Abstract
Background:
Both minimally invasive surgery (MIS) and open approaches for distal pancreatectomy are acceptable. MIS options include total laparoscopic/robotic (TLR) and hand-assist laparoscopy (HAL). When considering safety profile and specimen quality, the optimal approach is unknown.
Methods:
Patients who underwent distal pancreatectomy from 2010–2018 at two major academic institutions were included. Converted procedures were categorized into final approach. Ninety-day perioperative/pathologic outcomes of MIS and open were compared. Subset analyses between TLR vs HAL and HAL vs open were performed. Intent-to-treat analysis was performed.
Results:
Among 1006 patients, resection was performed by MIS in 35% (n = 352), open in 65%(n = 654). MIS had similar patient comorbidity profile as open but had increased operative time (183 vs 162 min; p < 0.01), lower estimated-blood-loss (EBL; 131 vs 341 mL; p < 0.01), fewer intraoperative blood transfusions (1.4 vs 5%; p < 0.01), shorter LOS (5.2 vs 7.2 days; p < 0.01). Tumor size was smaller (3.2 vs 4.4 cm; p < 0.01) with lower lymph node (LN) yield (14 vs 16; p < 0.01). When comparing HAL (n = 109) to TLR (n = 243), despite increased prior abdominal operations (60 vs 43%; p = 0.008), HAL had shorter operative time (167 vs 191 min; p < 0.01), similar length-of-stay (LOS; 5.4 vs 5.1 days; p = 0.27), and readmission rate (15 vs 13%; p = 0.47). When comparing HAL to open, the advantages of TLR approach persisted including lower EBL (171 vs 342 mL; p < 0.01), and shorter LOS (5.4 vs 7.2 days; p < 0.01). Although HAL had smaller tumors, it had a similar LN yield (16 vs 16; p = 0.80), and higher R0-rate (97 vs 83%; p < 0.01).
Conclusion:
Hand-assist laparoscopy is safe and feasible for distal pancreatectomy as operative time, complication profile, lymph node yield, and R0-rates are similar to open procedures, while maintaining the associated the advantages of a total laparoscopic/robotic approach with reduced blood loss and shorter length-of-stay.
Introduction
First described in 1913 by Mayo, distal pancreatectomy is commonly performed for tumors in the body or tail of the pancreas. The first successful laparoscopic distal resection of the pancreas was reported by Cuschieri in 1994, and its feasibility was further established by Gagner in his report of twelve patients with pancreatic neuroendocrine tumors.1,2 Since that time, the feasibility and safety of laparoscopic distal pancreatectomy have been well-documented in several studies, with similar or lower rates of operative and perioperative complications including pancreatic fistula, less operative blood loss, and decreased hospital length of stay compared to open distal pancreatectomy.3–5
The benefits of laparoscopic distal pancreatectomy have been replicated in benign, pre-malignant and malignant disease processes. Several studies have demonstrated its efficacy in the treatment of solid-pseudopapillary neoplasms and lymphoepithelial cysts.6,7 As the experience with laparoscopic distal pancreatectomy broadened, these results have been further recapitulated for malignant tumors and studies have proven oncologic adequacy of resection as defined by margin status and nodal assessment. In 2010, a large multicenter retrospective study by Kooby et al. demonstrated lower overall complications and surgical site infections associated with the laparoscopic approach compared to open when distal pancreatectomy was performed specifically for adenocarcinoma.8 Additionally, a meta-analysis in 2019 found no significant differences between minimally invasive and open approaches in terms of lymph nodes harvested, local recurrence rates, and overall survival.9
Despite apparent advantages and generalized acceptance of the laparoscopic technique, some have described several challenges including a steep learning curve, technical difficulty with increased proximity of the tumor to the celiac axis, and longer operative time.10 These challenges may be mitigated with the use of the laparoscopic hand-assist technique. Additionally, potential advantages to the hand-assist laparoscopic technique include those reported with open distal pancreatectomy including preservation of the surgeon’s ability to directly palpate the viscera, tumor, and surrounding structures. Furthermore, the ability to directly control hemorrhage may result in lower conversion rates, thus potentially preserving the advantages associated with a total laparoscopic approach.11,12
In review of the literature, no generalized accepted operative approach to distal pancreatectomy exists. There is very limited information on the outcomes of the laparoscopic hand-assist technique for benign, pre-malignant, and malignant tumors when compared to the total laparoscopic or open approaches. Given this gap, the primary aim of this study was to compare the hand-assist distal pancreatectomy technique to total minimally-invasive and open techniques. Intent-to-treat and final operative approach analyses were performed to evaluate the clinical impact of conversion.
Methods
Patient population
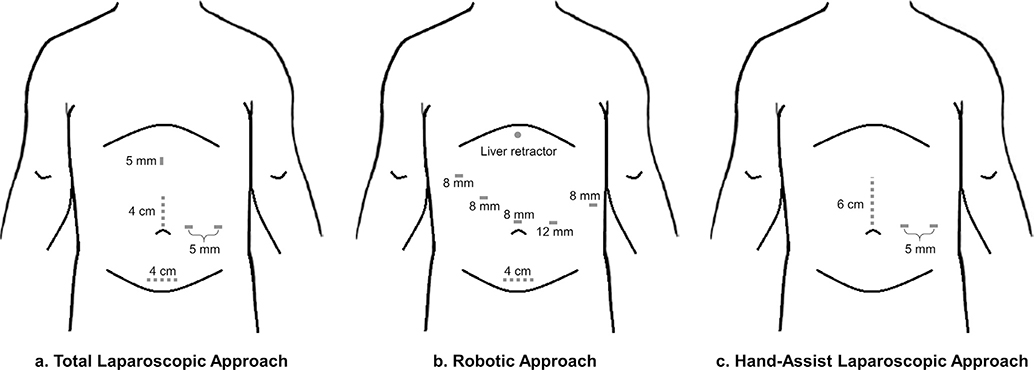
Participating institutions included Emory University Winship Cancer Institute and Memorial Sloan-Kettering Cancer Center. This study was approved by the Institutional Review Boards of each participating center. Patients who underwent distal pancreatic resection from 2010 to 2018 at each institution were identified. Patient demographics including sex, age and body mass index (BMI) were recorded. Histologic diagnoses were limited to pseudocyst, chronic pancreatitis, lymphoepithelial cyst, serous cystadenoma, solid pseudopapillary neoplasm, neuroendocrine tumor, mucinous cystic neoplasm, intraductal papillary mucinous neoplasm, adenocarcinoma, acinar cell carcinoma, cystic neoplasm with invasive carcinoma, and renal cell carcinoma. Operative details were obtained from dictated operative reports. Operative approach was defined as minimally invasive which included laparoscopic distal pancreatectomy and robotic distal pancreatectomy with an extraction port through a midline or Pfannenstiel incision (Fig. 1a,b), laparoscopic hand-assist with a hand port through an upper midline incision (Fig. 1c), or open performed through a midline laparotomy or left subcostal incision. Length of hospitalization, need for ICU admission or transfer, and need for perioperative blood transfusion were determined. Complications occurring within 90 days of operation were recorded.
Figure 1.
Schematic demonstrating port and incision sites for a) total laparoscopic approach b) robotic approach and c) hand-assist laparoscopic approach (extraction sites for laparoscopic and robotic approaches can be upper midline versus Pfannenstiel and some will add a third port for the hand-assist laparoscopic approach)
Patient characteristics, operative details, and postoperative outcomes of minimally invasive procedures (total laparoscopic, laparoscopic hand-assist, robotic-assisted) were compared with open approaches. Subset analyses of approaches were performed to compare the laparoscopic hand-assist technique with a total laparoscopic or robotic approach and with an open approach. Study results were evaluated on an as-treated basis and on an intention-to-treat basis. Reasons for conversion from a minimally invasive approach to a hand-assist or open approach were not captured.
Statistical analyses
All statistical analyses were performed using the SPSS 25.0 statistical package (IBM Inc., Armonk, NY). Statistical significance was pre-defined as 2-tailed p < 0.05. Chi-square or Fisher’s exact test were used for statistical analysis for categorical variables depending on the group size. Continuous variables were analyzed using t-tests or the Wilcoxon signed-rank test.
Results
Patient population
Among 1006 patients who underwent distal pancreatectomy for pancreatic lesions, mean age was 61 ± 14 years, 44% were male, and mean BMI was 28.4 ± 9.0 kg/m2. This cohort is further described in Table 1. Final pathologic diagnoses included cystic neoplasm with invasive carcinoma in 0.4% (n = 4), lymphoepithelial cyst in 0.6% (n = 6), acinar cell carcinoma in 0.9% (n = 10), pseudocyst in 1.7% (n = 17), chronic pancreatitis in 2.8% (n = 28), serous cystadenoma in 3.9% (n = 39), solid pseudopapillary neoplasm in 3.9% (n = 39), mucinous cystic neoplasm in 10.8% (n = 109), intraductal papillary mucinous neoplasm in 10.8% (n = 109), neuroendocrine tumor in 24.9% (n = 251), renal cell carcinoma in 24.9% (n = 251), and adenocarcinoma in 31.1% (n = 313).
Table 1.
Clinicopathologic factors, surgical variables, and outcomes of all patients
| Variable | All patients n = 1006 (%) |
|---|---|
| Baseline demographics | |
| Age (years, mean ± std) | 61 ± 14 |
| Male | 441 (44) |
| ASA class | |
| 1 | 16 (2) |
| 2 | 368 (37) |
| 3 | 588 (58) |
| 4 | 32 (3) |
| 5 | 0 (0) |
| BMI (kg/m2, mean ± std) | 28.4 ± 9.0 |
| Comorbidities | |
| Diabetes | 203 (20) |
| Congestive heart failure | 11 (1) |
| Severe COPD | 11 (1) |
| Chronic renal failure | 30 (3) |
| Prior abdominal operations | 461 (46) |
| Symptoms at presentation | |
| Incidental/asymptomatic | 575 (57) |
| Pancreatitis | 111 (11) |
| Jaundice | 1 (0.1) |
| Pain (not pancreatitis) | 188 (19) |
| Early satiety | 6 (0.6) |
| Bowel obstruction | 4 (0.4) |
| Other | 114 (11) |
| Intra-operative outcomes | |
| Pathologic diagnoses | |
| Benign | 129 (13) |
| Pre-malignant | 468 (47) |
| Malignant | 327 (33) |
| Missing | 82 (8) |
| Operative approach | |
| Open | 573 (57) |
| Total laparoscopic | 155 (15) |
| Laparoscopic hand-assist | 50 (5) |
| Laparoscopic converted to hand-assist | 43 (5) |
| Laparoscopic converted to open | 75 (8) |
| Robotic | 88 (9) |
| Robotic converted to laparoscopic hand-assist | 5 (0.5) |
| Robotic converted to open | 17 (2) |
| Length of surgery (minutes, mean ± std) | 170 ± 81 |
| Estimated blood loss (mL, mean ± std) | 269 ± 405 |
| Any pRBC transfusion | 39 (4) |
| Crystalloid (mL, mean ± std) | 2060 ± 1212 |
| Colloid (mL, mean ± std) | 224 ± 404 |
| Splenic resection | 973 (96) |
| Size of tumor (cm, mean ± std) | 4 ± 3 |
| Specimen length (cm, mean ± std) | 9 ± 3.6 |
| Lymph node resection | 944 (94) |
| Lymph nodes (#) | 15.6 ± 9.7 |
| Final resection statusa | |
| R0 | 670 (87) |
| R1b | 96 (12) |
| R2 | 5 (0.6) |
| Post-operative outcomes | |
| Complication | |
| Minor complication | 275 (27) |
| Major complication | 179 (18) |
| Drainage procedure | 114 (11) |
| Reoperation | 27 (3) |
| Length of stay (days, mean ± std) | 6.5 ± 4.7 |
| Thirty-day readmission | 164 (16) |
For pre-malignant and malignant tumors only.
Data reported as n (%) or mean ± SD; R1, microscopically positive margin.
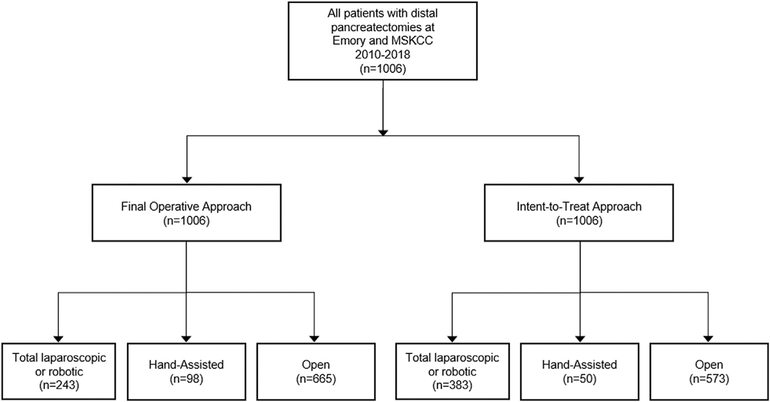
Final operative approach, considering conversions, for distal pancreatectomy was minimally invasive in 34% (n = 341 patients), of which 29% were laparoscopic hand-assist (n = 98), and 71% were total laparoscopic or robotic (n = 243). An open approach was used in 66% (n = 665). On intention-to-treat analysis, operative approach was minimally invasive in 43% (n = 433), of which 12% were laparoscopic hand-assist approach (n = 50), 63% were total laparoscopic (n = 273) and 25% were robotic approach (n = 110). Among the total laparoscopic cases, 16% were converted to a laparoscopic hand-assist approach (n = 43), and 27% were converted to an open approach (n = 75). Among the robotic cases, 5% were converted to a laparoscopic hand-assist approach (n = 5), and 15% were converted to an open approach (n = 17). No hand-assist cases were converted to open. An initial open approach was used in 57% (n = 573; Fig. 2).
Figure 2.
Flow diagram outlining final operative and intended operative approach
Minimally invasive versus open distal pancreatectomy
Patients undergoing a minimally invasive approach compared to open distal pancreatectomies were more likely to be younger (50 vs 62 years, p < 0.01), female (63% vs 53%, p < 0.01), have lower American Society of Anesthesiologists class (ASA 3: 56% vs 60%, p = 0.04), and have a higher BMI (29.5 vs 27.8 kg/m2, p = 0.02). However, both groups had a similar comorbidity profile, similar proportion of prior abdominal operations, and a similar disease presentation (all p > 0.05; Table 2). Intraoperatively, patients who underwent a minimally-invasive approach had a longer length of surgery (183 vs 162 min, p < 0.01), less operative blood loss (131 versus 341 ml, p < 0.01), and fewer blood transfusions (1.4% vs 5%, p < 0.01). However, they also had smaller mean tumor size (3.2 vs 4.4 cm, p < 0.01), fewer lymph nodes retrieved (14 vs 16, p < 0.01), a higher proportion of R0 resections for malignant/pre-malignant tumors (95% vs 83%, p < 0.01) and had more frequent drain placement (49% vs 33%, p < 0.01). Postoperatively, patients who underwent minimally-invasive distal pancreatectomy, had fewer complications (38% vs 48%, p = 0.02), fewer drainage procedures (3% vs 13%, p < 0.01), and shorter length of stay (5.2 vs 7.2 days, p < 0.01) when compared to the open approach. Both cohorts had similar rates of reoperations, ICU admission, and 30-day readmission (all p > 0.05).
Table 2.
Clinicopathologic factors, surgical variables, and outcomes associated with surgical approach
| Variable | Minimally invasive |
Open |
P value |
|---|---|---|---|
| n = 341 (%) | n = 665 (%) | ||
|
| |||
| Baseline demographics | |||
|
| |||
| Age (years, mean ± std) | 59 ± 14.7 | 62 ± 14 | <0.01 |
|
| |||
| Male | 130 (37) | 310 (47) | <0.01 |
|
| |||
| ASA class | |||
|
| |||
| 1 | 11 (3) | 5 (0.8) | 0.04 |
|
|
|||
| 2 | 130 (37) | 238 (36) | |
|
|
|||
| 3 | 197 (56) | 390 (60) | |
|
|
|||
| 4 | 11 (3) | 21 (3) | |
|
|
|||
| 5 | 0 | 0 | |
|
|
|||
| BMI (kg/m2, mean ± std) | 29.5 ± 13.2 | 27.8 ± 5.7 | 0.02 |
|
| |||
| Comorbidities | |||
|
| |||
| Diabetes | 67 (19) | 136 (21) | 0.56 |
|
| |||
| Congestive heart failure | 7 (2) | 4 (0.6) | 0.09 |
|
| |||
| Severe COPD | 4 (1) | 7 (1) | 1.0 |
|
| |||
| Chronic renal failure | 10 (3) | 20 (3) | 1.0 |
|
| |||
| Prior abdominal operations | 165 (48) | 296 (46) | 0.54 |
|
| |||
| Symptoms at presentation | |||
|
| |||
| Incidental/asymptomatic | 204 (59) | 370 (57) | 0.59 |
|
| |||
| Pancreatitis | 51 (15) | 60 (9) | 0.59 |
|
|
|||
| Jaundice | 1 (0.3) | 0 (0) | |
|
|
|||
| Pain (not pancreatitis) | 59 (17) | 129 (20) | |
|
|
|||
| Early satiety | 2 (0.6) | 4 (0.6) | |
|
|
|||
| Bowel obstruction | 0 (0) | 4 (0.6) | |
|
|
|||
| Other | 30 (9) | 84 (13) | |
|
|
|||
| Intra-operative outcomes | |||
|
| |||
| Length of surgery (minutes, mean ± std) | 183 ± 71 | 162 ± 85 | <0.01 |
|
| |||
| Operative blood loss (mL, mean ± std) | 131 ± 218 | 341 ± 459 | <0.01 |
|
| |||
| Any pRBC transfusion | 5 (1.4) | 34 (5) | <0.01 |
|
| |||
| Crystalloid (mL, mean ± std) | 2095 ± 1022 | 2041 ± 1303 | 0.47 |
|
| |||
| Colloid (mL, mean ± std) | 124 ± 274 | 277 ± 450 | <0.01 |
|
| |||
| Splenic resection | 328 (93) | 644 (99) | <0.01 |
|
| |||
| Size of tumor (cm, mean ± std) | 3.2 ± 2 | 4.4 ± 3.5 | <0.01 |
|
| |||
| Specimen length (cm, mean ± std) | 8.5 ± 3.8 | 9.8 ± 3.3 | <0.01 |
|
| |||
| Intraoperative drain placement | 171 (49) | 213 (33) | <0.01 |
|
| |||
| Lymph node resection | 308 (88) | 635 (97) | <0.01 |
|
| |||
| Lymph nodes (#) | 14.41 ± 9.6 | 16.27 ± 9.7 | <0.01 |
|
| |||
| Final resection statusa | |||
|
| |||
| R0 | 241 (95) | 429 (83) | <0.01 |
|
|
|||
| R1 | 10 (4) | 86 (16) | |
|
|
|||
| R2 | 3 (1) | 2 (0.4) | |
|
|
|||
| Post-operative outcomes | |||
|
| |||
| Complication | |||
|
| |||
| Minor complication | 89 (25) | 177 (27) | 0.02 |
|
|
|||
| Major complication | 45 (13) | 134 (20) | |
|
|
|||
| Drainage procedure | 28 (3) | 86 (13) | 0.02 |
|
| |||
| Reoperation | 7 (2) | 20 (3) | 0.42 |
|
| |||
| ICU admission | 14 (4) | 39 (6) | 0.23 |
|
| |||
| Length of stay (days, mean ± std) | 5.2 ± 2.3 | 7.2 ± 5.2 | <0.01 |
|
| |||
| Thirty-day readmission | 48 (14) | 116 (18) | 0.59 |
Bold indicates statistical significance.
For pre-malignant and malignant tumors only.
Total minimally invasive versus hand-assist distal pancreatectomy
In subset analysis, when comparing patients who underwent hand-assist distal pancreatectomy with total laparoscopic or robotic approach, patients were older (61.8 vs 57.5 years, p = 0.02), had a higher ASA class (ASA 3: 62% vs 55%, p = 0.04), more congestive heart failure (5% vs 1%, p = 0.02) and chronic renal failure (9% vs 0.4%, p < 0.01), and a higher proportion of prior abdominal operations (58% vs 43%, p = 0.02). However, they were well-matched for BMI, and disease presentation (all p > 0.05). Despite this more complicated preoperative profile, the hand-assist group had a shorter operative time (167 vs 191 min, p < 0.01), larger mean tumor size (3.7 vs 3.0 cm, p = 0.03), and an equal proportion of R0 resections for malignant/pre-malignant tumors (97% vs 94%, p = 0.41). Postoperatively, there were no significant differences in their complication profile, reoperation rates, need for ICU admission, hospital length of stay or readmission rates (all p > 0.05, Table 3).
Table 3.
Clinicopathologic factors, surgical variables, and outcomes associated with surgical approach: Subset analyses of surgical approaches
| Variable | Lap/Robotic |
Hand-Assist |
Open |
Lap/Rob vs Hand |
Open vs Hand |
|---|---|---|---|---|---|
| n = 243 (%) | n = 98 (%) | n = 665 (%) | P value | P value | |
|
| |||||
| Baseline demographics | |||||
|
| |||||
| Age (years, mean ± std) | 57.5 ± 15.1 | 61.8 ± 13.6 | 62.3 ± 13.7 | 0.02 | 0.63 |
|
| |||||
| Male | 70 (29) | 57 (58) | 313 (47) | <0.01 | 0.05 |
|
| |||||
| ASA class | |||||
|
| |||||
| 1 | 11 (5) | 0 (0) | 5 (0.8) | 0.04 | 0.6 |
|
|
|||||
| 2 | 90 (37) | 33 (34) | 245 (37) | ||
|
|
|||||
| 3 | 133 (55) | 60 (62) | 394 (59) | ||
|
|
|||||
| 4 | 7 (3) | 4 (4) | 21 (3) | ||
|
|
|||||
| 5 | – | – | – | ||
|
| |||||
| BMI (kg/m2, mean ± std) | 29 ± 15 | 30 ± 7 | 28 ± 6 | 0.63 | <0.01 |
|
| |||||
| Comorbidities | |||||
|
| |||||
| Diabetes | 43 (18) | 22 (22) | 138 (21) | 0.39 | 0.80 |
|
| |||||
| Congestive heart failure | 2 (1) | 5 (5) | 4 (0.6) | 0.02 | <0.01 |
|
| |||||
| Severe COPD | 3 (1) | 1 (1) | 7 (1) | 1.0 | 1.0 |
|
| |||||
| Chronic renal failure | 1 (0.4) | 9 (9) | 20 (30 | <0.01 | <0.01 |
|
| |||||
| Prior abdominal operations | 102 (43) | 55 (58) | 304 (46) | 0.02 | 0.05 |
|
| |||||
| Symptoms at presentation | |||||
|
| |||||
| Incidental/asymptomatic | 137 (57) | 59 (61) | 378 (57) | 0.18 | 0.02 |
|
|
|||||
| Pancreatitis | 33 (14) | 18 (19) | 60 (9) | ||
|
|
|||||
| Jaundice | 1 (0.4) | 0 (0) | 0 (0) | ||
|
|
|||||
| Pain (not pancreatitis) | 47 (20) | 10 (10) | 131 (20) | ||
|
|
|||||
| Early satiety | 2 (0.8) | 0 (0) | 4 (0.6) | ||
|
|
|||||
| Bowel obstruction | 0 (0) | 0 (0) | 4 (0.6) | ||
|
|
|||||
| Other | 19 (8) | 10 (10) | 86 (13) | ||
|
|
|||||
| Intra-operative outcomes | |||||
|
| |||||
| Pathologic diagnoses | |||||
|
| |||||
| Benign | 31 (13) | 14 (14) | 84 (13) | 0.23 | <0.01 |
|
|
|||||
| Pre-malignant | 156 (64) | 55 (56) | 257 (39) | ||
|
|
|||||
| Malignant | 33 (14) | 20 (20) | 274 (41) | ||
|
|
|||||
| Length of surgery (minutes, mean ± std) | 191 ± 70 | 167 ± 72 | 163 ± 84 | <0.01 | 0.65 |
|
| |||||
| Operative blood loss (mL, mean ± std) | 112 ± 138 | 143 ± 189 | 343 ± 467 | 0.15 | <0.01 |
|
| |||||
| Any pRBC transfusion | 3 (1) | 1 (1) | 35 (5) | 1.0 | 0.07 |
|
| |||||
| Crystalloid (mL) | 2154 ± 886 | 1944 ± 1303 | 2042 ± 1297 | 0.15 | 0.49 |
|
| |||||
| Colloid (mL) | 115 ± 239 | 130 ± 296 | 277 ± 452 | 0.62 | <0.01 |
|
| |||||
| Splenic resection | 220 (91) | 97 (99) | 655 (100) | 0.01 | 1.0 |
|
| |||||
| Size of tumor (cm) | 3.0 ± 1.7 | 3.7 ± 2.7 | 4.4 ± 3.5 | 0.03 | 0.03 |
|
| |||||
| Specimen length (cm) | 8.1 ± 3.9 | 9.3 ± 3.3 | 9.8 ± 3.4 | <0.01 | 0.17 |
|
| |||||
| Proximal margin (mm) | 15.2 ± 14.1 | 16.8 ± 12.7 | 17.8 ± 15.6 | 0.4 | 0.63 |
|
| |||||
| Intraoperative drain placement | 113 (47) | 53 (54) | 218 (33) | 0.25 | <0.01 |
|
| |||||
| Lymph node resection | 207 (85) | 90 (92) | 646 (97) | 0.14 | 0.02 |
|
| |||||
| Lymph nodes (#) | 14 ± 10 | 16 ± 9 | 16 ± 10 | 0.13 | 0.42 |
|
| |||||
| Method of pancreatic transection | |||||
|
| |||||
| Scalpel | 10 (4) | 2 (2) | 302 (47) | 0.43 | <0.01 |
|
|
|||||
| Staple | 239 (95) | 96 (98) | 327 (51) | ||
|
|
|||||
| Energy | 1 (0.4) | 0 (0) | 11 (2) | ||
|
|
|||||
| Final resection statusa | |||||
|
| |||||
| R0 | 170 (94) | 71 (97) | 429 (83) | 0.41 | <0.01 |
|
|
|||||
| R1 | 9 (5) | 1 (1) | 86 (17) | ||
|
|
|||||
| R2 | 2 (1) | 1 (1) | 2 (0.4) | ||
|
|
|||||
| Post-operative outcomes | |||||
|
| |||||
| Complication | 99 (41) | 38 (39) | 316 (48) | 0.83 | 0.12 |
|
| |||||
| Minor complication | 69 (28) | 26 (68) | 180 (57) | 1.0 | 0.23 |
|
|
|||||
| Major complication | 30 (12) | 12 (32) | 137 (43) | ||
|
|
|||||
| Drainage procedure | 21 (9) | 7 (7) | 86 (13) | 0.81 | 0.14 |
|
| |||||
| Reoperation | 5 (2) | 1 (1) | 21 (3) | 0.68 | 0.34 |
|
| |||||
| ICU admission | 7 (3) | 7 (7) | 39 (6) | 0.14 | 0.79 |
|
| |||||
| Length of stay (days, mean ± std) | 5.06 ± 2.3 | 5.2 ± 2.3 | 7.2 ± 5.3 | 0.68 | <0.01 |
|
| |||||
| Readmission | 38 (16) | 14 (14) | 136 (21) | 0.88 | 0.19 |
|
| |||||
| Thirty-day readmission | 32 (84) | 13 (93) | 119 (88) | 0.59 | 0.54 |
Bold indicates statistical significance.
For pre-malignant and malignant tumors only.
Hand-assist versus open distal pancreatectomy
When comparing patients who underwent hand-assist (n = 98) with open (n = 665) technique, patients had a higher mean BMI (30 vs 28 kg/m2, p < 0.01), more comorbidities including congestive heart failure (5% vs 0.6%, p < 0.01), and chronic renal failure (9% vs 3%, p < 0.01), and otherwise similar preoperative characteristics (Table 3). Intraoperatively, hand-assist had less operative blood loss (143 vs 343 ml, p < 0.01). Operative time, specimen length, and lymph node yield were similar between both techniques (all p > 0.05). The hand-assist approach had a higher proportion of R0 resections for malignant/pre-malignant tumors (97% vs 83%, p < 0.01). Postoperatively, there were no significant differences in their complication profile, reoperation rates, or need for ICU admission (all p > 0.05). However, patients who underwent hand-assist distal pancreatectomy had a significantly shorter length of stay (5.2 vs 7.2 days, p < 0.01; Table 3). When assessing only those patients who were converted from a total laparoscopic/robotic approach to hand-assist, the converted hand-assist group retained the significantly lower blood loss and length of stay associated with a total laparoscopic/robotic approach when compared to the open technique.
Intent-to-treat analysis: hand-assist versus minimally invasive and hand-assist versus open distal pancreatectomy
Subset analysis of initially intended approach comparing hand-assist (n = 50) versus minimally invasive technique (n = 383), demonstrated similar findings to the previous final operative approach analysis. When compared to the total laparoscopic or robotic technique, patients intended for the hand-assist technique had a higher proportion of chronic renal failure (12% vs 2%, p < 0.01), but were otherwise similar for preoperative factors. Again, hand-assist had a shorter operative time (157 vs 191 min, p < 0.01), less operative blood loss (110 vs 208 ml, p < 0.01), larger mean tumor size (3.7 vs 3.2 cm, p = 0.03), and an equally high rate of R0 resections for malignant/pre-malignant tumors (97% vs 93%, p = 0.15). Postoperatively, there were no significant differences in their complication profile, rates of pancreatic fistula, reoperation rates, need for ICU admission, hospital length of stay or readmission rates (all p > 0.05; Table 4).
Table 4.
Intent to treat analysis: Clinicopathologic factors, surgical variables, and outcomes associated with surgical approach
| Variable | Lap/Robotic |
Hand-Assist |
Open |
Lap/Rob vs Hand |
Open vs Hand |
|---|---|---|---|---|---|
| n = 383 (%) | n = 50 (%) | n = 573 (%) | P value | P value | |
|
| |||||
| Baseline demographics | |||||
|
| |||||
| Age (years, mean ± std) | 59 ± 15 | 62 ± 13 | 62 ± 14 | 0.25 | 0.70 |
|
| |||||
| Male | 145 (38) | 26 (52) | 269 (47) | 0.08 | 0.59 |
|
| |||||
| ASA class | |||||
|
| |||||
| 1 | 12 (3) | 0 (0) | 4 (0.7) | 0.16 | 0.66 |
|
|
|||||
| 2 | 149 (39) | 16 (33) | 203 (35) | ||
|
|
|||||
| 3 | 210 (55) | 30 (61) | 347 (61) | ||
|
|
|||||
| 4 | 10 (3) | 3 (6) | 19 (3) | ||
|
|
|||||
| 5 | 0 (0) | 0 (0) | 0 (0) | ||
|
|
|||||
| BMI (kg/m2, mean ± std) | 29 ± 13 | 30 ± 7 | 28 ± 6 | 0.51 | <0.01 |
|
| |||||
| Comorbidities | |||||
|
| |||||
| Diabetes | 72 (19) | 9 (18) | 122 (21) | 1.0 | 0.71 |
|
| |||||
| Congestive heart failure | 6 (2) | 3 (6) | 2 (0.3) | 0.08 | <0.01 |
|
| |||||
| Severe COPD | 3 (0.8) | 1 (2) | 7 (1) | 0.39 | 1.0 |
|
| |||||
| Chronic renal failure | 6 (20) | 6 (12) | 18 (3) | <0.01 | <0.01 |
|
| |||||
| Prior abdominal operations | 174 (46) | 29 (60) | 258 (46) | 0.09 | 0.07 |
|
| |||||
| Symptoms at presentation | |||||
|
| |||||
| Incidental/asymptomatic | 220 (58) | 30 (60) | 324 (57) | 0.53 | 0.23 |
|
|
|||||
| Pancreatitis | 48 (130 | 9 (18) | 54 (10) | ||
|
|
|||||
| Jaundice | 1 (0.3) | 0 (0) | 0 (0) | ||
|
|
|||||
| Pain (not pancreatitis) | 71 (19) | 5 (10) | 112 (20) | ||
|
|
|||||
| Early satiety | 2 (0.5) | 0 (0) | 4 (0.7) | ||
|
|
|||||
| Bowel obstruction | 0 (0) | 0 (0) | 4 (0.7) | ||
|
|
|||||
| Other | 36 (10) | 6 (12) | 72 (13) | ||
|
|
|||||
| Intra-operative outcomes | |||||
|
| |||||
| Length of surgery (minutes) | 191 ± 74 | 157 ± 64 | 157 ± 84 | <0.01 | 0.97 |
|
| |||||
| Operative blood loss (mL) | 208 ± 357 | 110 ± 153 | 323 ± 440 | <0.01 | <0.01 |
|
| |||||
| Any pRBC transfusion | 12 (3) | 0 (0) | 27 (5) | 0.37 | 0.23 |
|
| |||||
| Crystalloid (mL) | 2268 ± 1061 | 1632 ± 1023 | 1957 ± 1298 | <0.01 | 0.08 |
|
| |||||
| Colloid (mL) | 179 ± 345 | 109 ± 303 | 263 ± 443 | 0.13 | <0.01 |
|
| |||||
| Splenic resection | 358 (94) | 49 (98) | 565 (99) | 0.34 | 1.0 |
|
| |||||
| Size of tumor (cm) | 3.2 ± 2.0 | 3.7 ± 2.7 | 4.6 ± 3.6 | 0.25 | 0.11 |
|
| |||||
| Specimen length (cm) | 8.6 ± 3.8 | 9.1 ± 3.1 | 9.9 ± 3.4 | 0.36 | 0.12 |
|
| |||||
| Proximal parenchymal margin distance (mm) | 16.8 ± 14.9 | 18.0 ± 14.9 | 17.1 ± 15.1 | 0.66 | 0.74 |
|
| |||||
| Intraoperative drain placement | 171 (45) | 24 (48) | 189 (33) | 0.77 | 0.05 |
|
| |||||
| Lymph node resection | 343 (90) | 44 (88) | 556 (97) | 0.93 | <0.01 |
|
| |||||
| Lymph nodes (#) | 14.5 ± 9.9 | 15.3 ± 8.3 | 16.4 ± 9.7 | 0.59 | 0.45 |
|
| |||||
| Method of pancreatic transection | |||||
|
| |||||
| Scalpel | 38 (10) | 2 (4) | 274 (50) | 0.31 | <0.01 |
|
|
|||||
| Staple | 338 (89) | 48 (96) | 267 (49) | ||
|
|
|||||
| Energy | 3 (0.8) | 0 (0) | 9 (2) | ||
|
|
|||||
| Final resection statusa | |||||
|
| |||||
| R0 | 263 (93) | 38 (97) | 369 (82) | 0.15 | <0.01 |
|
|
|||||
| R1 | 18 (6) | 0 (0) | 78 (17) | ||
|
|
|||||
| R2 | 2 (1) | 1 (3) | 2 (0.4) | ||
|
| |||||
| Post-operative outcomes | |||||
|
| |||||
| Complication | 165 (43) | 22 (44) | 266 (46) | 0.90 | 0.84 |
|
| |||||
| Minor complication | 105 (64) | 15 (68) | 154 (58) | 0.69 | 0.47 |
|
|
|||||
| Major complication | 60 (36) | 7 (32) | 112 (42) | ||
|
|
|||||
| Drainage procedure | 37 (10) | 3 (6) | 74 (13) | 0.60 | 0.18 |
|
| |||||
| Reoperation | 9 (2) | 1 (2) | 17 (3) | 1.0 | 1.0 |
|
| |||||
| ICU admission | 14 (4) | 5 (10) | 34 (6) | 0.09 | 0.41 |
|
| |||||
| Length of stay (days, mean ± std) | 5.6 ± 2.9 | 5.1 ± 2.0 | 7.2 ± 5.4 | 0.26 | <0.01 |
|
| |||||
| Readmission | 67 (18) | 7 (14) | 114 (20) | 0.68 | 0.40 |
|
| |||||
| Thirty-day readmission | 57 (85) | 6 (86) | 101 (89) | 0.71 | 0.69 |
Bold indicates statistical significance.
For pre-malignant and malignant tumors only.
Similar to the previous analysis, comparison of intended hand-assist (n = 50) versus open technique (n = 573), demonstrated that patients who underwent resection with the hand-assist technique had a higher BMI (30 vs 28 kg/m2, p < 0.01), more comorbidities including congestive heart failure (6% vs 0.3%, p < 0.01), and chronic renal failure (12% vs 3%, p < 0.01). Intraoperatively, hand-assist had less operative blood loss (110 vs 323 ml, p < 0.01), and a higher proportion of R0 resections for malignant/pre-malignant tumors (97% vs 82%, p < 0.01). Operative time, tumor size, and lymph node yield were similar (all p > 0.05). Postoperatively, there were no significant differences in their complication profile, rates of pancreatic fistula, reoperation rates, or need for ICU admission (all p > 0.05). However, patients who underwent hand-assist distal pancreatectomy had a significantly shorter length of stay (5.1 vs 7.2 days, p < 0.01; Table 4).
Discussion
The aim of this study was to compare the short-term outcomes of the hand-assist laparoscopic technique to the total laparoscopic or robotic and open techniques in patients who underwent distal pancreatectomy performed by expert surgeons at two high-volume medical centers. The results confirm that the hand-assist laparoscopic approach maintains the intraoperative advantages of an open technique while employing the benefits of a total minimally invasive approach. As observed with open distal pancreatectomy, the hand-assist technique has a similar short operative time, and increased lymph node yield. Furthermore, hand-assist is associated with the same decreased blood loss and reduced length of stay as the total laparoscopic or robotic approach. As also evidenced in some early series on the hand-assist technique for distal pancreatectomy, this approach offers the ability to palpate the pancreatic tissue thus providing tactile feedback to the surgeon’s hand, allows for complex retraction and easier and more rapid control of bleeding by digital pressure which may lead to a lower open conversion rate (none in our series) than is observed with the total laparoscopic or robotic approach.13,14
Similar to the total laparoscopic or robotic approach, the hand-assist technique demonstrated favorable post-operative outcomes. Indeed, there were no differences in hospital length of stay between the hand-assist and the total laparoscopic or robotic technique (5.1 vs 5.2 days, p = 0.68). In the intent-to-treat analysis, inclusion of patients who underwent conversion to hand-assist in the laparoscopic cohort did not change these findings (5.6 vs 5.1 days, p = 0.26). Some have argued that the total laparoscopic approach uses smaller incisions which may result in decreased postoperative pain and improved cosmesis. However, a recent randomized-control trial in colorectal surgery was performed to evaluate post-operative outcomes with the use of single-port laparoscopy in which a single 4 cm incision is used when compared to multiple-port laparoscopy. Results demonstrated that total length of incision was shorter in the single-port laparoscopy group, both groups had similar postoperative pain, and patients were more satisfied with their cosmetic outcome in the single-port laparoscopy group.15 Similarly, in the hand-assist technique for distal pancreatectomy, only three total incisions are required including two to three 5 mm port incisions and a hand port incision which can vary in length from 4 to 7 cm depending on the surgeon’s hand size and specimen size resulting in a total incision length of approximately 5–8 cm (Fig. 1). Furthermore, the total laparoscopic/robotic approach still necessitates a specimen extraction site, which often approximates 4–5 cm in length. Although we were unable to quantify incision length in this study due to its retrospective nature, our institutions’ practices are similar to these reported estimates. Several other studies have also demonstrated similar postoperative pain scores and narcotic use between hand-assist and total laparoscopic techniques.16
Despite the advantages to total laparoscopic/robotic approaches, it is well known that patient factors such as body habitus, cardiac comorbidities, and a history of previous laparotomy are routine factors that may make a total laparoscopic/ robotic approach more challenging due to the longer operative time and effects of prolonged anesthesia and pneumoperitoneum on the patient’s cardiopulmonary status. Our results also suggest that these limitations may be overcome with the hand-assist technique as the patients in this cohort were older, had a higher ASA class, had more comorbidities such as congestive heart failure and chronic renal failure, and had a higher proportion of prior abdominal surgeries compared to the patients who underwent resection with a total laparoscopic or robotic approach (Table 3). Importantly, although the conversion rates from a total laparoscopic or robotic approach to either a hand-assist or open approach ranged from 5 to 27%, there were no cases converted from hand-assist approach to open. Despite these favorable results for the hand-assist technique, data from a study by Kneuertz et al. and Jayaraman et al. have suggested that although numbers of total laparoscopic operations are increasing, the frequency of hand access procedures have decreased over time.11,17
Furthermore, given the conversion rate of up to 27% from a total laparoscopic approach to either a hand-assist or open approach, the data from the current study suggests that the hand-assist approach may serve as a potential successive intermediary step in the conversion from total laparoscopy/robotic to open, as the converted hand-assist cases had the same significantly lower blood loss and length of stay as a total laparoscopic/robotic approach when compared to open. Additionally, the study by Jayaraman et al. demonstrated that conversion to open was associated with a higher rate of post-operative complications including pancreatic leak and a higher rate of hospital readmission.17 Although this observation is probably subject to confounding as the cases who undergo conversion are usually more technically difficult at baseline and thus prone to increased complications, this concept further supports the use of the hand-assist technique as an early successive step in which the surgeon deems laparoscopy not feasible. Given its zero-conversion rate to open in our study and its associated advantages, the hand-assist approach remains a viable option as the initial approach to performing distal pancreatectomy for any etiology.
This study was limited by its retrospective nature, and was subject to selection bias between minimally invasive and open techniques. Additionally, there is certainly surgeon bias as some may feel more comfortable with one particular technique. Furthermore, there were no inter-institutional standardized protocols used at both institutions during the course of this study. Although our analysis was histology non-specific due to a small sample size in each histologic category, the primary aim of this study was to assess the technical aspects of each operative approach; conducting a histology-specific study may serve as the aim of a future study. Additionally, robotic approaches are increasingly being used but only accounted for 8% of the cases in our cohort, thus limiting our ability to perform a subset analysis to evaluate outcomes for this particular approach. We did not report long-term outcomes including disease recurrence, survival and incisional hernia rates, although long-term oncologic outcome is not a valid outcome when grouping patients with mixed histologies. Notably, oncologic outcomes have not been demonstrated to be different between minimally invasive and open approaches for pancreatic adenocarcinoma and pathology alone should not influence choice of operative approach. The literature has demonstrated a hernia rate of nearly 10% after hand-assist laparoscopy. Due to this reason, some advocate for a total laparoscopic/robotic approach with specimen extraction through a Pfannenstiel incision as some studies have demonstrated that these are associated with significantly lower incisional hernia rates of 1–3%.18–20 Long-term follow-up is needed to determine the incisional hernia rates in our cohort.
Conclusion
Laparoscopic hand-assist distal pancreatectomy is a safe and effective technique whose advantages include those offered by a total minimally invasive approach including decreased blood loss and a shorter hospital length of stay while maintaining the advantages of an open procedure including faster operative time, and increased lymph node yield. The laparosopic hand-assist approach should be considered as either the initial approach or as a successive step prior to converting from laparoscopic/robotic to open distal pancreatectomy.
Financial support
Supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378/TL1TR002382 and the Katz Foundation.
Footnotes
Disclosures
The authors have no disclosures relevant to this study.
Conflicts of interest
None declared.
References
- 1.Cuschieri A (1994) Laparoscopic surgery of the pancreas. J R Coll Surg Edinb 39:178–184. [PubMed] [Google Scholar]
- 2.Gagner M, Pomp A, Herrera MF. (1996) Early experience with laparoscopic resections of islet cell tumors. Surgery 120:1051–1054. [DOI] [PubMed] [Google Scholar]
- 3.Mehrabi A, Hafezi M, Arvin J, Esmaeilzadeh M, Garoussi C, Emami G et al. (2015) A systematic review and meta-analysis of laparoscopic versus open distal pancreatectomy for benign and malignant lesions of the pancreas: it’s time to randomize. Surgery 157:45–55. [DOI] [PubMed] [Google Scholar]
- 4.Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. (2012) Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg 255:1048–1059. [DOI] [PubMed] [Google Scholar]
- 5.Eom BW, Jang JY, Lee SE, Han HS, Yoon YS, Kim SW. (2008) Clinical outcomes compared between laparoscopic and open distal pancreatectomy. Surg Endosc 22:1334–1338. [DOI] [PubMed] [Google Scholar]
- 6.Han HS, Min SK, Lee HK, Kim SW, Park YH. (2005) Laparoscopic distal pancreatectomy with preservation of the spleen and splenic vessels for benign pancreas neoplasm. Surg Endosc 19:1367–1369. [DOI] [PubMed] [Google Scholar]
- 7.Edwin B, Mala T, Mathisen O, Gladhaug I, Buanes T, Lunde OC et al. (2004) Laparoscopic resection of the pancreas: a feasibility study of the short-term outcome. Surg Endosc 18:407–411. [DOI] [PubMed] [Google Scholar]
- 8.Kooby DA, Hawkins WG, Schmidt CM, Weber SM, Bentrem DJ, Gillespie TW et al. (2010) A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg 210:779–785, 86–7. [DOI] [PubMed] [Google Scholar]
- 9.Yang DJ, Xiong JJ, Lu HM, Wei Y, Zhang L, Lu S et al. (2019) The oncological safety in minimally invasive versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Sci Rep 9:1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyman WB, Passeri M, Sastry A, Cochran A, Iannitti DA, Vrochides D et al. (2018) Robotic-assisted versus laparoscopic left pancreatectomy at a high-volume, minimally invasive center. Surg Endosc. [DOI] [PubMed] [Google Scholar]
- 11.Kneuertz PJ, Patel SH, Chu CK, Fisher SB, Maithel SK, Sarmiento JM et al. (2012) Laparoscopic distal pancreatectomy: trends and lessons learned through an 11-year experience. J Am Coll Surg 215:167–176. [DOI] [PubMed] [Google Scholar]
- 12.Postlewait LM, Kooby DA. (2015) Laparoscopic distal pancreatectomy for adenocarcinoma: safe and reasonable? J Gastrointest Oncol 6: 406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merchant NB, Parikh AA, Kooby DA. (2009) Should all distal pancreatectomies be performed laparoscopically? Adv Surg 43:283–300. [DOI] [PubMed] [Google Scholar]
- 14.D’Angelica M, Are C, Jarnagin W, DeGregoris G, Coit D, Jaques D et al. (2006) Initial experience with hand-assisted laparoscopic distal pancreatectomy. Surg Endosc 20:142–148. [DOI] [PubMed] [Google Scholar]
- 15.Maggiori L, Tuech JJ, Cotte E, Lelong B, Denost Q, Karoui M et al. (2018) Single-incision laparoscopy versus multiport laparoscopy for colonic surgery: a multicenter, double-blinded, randomized controlled trial. Ann Surg 268:740–746. [DOI] [PubMed] [Google Scholar]
- 16.Marcello PW, Fleshman JW, Milsom JW, Read TE, Arnell TD, Birnbaum EH et al. (2008) Hand-assisted laparoscopic vs. laparoscopic colorectal surgery: a multicenter, prospective, randomized trial. Dis Colon Rectum 51:818–826. Discussion 26–8. [DOI] [PubMed] [Google Scholar]
- 17.Jayaraman S, Gonen M, Brennan MF, D’Angelica MI, DeMatteo RP, Fong Y et al. (2010) Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg 211:503–509. [DOI] [PubMed] [Google Scholar]
- 18.Benlice C, Stocchi L, Costedio MM, Gorgun E, Kessler H. (2016) Impact of the specific extraction-site location on the risk of incisional hernia after laparoscopic colorectal resection. Dis Colon Rectum 59:743–750. [DOI] [PubMed] [Google Scholar]
- 19.Lee L, Abou-Khalil M, Liberman S, Boutros M, Fried GM, Feldman LS. (2017) Incidence of incisional hernia in the specimen extraction site for laparoscopic colorectal surgery: systematic review and meta-analysis. Surg Endosc 31:5083–5093. [DOI] [PubMed] [Google Scholar]
- 20.DeSouza A, Domajnko B, Park J, Marecik S, Prasad L, Abcarian H. (2011) Incisional hernia, midline versus low transverse incision: what is the ideal incision for specimen extraction and hand-assisted laparoscopy? Surg Endosc 25:1031–1036. [DOI] [PubMed] [Google Scholar]