Abstract
Background: This study aims to describe the prevalence of neurologic complications and hospital outcome in adult post-cardiotomy cardiogenic shock (PCS) patients receiving veno-arterial extracorporeal membrane oxygenation (V-A ECMO) support and factors associated with such adverse events.
Methods: Four hundred and fifteen adult patients underwent cardiac surgery and received V-A ECMO for more than 24 h because of PCS. Patients were divided into two groups: those who developed a neurological complication and those who did not (control group). Multivariable logistic regression was performed to identify factors independently associated with neurologic complications.
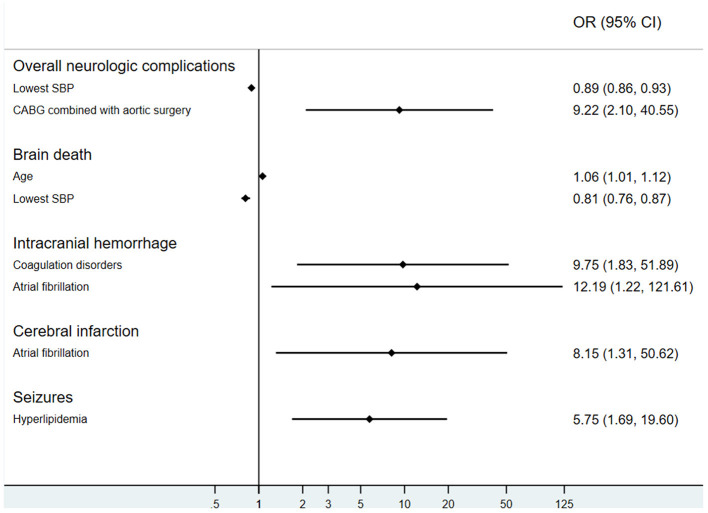
Results: Neurologic complications occurred in 87 patients (21.0%), including cerebral infarction in 33 patients (8.0%), brain death in 30 patients (7.2%), seizures in 14 patients (3.4%), and intracranial hemorrhage in 11 (2.7%) patients. In-hospital mortality in patients with neurologic complications was 90.8%, compared to 52.1% in control patients (p < 0.001). In a multivariable model, the lowest systolic blood pressure (SBP) level pre-ECMO (OR, 0.89; 95% CI: 0.86–0.93) and aortic surgery combined with coronary artery bypass grafting (OR, 9.22; 95% CI: 2.10–40.55) were associated with overall neurologic complications. Age (OR, 1.06; 95% CI: 1.01–1.12) and lowest SBP (OR, 0.81; 95% CI: 0.76–0.87) were correlative factors of brain death. Coagulation disorders (OR, 9.75; 95% CI: 1.83–51.89) and atrial fibrillation (OR, 12.19; 95% CI: 1.22–121.61) were shown to be associated independently with intracranial hemorrhage, whereas atrial fibrillation (OR, 8.15; 95% CI: 1.31–50.62) was also associated with cerebral infarction.
Conclusions: Neurologic complications in adult PCS patients undergoing V-A ECMO support are frequent and associated with higher in-hospital mortality. Identified risk factors of neurologic complications might help to improve ECMO management and might reduce their occurrence.
Keywords: extracorporeal membrane oxygenation, post-cardiotomy cardiogenic shock, neurological complication, lowest systolic blood pressure, in-hospital mortality
Introduction
Veno-arterial extracorporeal membrane oxygenation (V-A ECMO) is an effective technique to rescue patients with refractory cardiogenic shock or cardiac arrest (1–4). Despite the significantly increasing use and experience in recent years, V-A ECMO is still associated with very high in-hospital mortality (40–60%) and high rate of complications. Of these, bleeding, renal failure, infection, and neurologic complications, often result in poor outcomes or permanent disability (5–8). Previous studies have shown that the mortality in V-A ECMO patients associated with neurologic complications was high (9–13). However, the patients enrolled in these studies were from the Extracorporeal Life Support Organization (ELSO) registry or the complication profiles of detailed V-A ECMO indications were well not well-defined or including various V-A ECMO settings (9–13). One of the most common V-A ECMO indications is post-cardiotomy cardiogenic shock (PCS). Better understanding of the neurologic complications in PCS adult patients receiving V-A ECMO support might be meaningful to elucidate this peculiar aspect and improve the ECMO management in this challenging setting.
This study, therefore, aimed to assess the prevalence of cerebral injury and its influence on outcomes in adult PCS patients undergoing V-A ECMO support. Furthermore, independent risk factors of neurologic complications were also investigated.
Materials and Methods
ECMO Setting and Patient Profile
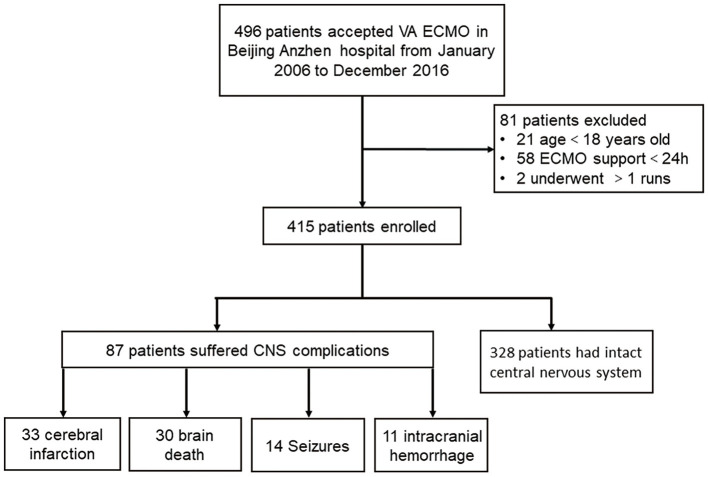
The present study was a retrospective cohort study conducted at Beijing Anzhen Hospital, Capital Medical University. Forty-two thousand six hundred and sixty-eight adult patients (>18 years old) received cardiac surgery in our center from January 2006 to December 2016. Four hundred and ninety-six underwent V-A ECMO because of PCS. Of those, 21 patients aged 17 years or younger were excluded. Fifty-eight patients undergoing ECMO for <24 h were excluded because of the lack of complete central nervous system (CNS) assessment. Two patients undergoing more than one ECMO run were also excluded to avoid bias from confounders contributing to the severity of illness. Finally, 415 adult patients requiring V-A ECMO were included in this study. Patients were categorized according to the in-hospital occurrence (the neurological complication group) or absence of neurologic complications (the control group), and the two groups were compared (Figure 1). Data were extracted from the prospective institutional registry database of ECMO patients. This study was approved by Beijing Anzhen Hospital human research ethics committee (Ethics number: 2016018X). Because this was a retrospective observational study, the individual patients' consent was waived.
Figure 1.
Study flow. CNS, central nervous system.
Post-cardiotomy cardiogenic shock patients included: (1) those who could not be weaned from cardiopulmonary bypass (CPB); (2) those presenting low cardiac output syndrome (LCOS) after CPB, cardiac arrest, or arrhythmias and hemodynamic instability despite satisfactory cardiac surgical procedure and conventional anti-arrhythmia therapy in the operating room; (3) those with delayed LCOS or cardiac arrest in intensive care unit (ICU) (14). Postoperative LCOS was defined as a systolic blood pressure (SBP) <90 mmHg for at least 30 min with a severe reduction in cardiac index (<1.8 L/min/m2) and elevated left or right ventricular filling pressures, or inadequate peripheral organ perfusion (pH < 7.3, serum lactate > 2 mmol/L, cool extremities, urine output <30 mL/h, and altered mental status), acute pulmonary congestion or edema despite adequate/appropriate fluid administration and pharmacologic agents or intra-aortic balloon pump (IABP) (14).
V-A ECMO Implantation Techniques
The decision to use V-A ECMO was made by the cardiac surgeon and ECMO team. V-A ECMO was setup by the experienced ECMO team members. The femoral vessels (vein and artery) were cannulated with Fr 17–21 draining cannulae, and Fr 15–19 perfusion cannulae (Medtronic, Minneapolis, MN) by surgical cut-down. An additional 7F catheter was systematically inserted distally to the cannulated femoral artery site to perfuse the limb. Intrathoracic cannulation strategy has not been used in the 415 patients.
Patient Management
The detailed management of patients under V-A ECMO was previously described (14, 15). Heparin was used for anticoagulation. A heparin bolus (5,000 IU) was injected before cannulation. After V-A ECMO initiation, if surgical site bleeding could be controlled or thoracic drainage was <0.5 ml/kg/min, continuous intravenous infusion of unfractionated heparin was given to the patients as early as possible to maintain the activated clotting time (ACT) between 180 and 220 s, or activated partial thromboplastin time (aPTT) in the range of 60–80 s. Therapeutic hypothermia (32–35°C) was initiated during the first 24 h in case of extracorporeal cardiopulmonary resuscitation (ECPR) patients. The partial pressure of carbon dioxide (PaCO2) was maintained between 35 and 45 mmHg before airway extubation.
Coagulation disorders could occur at any time of the perioperative period. In this study, we analyzed the coagulation disorders, defined as platelets <20 × 109/L, fibrinogen <1.5 g/L and prothrombin time <30% of the standard value (16), before and during ECMO support. The definition and/or treatment of ECMO-related complications, such as major bleeding, lower-limb ischemia or compartment syndrome requiring fasciotomy, renal failure requiring renal replacement therapy, and significant infection, were described in the previous reports (14, 17).
Definition, Monitoring, and Treatment of Neurologic Complications
We defined neurologic complications as any clinical event occurring during the V-A ECMO support, including any clinical sign suggestive of stroke, brain death, and seizures despite sedation (11–13, 17). Routine neurological examinations were performed at least twice a day by the ICU doctors and the nurses in charge of the patients during sedation interruption or after sedation withdrawal, including response to verbal orders or pain, tendon reflexes, brainstem reflexes, eye movement, and pupil size and their light reflection. When abnormal signs were detected (such as pupil dilatation, convulsion of the limbs, delirium confusion, no awakening after sedation withdrawal, etc.), a cerebral computed tomography (CT) scan was performed within 6 h, and a neurologist was consulted immediately to perform neurocognitive test (18). The diagnosis of cerebral infarction or intracranial hemorrhage was determined by a neurologist analyzing CT scan images. Brain death, an irreversible cessation of the functions of the entire brain, including the brain stem (19) was defined according to the diagnostic criteria set by the American Academy of Neurology (AAN) (19, 20). In addition to neurologic examination, EEG and transcranial Doppler were performed to confirm the electrical activity loss or the loss of cerebral blood flow. Seizures were identified by a neurologist by means of at least 30 min continuous EEG monitoring and clinical features. When a neurological lesion was diagnosed, it would be treated according to the neurologist's consultation.
ECMO Weaning
Weaning from V-A ECMO support was based on the clinical and laboratory evidences of recovery of cardiac and pulmonary function, including that pulsatile arterial waveform was maintained (pulse pressure >20 mmHg) for over 24 h, and LV ejection fraction was >20–25% or not worsened right heart function and no significant arterial blood O2 saturation when the ECMO flow was reduced to <1.5 L/min according to conventional protocol (15). Cardiac function and blood gas were continuously monitored during the weaning process. V-A ECMO was removed, and the femoral artery was primarily repaired in the operating room. Weaning off ECMO was considered successful when a patient survived V-A ECMO explantation for at least 48 h (15).
Statistical Analysis
Categorical data are reported as numbers and percentages. Continuous variables are expressed as mean ± standard deviations for normally distributed variables, or as median and interquartile range (IQR) for non-normally distributed variables. Normality of distribution was assessed by the Kolmogorov–Smirnov test. Categorical variables were compared with chi-square or Fisher's exact tests. Continuous variables were compared with two-tailed Student's t-test or Mann–Whitney U-test. Univariable and stepwise multivariate logistic regression analyses of baseline characteristics, pre-ECMO, and ECMO-related risk factors for neurologic complications were performed by calculating the odds ratio (OR) with 95% confidence interval (CI). Variables with p < 0.05 during univariable analysis were entered in multivariate logistic regression. Variables were retained in the model if the adjusted p-value was < 0.05. The maximum value of Youden's index was used to determine the threshold of lowest SBP before VA ECMO initiation. Analyses were performed using IBM SPSS Statistics v22.0 software (IBM Corp, Chicago, IL). p < 0.05 defined statistical significance.
Results
Two hundred and sixty-eight patients (64.6%) were successfully weaned off V-A ECMO, and 165 patients (39.8%) survived to discharge. Eighty-seven patients (21.0%) suffered from neurologic complications during V-A ECMO support (Figure 1). Thirty-three patients had preexisting neurological comorbidities (32 had a history of cerebral infarction, and 1 had suffered intracranial hemorrhage).
Baseline Characteristics
In comparing the baseline characteristics between the neurological complication group and the control group (Table 1), we found significant differences in age and body mass index (BMI). Patients experiencing neurological complications were older and had a higher ratio of BMI ≥ 30 (p < 0.05). The rates of hypertension and peripheral arterial disease were also higher in the neurological complication group (p < 0.01). Patients with atrial fibrillation appeared more frequently in the neurological complication group (p < 0.05). Patients in the neurological complication group had a higher ratio of cardiopulmonary resuscitation (CPR) before V-A ECMO initiation (43.7 vs. 20.7%, p < 0.001), and the lowest SBP before V-A ECMO start was lower in the neurological complication group [70.0 (65.0, 80.0) vs. 80.0 (75.0, 80.0) mmHg, p < 0.001]. Receiving aortic surgery combined with coronary artery bypass grafting (CABG) was more common in the neurological complication group (6.9 vs. 1.2%, p < 0.01). In our study, no heart transplantation recipients developed neurologic complications (p < 0.05; Table 1).
Table 1.
Demographics, baseline characteristics, and pre-extracorporeal membrane oxygenation information.
| Variable | Neurological complication group (n = 87) | Control group (n = 328) | p -value |
|---|---|---|---|
| Demographics and baseline characteristics | |||
| Age (years) | 61.0 [51.0, 66.0] | 55.0 [45.3, 63.8] | 0.002 |
| Older age (≥65 year) | 27 (31.0) | 80 (24.4) | 0.208 |
| Gender (male) | 56 (64.4) | 225 (68.6) | 0.453 |
| Body mass index (kg/m2) | 24.3 ± 3.7 | 23.7 ± 3.5 | 0.154 |
| Obesity (BMI ≥ 30) | 15 (17.2) | 30 (9.1) | 0.031 |
| Smoking | 35 (40.2) | 146 (44.5) | 0.460 |
| Comorbidities | |||
| Hypertension | 47 (54.0) | 117 (35.7) | 0.002 |
| Hyperlipidemia | 7 (8.0) | 23 (7.0) | 0.746 |
| Diabetes mellitus | 20 (23.0) | 63 (19.2) | 0.441 |
| Coronary artery disease | 48 (55.2) | 161 (49.1) | 0.325 |
| Peripheral arteria disease | 21 (24.1) | 36 (11.0) | 0.001 |
| COPD | 2 (2.3) | 4 (1.2) | 0.460 |
| Abnormal liver function | 0 | 4 (1.2) | 0.300 |
| Atrial fibrillation | 3 (3.4) | 2 (0.6) | 0.031 |
| Preexisting neurological comorbidities | 8 (9.2) | 25 (7.6) | 0.630 |
| Pre-ECMO situation | |||
| Intra-aortic balloon pump | 49 (56.3) | 187 (57.0) | 0.908 |
| CPR history before ECMO | 38 (43.7) | 68 (20.7) | <0.001 |
| Lowest SBP (mmHg) | 70.0 [65.0, 80.0] | 80.0 [75.0, 80.0] | <0.001 |
| Blood glucose (mg/dl) | 256.0 [198.0, 311.0] | 243.0 [189.0, 289.0] | 0.177 |
| Pre-ECMO cardiac procedures | |||
| CABG | 41 (47.1) | 122 (37.2) | 0.092 |
| Valve replacement | 20 (23.0) | 85 (25.9) | 0.577 |
| Aortic surgery | 7 (8.0) | 14 (4.3) | 0.153 |
| CHD repair | 2 (2.3) | 10 (3.0) | 0.711 |
| CABG and valve replacement | 7 (8.0) | 36 (11.0) | 0.425 |
| CABG and aortic surgery | 6 (6.9) | 4 (1.2) | 0.002 |
| CABG and CHD repair | 0 | 1 (0.3) | 0.606 |
| Valve replacement and CHD repair | 0 | 9 (2.7) | 0.118 |
| Valve replacement and aortic surgery | 0 | 5 (1.5) | 0.247 |
| CABG combined valve replacement and CHD repair | 0 | 1 (0.3) | 0.606 |
| CABG combined valve replacement and aortic surgery | 1 (1.1) | 2 (0.6) | 0.597 |
| CABG combined aortic surgery and CHD repair | 0 | 1 (0.3) | 0.606 |
| Heart transplantation | 0 | 23 (7.0) | 0.011 |
| Atrial/ventricular thrombus clearance | 3 (3.4) | 10 (3.0) | 0.849 |
| Pulmonary embolism | 0 | 10 (3.0) | 0.099 |
| Re-operation | 1 (1.1) | 9 (2.7) | 0.389 |
Results are expressed as mean ± SD, number (%) or median [27th−75th percentile interquartile range].
CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CPR, cardiopulmonary resuscitation; SBP, systolic blood pressure, CHD, congenital heart disease.
ECMO Indications and Outcomes of Patients With and Without Neurologic Complications
The proportion of patients receiving V-A ECMO for failure to wean off CPB was significantly lower in the neurological complication group (41.4 vs. 55.2% in the control group, p < 0.05). In addition, there were 61 ECPR patients, the incidence of neurological complications of these patients was 39.3%. The proportion of ECPR patients was significantly higher in the neurological complication group (27.6 vs. 11.3% in the control group, p < 0.01; Table 2).
Table 2.
VA-ECMO indications and outcomes.
| Variable | Neurological complication group (n = 87) | Control group (n = 328) | p -value |
|---|---|---|---|
| ECMO implantation | |||
| Failure to wean off CPB | 36 (41.4) | 181 (55.2) | 0.022 |
| LCOS in ICU | 27 (31.0) | 110 (33.5) | 0.659 |
| ECPR | 24 (27.6) | 37 (11.3) | <0.001 |
| Transfusion | |||
| RBC (U) | 26.0 [14.0, 35.0] | 23.5 [14.0, 32.0] | 0.417 |
| FFP (ml) | 2400.0 [1400.0, 3400.0] | 2000.0 [1400.0, 3000.0] | 0.309 |
| Complications | |||
| Renal failure need CRRT | 46 (52.9) | 155 (47.3) | 0.351 |
| Lower extremities ischemia | 15 (17.2) | 26 (7.9) | 0.010 |
| Femoral artery embolism | 1 (1.1) | 3 (0.9) | 0.842 |
| Cannulate site hemorrhage | 4 (4.6) | 28 (8.5) | 0.221 |
| Retroperitoneal hematoma | 0 | 1 (0.3) | 0.606 |
| Major bleeding of other reasons | 11 (12.6) | 43 (13.1) | 0.909 |
| Surgical incision infection | 3 (3.4) | 28 (8.5) | 0.113 |
| Sepsis | 16 (18.4) | 76 (23.2) | 0.327 |
| Lower phlebothrombosis | 0 | 1 (0.3) | 0.606 |
| Re-thoracotomy for hemostasis | 37 (42.5) | 136 (41.5) | 0.858 |
| Tracheostomy | 41 (47.1) | 126 (38.4) | 0.141 |
| Outcomes | |||
| Weaning from ECMO | 31 (35.6) | 237 (72.3) | <0.001 |
| Duration of ECMO (h) | 97.0 [50.0, 128.0] | 95.5 [65.0, 137.0] | 0.388 |
| Survival to discharge | 8 (9.2) | 157 (47.9) | <0.001 |
| Duration of MV (h) | 137.0 [72.0, 216.0] | 121.5 [52.5, 210.0] | 0.181 |
| ICU length of stay (h) | 170.0 [95.0, 245.0] | 169.5 [96.0, 264.0] | 0.386 |
| Hospital stay (d) | 17.0 [12.0, 25.0] | 25.0 [17.0, 36.75] | <0.001 |
Results are expressed as mean ± SD, number (%) or median [27th−75th percentile interquartile range].
CPB, cardiopulmonary bypass; LCOS, low cardiac output syndrome; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; CRRT, continuous renal replacement therapy; RBC, red blood cells, ICU, intensive care unit; FFP, fresh frozen plasma; MV, mechanical ventilation.
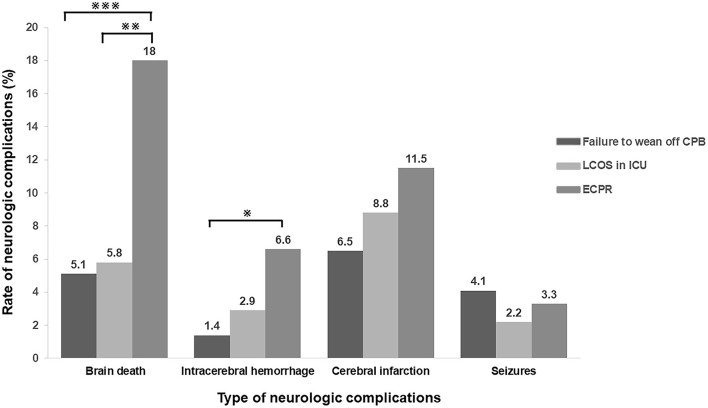
Further analysis was performed to detect the differences in the type of neurologic complications among the patients who experienced failure to wean off CPB, LCOS and ECPR. ECPR patients had a higher rate of brain death (18.0 vs. 5.1% in the patients failure to wean off CPB or 5.8% in the patients of LCOS, p < 0.01, respectively; Figure 2).
Figure 2.
Incidence of neurologic complications in different types of ECMO indications. ECPR patients had a higher rate of brain death, intracranial hemorrhage and cerebral infarction when compared with the patients with other indications. (※p < 0.05; ※※p < 0.01; ※※※P < 0.005) CPB, cardiopulmonary bypass; LCOS, low cardiac output syndrome; ICU, intensive care unit; ECPR, extracorporeal cardiopulmonary resuscitation.
Blood transfusion and severe bleeding were similar between the two groups (p > 0.05). Lower-extremity ischemia occurred more often in the neurological complication group (17.2 vs. 7.9% in the control group, p < 0.05). There were no significant differences in the duration of ECMO, mechanical ventilation (MV), and ICU stay. However, the overall hospital stay in the neurological complication group was significantly shorter than that in the control group [17.0 (12.0, 25.0) days vs. 25.0 (17.0, 36.75) days, p < 0.001] (Table 2).
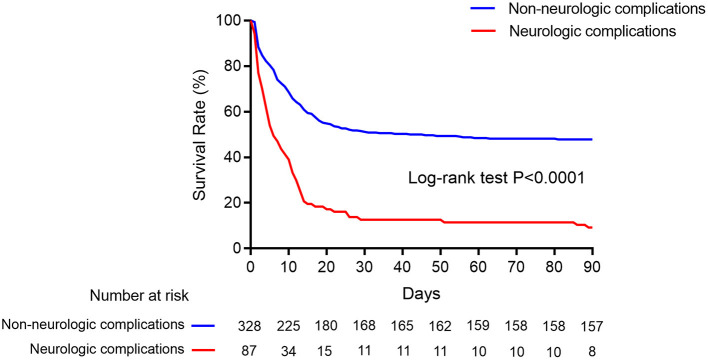
The rate of successful weaning from ECMO (35.6 vs. 72.3%, p < 0.001) and survival to discharge (9.2 vs. 47.9%, p < 0.001) in the neurological complication group were significantly lower than those in the control group (Table 2; Figure 3).
Figure 3.
Kaplan–Meier cumulative in-hospital mortality after ECMO initiation. Kaplan–Meier survival curves showed in-hospital mortality in ECMO patients with neurologic complications (red line) and without (blue line).
Prevalence and Prognosis of Different Type of Neurologic Complications
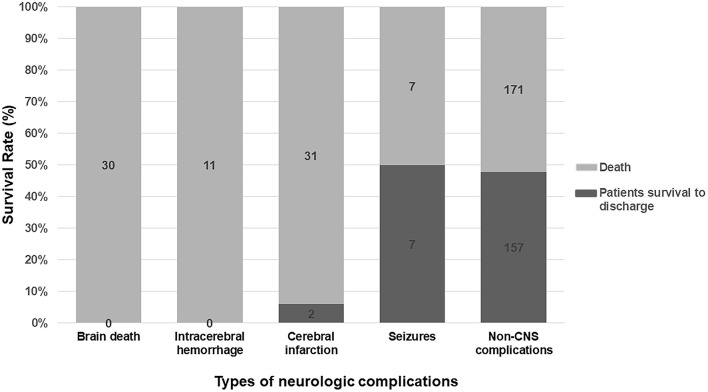
Among all kinds of neurologic complications, cerebral infarction was the most frequent (33 patients, 8.0%), followed by brain death (30, 7.2%), seizures (14, 3.4%), and intracranial hemorrhage (11, 2.7%), respectively. Two patients presented two kinds of neurologic complications at the same time.
Poor survival was observed in the patients with brain death, intracranial hemorrhage, and cerebral infarction (p < 0.001 compared with the control group). Patients with seizures had similar survival rate as compared to patients in the control group (Figure 4).
Figure 4.
Subgroup analysis for survival rate in different kinds of neurologic complications. Patients with brain death, intracranial hemorrhage, and cerebral infarction had a catastrophic outcome. Patients suffered seizures had a similar prognosis to patients in the control group.
Risks Factors Associated With Neurologic Complications
Multivariate logistic regression analysis showed that the lowest SBP level before VA ECMO initiation (OR, 0.89; 95% CI: 0.86–0.93; p < 0.001) and aortic vascular surgery combined with CABG (OR, 9.22; 95% CI: 2.10–40.55; p < 0.01) were associated with neurologic complications. Although ECPR was more common in the neurological complication group, it was not a risk factor at multivariable analysis (Table 3).
Table 3.
Univariable and multivariable analyses of factors associated with neurologic complications.
| Factor | Univariable analysis | P -value | Multivariable analysis | P -value | ||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Overall neurologic complications | ||||||
| Age | 1.03 | 1.01–1.05 | 0.003 | |||
| Obesity (BMI ≥ 30) | 2.07 | 1.06–4.05 | 0.034 | |||
| Hypertension | 2.11 | 1.31–3.40 | 0.002 | |||
| Peripheral arterial disease | 2.60 | 1.43–4.75 | 0.002 | |||
| CPR history before ECMO | 2.97 | 1.80–4.89 | <0.001 | |||
| Lowest SBP | 0.89 | 0.86–0.92 | <0.001 | 0.89 | 0.86–0.93 | <0.001 |
| CABG combined with aortic surgery | 6.00 | 1.65–21.76 | 0.006 | 9.22 | 2.10–40.55 | 0.003 |
| ECPR | 3.00 | 1.68–5.36 | <0.001 | |||
| Failure to wean off CPB | 0.57 | 0.36–0.93 | 0.023 | |||
| Brain death | ||||||
| Age | 1.06 | 1.02–1.11 | 0.002 | 1.06 | 1.01–1.12 | 0.022 |
| Obesity (BMI ≥ 30) | 2.78 | 1.12–6.90 | 0.028 | |||
| Peripheral arterial disease | 3.14 | 1.35–7.30 | 0.008 | |||
| CPR history before ECMO | 6.95 | 3.14–15.42 | <0.001 | |||
| Lowest SBP | 0.81 | 0.77–0.86 | <0.001 | 0.81 | 0.76–0.87 | <0.001 |
| CABG | 2.89 | 1.34–6.25 | 0.007 | |||
| ECPR | 3.88 | 1.74–8.63 | 0.001 | |||
| Intracranial hemorrhage | ||||||
| Coagulation disorders | 8.76 | 1.67–45.85 | 0.010 | 9.75 | 1.83–51.89 | 0.008 |
| Atrial fibrillation | 10.00 | 1.02–97.71 | 0.048 | 12.19 | 1.22–121.61 | 0.033 |
| Cerebral infarction | ||||||
| Atrial fibrillation | 8.15 | 1.31–50.62 | 0.024 | |||
| Seizures | ||||||
| Hyperlipidemia | 5.75 | 1.69–19.60 | 0.005 | |||
BMI, body mass index; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; SBP, systolic blood pressure.
Age (OR, 1.06; 95% CI: 1.01–1.12, p < 0.05) and lowest SBP (OR, 0.81, 95% CI: 0.76–0.87, p < 0.001) were correlative factors of brain death. Coagulation disorders (OR, 9.75, 95% CI: 1.83–51.89, p < 0.01) and atrial fibrillation (OR, 12.19, 95% CI: 1.22–121.61, p < 0.05) could influence the incidence of intracranial hemorrhage. The occurrence of cerebral infarction might also be affected by atrial fibrillation (OR, 8.15, 95% CI: 1.31–50.62, p < 0.05). Hyperlipidemia patients had increased odds for seizures (OR, 5.75, 95% CI: 1.69–19.60, p < 0.01). It is noteworthy that preexisting neurological comorbidities were not risk factors of neurologic complications on ECMO (OR, 1.23, 95% CI: 0.53–2.83, p > 0.05; Figure 5).
Figure 5.
Risk factors of neurologic complications during extracorporeal membrane oxygenation. SBP, systolic blood pressure; CABG, coronary artery bypass grafting.
Threshold of the Lowest SBP Level Before V-A ECMO Initiation in the Prediction of Neurologic Complications
In converting the lowest SBP level before V-A ECMO initiation from a continuous to a categorical variable, 72.5 mmHg was chosen as a threshold. It offered a sensitivity and specificity of 86.6 and 50.6% for prediction, respectively. The odds ratio of neurologic complications associated with SBP lower than 72.5 mmHg was 6.61 (95% CI: 3.90–11.19, p < 0.001; Figure 6).
Figure 6.
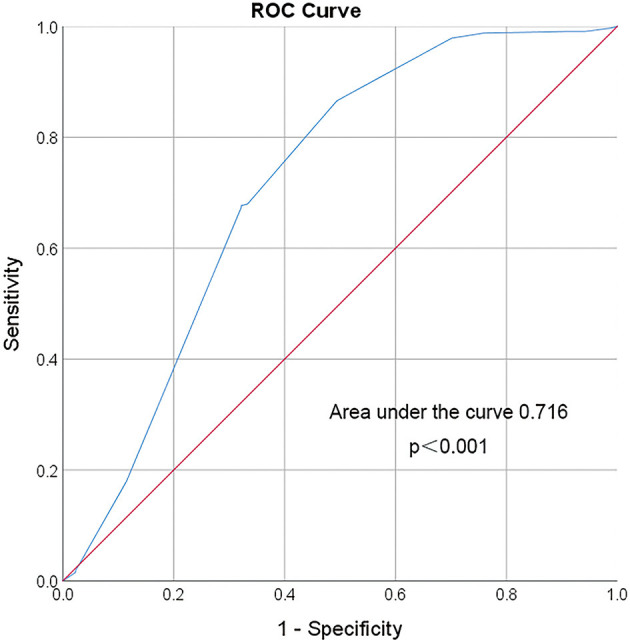
ROC curve of the lowest SBP before VA ECMO initiation. Area under the ROC Curve of the lowest systolic blood pressure (72.5 mmHg) before VA ECMO initiation for predicting incidence of neurologic complications during ECMO is 0.716 (95% CI 0.647–0.786). The red line indicates reference values.
Discussion
This study shows the neurologic complications in adult PCS patients receiving V-A ECMO support. We found that the rate of successful weaning from V-A ECMO and survival to discharge were significantly lower in patients with neurologic complications. The lowest SBP level pre-ECMO and aortic surgery combined with CABG were identified as correlative factors independently associated with overall neurologic complications in these patients.
In this study, neurologic complications occurred in 21% of cases in adult PCS patients supported with V-A ECMO, a slightly higher rate reported by previous investigations (6–17%) (12, 15, 21, 22). This perhaps was due to the type of cardiac surgery or by neurologic patient examination protocols. In previous studies, owing to the difficulties of getting an imaging examination during V-A ECMO support or patients dying from severe neurologic complications without an imaging examination, the true incidence of neurologic complications might have been underestimated (14–16, 18, 21–25).
Matteen et al. (23) found that increased age was associated with higher rates of death and neurological morbidity. We also found that patients with neurologic complications were older, obese, and with more comorbidities, especially hypertension, hyperlipidemia, and peripheral arterial disease. All of these conditions indicate patients' worse general status and vascular condition in our series, which may lead to increased rate of neurologic complications.
We found that the incidence of neurologic complications was associated to pre-ECMO low perfusion situations. ECPR was also frequently present in patients with neurologic complications. The incidence of neurologic complications in ECPR patients was higher than that in the patients failure to wean off CPB or the patients of postoperative LCOS. The reasons may be that the patients failing to wean off CPB (or postoperative LCOS patients) could promptly transition to V-A ECMO and whereas the majority of ECPR patients may have experienced cerebral hypo-perfusion, hypoxia, and reperfusion injury prior to ECMO implantation (23). Moreover, aortic surgery combined with CABG was shown to be an independent risk factor of the neurologic complications in the current study. The possible reason could be that the patients in our study had type A aortic dissection involving coronary artery and multiple organs, requiring intraoperative deep hypothermic circulatory arrest and long CPB times, conditions more likely associated with postoperative neurological injury.
Coagulation disorders can be induced by many factors, including the ECMO circuit, the surgical procedures, and the severity of the disease. Coagulation disorders were important factors affecting the integrity of the neurologic system and might lead to intracranial hemorrhage (12). In addition, we found that a history of atrial fibrillation was an independent risk factor of both intracranial hemorrhage and cerebral infarction. Atrial fibrillation is a well-known reason for thromboembolism in PCS patients (26). On the other hand, the patients with atrial fibrillation need anticoagulants to prevent thrombosis, condition favoring the occurrence of intracranial hemorrhage. Our results are in accordance with the previous literature regarding intracranial bleeding occurring in ECMO patients (27–29). Hyperlipidemia was associated with seizures which may be due to asymptomatic cerebrovascular disease secondary to dyslipidemia, but the underlying mechanism is yet less defined and warrants further research. A threshold of lowest SBP before V-A ECMO initiation, which may predict prognosis and assist doctors in managing patients, was defined in our investigation. When patients' blood pressure cannot be sustained by vasopressors, ECMO should be used prior to severe and refractory hypotension. In the clinical setting, neurologic complications may be induced by multiple factors during V-A ECMO support. Similarly, risk factor identification may help initiate steps to lower the risk of such complications in PCS patients undergoing temporary ECMO assistance.
Limitations
This single-center study is limited by its retrospective nature. The exact timing of neurologic complication in relation to ECMO and information regarding neurologic impairment before ECMO were uncertainty. In addition, full neuroimaging assessment was not performed on every patient. Even though we could perform CT scan, we had no MRI results because of the restrictions of the ECMO device, and, therefore, some subtle abnormalities, such as cerebral microbleeds, might have been not objectivated (30). Therefore, neurological complications were likely underestimated. However, routine neurological examinations were performed at least twice a day by the ICU staffs. It was unlikely to miss neurological complications with positive clinical manifestations in this study. Neurological events after ECMO weaning were not involved in this study, because of confounding factors. Another limitation is that we did not have long-term follow-up on survivors.
Conclusions
Neurologic complications are frequent in adult PCS patients treated with VA ECMO, and are associated with increased in-hospital mortality. We identified the lowest SBP level before V-A ECMO initiation, CABG combined with aortic surgery, age, coagulation disorders, atrial fibrillation and hyperlipidemia as independent risk factors for different neurologic complications during V-A ECMO support in PCS patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by Beijing Anzhen Hospital human research Ethics Committee (Ethics number: 2016018X). Because this was a retrospective observational study, the individual patients' consent was waived.
Author Contributions
DH and HW participated in the design of the study, analyzed the data, and drafted the manuscript. FY interpreted the data and revised the manuscript. XH conceived the study, participated in its design, and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- ACT
activated clotting time
- aPTT
activated partial thromboplastin time
- BMI
body mass index
- CABG
coronary artery bypass grafting
- CI
confidence interval
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disease
- CPB
cardiopulmonary bypass
- CPR
cardiopulmonary resuscitation
- CRRT
continuous renal replacement therapy
- CT
computed tomography
- ECMO
extracorporeal membrane oxygenation
- ECPR
extracorporeal cardiopulmonary resuscitation
- FFP
fresh frozen plasma
- IABP
intra-aortic balloon pump
- ICU
intensive care unit
- IQR
interquartile range
- LCOS
low cardiac output syndrome
- MV
mechanical ventilation
- OR
odds ratio
- PCS
post-cardiotomy cardiogenic shock
- RBC
red blood cells
- SBP
systolic blood pressure
- VA ECMO
veno-arterial extracorporeal membrane oxygenation.
Footnotes
Funding. This project was supported by National Natural Science Foundation of China (Grant No. 81870305).
References
- 1.Pineton de Chambrun M, Bréchot N, Lebreton G, Schmidt M, Hekimian G, Demondion P, et al. Venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock post-cardiac arrest. Intensive Care Med. (2016) 42:1999–2007. 10.1007/s00134-016-4541-y [DOI] [PubMed] [Google Scholar]
- 2.Karagiannidis C, Brodie D, Strassmann S, Stoelben E, Philipp A, Bein T, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. (2016) 42:889–96. 10.1007/s00134-016-4273-z [DOI] [PubMed] [Google Scholar]
- 3.Tramm R, Ilic D, Davies AR, Pellegrino VA, Romero L, Hodgson C. Extracorporeal membrane oxygenation for critically ill adults. Cochrane Database Syst Rev. (2015) 1:CD010381. 10.1002/14651858.CD010381.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J. (2015) 36:2246–56. 10.1093/eurheartj/ehv194 [DOI] [PubMed] [Google Scholar]
- 5.Lawler PR, Silver DA, Scirica BM, Couper GS, Weinhouse GL, Camp PC, Jr. Extracorporeal membrane oxygenation in adults with cardiogenic shock. Circulation. (2015) 131:676–80. 10.1161/CIRCULATIONAHA.114.006647 [DOI] [PubMed] [Google Scholar]
- 6.Chang CH, Chen HC, Caffrey JL, Hsu J, Lin JW, Lai MS, et al. Survival analysis after extracorporeal membrane oxygenation in critically ill adults: a nationwide cohort study. Circulation. (2016) 133:2423–33. 10.1161/CIRCULATIONAHA.115.019143 [DOI] [PubMed] [Google Scholar]
- 7.Ouweneel DM, Schotborgh JV, Limpens J, Sjauw KD, Engström AE, Lagrand WK, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. (2016) 42:1922–34. 10.1007/s00134-016-4536-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. (2008) 372:554–61. 10.1016/S0140-6736(08)60958-7 [DOI] [PubMed] [Google Scholar]
- 9.Nasr DM, Rabinstein AA. Neurologic complications of extracorporeal membrane oxygenation. J Clin Neurol. (2015) 11:383–9. 10.3988/jcn.2015.11.4.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta A, Ibsen LM. Neurologic complications and neurodevelopmental outcome with extracorporeal life support. World J Crit Care Med. (2013) 2:40–7. 10.5492/wjccm.v2.i4.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutter R, Tisljar K, Marsch S. Acute neurologic complications during extracorporeal membrane oxygenation: a systematic review. Crit Care Med. (2018) 46:1506–13. 10.1097/CCM.0000000000003223 [DOI] [PubMed] [Google Scholar]
- 12.Lorusso R, Barili F, Mauro MD, Gelsomino S, Parise O, Rycus PT, et al. In-hospital neurologic complications in adult patients undergoing venoarterial extracorporeal membrane oxygenation: results from the Extracorporeal Life Support Organization Registry. Crit Care Med. (2016) 44:e964–e72. 10.1097/CCM.0000000000001865 [DOI] [PubMed] [Google Scholar]
- 13.Lorusso R, Gelsomino S, Parise O, Di Mauro M, Barili F, Geskes G, et al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: findings from the Extracorporeal Life Support Organization Database. Crit Care Med. (2017) 45:1389–97. 10.1097/CCM.0000000000002502 [DOI] [PubMed] [Google Scholar]
- 14.Li CL, Wang H, Jia M, Ma N, Meng X, Hou XT. The early dynamic behavior of lactate is linked to mortality in postcardiotomy patients with extracorporeal membrane oxygenation support: a retrospective observational study. J Thorac Cardiovasc Surg. (2015) 149:1445–50. 10.1016/j.jtcvs.2014.11.052 [DOI] [PubMed] [Google Scholar]
- 15.Cavarocchi NC, Pitcher HT, Yang Q, Karbowski P, Miessau J, Hastings HM, et al. Weaning of extracorporeal membrane oxygenation using continuous hemodynamic transesophageal echocardiography. J Thorac Cardiovasc Surg. (2013) 146:1474–9. 10.1016/j.jtcvs.2013.06.055 [DOI] [PubMed] [Google Scholar]
- 16.Luyt CE, Bréchot N, Demondion P, Jovanovic T, Hékimian G, Lebreton G, et al. Brain injury during venovenous extracorporeal membrane oxygenation. Intensive Care Med. (2016) 42:897–907. 10.1007/s00134-016-4318-3 [DOI] [PubMed] [Google Scholar]
- 17.Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. (2014) 97:610–6. 10.1016/j.athoracsur.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 18.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2016) 374:1609–20. 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 19.Rizvi T, Batchala P, Mukherjee S. Brain death: diagnosis and imaging techniques. Semin Ultrasound CT MR. (2018) 39:515–29. 10.1053/j.sult.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 20.Wijdicks EF. Determining brain death in adults. Neurology. (1995) 45:1003–11. 10.1212/WNL.45.5.1003 [DOI] [PubMed] [Google Scholar]
- 21.Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. (2010) 139:302–11:311.e1. 10.1016/j.jtcvs.2009.10.043 [DOI] [PubMed] [Google Scholar]
- 22.Wu MY, Lin PJ, Lee MY, Tsai FC, Chu JJ, Chang YS, et al. Using extracorporeal life support to resuscitate adult postcardiotomy cardiogenic shock: treatment strategies and predictors of short-term and midterm survival. Resuscitation. (2010) 81:1111–6. 10.1016/j.resuscitation.2010.04.031 [DOI] [PubMed] [Google Scholar]
- 23.Mateen FJ, Muralidharan R, Shinohara RT, Parisi JE, Schears GJ, Wijdicks EF. Neurological injury in adults treated with extracorporeal membrane oxygenation. Arch Neurol. (2011) 68:1543–9. 10.1001/archneurol.2011.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anselmi A, Flécher E, Corbineau H, Langanay T, Le Bouquin V, Bedossa M, et al. Survival and quality of life after extracorporeal life support for refractory cardiac arrest: a case series. J Thorac Cardiovasc Surg. (2015) 150:947–54. 10.1016/j.jtcvs.2015.05.070 [DOI] [PubMed] [Google Scholar]
- 25.Kasirajan V, Smedira NG, McCarthy JF, Casselman F, Boparai N, McCarthy PM. Risk factors for intracranial hemorrhage in adults on extracorporeal membrane oxygenation. Eur J Cardiothorac Surg. (1999) 15:508–14. 10.1016/S1010-7940(99)00061-5 [DOI] [PubMed] [Google Scholar]
- 26.Hogue CW, Jr, Murphy SF, Schechtman KB, Dávila-Román VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation. (1999) 100:642–7. 10.1161/01.CIR.100.6.642 [DOI] [PubMed] [Google Scholar]
- 27.Fletcher-Sandersjöö A, Bartek J, Jr, Thelin EP, Eriksson A, Elmi-Terander A, Broman M, et al. Predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: an observational cohort study. J Intensive Care. (2017) 5:27. 10.1186/s40560-017-0223-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokolovic M, Pratt AK, Vukicevic V, Sarumi M, Johnson LS, Shah NS. Platelet count trends and prevalence of heparin-induced thrombocytopenia in a cohort of extracorporeal membrane oxygenator patients. Crit Care Med. (2016) 44:e1031–e7. 10.1097/CCM.0000000000001869 [DOI] [PubMed] [Google Scholar]
- 29.Opfermann P, Bevilacqua M, Felli A, Mouhieddine M, Bachleda T, Pichler T, et al. Prognostic impact of persistent thrombocytopenia during extracorporeal membrane oxygenation: a retrospective analysis of prospectively collected data from a cohort of patients with left ventricular dysfunction after cardiac surgery. Crit Care Med. (2016) 44:e1208–e18. 10.1097/CCM.0000000000001964 [DOI] [PubMed] [Google Scholar]
- 30.Cavayas YA, Del Sorbo L, Fan E. Intracranial hemorrhage in adults on ECMO. Perfusion. (2018) 33:42–50. 10.1177/0267659118766435 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.