Abstract
Background:
Ischemia and reperfusion (I/R) is a pathological condition characterized by an initial restriction of blood supply to an organ followed by the subsequent restoration of perfusion and concomitant reoxygenation.
Objective:
The aim of the study is to assess the possible cardioprotective potential effect of berberine in myocardial ischemia reperfusion injury induced by ligation of coronary artery in a male rat model.
Methods:
Total amount of 28 adult male albino rats were randomized into 4 equal groups: 1) Sham group, rats underwent the same anesthetic and surgical procedure as the control group except for LAD ligation; 2), Active control group, rats subjected to regional ischemia for 30 min by ligation of LAD coronary artery and reperfusion for 2 hours, 3), Control vehicle group, rats received dimethyl sulphoxide (DMSO) (vehicle of berberine) via IP route and subjected to ischemia for 30 minutes before ligation of LAD coronary artery & reperfusion for 2 hr; 4), Berberine treated group, rats pretreated with berberine10 mg/kg via IP injection 30minutes before ligation of LAD coronary artery & then subjected to reperfusion for 2 hr.
Results:
In the control group, as compared with sham, tissue TNF-α, IL-6, IL-10, caspase-3 and BAX, plasma cTn-T and serum MDA significantly increased (P<0.05), while serum GSH significantly decreased (P<0.05). The histopathological control group showed a significant cardiac injury (P<0.05) compared with the sham group. Berberine significantly counteracted (P<0.05) the increase of TNF-α, IL-6, caspase-3 and BAX and counteracted the increase in plasma cTn-T and serum MDA. Berberine produces a significant elevation (P<0.05) in cardiac IL-10 and serum GSH with a significant reduction in (P<0.05) cardiac injury.
Conclusion:
Berberine attenuates myocardial I/R injury in male rats via interfering with inflammatory reactions and apoptosis which were induced by I/R injury.
Keywords: Berberine, Ischemia/reperfusion, Apoptosis, Inflammatory reactions
1. BACKGROUND
Coronary heart disease (CHD) has become the chief cause of human death, accounting for 13.2% of the top 10 causes (1). Ischemia and reperfusion (I/R) is a pathological condition characterized by an initial restriction of blood supply to an organ followed by the subsequent restoration of perfusion and concomitant reoxygenation (2). Prolonged organic ischemia is characterized by insufficient oxygen supply resulting in tissue ATP depletion with a transition to activation of anaerobic metabolic pathways which cannot maintain cellular function for prolonged periods lastly leading to cell death (3). Within myocardial ischemia, tissue pH significantly declines and returns to normal after reperfusion (4). A difference in metabolic supply and demand within the ischemic organ results in deep tissue hypoxia and microvascualar dysfunction (5). Neutrophils induce inflammatory mediators that amplify recruitment of Neutrophil in the ischemic reperfused myocardium, so expanding myocardial damage (6). Furthermore, I/R leads to the triggering of cell death programs, involving apoptosis and necrosis (7). Myocardial ischemia is differentiated with anaerobic metabolism and intracellular acidosis (8). During reperfusion, the electron transport chain is reactivated, generating ROS. ROS mediate myocardial reperfusion injury by inducing the opening of the MPTP, acting as a Neutrophil chemoattractant. This contributes to intracellular Ca2+ overload and damages the cell membrane by lipid peroxidation, inducing enzyme denaturation and causing direct oxidative damage to DNA. Several hours after the onset of myocardial reperfusion, Neutrophils accumulate in the infracted myocardial tissue in response to the release of the chemoattractant ROS, cytokines, and activated complement (9). Actually the reperfusion can be more injurious than the pre-reperfusion ischemia. Several clinical and experimental studies have established that berberine has protective effects on MIRI (myocardial ischemia reperfusion injury) (10-12). By decreasing the level of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, berberine could diminish oxidative stress that was the main source of ROS generation within cells (13). Through induction of the nuclear factor erythroid-2-related factor-2 (Nrf 2) pathway, berberine inhibited the production of oxidative stress (14). The activation of phosphatidylinositol 3-kinase (PI3K)/Akt pathway, AMPK pathway also the P38 pathway implicated in the activity of berberine on Nrf2 which could trigger the expression of antioxidant enzymes, elevate GSH and SOD level within cells and decrease the production of oxidative stress in addition to ROS (14). The NF-κB pathway plays a key role in controlling inflammation (15). As a transcription factor, NF-κB (16) and AP-1 (17) stimulated the expression of different proinflammatory mediators like IL-6, TNF-α, COX2 as well as iNOS. Through activation of PPARγ, berberine decreases the generation of proinflammatory mediators partly (18).
The activation of P38 and AMPK by therapy of berberine induces nuclear translocation of Nrf 2 and stops the generation of proinflammatory mediators moreover to antioxidative activity (19). Autophagy is involved in a wide range of physiological processes and the pathogenesis of a variety of diseases, such as MIRI, acute lung injury, and various types of infections (20,21). Huang et al., (22) found that berberine treatment significantly enhanced H/R-induced cell viability and reduced I/R-induced myocardial infarct size and cellular Autophagy levels (22) confirmed that berberine attenuates mitochondrial dysfunction by inducing autophagic flux in myocardial H/R injury. Chen et al., (23) demonstrate that berberine exerts an anti-apoptotic effect and improves cardiac functional recovery following myocardial I/R through activating AMPK and PI3K–Akt–eNOS signaling.
2. OBJECTIVE
The aim of the study is to assess the possible cardioprotective potential effect of berberine in myocardial ischemia reperfusion injury induced by ligation of coronary artery in a male rat model.
3. MATERIAL AND METHODS
Materials
Pure berberine (>98%) Santacruz Biotechnology (USA), normal saline (KSA), ketamine (Hikma, Jordan), Xylazine (Rompun TM 2% vials, Bayer AG, Leverkusen, Germany). Rat TNF-α, IL-6, IL-10, caspase3, BAX and cTnT (ELISA) kits were purchased from Biotangusa, USA. Trichloroacetic acid (TCA)Merck-Germany, Ethylene diaminetetraacetic acid disodium (EDTA)BDH, U.K. Thiobarbituric acid (TBA) Fluka company, Switzerland 5,5-Dithiobis (2-nitrobenzoic acid) DTNB Sigma company Ltd. Reduced glutathione Biochemical, USA and Methanol Fluka company, Switzerland. Regarding instruments, High Intensity Ultrasonic Liquid Processor (Sonics & materials Inc., USA), Digital Spectrophotometer EMCLAB/ Germany, Bio-Elisa Reader, BioTek Instruments, USA and ventilator (Harvard USA).
Animals
After the approval that has been established by the Institutional Animal Care and Use Committee (IACUC) and submit the required applications, 28 male albino rats weighting (180-320 g) were purchased from Animal Resource Center. They were housed in the animal house (for one week) in a temperature-controlled (25°±1C) room (humidity was kept at (60–65%) with alternating 12-h light/12-h dark cycles and were allowed to access freely regarding water and chow diet until the time of starting the experimental study.
Study design
After the 1st week of accommodation, the 28 rats were randomly divided into 4 groups (7 rats in each) as follow:
Sham group: Rats underwent the same anesthetic and surgical procedures but without ligation for the LAD.
Active control (MI/R) group: rats followed a surgical operation for LAD ligation and they were subjected to 30 min of ischemia and 120 min of reperfusion. MI/R + Vehicle pretreated group: rats were pretreated with DMSO via intraperitoneal injection 30 minutes before ligation of LAD, then underwent surgical LAD ligation, and subjected to 30min of ischemia followed by 120 min of reperfusion. MI/R + Berberine pretreated group: rats of this group take a single I.P injection of berberine in a concentration of 10 mg/kg dissolved in 0.1% DMSO 1 hour immediately before ligation of LAD, then subjected to surgical LAD ligation with 30 minutes of ischemia followed by 120 min of reperfusion (24).
Statistical ligation of the LAD
Rats were anesthetized with (IP) injection of 100 mg/kg ketamine and 10 mg/kg xylazine (25). After intubation of the trachea by a 20 G cannula and the Endotracheal tube was connected tightly to the ventilation machine. The ventilation rate was fixed from 120-135 breath/minute with tidal volume 20 ml/kg body weight, with 100% oxygen. Pericardial layer incision was made by administration round end scissors to open the space. The LAD coronary artery was transient ligated 1 to 2 mm below the tip of the left auricle using a tapered needle and an 8-0 polypropylene ligature. Tightening the ligature could then occlude the artery for a 30-minute ischemic period (26). The chest cavity was closed by bringing together the fourth and fifth ribs with one 2-0 silk suture. Cardiac reperfusion was achieved by releasing the tension applying to the ligature for 120 minutes (27). The rats were euthanized after reperfusion via injection high dose of anesthesia and the chest was re-opened then the right ventricle was punctured with a syringe needle so that about 3 ml of blood was aspirated for later blood analysis. After that, the heart was isolated and divided into 2 pieces, the apical part used for histological examination and the basal was used for measuring the tissue parameters.
Blood sampling for measurement of plasma cTn-T, serum MDA and serum reduced GSH
At the end of experiment, about 2-3 ml of blood sample was placed in a tube containing disodium ethylene diamine tetra acetic acid (EDTA) (22 mg/mL) as anticoagulant and mixed thoroughly and then centrifuged at 3000 rpm for 15 min then the supernatant was used for determination of plasma cTn-T level, whereas the remaining blood was allowed to clot in an ordinary tube at 37 oC then it was centrifuged at 3000 rpm for 15 minutes then the supernatant was taken for MDA and GSH serum levels determination.
Tissue preparation for TNF-α, IL-6, IL-10, caspase 3 and BAX measurements
The upper parts of the ventricles were washed with cold normal saline to remove any blood, stored in deep freeze (-20°C), and then homogenized with high intensity liquid processor in 1:10 (w/v) phosphate buffered saline that contain 1% triton X-100 and protease inhibitor cocktail (28). The homogenate was centrifuged at 14000 rpm 4°C for 20 min. The supernatant was collected for determination of TNF-α, IL-10, IL-6, Bax, and Caspase- 3 by ELISA with a commercially available ELISA kit (Literature of kit by life Diagnostic, USA) according to the manufacturer’s instructions.
Preparation for Histopathology
the apical parts of the heart were excised immediately, rinsed using ice-cold 0.9% saline and fixed in 10% formalin solution pH 7.4 (29) embedded in paraffin wax. The paraffin-embedded tissues were sectioned (4-μm thick), stained with hematoxylin and eosin (H&E). Damage scores were evaluated according to the following morphological criteria that have been used to evaluate the histopathological damage (30) as follow:
Score 0, no damage; score 1 (mild), interstitial edema and focal necrosis; score 2 (moderate), diffuse myocardial cell swelling and necrosis; score 3 (severe), necrosis with presence of contraction bands and Neutrophil infiltrate; score 4 (highly severe), widespread necrosis with presence of contraction bands, Neutrophil infiltrate, and hemorrhage.
Statistical analysis
Data were expressed as mean ± SEM. An expert statistical advice was considered for data analysis which was aided by a computer. Statistical analysis was done using SPSS version 20.0 computer software (Statistical Package for Social Science). ANOVA (analysis of variance) had been used for measurement (numerical data). Mann-Whitney test had been used for myocardial damage score, P-value <0.05 regarded as significant.
4. BIOCHEMICAL RESULTS
Effect on Pro-inflammatory cytokines (TNF-α, IL-6):
Results revealed a significant increase (P<0.05) in (TNF-α and IL-6) cardiac tissue levels in the MI/R group as compared with the sham group, while in the MI/R + berberine pretreated group, berberine produce a significant decrease (P<0.05) in the (TNF-α and IL-6) cardiac tissue levels as compared with the MI/R group as shown in Table 1 and Figures 1 and 2.
Table 1. Comparison according to Mann-Whitney test for scoring regarding histopathological changes.
| GROUP | P value |
|---|---|
| 1. Sham | |
| 2. Control | <0.05* |
| 3. Berberine | <0.05#* |
Figure 1. The mean of myocardial TNF-α (pg/mg) in the four experimental groups at the end of the experiment.*P<0.05 vs. sham; #P<0.05 vs. Control group.

Figure 2. The mean of myocardial TNF-α (pg/mg) in the four experimental groups at the end of the experiment.*P<0.05 vs. sham; #P<0.05 vs. Control group.

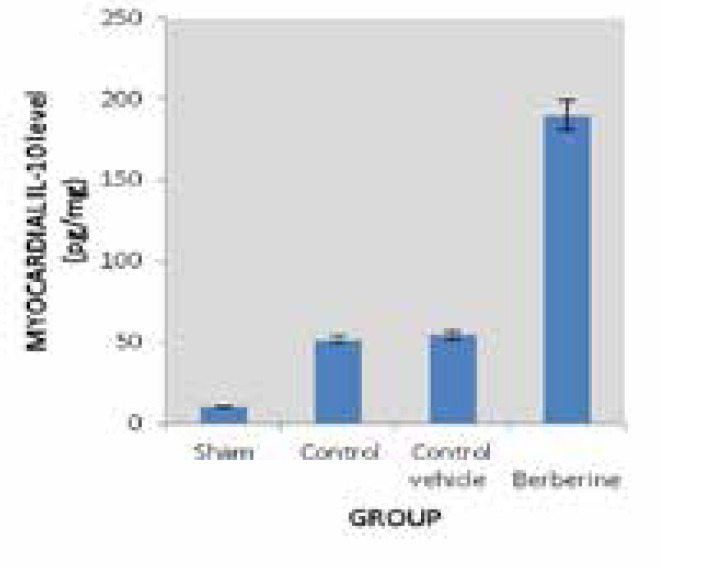
Effect on anti-inflammatory cytokine (IL-10):
Results revealed a significant increase (P<0.05) in (IL-10) cardiac tissue level in the MI/R group as compared with the sham group, while in the MI/R + berberine pretreated group, berberine produce a significant elevation (P<0.05) in the (IL-10) cardiac tissue level as compared with all other groups (sham group, the MI/R group and MI/R +vehicle group as shown in Table 1 and Figure 3.
Figure 3. The myocardial mean ofIL-10 (pg/mg) in the four experimental groups at the end of the experiment *P<0.05 vs. sham group; #P<0.05 vs. Control group.
Effect on apoptotic markers (caspase-3 BAX):
Results revealed a significant increase (P<0.05) in (caspase-3 and BAX) cardiac tissue levels in the MI/R group as compared with the sham group, while in the MI/R + berberine pretreated group, berberine produce a significant reduction (P<0.05) in the (caspase-3 and BAX) cardiac tissue levels as compared with the MI/R group as shown in Table 1 and Figures 4 and 5.
Figure 4. The myocardial mean of BAX (pg/mg) in the four experimental groups at the end of the experiment *P<0.05 vs. sham group; #P<0.05 vs. Control group.

Figure 5. The myocardial mean ofCaspase-3 (pg/mg) in the four experimental groups at the end of the experiment. *P<0.05 vs. sham group, # P<0.05 vs. Control group.

Effect on Plasma Level of Troponin T (cTnT):
Results revealed a significant increase (P<0.05) in (cTnT) plasma level in the MI/R group as compared with the sham group, while in the MI/R + berberine pretreated group, berberine produce a significant reduction (P<0.05) in the (cTnT) plasma level as compared with the MI/R group as shown in Table 1 and Figure 6.
Figure 6. The mean of plasma cTn-T level (pg/ml) in the four experimental groups at the end of the experiment. P<0.05 vs. sham group # P<0.05 vs. Control group.

Effect on the serum level of oxidative stress markers (MDA and GSH):
Results revealed a significant increase (P<0.05) in the serum level of MDA in the MI/R group as compared with the sham group, while in the MI/R +berberine pretreated group, berberine produces a significant reduction (P<0.05) in MDA serum level as compared with the MI/R group. About GSH, results revealed a significant decrease (P<0.05) in the serum level of GSH in the MI/R group as compared with the sham group, while in the MI/R +berberine pretreated group, berberine produces a significant increase (P<0.05) in GSH serum level as compared with the MI/R group as shown in Table 1 and Figures 7 and 8.
Figure 7. The myocardial mean of MDA (μmol/L) in the four experimental groups at the end of the experiment. *P<0.05 vs. sham group, # P<0.05 vs. Control group.

Figure 8. The myocardial mean of GSH (μmol/L) in the four experimental groups at the end of the experiment. *P<0.05 vs. sham group, # P<0.05 vs. Control group.

Histopathological Findings
Histological, the MI/R group revealed a significant cardiac tissue injury (P<0.05) compared with the sham group, and this injury was showing severe hemorrhage, presence of interstitial edema, necrosis and Neutrophil infiltration in contrast with the cross-section of the sham group which showed a 100% normal structure of cardiac tissue with no interstitial edema, no diffuse myocardial cell swelling and necrosis, no Neutrophils infiltration, no hemorrhage, no capillary compression and no evidence of apoptosis. Treatment of rats with berberine significantly improved (P<0.05) the injury of cardiac tissue as compared with control group and cross section from this group (MI/R+ berberine) showed mild cardiac injury with absence of necrosis and few interstitial odema and PMN infilteration while there was no significant difference between the MI/R and MI/R +vehicle groups as shown in Figure 9 (A, B, C, D).
Figure 9. Representative photomicrograph of a section of the heart tissue section stained with Haematoxylin and Eosin (X 40). A)The control group showing hemorrhage ,interstitial edema, necrosis and Neutrophil infiltration .B) The sham group showing normal architecture C) The vehicle group showing sever hemorrhage and extravasations of RBC, presence of sever interstitial edema, presence of Neutrophil infiltration and necrosis. D) The Berberine pretreated group showing mild cardiac injury with absence of necrosis and few interstitial odema and PMN infilteration.
5. DISCUSSION
The common origin of myocardial infarction is occlusion of the coronary artery as a result of the embolization of an unstable coronary plaque (31). Activation of PMN’s, eicosanoid, cytokines, ROS and complement products have been shown to be involved in the initial ischemic period (32). The intracellular and extracellular accumulation of these products triggers homeostatic pathways involving necrosis, apoptosis and inflammation that initially occur during acute myocardial infarction. The apoptotic response may then lead to potential permanent tissue or end organ dysfunction. Restoration of blood flow to ischemic myocardium is the current therapy, yet is associated with ischemia/reperfusion injury (33).
Numerous studies suggest that the treatment of rats with berberine can significantly drop myocardial I/R injury and posterior to induction of ventricular arrhythmias and myocardial histological changes (34, 35). Several studies have also discovered that certain berberine derivatives exert a cardioprotective effect by reducing oxidative damage. Yu et al . (36) showed that berberine significantly reduced myocardial damage against ischemia/reperfusion injury possibly due to its strong antioxidant and anti-inflammatory activities via SIRT1 signaling that plays a key role in this state. These results reveal that berberine may be a promising candidate for the treatment of myocardial ischemia/reperfusion injury in cardiac surgery and ischemic heart diseases.
Effects of Berberine on pro-inflammatory cytokines (TNF-α, IL-6) and on the anti-inflammatory cytokine (IL-10)
Pretreatment with berberine before induction of myocardial ischemia produced a significant reduction (P<0.05) in the myocardial tissue levels of pro-inflammatory cytokines (TNF-α, IL-6 ), with a significant elevation (P<0.05) in the level of anti-inflammatory cytokine IL-10 compared to control. Zhang et al., (37) found that serum TNF-α and IL-6 levels augmented considerably in the control rats in comparison to the sham group. Pretreatment with berberine decreased serum concentration of IL-6 also TNF-α compared with the control rats in acute ischemic cardiac tissue insult incited via isoproterenol. Chen et al., (38) observed that the anti-inflammatory action of berberine was noted by the reduction of proinflammatory cytokines. The generation of IL-6 as well as TNF-α diminished through a therapy of berberine. There is no data yet available on the effect of berberine on anti-inflammatory cytokine, IL-10.
Effect of Berberine on Caspase 3 and BAX
The level of caspase 3 and BAX in cardiac tissue was significantly decreased (P<0.05) in the berberine pretreated group compared to the control group. Chen et al., (23) reported that berberine minimized hypoxia/reoxygenation-induced myocardial apoptosis, increased Bcl-2/Bax ratio and decreased caspase-3 expression, together with enhanced activation of PI3K-Akt and increased AMPK +and eNOS phosphorylation. Zhang et al., (37) showed that berberine treated group significantly reduced TNF-α level and the protein levels of Bax in comparing to control rats in myocardial ischemia produced in rats by isoproterenol. Lv et al., (39) found that berberine attenuated doxorubicin (DOX)-induced cardiomyocyte apoptosis that cause cardiac injury via decreasing caspase-3. Zhu et al, (40) established that berberine could promote mitochondrial Autophagy, decrease myocardial enzyme activity, induce cardiomyocytes proliferation, inhibit cardiomyocytes apoptosis, and protect the heart from myocardial I/R injury, possibly through the HIF-1α/BNIP3 pathway.
Effect of Berberine on cTnT level
The cTnT plasma level of the berberine pretreated group was significantly decreased (P<0.05) compared to the control group. To the best of our knowledge, there is no study that measured the effect of berberine on cTnT in myocardial ischemia reperfusion injury.
Effect of Berberine on MDA and reduced GSH level
There was a significant decrease (P<0.05) in serum MDA level with a significant elevation (P<0.05) of GSH serum level in the berberine pretreated group compared to the active group. Berberine increases the level of reduced GSH but decreases MDA concentration that helps to overcome oxidative stress and increase the scavenging ability of free radicals (41). Germoush and Mahmoud, (42) showed that cyclophosphamide-administration incited oxidative stress in the liver. Moreover to its anti-inflammatory and antioxidant activities, berberine exhibited significant hepatoprotection against hepatotoxicity provoked by CP via lessening of lipid peroxidase enzyme.
6. CONCLUSION
It can be concluded that pretreatment with berberine modulates myocardial ischemia reperfusion injury via interfering with inflammatory, oxidative pathways and apoptosis.
Author’s contribution:
All author were involved in all steps of preparation of this article. Final proofreading was made by the first author.
Conflicts of interest:
None declared.
Financial support and sponsorship:
Nil.
REFERENCES
- 1.Lang IM, Badr-Eslam R, Greenlaw N, Young R, Steg PG. Management and clinical outcome of stable coronary artery disease in Austria. Wien Klin Wochenschr. 2017;129(23):879–892. doi: 10.1007/s00508-017-1248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007 Sep 13;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 3.Datta G, Fuller BJ, Davidson BR. Molecular mechanisms of liver ischemia reperfusion injury: insights from transgenic knockout models. World J Gastroenterol. 2013 Mar 21;19(11):1683–1698. doi: 10.3748/wjg.v19.i11.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MV, Downey JM. Ischemic postconditioning: from receptor to end-effector. Antioxid Redox Signal. 2011 Mar 1;14(5):821–831. doi: 10.1089/ars.2010.3318. [DOI] [PubMed] [Google Scholar]
- 5.Eltzschig HK, Eckle T. Ischemia and reperfusion - from mechanism to translation. Nat Med. 2011 Nov 7;17(11):1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, Zhang B, Fan R, Zhao L, Wang Y, Zhang S, et al. U50, 488H inhibits Neutrophil accumulation and TNF-α induction induced by ischemia-reperfusion in rat heart. Cytokine. 2011 Nov;56(2):503–507. doi: 10.1016/j.cyto.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009 Oct 15;361(16):1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacaksiz A, Teker ME, Buyukpinarbasili N, Inan O, Tasal A, Sonmez O, et al. Does pantoprazole protect against reperfusion injury following myocardial ischemia in rats? Eur Rev Med Pharmacol Sci. 2013 Jan;17(2):269–275. [PubMed] [Google Scholar]
- 9.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013 Jan;123(1):92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Z, Han Z, Ye B, Dai Z, Shan P, Lu Z, et al. Berberine alleviates cardiac ischemia/reperfusion injury by inhibiting excessive Autophagy in cardiomyocytes. Eur J Pharmacol. 2015 Sep 5;762:1–10. doi: 10.1016/j.ejphar.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Liu J, Ma A, Chen Y. Cardioprotective effect of berberine against myocardial ischemia/reperfusion injury via attenuating mitochondrial dysfunction and apoptosis. Int J Clin Exp Med. 2015 Aug 15;8(8):14513–14519. eCollection 2015. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Bian H, Guo L, Zhu H. Pharmacologic preconditioning with berberine attenuating ischemia-induced apoptosis and promoting Autophagy in neuron. Am J Transl Res. 2016;8(2):1197–1207. [PMC free article] [PubMed] [Google Scholar]
- 13.Sarna LK, Wu N, Hwang SY, Siow YL, Karmin O. Berberine inhibits NADPH oxidase mediated superoxide anion production in macrophages. Can J Physiol Pharmacol. 2010 Mar;88(3):369–378. doi: 10.1139/Y09-136. [DOI] [PubMed] [Google Scholar]
- 14.Hsu YY, Chen CS, Wu SN, Jong YJ, Lo YC. Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mechanism in NSC34 motor neuron-like cells. Eur J Pharm Sci. 2012 Aug 15;46(5):415–425. doi: 10.1016/j.ejps.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Gratas-Delamarche A, Derbré F, Vincent S, Cillard J. Physical inactivity, insulin resistance, and the oxidative-inflammatory loop. Free Radic Res. 2014 Jan;48(1):93–108. doi: 10.3109/10715762.2013.847528. [DOI] [PubMed] [Google Scholar]
- 16.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006 Aug 8;114(6):597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 17.Schonthaler HB, Viniegra JG, Wagner EF. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann Rheum Dis. 2011 Mar;70(Suppl 1):i109–12. doi: 10.1136/ard.2010.140533. [DOI] [PubMed] [Google Scholar]
- 18.Feng AW, Gao W, Zhou GR, Yu R, Li N, Huang XL, Li QR, et al. Berberine ameliorates COX-2 expression in rat small intestinal mucosa partially through PPARγ pathway during acute end toxemia. Int Immunopharmacol. 2012 Jan;12(1):182–188. doi: 10.1016/j.intimp.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Mo C, Wang L, Zhang J, Numazawa S, Tang H, Tang X, et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and end toxin-shocked mice. Antioxid Redox Signal. 2014 Feb 1;20(4):574–588. doi: 10.1089/ars.2012.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin L, Zhang L, Yu L, Han L, Ji W, Shen H, et al. Time-dependent changes of Autophagy and apoptosis in lipopolysaccharide-induced rat acute lung injury. Iran J Basic Med Sci. 2016 Jun;19(6):632–637. [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Chang G, Gao L, Jiang D, Wang L, Li G, et al. Trimetazidine protects against myocardial ischemia/reperfusion injury by inhibiting excessive Autophagy. J Mol Med (Berl) 2018 Aug;96(8):791–806. doi: 10.1007/s00109-018-1664-3. [DOI] [PubMed] [Google Scholar]
- 22.Kwak HJ, Park KM, Choi HE, Park HY. Protective mechanisms of NO preconditioning against NO-induced apoptosis in H9c2 cells: role of PKC and COX-2. Free Radic Res. 2009 Aug;43(8):744–52. doi: 10.1080/10715760903040602. [DOI] [PubMed] [Google Scholar]
- 23.Wei ZQ, Guang LY. Berberine attenuates myocardial ischemia reperfusion injury by suppressing the activation of PI3K/AKT signaling. Exp Ther Med. 2016 Mar;11(3):978–984. doi: 10.3892/etm.2016.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domitrović R, Jakovac H, Blagojević G. Hepatoprotective activity of berberine is mediated by inhibition of TNF-α, COX-2, and iNOS expression in CCl(4)-intoxicated mice. Toxicology. 2011 Feb 4;280(1-2):33–43. doi: 10.1016/j.tox.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z, Shen Y, Sun A, Huang G, Zhu H, Huang B, et al. Magnetic targeting enhances retrograde cell retention in a rat model of myocardial infarction. Stem Cell Research & Therapy. 2013;4(149) doi: 10.1186/scrt360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 2000 Aug 18;47(3):549–555. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- 27.Ivanov AV, Gorodetskaya EA, Kalenikova EI, Medvedev OS. Single intravenous injection of coenzyme Q10 protects the myocardium after irreversible ischemia. Bull Exp Biol Med. 2013 Oct;155(6):771–774. doi: 10.1007/s10517-013-2249-3. [DOI] [PubMed] [Google Scholar]
- 28.Rossoni G, Gomaraschi M, Berti F, Sirtori CR, Franceschini G, Calabresi L. Synthetic high-density lipoproteins exert cardioprotective effects in myocardial ischemia/reperfusion injury. J Pharmacol Exp Ther. 2004 Jan;308(1):79–84. doi: 10.1124/jpet.103.057141. [DOI] [PubMed] [Google Scholar]
- 29.Chang R, Li Y, Yang X, Yue Y, Dou L, Wang Y, et al. Protective Role of Deoxyschizandrin and Schisantherin A against Myocardial Ischemia–Reperfusion Injury in Rats. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med. 2004 Jan-Jun;10(1-6):55–62. doi: 10.2119/2004-00032.aneja. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White HD, Chew DP. Acute myocardial infarction. Lancet. 2008 Aug 16;372(9638):570–84. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- 32.Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock. 2004 May;21(5):401–9. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Keeley EC, Hillis LD. Primary PCI for myocardial infarction with ST-segment elevation. N Engl J Med. 2007 Jan 4;356(1):47–54. doi: 10.1056/NEJMct063503. [DOI] [PubMed] [Google Scholar]
- 34.Sun X, Zhong J, Wang D, Xu J, Su H, An C, et al. Increasing glutamate promotes ischemia-reperfusion-induced ventricular arrhythmias in rats in vivo. Pharmacology. 2014;93:4–9. doi: 10.1159/000356311. [DOI] [PubMed] [Google Scholar]
- 35.Briest F, Grabowski P. PI3K-AKT-mTOR-signaling and beyond: the complex network in gastroenteropancreatic neuroendocrine neoplasms. Theranostics. 2014 Jan 29;4(4):336–365. doi: 10.7150/thno.7851. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu L, Li Q, Yu B, Yang Y, Jin Z, Duan W, et al. Berberine attenuates myocardial ischemia/reperfusion injury by reducing oxidative stress and inflammation response: role of silent information regulator 1. Oxidative Medicine and Cellular Longevity. 2016;2016 doi: 10.1155/2016/1689602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang T, Yang S, Du J. Protective effects of berberine on isoproterenol-induced acute myocardial ischemia in rats through regulating HMGB1-TLR4 axis. Evid Based Complement Alternat Med. 2014;2014:849783. doi: 10.1155/2014/849783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Wang Y, Zhang J, Sun C, Lopez A. Berberine improves glucose homeostasis in streptozotocin-induced diabetic rats in association with multiple factors of insulin resistance. International Scholarly Research Notices. 2011;2011 doi: 10.5402/2011/519371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lv X, Yu X, Wang Y, Wang F, Li H, Wang Y, et al. Berberine inhibits doxorubicin-triggered cardiomyocyte apoptosis via attenuating mitochondrial dysfunction and increasing Bcl-2 expression. PloS one. 2012;7:10. doi: 10.1371/journal.pone.0047351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu N, Li J, Li Y, Zhang Y, Du Q, Hao P, et al. Berberine protects against simulated ischemia/reperfusion injury-induced H9C2 cardiomyocytes apoptosis in vitro and myocardial ischemia/reperfusion-induced apoptosis in vivo by regulating the mitophagy-mediated HIF-1α/BNIP3 pathway. Front. Pharmacol. 2020 Mar 27; doi: 10.3389/fphar.2020.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 42.Germoush MO, Mahmoud AM. Berberine mitigates cyclophosphamide-induced hepatotoxicity by modulating antioxidant status and inflammatory cytokines. J Cancer Res Clin Oncol. 2014 Jul;140(7):1103–1109. doi: 10.1007/s00432-014-1665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]



