Abstract
Background
Hepatoid adenocarcinoma of the stomach (HAS) is a rare type of primary gastric cancer, and most previous studies have reported that HAS has a poor prognosis due to its aggressive biological behavior. The aim of this study was to compare the prognosis of HAS to that of gastric signet ring cell carcinoma (SRC).
Methods
This was a single-center, retrospective, observational cohort study (January 2010 to January 2016) of gastric cancer patients with pathological HAS and SRC. Overall survival was compared between HAS and SRC patients. We used univariate Cox regression, multivariate Cox regression, propensity score matching (PSM), inverse probability of treatment weighting, standardized mortality ratio weighting, standardized mortality ratio weighting, and overlap weighting to perform a prognostic analysis.
Results
A total of 725 (672 SRC and 53 HAS) patients were included. After nearest-neighbor 1:4 PSM, 200 SRC patients and 50 HAS patients were matched. Only in univariate Cox regression analysis with the cohort before PSM did HAS show a significantly worse prognosis than SRC [hazard ratio (HR), 1.66; 95% confidence interval (CI), 1.02–2.69, p = 0.040]. However, in the analysis of multivariate Cox regression with the cohort before PSM and series analysis based on the propensity score, all of the results indicated that there was no statistically significant difference in overall survival between HAS and SRC (all p > 0.05). Furthermore, in the subgroup of proximal location (p = 0.027), T stage 4a & 4b (p = 0.001), N stage 3a & 3b (p = 0.022), with cancer nodules (p = 0.026), serum CEA higher than the normal value (p = 0.038), and serum CA199 higher than the normal value (p = 0.023), the prognosis of HAS was significantly worse than that of SRC.
Conclusion
Based on our study, there was no statistically significant difference in overall survival between HAS and gastric SRC patients. However, in patients with an advanced tumor stage, HAS may have a worse overall survival than SRC.
Keywords: hepatoid adenocarcinoma of the stomach, signet ring cell carcinoma, overall survival, propensity score matching, prognosis
Introduction
Hepatoid adenocarcinoma of the stomach (HAS) is a rare type of primary gastric cancer (GC), and most previous studies have reported that the incidence of HAS is less than 1% of all GC (1, 2). According to the World Health Organization (WHO) gastrointestinal tumor sample classification, hepatoid adenocarcinoma (HAC) is defined as adenocarcinoma of extrahepatic origin with morphological features of liver cell differentiation, composed of large polygonal eosinophilic hepatocytes such as neoplastic cells (3). The etiology of HAS is not clear, and some studies suggest that the occurrence of HAS may be related to the common embryonic origin of the stomach and liver from the foregut (4). HAS is considered to have a poor prognosis due to its aggressive biological behavior (5, 6). However, the prognosis of HAS remains controversial; for example, in the study of Zhou et al., there was no significant difference in the prognosis between HAS and non-HAS GC (7).
According to the WHO Classification of Tumors of the Digestive System, the main categories of gastric adenocarcinoma are tubular and papillary adenocarcinoma (T&PAC), mucinous adenocarcinoma (MAC), signet ring cell carcinoma (SRC), mixed carcinomas, and rare histological variants (8). The incidence of gastric SRC is 15.9%–17% of all GCs (9); moreover, the prognosis of SRC is considered worse than that of other types of GC, especially among patients with advanced cancer stages (10, 11). Therefore, the proportion of SRC in the non-HAS GC population will directly affect the prognosis of non-HAS and thus affect the comparison of the prognosis of HAS and non-HAS GC.
The objective of this study was to compare the prognosis of HAS to that of gastric SRC, explore whether HAS really does have a worse prognosis than SRC, and confirm whether HAS is a subtype of GC with a poor prognosis.
Materials and Methods
Study Design and Patient Selection
A single-center retrospective cohort study was conducted utilizing the database of the First Medical Center of Chinese PLA General Hospital from January 1, 2010, to January 1, 2016. A total of 3,095 patients with GC were admitted. The inclusion criteria were SRC or HAS patients according to the final pathology report after radical surgery. The exclusion criteria included the following: patients younger than 18 years or older than 80 years at the time of diagnosis; lack of a pathological diagnosis; the pathological diagnosis was tubular adenocarcinoma, papillary adenocarcinoma, mucinous adenocarcinoma, or another rare type of GC; the tumor tissue contains both SRC and HAS components; with distant metastasis; with palliative surgery; history of prior or concurrent other malignancies; incomplete clinical data; and missing follow-up. The database included information on demographics, clinical and pathological characteristics, and follow-up visits. The follow-up ended on March 1, 2019, and the data were obtained by reviewing medical records and telephone follow-up. Our primary outcome was overall survival (OS), defined as the time from surgery to death from cancer or any other cause. This study was approved by the ethics committee of the Chinese PLA General Hospital and was conducted in accordance with the recommendations of the institutional review board, which waived the requirement for informed consent due to its retrospective nature.
Diagnosis of Patients
The diagnosis of all patients was confirmed by postoperative pathological diagnosis. SRC was defined as a tumor that only had a signet ring cell carcinoma component or was only mixed with a tubular adenocarcinoma component and/or a papillary adenocarcinoma component. HAS was defined as a tumor that only had a hepatoid adenocarcinoma component or was only mixed with a tubular adenocarcinoma component and/or a papillary adenocarcinoma component.
Statistical Analysis
To minimize the potential bias of basic clinical characteristics, multivariate Cox regression with propensity score matching (PSM) (12) was used to compare the prognosis between HAS and SRC. A 1:4 nearest-neighbor matching algorithm was applied using a caliper width of 0.2. Fifteen independent variables thought to be confounders were selected to generate the propensity score, and these variables are marked in Table 1. A standardized mean difference (SMD) was used to examine the degree of PSM, and a threshold of less than 0.1 was considered acceptable. Survival curves were plotted by Kaplan–Meier and log-rank analyses.
Table 1.
Baseline characteristics of participants.
| Characteristics | Unmatched patients | PSM patients | ||||
|---|---|---|---|---|---|---|
| SRC | HAS | SMD | SRC | HAS | SMD | |
| 672 | 53 | 200 | 50 | |||
| No. (%) | No. (%) | No. (%) | No. (%) | |||
| Gender (female) | 152 (22.6) | 12 (22.6) | 0.001 | 42 (21.0) | 12 (24.0) | 0.072 |
| Age ≥ 60 years old (yes) | 310 (46.1) | 31 (58.5) | 0.249 | 115 (57.5) | 28 (56.0) | 0.030 |
| Neoadjuvant chemotherapy (no) | 622 (92.6) | 48 (90.6) | 0.072 | 184 (92.0) | 46 (92.0) | <0.001 |
| BMI ≥ 24 (no) | 369 (54.9) | 28 (52.8) | 0.042 | 112 (56.0) | 25 (50.0) | 0.120 |
| Location | 0.179 | 0.050 | ||||
| Proximal | 196 (29.2) | 15 (28.3) | 52 (26.0) | 14 (28.0) | ||
| Middle | 176 (26.2) | 18 (34.0) | 68 (34.0) | 17 (34.0) | ||
| Distal | 300 (44.6) | 20 (37.7) | 80 (40.0) | 19 (38.0) | ||
| Tumor size ≥ 4 cm (no) | 314 (46.7) | 23 (43.4) | 0.067 | 92 (46.0) | 22 (44.0) | 0.040 |
| T stage | 0.496 | 0.028 | ||||
| T1 | 140 (20.8) | 5 (9.4) | 20 (10.0) | 5 (10.0) | ||
| T2 | 134 (19.9) | 10 (18.9) | 38 (19.0) | 10 (20.0) | ||
| T3 | 144 (21.4) | 22 (41.5) | 78 (39.0) | 19 (38.0) | ||
| T4 | 254 (37.8) | 16 (30.2) | 64 (32.0) | 16 (32.0) | ||
| N stage | 0.280 | 0.078 | ||||
| N0 | 300 (44.6) | 17 (32.1) | 70 (35.0) | 16 (32.0) | ||
| N1 | 90 (13.4) | 9 (17.0) | 32 (16.0) | 8 (16.0) | ||
| N2 | 115 (17.1) | 13 (24.5) | 48 (24.0) | 12 (24.0) | ||
| N3 | 167 (24.9) | 14 (26.4) | 50 (25.0) | 14 (28.0) | ||
| Lymph nodes examined ≥16 (yes) | 532 (79.2) | 44 (83.0) | 0.098 | 158 (79.0) | 41 (82.0) | 0.076 |
| Perineural invasion (no) | 548 (81.5) | 37 (69.8) | 0.276 | 142 (71.0) | 36 (72.0) | 0.022 |
| Vascular invasion (no) | 505 (75.1) | 36 (67.9) | 0.161 | 141 (70.5) | 34 (68.0) | 0.054 |
| Cancer nodules (no) | 619 (92.1) | 46 (86.8) | 0.174 | 176 (88.0) | 44 (88.0) | <0.001 |
| CEA > 5.0 μg/L (no) | 96 (14.3) | 9 (17.0) | 0.074 | 27 (13.5) | 8 (16.0) | 0.071 |
| CA199 > 37.0 U/ml (no) | 81 (12.1) | 5 (9.4) | 0.085 | 18 (9.0) | 5 (10.0) | 0.034 |
| CA724 > 10.0 U/ml (no) | 80 (11.9) | 5 (9.4) | 0.080 | 19 (9.5) | 5 (10.0) | 0.017 |
BMI, Body mass index; CEA, Carcinoembryonic antigen; CA, Carbohydrate antigen; HAS, Hepatoid adenocarcinoma of the stomach; PSM, Propensity score matching; SMD, Standardized mean difference; SRC, Signet ring cell carcinoma.
To more reliably compare the differences in overall survival between HAS and SRC, the following survival analysis method and weighting method were performed (1): univariate survival analysis was performed using Cox univariate regression analysis before PSM (2); multivariate Cox regression analysis was performed with adjustments for all covariates shown in Table 1 before PSM; (3) multivariate Cox regression analysis was conducted with the same strata and covariates after matching according to the propensity score; (4) multivariate Cox regression analysis was conducted with the same strata and covariates and inverse probability of treatment weighting (IPTW) according to the propensity score (13); (5) multivariate Cox regression analysis was conducted with the same strata and covariates and overlap weighting (OW) according to the propensity score (14).
Subgroup analysis was performed with univariate Cox regression analysis after PSM to explore the consistency of the prognostic differences between HAS and SRC in the different subgroups.
All statistical analyses were performed using R-4.0 software (http://www.r-project.org), and p < 0.05 (two-sided) was considered statistically significant.
Results
Participants
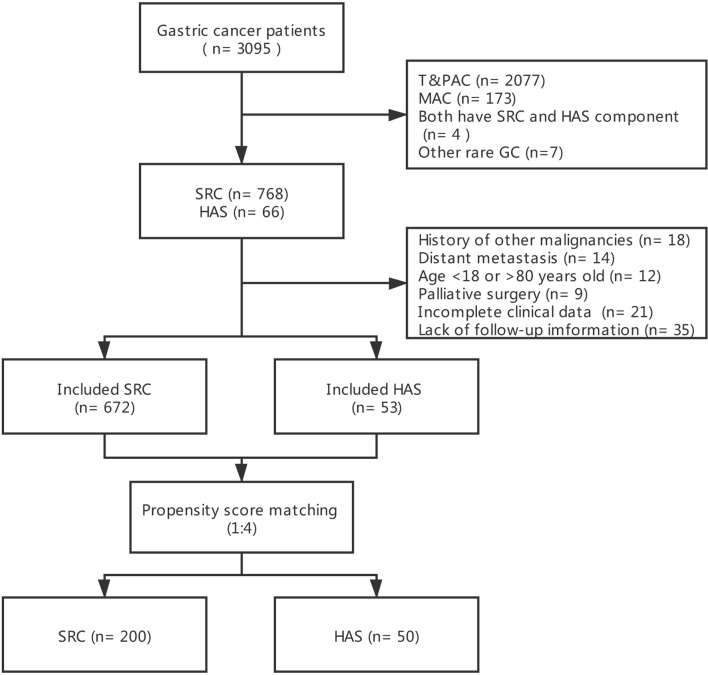
Between January 1, 2010, and January 1, 2016, there were 3,095 GC registrations in our medical center. After screening by the inclusion and exclusion criteria, 672 SRC patients and 53 HAS patients remained. After 1:4 PSM, 200 SRC patients and 50 HAS patients were matched. The flow chart depicting the selection of the study population is presented in Figure 1.
Figure 1.
Flow chart depicting the selection of the study population.
Baseline Characteristics
In the crude cohort before PSM, the two groups (HAS and SRC) were consistent in 8 of a total of 15 variables, but there was no consistency in the distribution of age (SMD = 0.249), tumor location (SMD = 0.179), T stage (SMD = 0.496), N stage (SMD = 0.280), perineural invasion (SMD = 0.276), vascular invasion (SMD = 0.161), or cancer nodules (SMD = 0.174). In the matched cohort after PSM, except for body mass index (SMD = 0.120), the other 14 variables were consistent between the two groups (Table 1).
Outcome Analysis
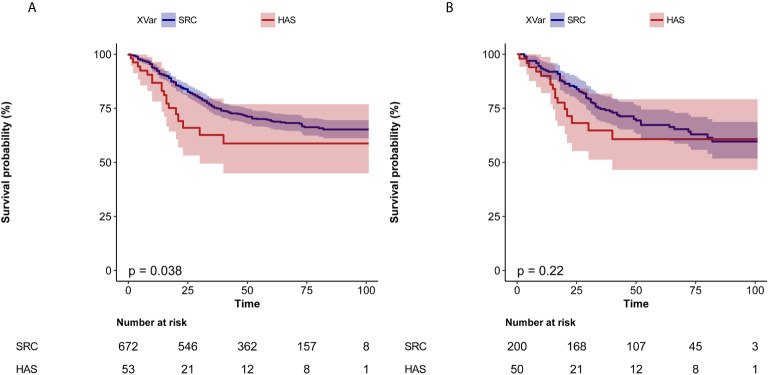
In the crude cohort, the median follow-up was 52 [interquartile range (IQR), 33–74.0] months in the SRC group and 19 (IQR, 16–47) months in the HAS group. The crude 1-year survival was 92.3% [95% confidence interval (CI), 90.3%–94.4%] vs. 86.8% (95% CI, 78.1%–96.4%), and the 3-year survival was 75.3% (95% CI, 72.0%–78.7%) vs. 62.7% (95% CI, 49.4%–79.5%) in the SRC vs. HAS group, respectively. Comparing OS, the HAS group had a worse prognosis. In univariate Cox regression analysis, the hazard ratio (HR) was 1.66 (95% CI, 1.02–2.69, p = 0.040) (Table 2). Kaplan–Meier curves showed the same outcome (log-rank test: p = 0.038) (Figure 2A). However, in multivariate Cox regression analysis, OS was not significantly different between the two groups, and the HR was 1.63 (95% CI, 0.99–2.70, p = 0.056) (Table 2).
Table 2.
Different analysis methods compare the prognostic differences between HAS and SRC patients in overall survival (HAS vs. SRC).
| Analysis | HR (95% CI) | p-value |
|---|---|---|
| Unmatched univariate analysis | 1.66 (1.02, 2.69) | 0.040 |
| Multivariate adjusted | 1.63 (0.99, 2.70) | 0.056 |
| Propensity score matched | 1.35 (0.96, 1.91) | 0.087 |
| Weighted IPTW | 1.22 (0.73, 2.05) | 0.446 |
| Weighted OW | 1.31 (0.65, 2.61) | 0.448 |
IPTW, Inverse probability of treatment weighting; OW, Overlap weighting.
Figure 2.
Kaplan–Meier curves for overall survival according to lymph node ratio. (A) Before PSM. (B) After PSM.
In the matched cohort, the median follow-up was 52 (IQR, 23–73) months in the SRC group and 20 (IQR, 16–48) months in the HAS group. The 1-year survival was 92.4% (95% CI, 88.8%–96.2%) vs. 90.0% (95% CI, 82.1%–98.7%), and the 3-year survival was 74.7% (95% CI, 68.8%–81.0%) vs. 64.8% (95% CI, 51.3%–81.8%) in the SRC vs. HAS group. Multivariate Cox regression analysis after PSM showed that the difference in prognosis between the two groups was not statistically significant (Table 2). Kaplan–Meier curves showed the same outcome (log-rank test: p = 0.220) (Figure 2B).
Sensitivity Analysis
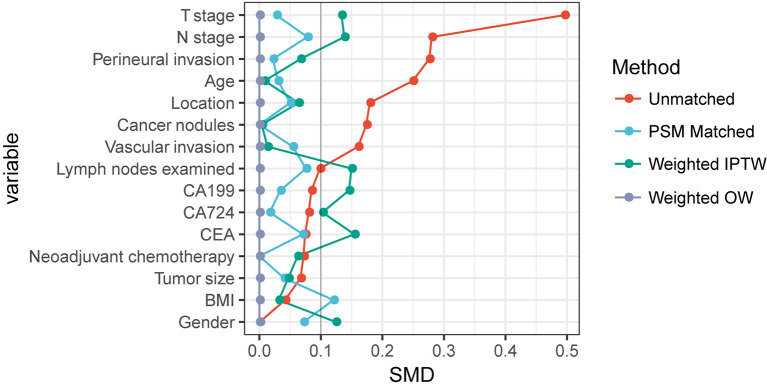
To further verify the stability of the results, IPTW and OW weighted adjusted multivariate Cox regression analysis according to the propensity score was performed. The baseline characteristics of the two groups were better balanced in these analyses (Figure 3). Although all of the results showed that HAS had a worse prognosis than SRC (all HR > 1), the difference was not statistically significant (all p > 0.05) (Table 2).
Figure 3.
Comparability of baseline characteristics based on standardized mean difference in different survival analysis method.
Subgroup Analysis
In the subgroup analysis stratified by the 15 variables, in most subgroups, the prognosis of HAS and SRC was not significantly different. However, in the subgroup of proximal location (p = 0.027), T stage 4a & 4b (p = 0.001), N stage 3a & 3b (p = 0.022), with cancer nodules (p = 0.026), serum CEA higher than the normal value (p = 0.038), and serum CA199 higher than the normal value (p = 0.023), the prognosis of HAS was significantly worse than that of SRC (Table 3).
Table 3.
Subgroup analysis with univariate Cox regression analysis of overall survival between HAS and SRC after PSM.
| Subgroup | SRC | HAS | p-value | ||||
|---|---|---|---|---|---|---|---|
| n total | n event (%) | HR (95% CI) | n total | n event (%) | HR (95% CI) | ||
| Gender | |||||||
| Male | 158 | 54 (34.2) | 1 (Ref) | 38 | 12 (31.6) | 1.43 (0.76–2.69) | 0.263 |
| Female | 42 | 14 (33.3) | 1 (Ref) | 12 | 4 (33.3) | 1.3 (0.43–3.99) | 0.643 |
| Age ≥ 60 years old | |||||||
| No | 85 | 29 (34.1) | 1 (Ref) | 22 | 5 (22.7) | 0.99 (0.38–2.57) | 0.979 |
| Yes | 115 | 39 (33.9) | 1 (Ref) | 28 | 11 (39.3) | 1.77 (0.9–3.48) | 0.098 |
| Neoadjuvant chemotherapy | |||||||
| Yes | 16 | 6 (37.5) | 1 (Ref) | 4 | 1 (25) | 0.74 (0.09–6.18) | 0.782 |
| No | 184 | 62 (33.7) | 1 (Ref) | 46 | 15 (32.6) | 1.45 (0.82–2.56) | 0.201 |
| BMI ≥ 24 | |||||||
| Yes | 88 | 32 (36.4) | 1 (Ref) | 25 | 9 (36) | 1.49 (0.71–3.14) | 0.297 |
| No | 112 | 36 (32.1) | 1 (Ref) | 25 | 7 (28) | 1.26 (0.56–2.85) | 0.580 |
| Location | |||||||
| Proximal | 52 | 14 (26.9) | 1 (Ref) | 14 | 6 (42.9) | 3.05 (1.13–8.2) | 0.027 |
| Middle | 68 | 21 (30.9) | 1 (Ref) | 17 | 5 (29.4) | 1.56 (0.58–4.18) | 0.381 |
| Distal | 80 | 33 (41.2) | 1 (Ref) | 19 | 5 (26.3) | 0.7 (0.27–1.8) | 0.456 |
| Tumor size ≥ 4 cm | |||||||
| Yes | 108 | 42 (38.9) | 1 (Ref) | 28 | 9 (32.1) | 1.14 (0.55–2.35) | 0.723 |
| No | 92 | 26 (28.3) | 1 (Ref) | 22 | 7 (31.8) | 1.9 (0.81–4.43) | 0.137 |
| T stage | |||||||
| T 1a & 1b | 20 | 1 (5) | 1 (Ref) | 5 | 0 (0) | 0 (0–Inf) | 0.999 |
| T 2 | 38 | 15 (39.5) | 1 (Ref) | 10 | 1 (10) | 0.41 (0.05–3.15) | 0.393 |
| T 3 | 78 | 32 (41) | 1 (Ref) | 19 | 4 (21.1) | 0.7 (0.25–2) | 0.511 |
| T 4a & 4b | 64 | 20 (31.2) | 1 (Ref) | 16 | 11 (68.8) | 3.69 (1.75–7.79) | 0.001 |
| N stage | |||||||
| N 0 | 70 | 8 (11.4) | 1 (Ref) | 16 | 1 (6.2) | 0.6 (0.07–4.83) | 0.633 |
| N 1 | 32 | 11 (34.4) | 1 (Ref) | 8 | 1 (12.5) | 0.86 (0.11–6.97) | 0.887 |
| N 2 | 48 | 19 (39.6) | 1 (Ref) | 12 | 5 (41.7) | 1.66 (0.61–4.48) | 0.318 |
| N 3a &3b | 50 | 30 (60) | 1 (Ref) | 14 | 9 (64.3) | 2.54 (1.14–5.65) | 0.022 |
| Lymph nodes examined ≥16 | |||||||
| Yes | 42 | 15 (35.7) | 1 (Ref) | 9 | 1 (11.1) | 0.36 (0.05–2.75) | 0.325 |
| No | 158 | 53 (33.5) | 1 (Ref) | 41 | 15 (36.6) | 1.73 (0.97–3.09) | 0.062 |
| Perineural invasion | |||||||
| Yes | 58 | 29 (50) | 1 (Ref) | 14 | 4 (28.6) | 0.78 (0.27–2.22) | 0.639 |
| No | 142 | 39 (27.5) | 1 (Ref) | 36 | 12 (33.3) | 1.91 (0.99–3.67) | 0.052 |
| Vascular invasion | |||||||
| Yes | 59 | 23 (39) | 1 (Ref) | 16 | 8 (50) | 2.1 (0.92–4.78) | 0.078 |
| No | 141 | 45 (31.9) | 1 (Ref) | 34 | 8 (23.5) | 1.06 (0.5–2.25) | 0.888 |
| Cancer nodules | |||||||
| Yes | 24 | 12 (50) | 1 (Ref) | 6 | 5 (83.3) | 3.52 (1.16–10.72) | 0.026 |
| No | 176 | 56 (31.8) | 1 (Ref) | 44 | 11 (25) | 1.17 (0.61–2.24) | 0.636 |
| CEA > 5.0 μg/L | |||||||
| No | 173 | 58 (33.5) | 1 (Ref) | 42 | 11 (26.2) | 1.1 (0.58–2.11) | 0.766 |
| Yes | 27 | 10 (37) | 1 (Ref) | 8 | 5 (62.5) | 3.61 (1.07–12.16) | 0.038 |
| CA199 > 37.0 U/ml | |||||||
| No | 182 | 62 (34.1) | 1 (Ref) | 45 | 12 (26.7) | 1.14 (0.61–2.12) | 0.681 |
| Yes | 18 | 6 (33.3) | 1 (Ref) | 5 | 4 (80) | 4.69 (1.24–17.78) | 0.023 |
| CA724 > 10.0 U/ml | |||||||
| No | 181 | 59 (32.6) | 1 (Ref) | 45 | 14 (31.1) | 1.57 (0.87–2.82) | 0.134 |
| Yes | 19 | 9 (47.4) | 1 (Ref) | 5 | 2 (40) | 0.71 (0.15–3.28) | 0.659 |
AFP, Alpha fetoprotein; BMI, Body mass index; CA Carbohydrate antigen; CEA, Carcinoembryonic antigen; HAS, Hepatoid adenocarcinoma of the stomach; HR, Hazard ratio; SRC, Signet ring cell carcinoma.
Discussion
HAS is a rare neoplasm, and the annual incidence of HAS is approximately 0.58–0.83 cases per million people (6, 15). Previous studies were mainly case reports or case series from a single medical center and mainly came from Asian regions (1, 2, 16). In these previous studies, HAS patients often were reported to have a worse prognosis than non-HAS patients (17, 18). In this study, a relatively large number of HAS patients were included. Although the prognosis of HAS was significantly worse than that of SRC in the survival analysis without adjusting for confounders, the prognosis of HAS and SRC did not show a significant difference in multivariate regression analysis. In addition, in other analyses based on propensity scores, the results were consistent, and the prognosis of HAS was not statistically worse than that of SRC. Therefore, based on these results, we inferred that there was no difference in overall survival between HAS and SRC.
In the subgroup analyses, the results were very interesting. Although in most subgroups HAS did not show a difference in prognosis from SRC, in some subgroups of indicators suggesting an advanced stage of the tumor (T stage 4a & 4b, N stage 3a & 3b, with cancer nodules, serum CEA higher than the normal value, and serum CA199 higher than the normal value), HAS had a worse overall survival than SRC. At present, the controversy about the prognosis of SRC lies in previous studies showing that the prognosis of SRC in early-stage patients may be better than that of non-SRC (19, 20), while the prognosis of SRC in advanced-stage patients is worse (10, 11). The reason for the worse prognosis of overall SRC patients has been suggested to be caused by a greater proportion of patients in an advanced stage (21). However, in our study, it seems that in patients with an advanced tumor stage, the overall survival of HAS was worse than that of SRC.
In a subgroup analysis based on tumor location, in the proximal GC group, the comparison of the prognosis between HAS and SRC was significantly different. Analyzing the reasons for this result, it is unavoidable that the reliability of the result is limited due to the scant sample size, but at the same time, we should also consider the impact of the differences in biological characteristics between proximal GC and distal GC. Previous studies have shown that proximal GC and distal GC have differences in their expression of some oncogenes and antioncogenes, such as HER2 (22), Smad4 (23), p53 (24), and p16 (23). This reminds us that in follow-up studies, these factors should be included in the analysis of prognosis.
The lymph nodes examined were related to the prognosis of GC (25), but the optimal number of lymph nodes examined remains controversial (26). The AJCC 8th GC staging system recommends that at least 16 lymph nodes should be examined (27). Obviously, not all patients can have a sufficient number of lymph nodes detected due to the surgical methods applied and for other reasons. In our study, after adjusting for this important confounder, in the subgroup analysis stratified by the lymph nodes examined <16 or ≥16, no prognostic difference was observed between HAS and SRC. This result verified the reliability of our speculation that the two groups had no significant difference in overall survival.
It should be pointed out that after PSM, the covariate BMI did not match well (SMD > 0.1). However, in the subsequent subgroup analysis stratified by BMI, the results were consistent in both layers, and the prognoses of HAS and SRC were not significantly different. This result indicated that the poor matching of BMI did not significantly affect the reliability of the result.
There were several limitations of this study. First, although we adjusted for as many possible confounders as we could and performed a propensity score-matched cohort to balance these confounders between groups, some residual confounders may still exist. Second, the sample size might be small for robust statistical analyses, but HAS is a rare subtype of GC. Most previous studies were case reports or case series, and as far as we know, the largest sample size in a single center report is only 75 cases (7); therefore, further multicenter studies of HAS are necessary. Third, this study only focused on overall survival, not investigating other indicators, such as complications, and the lack of data on local recurrence precluded us from assessing the difference in disease recurrence and disease-free survival. Fourth, some inevitable issues, such as information biases, might exist owing to its retrospective design.
In conclusion, this is the first study to our knowledge to investigate the difference in prognosis between HAS and gastric SRC. Our data suggested that there was no statistically significant difference in the overall survival between patients with HAS and gastric SRC. However, in patients with advanced tumor stages, HAS may have a worse overall survival than SRC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the ethics committee of the Chinese PLA General Hospital and was conducted in accordance with the recommendations of the institutional review board, which waived the requirement for informed consent due to its retrospective nature.
Author Contributions
The authors that contributed to the study conception and design were YY, YL, and XD. Data acquisition and interpretation were performed by YY and YL. The first draft of the manuscript was written by YY. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks to every patient included for his/her scientific contributions to this study.
References
- 1.Xia R, Zhou Y, Wang Y, Yuan J, Ma X. Hepatoid Adenocarcinoma of the Stomach: Current Perspectives and New Developments. Front Oncol (2021) 11:633916. 10.3389/fonc.2021.633916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Søreide JA. Therapeutic Approaches to Gastric Hepatoid Adenocarcinoma: Current Perspectives. Ther Clin Risk Manage (2019) 15:1469–77. 10.2147/TCRM.S204303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li T, Liu T, Wang M, Zhang M. A-Fetoprotein Producing Hepatoid Gastric Adenocarcinoma With Neuroendocrine Differentiation: A Case Report. Med (Baltimore) (2018) 97:e12359. 10.1097/MD.0000000000012359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Sun L, Li Z, Gao J, Ge S, Zhang C, et al. Hepatoid Adenocarcinoma of the Stomach: A Unique Subgroup With Distinct Clinicopathological and Molecular Features. Gastric Cancer (2019) 22:1183–92. 10.1007/s10120-019-00965-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valle L, Thomas J, Kim C, Szabo E, Brown GT, Citrin D, et al. Hepatoid Adenocarcinoma of the Lung Metastasizing to the Tonsil. Mol Clin Oncol (2017) 6:705–7. 10.3892/mco.2017.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng XY, Yin YP, Xiao H, Zhang P, He J, Liu WZ, et al. Clinicopathological Characteristics and Prognosis of Hepatoid Adenocarcinoma of the Stomach: Evaluation of a Pooled Case Series. Curr Med Sci (2018) 38:1054–61. 10.1007/s11596-018-1983-1 [DOI] [PubMed] [Google Scholar]
- 7.Zhou K, Wang A, Ao S, Chen J, Ji K, He Q, et al. The Prognosis of Hepatoid Adenocarcinoma of the Stomach: A Propensity Score-Based Analysis. BMC Cancer (2020) 20:671. 10.1186/s12885-020-07031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology (2020) 76:182–8. 10.1111/his.13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Zong Z, Zhou T, Sun L, Wang A, Zhang K, et al. Trends of Incidence and Survival in Patients With Gastroenteropancreatic Signet Ring Cell Carcinoma: An Analysis From the Surveillance, Epidemiology, and End Results Program. J Gastrointest Oncol (2019) 10:979–88. 10.21037/jgo.2019.06.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bamboat ZM, Tang LH, Vinuela E, Kuk D, Gonen M, Shah MA, et al. Stage-Stratified Prognosis of Signet Ring Cell Histology in Patients Undergoing Curative Resection for Gastric Adenocarcinoma. Ann Surg Oncol (2014) 21:1678–85. 10.1245/s10434-013-3466-8 [DOI] [PubMed] [Google Scholar]
- 11.Taghavi S, Jayarajan SN, Davey A, Willis AI. Prognostic Significance of Signet Ring Gastric Cancer. J Clin Oncol (2012) 30:3493–8. 10.1200/JCO.2012.42.6635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao XI, Wang X, Speicher PJ, Hwang ES, Cheng P, Harpole DH, et al. Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies. J Natl Cancer Inst (2017) 109:djw323. 10.1093/jnci/djw323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas L, Li F, Pencina M. Using Propensity Score Methods to Create Target Populations in Observational Clinical Research. JAMA (2020) 323:466–7. 10.1001/jama.2019.21558 [DOI] [PubMed] [Google Scholar]
- 14.Thomas LE, Li F, Pencina MJ. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA (2020) 323:2417–8. 10.1001/jama.2020.7819 [DOI] [PubMed] [Google Scholar]
- 15.Lin CY, Yeh HC, Hsu CM, Lin WR, Chiu CT. Clinicopathologial Features of Gastric Hepatoid Adenocarcinoma. BioMed J (2015) 38:65–9. 10.4103/2319-4170.126860 [DOI] [PubMed] [Google Scholar]
- 16.Inagawa S, Shimazaki J, Hori M, Yoshimi F, Adachi S, Kawamoto T, et al. Hepatoid Adenocarcinoma of the Stomach. Gastric Cancer (2001) 4:43–52. 10.1007/s101200100016 [DOI] [PubMed] [Google Scholar]
- 17.Zhang JF, Shi SS, Shao YF, Zhang HZ. Clinicopathological and Prognostic Features of Hepatoid Adenocarcinoma of the Stomach. Chin (Engl) (2011) 124:1470–6. 10.3760/cma.j.issn.0366-6999.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 18.Inoue M, Sano T, Kuchiba A, Taniguchi H, Fukagawa T, Katai H. Long-Term Results of Gastrectomy for Alpha-Fetoprotein-Producing Gastric Cancer. Br J Surg (2010) 97:1056–61. 10.1002/bjs.7081 [DOI] [PubMed] [Google Scholar]
- 19.Imamura T, Komatsu S, Ichikawa D, Kawaguchi T, Kosuga T, Okamoto K, et al. Early Signet Ring Cell Carcinoma of the Stomach is Related to Favorable Prognosis and Low Incidence of Lymph Node Metastasis. J Surg Oncol (2016) 114:607–12. 10.1002/jso.24377 [DOI] [PubMed] [Google Scholar]
- 20.Hyung WJ, Noh SH, Lee JH, Huh JJ, Lah KH, Choi SH, et al. Early Gastric Carcinoma With Signet Ring Cell Histology. Cancer (2002) 94:78–83. 10.1002/cncr.10120 [DOI] [PubMed] [Google Scholar]
- 21.Lu M, Yang Z, Feng Q, Yu M, Zhang Y, Mao C, et al. The Characteristics and Prognostic Value of Signet Ring Cell Histology in Gastric Cancer: A Retrospective Cohort Study of 2199 Consecutive Patients. Med (Baltimore) (2016) 95:e4052. 10.1097/MD.0000000000004052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, et al. Amplification of HER-2 in Gastric Carcinoma: Association With Topoisomerase IIalpha Gene Amplification, Intestinal Type, Poor Prognosis and Sensitivity to Trastuzumab. Ann Oncol (2005) 16:273–8. 10.1093/annonc/mdi064 [DOI] [PubMed] [Google Scholar]
- 23.Kim MA, Lee HS, Yang HK, Kim WH. Clinicopathologic and Protein Expression Differences Between Cardia Carcinoma and Noncardia Carcinoma of the Stomach. Cancer (2005) 103:1439–46. 10.1002/cncr.20966 [DOI] [PubMed] [Google Scholar]
- 24.Tang H, Hokita S, Che X, Baba M, Aridome K, Kijima F, et al. Comparison of P53 Expression in Proximal and Distal Gastric Cancer: Histopathologic Correlation and Prognostic Significance. Ann Surg Oncol (1997) 4:470–4. 10.1007/BF02303670 [DOI] [PubMed] [Google Scholar]
- 25.Bunt AM, Hogendoorn PC, van de Velde CJ, Bruijn JA, Hermans J. Lymph Node Staging Standards in Gastric Cancer. J Clin Oncol (1995) 13:2309–16. 10.1200/JCO.1995.13.9.2309 [DOI] [PubMed] [Google Scholar]
- 26.Yang ZL, Zhu MH, Shi Q, Lu FM, Wang CX. Prognostic Value of the Number of Lymph Nodes Examined in Patients With Node-Negative Gastric Cancer. J Gastrointest Surg (2019) 23:460–7. 10.1007/s11605-018-3947-y [DOI] [PubMed] [Google Scholar]
- 27.Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, et al. Proposal of a New Stage Grouping of Gastric Cancer for TNM Classification: International Gastric Cancer Association Staging Project. Gastric Cancer (2017) 20:217–25. 10.1007/s10120-016-0601-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.