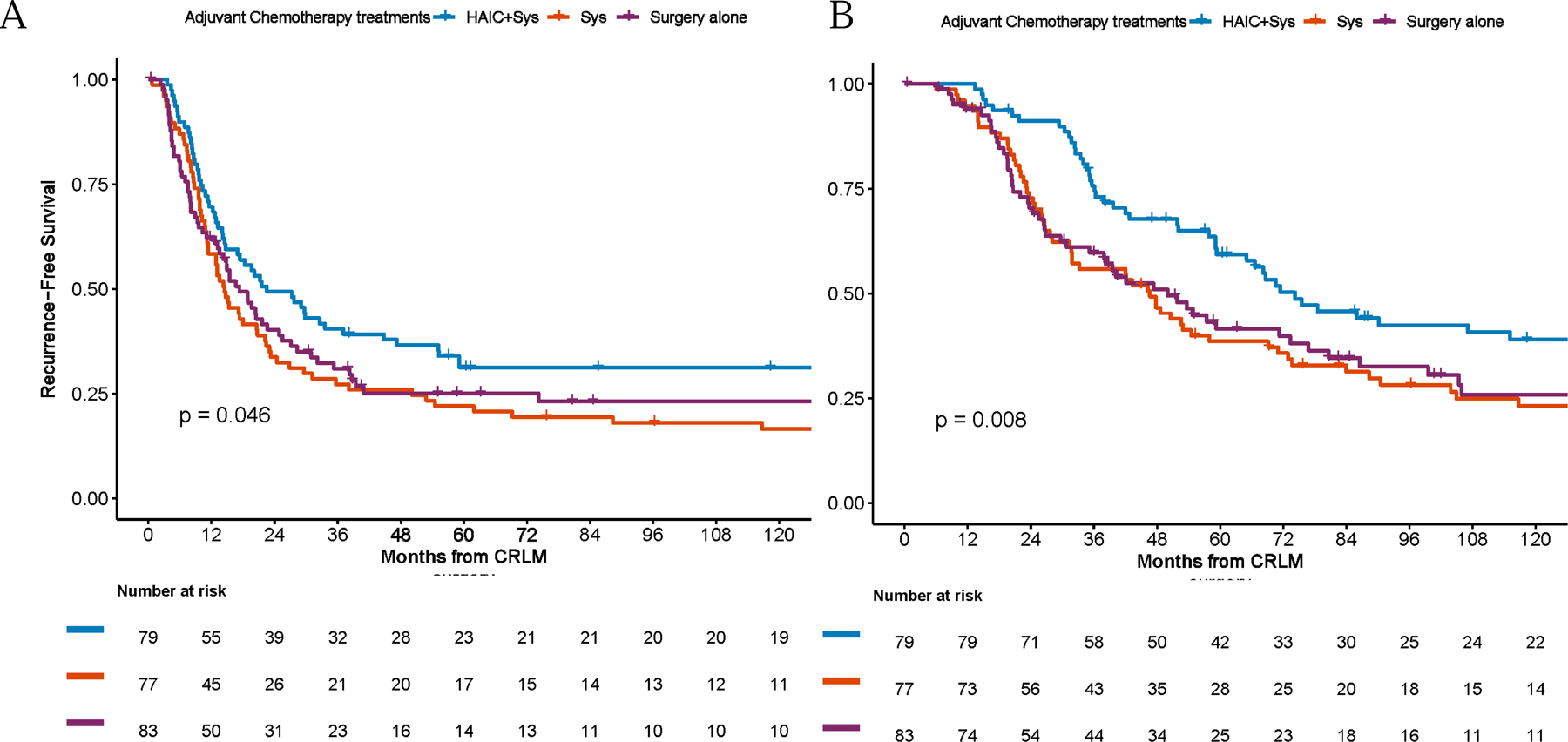
Recurrence-free (A) and overall-survival (B) by treatment groups
HAIC=hepatic arterial infusion chemotherapy; Sys=systemic chemotherapy; CLM=colorectal liver metastasis.
On multivariable analysis, after controlling for potential confounders, patients who received HAIC+Sys had a reduced risk of RFS events as compared to patients who had received Surgery alone (HRadj: 0.64, 95%CI: 0.44–0.94, p=0.022) (Table 2). In contrast, relative to the Surgery alone group, an elevated risk of RFS events among patients in the Sys group (HRadj: 1.06, 95%CI: 0.74–1.53, p=0.751) was not observed. Other factors that were independently associated with increased risk of RFS included largest tumor size >5 cm (HRadj: 1.64, 95%CI: 1.15–2.32, p=0.006), multiple CLM (HRadj: 1.53, 1.13–2.08, p=0.006), and right-sided primary tumors (HRadj: 1.76, 95%CI: 1.21–2.58, p=0.003).
Table 2.
Association between adjuvant chemotherapy and recurrence-free survival and overall survival after controlling for potential confounders – multivariable analysis
| Variable | Comparison | Recurrence-free survival | Overall survival | ||
|---|---|---|---|---|---|
| Hazard Ratio (95%CI) |
p value | Hazard Ratio (95%CI) |
p value | ||
| HAIC+Sys | Surgery alone | 0.64 (0.44 −0.94) | 0.022 | 0.54 (0.36 −0.81) | 0.003 |
| Sys | Surgery alone | 1.06 (0.74 −1.53) | 0.751 | 1.02 (0.69 −1.50) | 0.917 |
| Largest size of CLM (cm) | >5 vs. ≤5 | 1.64 (1.15 −2.32) | 0.006 | 2.03 (1.41 −2.93) | <0.001 |
| Number of CLM | 1 vs. <1 | 1.53 (1.13 −2.08) | 0.006 | 1.32 (0.95 −1.82) | 0.094 |
| CEA prior to hepatectomy (µg/L) | >200 vs. ≤200 | 1.77 (0.98 −3.21) | 0.060 | 1.62 (0.86 −3.05) | 0.132 |
| Disease-free interval (DFI) (months) | <12 vs. ≥12 | 1.13 (0.84 −1.53) | 0.419 | 0.96 (0.69 −1.34) | 0.829 |
| Nodal status of primary | N2 vs. N1 | 1.19 (0.87 −1.64) | 0.276 | 1.17 (0.83 −1.65) | 0.386 |
| Right-sided primary | Left-sided primary | 1.76 (1.21 −2.58) | 0.003 | 1.93 (1.29 −2.89) | 0.002 |
| Rectal cancer | Left-sided primary | 1.18 (0.82 −1.71) | 0.380 | 1.08(0.72 −1.62) | 0.714 |
| Surgical era | 2002–2014 vs 1992–2001 | 1.09 (0.80 −1.48) | 0.602 | 0.83 (0.69 −1.17) | 0.283 |
HAIC=hepatic arterial infusion chemotherapy; Sys=systemic chemotherapy; CLM=colorectal liver metastasis; CEA=carcinoembryonic antigen; pos=positive; neg=negative; CI=confidence interval
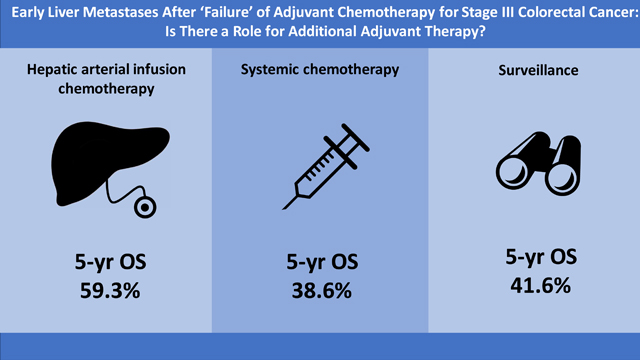
For the whole cohort, there were a total of 166 deaths at the time of analysis (49 in HAIC+Sys, 61 in Sys and 56 in Surgery alone). The median OS was 54.7 months (95%CI: 47.3–71.3), with a 5-year OS of 47% (95%CI: 40–54%). The median OS for patients treated with HAIC+Sys was longer than for patients in the Sys or Surgery alone groups (HAIC+Sys: 74.3 months [95%CI: 59.3–131.6] vs. Sys: 46.6 months [95%CI: 31.7–69.2] vs. Surgery alone: 50.0 months [95%CI: 35.2–76.8]; p=0.008) (Figure 1B). Other factors associated with improved OS from univariate analyses are shown in Supplemental Table 2.
Adjuvant treatment with HAIC+Sys remained independently associated with improved OS in multivariable analysis, and patients who received HAIC+Sys had a reduced risk of all-cause mortality as compared to patients in the Surgery alone group (HRadj: 0.54, 95%CI: 0.36–0.81; p=0.003) (Table 2). In addition, largest tumor size >5 cm (HRadj: 2.03, 95%CI: 1.41–2.93, p<0.001) and right-sided primary tumors (HRadj: 1.93, 95%CI: 1.29–2.89, p=0.002) were also independently associated with worse OS.
DISCUSSION
Patients who receive adjuvant systemic chemotherapy for resected node-positive stage III CRC and fail during or shortly after completion of treatment have limited chemotherapy options. Many of these patients are treated with further systemic chemotherapy, despite data from several trials that demonstrate that second-line agents have very limited activity 21–27. By contrast, HAIC does have activity in the second-line setting and is a proven adjuvant therapy for resected CLM 16,17,28. No prior study has addressed adjuvant therapy in the context of early CLM with prior adjuvant chemotherapy for the primary.
This study analyzed 239 consecutive patients who underwent complete resection for early metachronous CLM and found that adjuvant therapy with HAIC+Sys was independently associated with prolonged RFS and OS. Furthermore, the outcomes for patients receiving adjuvant systemic chemotherapy did not differ from those receiving surgery alone. These results are consistent with previously published studies on surgery for CLM 16,17,28. In a recent large study of 2,368 consecutive patients who underwent complete resection of CLM, Groot Koerkamp et al. demonstrated that adjuvant HAIC was independently associated with improved OS, despite more advanced disease in the HAIC group 28. The OS rates for patients in the present study receiving adjuvant systemic chemotherapy alone was similar to the OS found in other large studies. Hamady et al. reported a median OS of 45 months among 2,715 patients after resection of CLM 29. These results were nearly identical to the 48 months reported in this study.
It is noteworthy that no significant difference in OS was found between patients treated with adjuvant systemic chemotherapy and those receiving surgery alone. These findings are consistent with previously published results from several prospective RCT 11–14. For example, Nordlinger et al. found no difference in OS comparing the addition of perioperative FOLFOX4 chemotherapy (n=182) with surgery alone (n=182) for patients with resectable CLM 11. In the present analysis there was a shorter median follow-up time for patients undergoing surgery alone than that in the two other groups. However, 55 months, is an adequate follow up time given that recurrence usually occurs within 2 years after resection of CLM 11–14,29.
Among patients who develop a recurrence after resection of CLM, the liver is the only site of initial recurrence in approximately half of the patients 30. Therefore, HAIC+Sys has been investigated as an adjuvant strategy. HAIC takes advantage of the fact that CLM are perfused by the hepatic artery and drugs such as floxuridine have a high first-pass extraction in the liver31,32. A phase III RCT performed at MSKCC by Kemeny et al. found a 2-year OS and PFS benefit for patients treated with systemic 5-FU and HAIC compared to systemic 5-FU alone after resection of CLM16,17. An additional multicenter RCT found a significant reduction in RFS and hepatic RFS after resection in patients treated with adjuvant HAIC+Sys as compared with surgery alone33. The current study demonstrates that adjuvant HAIC is associated with improved outcomes in the specific subset of patients who “fail” adjuvant chemotherapy for their primary tumor.
The specific adjuvant chemotherapy regimen after primary resection was not associated with outcome in the analysis (Supplemental Table 1–2). Modern more effective chemotherapy may exert a selective pressure on its own, and patients with early failure may develop therapeutic resistant disease 34,35. A study by Andreou et al. showed that adjuvant FOLFOX for primary CRC was associated with a high rate of somatic mutations in liver metastases and inferior outcomes after hepatectomy for metachronous CLM 34. However, the use of adjuvant regional therapy with HAIC may be an effective therapeutic approach overcome this potential chemoresistance. As regional therapy gains more acceptance in the adjuvant therapy of CLM 36,37, further studies of this topic are hopefully forthcoming.
The role of resection in patients with extrahepatic metastatic disease is controversial. In this study, 6 patients (2.5%) had incidental portal lymph node metastasis found at the time of surgery but without any presence of other extrahepatic metastatic disease. These patients were included since they are typically included in most series on this topic 13,28.
In the present study a Cox hazards model was used to evaluate independent associations between treatment groups and outcomes. This method was chosen to avoid the difficulties of matching across three groups and excluding a number of unmatched patients, a source of bias itself. Research suggests that regression can be more powerful than matching in dealing with confounders 38. The covariates chosen to be included in the reported OS and RFS models were known confounders in this disease group 18,19 and included the individual component of CRS, tumor location and surgical era. To address the concerns that propensity score frame work and Inverse-probability-of-treatment-weighted (IPTW) method might yield a more robust result, a sensitivity analysis using IPTW methodology was performed which adjusted for baseline covariates (age, body mass index, number of resected liver segment, gender, ASA score, exposure to neoadjuvant chemotherapy prior hepatectomy, size of largest tumor at pathology, preoperative CEA, number of liver metastasis, nodal status from primary disease, DFI, location of primary tumor, any complication and surgical era) and the results and conclusion of the adjusted mean treatment effect on OS and RFS didn’t change substantially (data not shown).
The current study has several limitations. Most importantly, the difference in outcome between HAIC+Sys and Sys may be explained by selection bias. There are no specific selection criteria for the use of adjuvant HAIC at MSKCC and it is considered in nearly all cases. The decision to proceed with adjuvant HAIC is the result of extensive consultation with our own and external physicians and ultimately is at the discretion of the treating physicians and patients. Another source of bias was that the study covered a long-time period of 21 years, spanning the introduction of modern chemotherapy. Patients were more likely to have HAIC in the modern era, while the rate of surgery alone decreased over time. However, the surgical era was not associated with improved outcome multivariable analyses. The creation of a homogenous cohort that was limited to patients with stage histologically proven stage III CRC required a long time period to have an adequate number of patients. An intent to treat principle is important when studying adjuvant chemotherapy. Unfortunately, in the setting of a retrospective study, an intention-to-treat analysis was not feasible, since it is unknown what the preoperative intention was in the Sys and Surgery alone groups.
CONCLUSIONS
Adjuvant HAIC was independently associated with an improved RFS and OS in patients presenting with early CLM after adjuvant chemotherapy for node-positive CRC. This significant association remained after adjustment for known confounding factors. Patients who received adjuvant HAIC lived over 2 years longer than patients treated with systemic chemotherapy alone. Adjuvant systemic therapy alone was not associated with improved outcomes compared to surgery alone. Adjuvant HAIC+Sys is a promising therapy for patients with early metachronous CLM who have received prior systemic chemotherapy.
Supplementary Material
Acknowledgments:
Erin Patterson and Crystal Tran (Memorial Sloan Kettering Cancer Center) provided editorial support.
Funding:
This work was supported in part by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30CA008748. This funding source has no such involvement in the study design; collection, analysis, and interpretation of data; writing of the manuscript; and decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: The authors have nothing to disclose.
Prior presentation: This work was presented in part at the 2018 International Hepato-Pancreato-Biliary Annual Meeting (IHPBA).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67(1):7–30. 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Andre T, Boni C, Navarro M, et al. Improved Overall Survival With Oxaliplatin, Fluorouracil, and Leucovorin As Adjuvant Treatment in Stage II or III Colon Cancer in the MOSAIC Trial. Journal of Clinical Oncology 2009;27(19):3109–16. 10.1200/Jco.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 3.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322(6):352–8. 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 4.Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22(10):1797–806. 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 5.Quasar Collaborative G, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370(9604):2020–9. 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 6.Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27(19):3109–16. 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 7.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25(16):2198–204. 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 8.Schmoll HJ, Tabernero J, Maroun J, et al. Capecitabine Plus Oxaliplatin Compared With Fluorouracil/Folinic Acid As Adjuvant Therapy for Stage III Colon Cancer: Final Results of the NO16968 Randomized Controlled Phase III Trial. J Clin Oncol 2015;33(32):3733–40. 10.1200/JCO.2015.60.9107. [DOI] [PubMed] [Google Scholar]
- 9.Schmoll HJ, Twelves C, Sun W, et al. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol 2014;15(13):1481–92. 10.1016/S1470-2045(14)70486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong YS, Nam BH, Kim KP, et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol 2014;15(11):1245–53. 10.1016/S1470-2045(14)70377-8. [DOI] [PubMed] [Google Scholar]
- 11.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14(12):1208–15. 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 12.Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 2006;24(31):4976–82. 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 13.Primrose J, Falk S, Finch-Jones M, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol 2014;15(6):601–11. 10.1016/S1470-2045(14)70105-6. [DOI] [PubMed] [Google Scholar]
- 14.Ychou M, Hohenberger W, Thezenas S, et al. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol 2009;20(12):1964–70. 10.1093/annonc/mdp236. [DOI] [PubMed] [Google Scholar]
- 15.Khoo E, O’Neill S, Brown E, Wigmore SJ, Harrison EM. Systematic review of systemic adjuvant, neoadjuvant and perioperative chemotherapy for resectable colorectal-liver metastases. HPB (Oxford) 2016;18(6):485–93. 10.1016/j.hpb.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med 2005;352(7):734–5. 10.1056/NEJM200502173520723. [DOI] [PubMed] [Google Scholar]
- 17.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 1999;341(27):2039–48. 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 18.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LHJAos. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230(3):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creasy JM, Sadot E, Koerkamp BG, et al. The impact of primary tumor location on long-term survival in patients undergoing hepatic resection for metastatic colon cancer. Ann Surg Oncol 2018;25(2):431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss JM, Pfau PR, O’Connor ES, et al. Mortality by stage for right-versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results–Medicare data. J Clin Oncol 2011;29(33):4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22(2):229–37. 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 22.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25(12):1539–44. 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 23.Bennouna J, Hiret S, Bertaut A, et al. Continuation of Bevacizumab vs Cetuximab Plus Chemotherapy After First Progression in KRAS Wild-Type Metastatic Colorectal Cancer: The UNICANCER PRODIGE18 Randomized Clinical Trial. JAMA Oncol 2019;5(1):83–90. 10.1001/jamaoncol.2018.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30(28):3499–506. 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 25.Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 2015;16(5):499–508. 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 26.Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28(31):4706–13. 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 27.Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008;26(14):2311–9. 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 28.Groot Koerkamp B, Sadot E, Kemeny NE, et al. Perioperative Hepatic Arterial Infusion Pump Chemotherapy Is Associated With Longer Survival After Resection of Colorectal Liver Metastases: A Propensity Score Analysis. J Clin Oncol 2017;35(17):1938–44. 10.1200/JCO.2016.71.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamady ZZ, Lodge JP, Welsh FK, et al. One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach. Ann Surg 2014;259(3):543–8. 10.1097/SLA.0b013e3182902b6e. [DOI] [PubMed] [Google Scholar]
- 30.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 2009;250(3):440–8. 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 31.Ackerman NB. The blood supply of experimental liver metastases. IV. Changes in vascularity with increasing tumor growth. Surgery 1974;75(4):589–96. [PubMed] [Google Scholar]
- 32.Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol 1983;10(2):176–82. [PubMed] [Google Scholar]
- 33.Kemeny MM, Adak S, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy--an intergroup study. J Clin Oncol 2002;20(6):1499–505. 10.1200/JCO.2002.20.6.1499. [DOI] [PubMed] [Google Scholar]
- 34.Andreou A, Kopetz S, Maru DM, et al. Adjuvant chemotherapy with FOLFOX for primary colorectal cancer is associated with increased somatic gene mutations and inferior survival in patients undergoing hepatectomy for metachronous liver metastases. Ann Surg 2012;256(4):642–50. 10.1097/SLA.0b013e31826b4dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greaves M, Maley CCJN. Clonal evolution in cancer. Nature 2012;481(7381):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chouliaras K, Russell G, Levine E, et al. Hepatic arterial infusion chemotherapy for colorectal liver metastases revisited. HPB : the official journal of the International Hepato Pancreato Biliary Association 2020. [DOI] [PMC free article] [PubMed]
- 37.Buisman FE, Grünhagen DJ, Homs MY, et al. Adjuvant Hepatic Arterial Infusion Pump Chemotherapy After Resection of Colorectal Liver Metastases: Results of a Safety and Feasibility Study in The Netherlands. Ann Surg Oncol 2019;26(13):4599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brazauskas R, Logan BR. Observational Studies: Matching or Regression? Biol Blood Marrow Transplant 2016;22(3):557–63. 10.1016/j.bbmt.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


