Abstract
A case of recurrent coronavirus disease 2019 (COVID-19) with neurovestibular symptoms was reported. In March 2020, a physician working in an Italian pediatric hospital had flu-like symptoms with anosmia and dysgeusia, and following a reverse transcription PCR (RT/PCR) test with a nasopharyngeal swab tested positive for SARS-CoV-2. After home quarantine, 21 days from the beginning of the symptoms, the patient tested negative in two subsequent swabs and was declared healed and readmitted to work. Serological testing showed a low level of immunoglobulin G (IgG) antibody title and absence of immunoglobulin M (IgM). However, 2 weeks later, before resuming work, the patient complained of acute vestibular syndrome, and the RT/PCR test with mucosal swab turned positive. On the basis of the literature examined and reviewed for recurrence cases and vestibular symptoms during COVID-19, to our knowledge this case is the first case of recurrence with vestibular impairment as a neurological symptom, and we defined it as probably a viral reactivation. The PCR retest positivity cannot differentiate re-infectivity, relapse, and dead-viral RNA detection. Serological antibody testing and viral genome sequencing could be always performed in recurrence cases.
Keywords: recurrence, occupational medicine, neuroCOVID, neurologic symptoms, vestibular syndrome, healthcare workers, case report
Introduction
In China, in December 2019, the epidemic caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) rapidly diffused all over the world leading to a pandemic. While the coronavirus disease 2019 (COVID-19) typically presents as a self-limiting respiratory disease, and in hospitalized patients, the clinical picture is dominated by respiratory distress (Del Sole et al., 2020), progression to severe illness with multiorgan involvement, including the blood vessels, heart, gut, kidneys, testicles, and brain has been reported (Asadi-Pooya and Simani, 2020; Chen X. et al., 2020; Ibrahim, 2020; Leonardi et al., 2020; Nepal et al., 2020). Patients suffering from COVID-19 can develop acute or long-term neurological sequelae (Ellul et al., 2020). The prevalence of neuro-COVID varies considerably between individual studies ranging from 4.1% (Xiong et al., 2020) to 57.4% (Romero-Sánchez et al., 2020) and even 84% in COVID-19 with acute respiratory distress syndrome (Helms et al., 2020). The onset of nervous system damage can be asynchronous with systemic manifestations and the typically salient severe respiratory disease (Vavougios, 2020).
In our pediatric hospital, between March and May 2020, there were 25 cases of COVID-19 among healthcare workers (HCWs), equal to 1.1% of the total staff. Two of them had at least one symptom, namely, headache, anosmia, and dysgeusia or all the three symptoms at the same time. These neurological symptoms indicate that the virus, like other respiratory viruses (Bohmwald et al., 2018), enters the central nervous system (CNS) through the olfactory bulb causing inflammation. Furthermore, SARS-CoV-2 viruses can spread from the mechano- and chemo-receptors in the lungs and lower respiratory airways to the medullary cardiorespiratory center via a synapse-connected route (Li Y. C. et al., 2020).
In this study, we report a case of recurrent SARS-CoV-2 infection with neurovestibular involvement, review literature cases with vestibular involvement, and discuss the neurotropism of this virus based on literature data.
Case Description
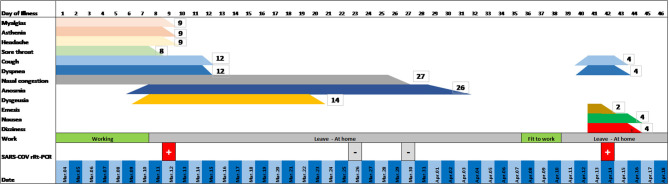
A 48-year-old female physician in a pediatric hospital presented cough, slight dyspnea, severe myalgia, asthenia, and headache on March, 2020, followed by anosmia and dysgeusia over the following few days (Figure 1) without fever. When symptoms appeared the worker self-isolated at home. The allergic rhinitis the patient suffers from led to a short delay in diagnosis; however, a swab carried out 8 days from the beginning of the symptoms tested positive for SARS-CoV-2 (Allplex™2019-nCoV Assay). In subsequent days, the patient felt better and became asymptomatic. On days 23 and 27 the patient was retested and was negative both times. On day 37, serology was performed by ELISA and a low level of immunoglobulin G (IgG) against SARS-CoV-2 was detected. Immunoglobulin M (IgM) search was negative. She was feeling well and was declared fit to work by the occupational physician of the hospital. However, before returning to work, on day 39 the patient woke up experiencing intensive dizziness, described as subjective vertigo, associated with vomiting and bilateral aural fullness (Figure 1). The dizziness quickly got worse as the patient lied supine on the right side. She was transported by ambulance to the emergency room of a hospital where dizzying syndrome was diagnosed and treated with metoclopramide. Physical examination revealed normal vital signs, while the patient was breathing ambient air. Some relevant auxiliary examinations such as blood routine, coagulation function, liver and renal function, electrolytes, and inflammation indicators were completed, and the results were normal. After 3 h and the improvement of the symptoms, she was discharged home, cared by health public service, and treated with betahistine dihydrochloride for 1 week. The day after, on day 40, a nasal swab was obtained, which tested positive.
Figure 1.
Clinical evolution of the patient: timeline.
In the following days, the dizziness disappeared and only a mild postural instability persisted, whereas aural fullness was unchanged. The only pathology that she suffered from was allergic rhinitis. She had never suffered from dizziness in the past.
An ear, nose, and throat (ENT) visit took place on day 44. Otoscopy was negative. Romberg, Unterberger (March-in-place), and finger-to-nose tests were negative. Neither bedside vestibular examination with Frenzel glasses showed spontaneous nystagmus, nor was nystagmus evoked by supine position, Dix-Hallpike maneuver, lateral head rotation, and Rose position. The head-shaking test was negative. The only relevant finding of the vestibular examination was the patient complaining about slight dizziness during the Dix-Hallpike maneuver on the right side (supine position with the head hyperextended and tilted to the right side). The patient did not develop fever both in the first and in the second phase of the symptoms related to COVID-19. Despite fever being one of the most common symptoms of COVID-19, it may be absent in some individuals. Finally, the patient was completely healed without any deficit and was able to return to work.
Discussion
The case reported presents numerous points of interest. First, the recurring nature of the symptoms must be emphasized along with the neurological origin of the symptoms themselves. Second, the neurotropism and neuroinvasive potential of the virus into recurrence mode should be considered. Finally, the observation that the presence of anti-spike IgG has been followed by a positive PCR test; this feature is rather infrequent, having been found in 0.13 per 10,000 days at risk in HCWs, whereas the probability of having a positive PCR test in the anti-spike–seronegative HCWs is significantly higher (1.09 per 10,000 days at risk) (Lumley et al., 2020).
The presence of at least two consecutive negative RT-PCR tests in respiratory samples (with samples taken at least 24 h apart) and the appearance of specific IgG at serological test, according to the discharge criteria of the European Centre for Disease Prevention and Control (Yahav et al., 2021), permits to classify the case exposed as recurrent COVID-19 with multiple district neurological symptoms (vestibular symptoms, previously anosmia and dysgeusia). Indeed reinfection should be considered during the first 90 days if clinical symptoms of the first episode resolve and two PCR tests were negative before the new episode (Yahav et al., 2021).
Currently, there are numerous reports that a number of patients tested positive again after two consecutive negative PCR tests or after clinical recovery (Table 1) (Falahi and Kenarkoohi, 2020). Nasopharyngeal swabs tend to have a higher sensitivity than the other samples and are the most common method for diagnosis of COVID-19 recurrence, regardless of the clinical disease manifestation. With the progression of SARS-CoV-2 infection, the virus could migrate from the upper respiratory tract to the lower respiratory tract and lungs, resulting in insufficient viral load in the upper respiratory tract, which may explain the negative result of the nasopharyngeal swab test. The detection rate and sensitivity have been improved by multisite specimen collection and serological assays (Table 1). The prevalence of disease recurrence among COVID-19 recovered patients was approximately 14.8%, ranging from 7.35 to 21.4% (Azam et al., 2020; Hoang, 2020). The interval duration from the last negative PCR tests to recurrent positive results ranged from 1 to 123 days (min average 16.16 ± 20.93 ds, max average 25.39 ± 23.00 ds) for 1,038 cases in a selected population of 13,565 patients (Table 1). The case described experienced at the onset two of the three typical symptoms in the first phase of infection, namely, cough (68%) and shortness of breath (66%) without fever (69%). About 92% of the patients experienced at least one of these, less frequently in women (66, 64, 66 respectively; 90% at least one of these; vs. men 70, 67, 71; 93%, each p < 0.001) (ISARIC Clinical Characterisation Group, 2021). She presented symptoms of recurrence 17 days from the last negative PCR tests, in accordance with the literature. True reinfection has criteria that must be considered, including isolation of the complete genome of the virus (and not just genomic fragments) in the second episode (Falahi and Kenarkoohi, 2020), but we did not have this data. In our patient, we could suspect a viral reactivation due to low level of IgG against SARS-CoV-2 detected, even without lymphopenia. It is also possible that the immune responses can suppress, but not completely eradicate, SARS-CoV-2, which may have led to the false-negative results due to lower viral loads (Chen J. et al., 2020). Once the virus starts replicating again, the RT-PCR results reverted to positive and expressed as a new neuroinvasion in the vestibular system. NeuroCovid is now well-known (Whittaker et al., 2020), but recurrence of positive RT-PCR with neurological symptoms is very rare and no further instances of vestibular symptoms are described as recurrence (Table 1). To our knowledge, this is the first case of vestibular symptoms as recurrence of positive RT-PCR SARS-CoV-2. This case shows the neuroinvasive potentials of SARS-CoV-2 and the possibility of disease reactivation after clinical and analytic recovery. SARS-CoV-2 might be latent in some neurons to hide from immune surveillance (Brandt and Dieterich, 2017; Zhang M. et al., 2020). For reasons unclear, after an apparent remission the virus reactivated and was again identifiable in the respiratory tract. At the same time, the multiplication in the nervous system caused recurrence with intense neurological disturbance, clinically highlighted by dizziness associated with slight dyspnea.
Table 1.
Recurrent cases reported in literature.
| Reference | Patients (n°) | Days from recurrence of COVID-19 | Test for recurrence of SARS-CoV-2 positive | Kit type (sensitivity and specificity) | Clinical symptoms | Recurrence with neurological signs |
|---|---|---|---|---|---|---|
| Abdullah et al., 2020 | 27/138 pt | 11 days | Nasopharyngeal and oropharynx swabs for RT-PCR | No specified | 6 pts mild symptoms, 21 pts no clinical symptoms | |
| Alonso et al., 2020 | 26-year-old man | 1 month later | Nasopharyngeal and throat swabs for RT-PCR | Allplex™2019-nCoV Assay [sensitivity (95% CI) 98.2 (90.3–100.0) % specificity (95% CI) 100.0 (94.9–100.0)] | A more vigorous COVID-19 recurrence | |
| An et al., 2020 | 38/242 pts | 5–7 days | Digestive (anal swab) and respiratory RT-PCR tests for the S gene and for ORF genes. Ct ≤ 37.0 Next-generation sequencing of samples Specific total antibody IgG, IgA, and IgM |
QIAamp RNA Viral Kit (hyper-sensitive kit compares to commercial kit) | Fewer respiratory and digestive tract symptoms | |
| Bentivegna et al., 2020 | 1 pt | 23 days | Nasopharyngeal swab RT-PCR IgM seroconversion | No specified | Asymptomatic | |
| Bongiovanni et al., 2020 | 125/1,146 pts | Mean 19.9 (3–43) days | Nasopharyngeal swabs RT-PCR | No specified | Asymptomatic (96, 76.8%), general sign (25, 20.0%), and respiratory failure (4, 3.2%) | |
| Cao et al., 2020 | 8/108 pts | 6–28 days | Deep nasal cavity swab samples or throat swab samples RT-PCR | No specified | Asymptomatic | |
| Cento et al., 2020 | 264/2,521 pts after one negative results by RT-PCR assay | 20–30 days | Nasopharyngeal swabs RT-PCR Cts-values ≥ 24 |
No specified | No clinical symptoms | |
| Chae et al., 2020 | 1 pt | 6 days | Naso- and oropharyngeal swab RT-PCR | No specified | Ground-glass opacities in the right upper lobe | |
| Chen D. et al., 2020 | 46-year-old woman | 2 days after 2 negative tests | Oropharyngeal swab test RT-PCR | No specified | Respiratory symptoms had already improved | |
| Chen J. et al., 2020 | 81/1,067 pts | 7–10 days | Throat-swabs RT-PCR tests | No specified | 84.0% (68) mild, 14.8% (12) severe, and 1.2% (1) critical of the cases with pulmonary, liver, kidney, and myocardial damage | |
| Chen M. et al., 2020 | 6/11 pts | 6–27 days | Oropharyngeal swab RT-PCR | No specified | Mild to moderate | |
| Chen S. L. et al., 2020 | 189/1,282 pts | 28 days | Nasopharyngeal and anal swabs specimens RT-PCR ORF1ab and N genes |
Catalog no. DA0931; DaAn Gene, Guangzhou, China (unavailable) | Cough (15.87%), Diarrhea (0.53%), Dyspnea (3.70%) | Fatigue (1.06%), Myalgia (1.06%), |
| Chen Y. et al., 2020 | 4 pts | 3 days after discharge | Nasopharyngeal, oropharyngeal, and anal swabs RT-PCR test | Kits from different manufacturers. No specified |
No clinical symptoms | |
| Crouwel et al., 2020 | 28-year-old female | 50 days | Nasopharyngeal swabs for the RT-PCR test | No specified | Diarrhea, nausea, coughing, sneezing | Headache, myalgia, anosmia, and dysgeusia |
| Deng et al., 2020 | 61 pts | Ranged from 3 to 35 days (median, 10 days) | Nasal and pharyngeal swab specimens, stool and sputum specimens RT-PCR | No specified | Mild 38 (62.3%) General 20 (32.8%) Severe 3 (4.9%) |
Headache 5 pts (8.2%) |
| Dou C. et al., 2020 | 1 pt | 15 days | Oropharyngeal swab RT-PCR | No specified | Asymptomatic | |
| Dou P. et al., 2020 | 2 pts | 17 days | Throat and anus swab RT-PCR | No specified | Asymptomatic | |
| Du et al., 2020 | 3/126 pts | 10–18 days | Nasopharynx and oropharynx swab RT-PCR targeting the ORF1ab gene and N gene Ct ≤ 37 |
Bio-Germ, Shanghai, China (sensitivity 96.15% specificity 100.0%) | Asymptomatic | |
| Duggan et al., 2021 | 1 pt | 10 days | Nasopharyngeal swabs for the RT-PCR test | No specified | Critical | |
| Fu et al., 2020 | 3 | 1–5 days | Nasopharyngeal swab RT-PCR; IgM and IgG antibodies | No specified | Asymptomatic | |
| Gao et al., 2020 | 70-year-old male patient | 15 days | Nasopharyngeal, blood, and rectal swab RT-PCR ORF1ab and N genes |
No specified | No symptoms | |
| Geling et al., 2020 | 24-year-old male | 7 days after discharge | Sputum specimen RT-PCR for ORF1ab and the N gene | No specified | No clinical symptoms | |
| Gidari et al., 2021 | 9 pts | 14–50 days | Respiratory samples RT-PCR for E gene, gene N Ct ≤ 40 |
Allplex™ 2019-nCoV Assay [sensitivity (95% CI) 98.2 (90.3–100.0) % specificity (95% CI) 100.0 (94.9–100.0)] | No clinical symptoms, retrosternal sense of weight | Headache, arthro-myalgias, asthenia, and insomnia |
| Gousseff et al., 2020 | 11 pts | 21–49 days after a symptom (quarantine) | Naso-pharyngeal swabs RT-PCR | No specified | Median duration of symptoms was 18 days for the first episode and 10 days for the second one | |
| Guo et al., 2020 | 27-year-old man | 3 days after discharge | Throat swab specimens A fluorescent immunochromatography detection kit specific to the IgM and IgG antibodies against SARS-CoV-2 |
Zhongshan Chuangyi Biochemical Engineering Co. (unavailable) | No clinical symptoms | |
| Habibzadeh et al., 2020 | 9/13 pts | 15–48 days | Nasopharyngeal swabs RT-PCR tested the E and RdRP genes | Invitrogen ChargeSwitch Total RNA Cell Kit, Invitrogen Co. (unavailable) | No clinical symptoms | |
| Hao et al., 2020 | 24/104 pts | Quarantine | Respiratory specimens (nasal/throat swab or sputum) RT-PCR tests of | No specified | No clinical symptoms | |
| He et al., 2020 | 1 pt | 8 days | Throat swab samples RT-PCR | No specified | Dry cough, arthralgia | Headache |
| Hu R. et al., 2020 | 11/69 pts | 14 days (range, 9–17 days). | Nasopharyngeal swabs RT-PCR | No specified | No clinical symptoms | |
| Huang et al., 2020 | 69/414 pts | Median 19 days, range 6–52 days | Nasopharyngeal and anal swabs qRT-PCR ORF 1ab and N genes. Ct ≤ 37 Antibody Chemiluminescent microparticle immunoassay kit IgM and IgG in plasma. |
QIAamp RNAViralKit (hyper-sensitive kit compares to commercial kit code. GZ-D2RM25, Shanghai GeneoD) | Respiratory symptoms including cough and increased sputum | |
| Jiang et al., 2020 | 6/35 pts | 9–10 days | Throat swabs or sputum samples for RT-PCR | No specified | 1 expectoration, nausea, 1 cough, 4 asymptomatic | 1 fatigue, sore muscles |
| Kang, 2020; KCDA, 2020 | 292/8,922 pts | 1–37 days | Nasopharyngeal swabs RT-PCR | No specified | Asymptomatic to minor symptoms | |
| Landi et al., 2020 | 6/29 pts | 13–24 days | Nasopharyngeal swabs RT-PCR | No specified | Asymptomatic or mild | |
| Lan et al., 2020 | 4 pts | 5–13 days | Throat swabs RT-PCR | BioGerm (sensitivity 96.15% specificity 100.0%) | Asymptomatic | |
| Li C. et al., 2020 | 15/85 pts | 9–30 days | Nasopharyngeal swabs RT-PCR | No specified | Two patients (13.3%) had cough, one (6.6%) had dyspnea | |
| Li J. et al., 2020 | 50-year-old man | Days 34 and 38 during quarantine | Nasopharyngeal swabs RT-PCR | No specified | No clinical symptoms | |
| Li Y. et al., 2020 | 6/13 pts | 6–14 days | Respiratory swabs qRT-PCR for RdRP, E, and N gene | Liferiver detection kit (sensitivity 90% specificity 100.0%) | Asymptomatic | |
| Ling et al., 2020 | 11/66 | 6–11 days | Oropharyngeal swab, stool, urine, and serum RT-PCR | Biosystems 251658240 7500 Real-Time PCR Systems (sensitivity 85.3% specificity 100.0%) | Asymptomatic | |
| Liu B. et al., 2020 | 8/47 pts | From 8 to 39 days after viral shedding | Anal and throat swab samples RT-PCR Antibodies against the spike glycoprotein (S); the receptor-binding domain (RBD); conserved heptad repeats (HR1–HR2) in the S2 domain; and the N, membrane (M), and E proteins. |
Kit from a different manufacturer (no specified) | No clinical symptoms | |
| Liu et al., 2020a | 9/51 pts | 7–14 days | Oropharyngeal-swab RT-PCR | BioGerm (sensitivity 96.15% specificity 100.0%) | Asymptomatic (6, 66.7%), mild (3, 33.3%) | |
| Liu F. et al., 2020 | 35-years old man | Positive during quarantine and returned positive after second quarantine (three hospitalizations) | Nasopharyngeal swabs RT-PCR E gene, RdRP gene, and N gene Ct ≤ 43 |
Liferiver detection kit (sensitivity 90% specificity 100.0%) | Mild clinical symptoms deteriorations | |
| Liu J. et al., 2020 | 15/62 pts | 14 days | Respiratory tract samples RT-PCR spike receptor-binding domain (S-RBD) and N spike protein as antigens. ORF1ab, NP genes fragments, Ct ≤ 38 COVID-19 IgG or IgM antibody |
BioGerm (sensitivity 96.15% specificity 100.0%) | Mild cases 5 (33.3%) Common cases 9 (60.0%) Severe cases 1 (6.6%) | |
| Liu T. et al., 2020 | 11/150 pts | 38 days, range 35–44 days | Throat swabs RT-PCR and serum IgM/IgG rapid test | BioGerm (sensitivity 96.15% specificity 100.0%) | No clinical symptoms reported | |
| Loconsole et al., 2020 | 48-year-old man | 30 days after 2 negative tests | Nasopharyngeal swab RT-PCR 2 targeting E-gene, RdRP-gene and N-gene | No specified | New symptoms, i.e., dyspnea and chest pain. | |
| Lu et al., 2020 | 87/619 pts | 2–19 days | Nasopharyngeal swabs, throat swabs and anal swabs RT-PCR and multiplex PCR sequencing including targeting the ORF1ab, N, RdRp, E. Microneutralization antibody assays for SARS-CoV-2 |
Three kits DAAN GENE (unavailable) BioGerm (sensitivity 96.15% specificity 100.0%) Liferiver detection kit (sensitivity 90% specificity 100.0%) |
Asymptomatic (77, 88.5%), mild (10, 11.5%) | |
| Luciani et al., 2020 | 69-year-old man | 41 days | Nose-pharyngeal swab RT-PCR | No specified | Fever, dyspnea, anemia | |
| Mardani et al., 2020 | 64-year-old woman | 21 days | Nasopharyngeal swabs RT-PCR | QiaSymphony; Qiagen, Hilden, Germany (hyper-sensitive kit compares to commercial kit) | Consciousness suddenly decreased, associated with respiratory distress | Meningoencephalitis |
| Mei et al., 2020 | 23/651 pts | 4–38 days | Nasopharyngeal and oropharyngeal swabs qRT-PCR immunochromatographic strip assay for anti-SARS-CoV-2 viral immunoglobulins | No specified | 15 (65%) were asymptomatic, 8 presented mild to moderate symptoms | |
| Peng et al., 2020) | 7 pts | During quarantine | Throat or anal swab on qRT-PCR | No specified | Milder symptoms | |
| Qiao et al., 2020 | 1/15 pts | 16 days | Throat swabs RT-PCR | No specified | Mild (itchy throat) | |
| Ravioli et al., 2020 | 2 pts | 14–21 days | Nasopharyngeal swab RT-PCR | No specified | Moderate (1, 50.0%) and death (1, 50.0%) | |
| Salcin and Fontem, 2020 | 62 year old female | 120 days | Nasopharyngeal swabs RT-PCR | No specified | Acute Respiratory Distress Syndrome | |
| Sen et al., 2020 | 5 pts | 5–43 days | Nasopharyngeal swabs RT-PCR | No specified | 1 pt asymptomatic/ 4 pts acute febrile illness | |
| Sharma et al., 2020 | 57-year-old man | 48 days | Nasopharyngeal swabs RT-PCR RdRp gene and E gene. Ct ≤ 30 Rapid COVID-19 IgM and IgG |
Cephpeid Xpert® Xpress (unavailable) | Fever, and a productive cough | Myalgia, headache |
| Fernandes Valente Takeda et al., 2020 | 6 pts health professionals | ranged from 53 to 70 days (median, 56.5 days) | Naso and/or oropharyngeal swab samples RT-PCR | No specified | Symptomatic second episode | 2 pts anosmia |
| Tian et al., 2020 | 20/147 pts | 17.25 days, ranging 7–47 days after discharge | RT-PCR ORF1ab gene and N gene |
DAAN GENE, Guangzhou, China (unavailable) | No clinical symptoms | |
| To et al., 2020 | 1 pt | 123 days | Respiratory specimens RT-PCR whole genome sequencing | LightMix® E-gene kit (highly sensitive, specificity 100%). | Asymptomatic | |
| Wang H. et al., 2020 | 1 pt | 15 days | Sputum, nasopharyngeal swabs RT-PCR | No specified | Mild | |
| Wang X. et al., 2020 | 8/131 pts | 7–30 days | Nose and throat RT-PCR ORF1b and N |
No specified | 2 pts fever, 6 pts no clinical symptoms | |
| Wong et al., 2020 | 21/106 pts | 13–16 days | Nasopharyngeal swab RT-PCR Orf1ab and N Ct <40 | BGI Genomics [sensitivity 88.2%e (78.1–94.8), specificity 100% (95.8–100)] | 1 a mild cough, 20 asymptomatic | |
| Wu F. et al., 2020 | 1 pt | 6 days | Throat swab RT-PCR | No specified | Mild | |
| Wu J. et al., 2020 | 10/60 pts | In-home 2-week quarantine | Nasopharyngeal and anal swab samples RT-PCR | No specified | 2 occasionally cought, 8 no clinical symptoms | |
| Xiao A. T. et al., 2020 | 15 of 70 patients | 45 days after symptoms onset | Throat swab samples or deep nasal cavity swab samples RT-PCR | Shanghai Huirui Biotechnology Co., Ltd. (unavailable) | No symptoms | |
| Xiao Y. et al., 2020 | 40/116 pts | During quarantine | Nasopharyngeal swab RT-PCR | Shanghai Huirui Biotechnology Co. (unavailable) | No symptoms | |
| Xie et al., 2020 | 22/161 pts | 1–14 days | Throat swabs and anal swabs RT-PCR | No specified | No data | |
| Xing et al., 2020 | 2/62 pts | 6–14 days | Throat swab samples RT-PCR for ORF1ab and N. Ct ≤ 37 |
BioGerm (sensitivity 96.15% specificity 100.0%) | Asymptomatic | |
| Yang et al., 2020 | 93/479 pts | 7-90 days | RT-qPCR Ct ≤ 40 IgM, IgG, and total antibody |
Zhongshan Daan Biotech. (sensitivity and specificity 100%) | 67 (72%) no symptoms, while 26 (28%) mild symptoms, including slight cough (18/93 [19%]) and chest tightness (3/93 [3%]). | |
| Ye et al., 2020 | 5/55 pts | 30 days | Throat swab samples qRT-PCR | No specified | Fever | |
| Yoo et al., 2020 | 1 pt | 14 days | Upper airway (nasopharyngeal swab), lower airway (sputum), urine, stool, saliva, and serum. qRT-PCR RdRP, N genes, and E gene Ct-values <35 |
Allplex™2019-nCoV Assay (Seegene Inc., Seoul, Korea) [sensitivity (95% CI) 98.2 (90.3–100.0) % specificity (95% CI) 100.0 (94.9–100.0)] | Mild | |
| Yuan B. et al., 2020 | 20/182 recovered patients | During a 14-day medical isolation | Blood, nasopharyngeal swabs, and anal swabs RT-PCR Antibody detection Ct-values <37 |
Bio-Germ, Shanghai (sensitivity 96.15% specificity 100.0%) | No clinical symptoms | |
| Yuan J. et al., 2020 | 25/172 discharged patients | 14 days, 5.23 ±4.13 days | Cloacal swab and nasopharyngeal swab samples RT-PCR Ct-value ≤ 40 |
AM1005; Thermo Fisher Scientific [sensitivity 85.3% (74.6–92.8) Specificity 100% (95.8–100)] | No clinical symptoms | |
| Zhang B. et al., 2020 | 7 pts | 7–11 days | Throat or rectal swabs RT-PCR | No specified | Asymptomatic | |
| Zhang J.-F. et al., 2020 | 1pt | 4 days | Throat swab sample RT-PCR | No specified | Asymptomatic | |
| Zhang R. Z. et al., 2020 | 4 pts | 14–21 days | Nasopharyngeal swab RT-PCR | No specified | Asymptomatic | |
| Zhao et al., 2020 | 7/14 pts | 7–17 days | Nasopharyngeal swab samples RT-PCR Ct-value ≤ 40 |
Zhongshan Daan Biotech (sensitivity and specificity 100%) | Asymptomatic | |
| Zheng et al., 2020 | 3/20 pts after hospital discharge | 7 days | Salivary tests RT-PCR and fecal nucleic acid (RNA) test | No specified | No symptoms | |
| Zhou H. et al., 2020 | 40-year-old male | 5 days after | Sputum or nasopharyngeal swab specimens RT-PCR ORF 1a/1b and nuclear gene Ct-values ≤ 40 |
No specified | Higher density of consolidation on chest CT | |
| Zhou X. et al., 2020 | 17/98 pts | 3–8.5 days | Oropharyngeal swab RT-PCR. Total exon sequencing | No specified | 12 fever, 8 cough, 1 diarrhea | 4 fatigue |
| Zhou Y. et al., 2020 | 53/257 pts | During quarantine | Throat swabs RT-PCR | No specified | Two patients developed clinical symptoms: one of the patients developed a cough, while the other patient had diarrhea |
RT-PCR, reverse transcriptase polymerase chain reaction; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; Ct, cycle threshold; ORF1ab, open reading frame1ab; N, nucleocapsid gene; E, envelope gene; RdRP, RNA-dependent RNA polymerase genes.
The clinical picture and subsequent ENT are compatible with a diagnosis of the spontaneous acute vestibular syndrome. The most common cause is an acute peripheral vestibulopathy known as vestibular neuritis, affecting the vestibular nerve or “pseudoneuritis” if the acute lesions affect the root entry zone of the eighth nerve or the vestibular nucleus (Wu Y. et al., 2020).
The neuroinvasive potential of SARS-CoV-2 is highlighted by some studies (Baig, 2020; Magnavita et al., 2020).
A relapse of the disease with the involvement of the nervous system may indicate that the virus can be neurotropic since the beginning of the disease or in its recurrence form.
The virus may reach the central nervous system via the olfactory nerve. Olfactory and gustatory dysfunctions without rhinorrhea or nasal obstruction are distinctive of patients with mild-to-moderate COVID-19 infection (Baig, 2020; Cooper et al., 2020; Magnavita et al., 2020; Paniz-Mondolfi et al., 2020; Wu Y. et al., 2020), leading to speculation regarding the olfactory nerve as a possible route of the central nervous system entry.
Dizziness is a common onset symptom of COVID-19 (Table 2). This symptom is often considered a non-specific neurological manifestation and is not actively researched or detailed in the description of the clinical picture. This can lead to variability of prevalence estimates, ranging from 3 to 16% between studies. Dizziness such as headache, fatigue, and myalgia are all likely to be caused by the systemic condition if not well-characterized. Specific vestibular or hearing impairment is rarely reported (Table 2). Vertigo should be investigated in SARS-CoV-2 patients and considered along with neurological signs induced by the invasion of the vestibular pathway from the nerve to the vestibular nuclei complex. It is plausible to hypothesize that if the SARS-CoV-2 can also reach the brain from the lungs through the vagus nerve, the virus will invade the brainstem starting with the vagal nucleus and surrounding sites, including the respiratory control center and more, which can lead to more respiratory dysfunction that further exacerbates the damage caused by the primary infection in the lungs or others neurological symptoms (Lukiw et al., 2020; Yachou et al., 2020), such as vestibular impairment. This hypothesis is supported by the evidence of the presence of a consistent angiotensin-converting enzyme (ACE2) expression across the cerebral cortex. The highest ACE2 expression was found in the pons and the medulla oblongata (Guan et al., 2020). Indeed, SARS-CoV-2 appears to bind exclusively to the ACE2 protein, a single-pass type 1 transmembrane receptor with its enzymatically active domain exposed on the surface of multiple cell types, such as type II alveolar cells of the respiratory system, enterocytes and intestinal epithelial cells, endothelial cells, epithelial cells of the conjunctival epithelium, kidney cells (renal tubules), and certain immune cells, such as the alveolar monocytes/macrophages and certain cells of the CNS including those of the cerebral cortex, especially the brainstem (Zubair et al., 2019; Chigr et al., 2020; Kabbani and Olds, 2020; Li C. et al., 2020; Li M. et al., 2020; Panupattanapong and Brooks, 2020; Zhou L. et al., 2020; 154). The highest levels of ACE2-expression in the brain were found in the pons and medulla oblongata, the breathing centers of the brain, which may in part explain the unusually strong ability of SARS-CoV-2 to disrupt normal respiration and pulmonary manifestations including shortness of breath, impaired breathing, and severe respiratory distress. Significant neuroinvasion involving SARS-CoV-2 has been reported from both patients and experimental animals, where the brainstem was heavily infected from apparent spreading via a synapse-connected route to the medullary cardiorespiratory centers (Panupattanapong and Brooks, 2020).
Table 2.
Dizziness as clinical onset symptom reported in literature.
| References | Study | Dizziness | % | Hearing or Vestibular impairment |
|---|---|---|---|---|
| Chen T. et al., 2020 | Retrospective study | Dizziness | 7.66% | |
| Chern et al., 2021 | Case report | Bilateral sudden sensorineural hearing loss, bilateral aural fullness, and vertigo | ||
| Chirakkal et al., 2020 | Case report | Hearing loss and tinnitus | ||
| Correia et al., 2020 | Systematic review | Dizziness | 13.9 % | |
| Degen et al., 2020 | Case report | Asymmetric and bilateral sudden hearing loss and tinnitus | ||
| Di Carlo et al., 2020 | Systematic review | Dizziness | 13.9 % | |
| Fadakar et al., 2020 | Case report | Progressive vertigo in cerebellitis | ||
| Fidan, 2020 | Case report | Acute otitis media, hearing loss, and tinnitus | ||
| Hu Z. et al., 2020 | Retrospective study | Dizziness | 4% | |
| Iltaf et al., 2020 | Cross-sectional study | Vertigo | 3.4% | |
| Sia, 2020 | Case Report | Dizziness | ||
| Karadaş et al., 2020 | Prospective clinical study | Dizziness | 6.7% | |
| Karimi-Galougahi et al., 2020 | Case series | 6 pts with acute-onset hearing loss and/or vertigo | ||
| Kilic et al., 2020 | Case report | Sudden hearing loss | ||
| Klironomos et al., 2020 | Retrospective study (one case) | Vestibular neuronitis | ||
| Lamounier et al., 2020 | Case report | Sudden hearing loss | ||
| Liu et al., 2020b | Case report | Vertigo | ||
| Lon et al., 2020 | Cross-sectional | Dizziness | 20% | |
| Maharaj and Hari, 2020 | Case report | Vertigo and tinnitus | ||
| Maharaj et al., 2020 | Systematic review | 100% hearing loss, 10% associated vestibular symptoms | ||
| Malayala and Raza, 2020 | Case report | Acute vestibular neuritis | ||
| Mao et al., 2020 | Retrospective study | Dizziness | 16.8% | |
| Mi et al., 2020 | Retrospective study | Dizziness | 33% | |
| Özçelik Korkmaz et al., 2020 | Case series | Dizziness | 31.8% | Tinnitus (11%), true vertigo (6%), hearing impairment (5.1%) |
| Qin et al., 2020 | Case series | Dizziness | 8.1% | |
| Romero-Sánchez et al., 2020 | Retrospective study | Dizziness | 6.1% | |
| Shahriarirad et al., 2020 | Case series | Dizziness/vertigo | 39.8% | |
| Sriwijitalai and Wiwanitkit, 2020 | Case series | Six cases of patients with sudden hearing loss | ||
| Sun et al., 2020 | Case report | Bilateral hearing loss and tinnitus | ||
| Viola et al., 2020 | Multicentric study | Dizziness | 94.1% | Thirty-four patients (18.4%) reported equilibrium disorders after COVID-19 diagnosis. Of these, 32 patients reported dizziness (94.1%) and 2 (5.9%) reported acute vertigo attacks. Forty-three patients (23.2%) reported tinnitus; 14 (7.6%) reported both tinnitus and equilibrium disorders. |
| Wang D. et al., 2020 | Case series | Dizziness | 6.5% | |
| Zhong et al., 2020 | Retrospective study | Dizziness | 10.4% |
A limitation of this case consists of the absence of magnetic resonance documentation of vestibular impairment and the genetic characterization of the viruses at the onset and recurrence of COVID-19. The rapid resolution of clinical symptoms within a few days and the trend of not submitting the non-hospitalized patient to neuroimaging exam above all in patients with low suspicions of CNS disease and plan for outpatient ENT visit in the pandemic period, prompted the emergency physician not to proceed. Several causes for repositive tests for SARS-CoV-2 in COVID-19 patients during the recovery period have been described. They include false RT-PCR results or positive due to traces of the RNA genome, intermittent virus shedding, viral reactivation in people with low antibody levels or immunity, reinfection with another SARS-CoV-2 strain, an acute severe systemic inflammatory response known as cytokine release syndrome (CRS), or exposure to a contaminated environmental surface after discharge (Yang et al., 2020; Dao et al., 2021). Various molecular diagnostic assays have been developed and used worldwide, but the differences in their diagnostic performances remain poorly understood (Matsumura et al., 2020; Liotti et al., 2021; Wang M. et al., 2021). Most of the articles do not report the commercial kit used for RT-PCR, and, where reported, the sensitivity and specificity data for the kit is not often available in the literature (Table 1). All the assays exhibited a specificity of 100%, while sensitivity varied (Table 1). The RT-PCR test cannot distinguish between live and dead viruses, but most recurrence of positive RT-PCR is expressed in an asymptomatic way; therefore likely due to dead viruses. We did not perform a genetic characterization of the viruses in order to distinguish between reinfection and reactivation of SARS-CoV-2 in our repositive patient.
Conclusions
This case is suggestive of colonization of the nervous system that can also result in clinical manifestations in cases of recurrence witnessing the diffusion or permanence of SARS-CoV-2 in the nervous system. It also suggests the neurotrophic hypothesis with the possibility of brainstem invasion (pons and medulla oblongata) and the possibility of recurrence with a SARS-CoV-2 positive RT-PCR test and of clinical recurrence with specific neurological symptoms.
Neurological symptoms should be sought and typified in each SARS-CoV-2 patient.
With the outbreak of COVID-19, to better manage the current phase of the pandemic, we should be vigilant for the presence of any neurological symptoms, both as an onset and as a recurrence of infection.
On the basis of the literature examined and reviewed, for recurrence cases and vestibular symptoms during COVID-19, to our knowledge, this is the first case of recurrence with vestibular impairment as neurological symptom, and we suspect it is likely due to viral reactivation. The PCR retest positivity cannot differentiate between reinfectivity and relapse, and dead-viral RNA detection, serological antibody testing, and viral genome sequencing could be always performed in recurrence cases.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors equally contributed to the conception and design of the study. All the authors agreed on the previous version of the manuscript, read, and approved the final manuscript.
Author Disclaimer
As for FG, Medical Director of the Italian Ministry of Health, the opinion and contents expressed in the study are the sole responsibility of the author, and they are not attributable in any way to the institutional and functional positions held by the same at the Italian Ministry of Health (Article 12, paragraph 6, of the Code of Conduct of the Italian Ministry of Health, adopted with DM March 6, 2015 and later).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdullah M. S., Chong P. L., Asli R., Momin R. N., Mani B. I., Metussin D., et al. (2020). Post discharge positive re-tests in COVID-19: common but clinically non-significant. Infect. Dis. 24, 743–7485. 10.1080/23744235.2020.1780309 [DOI] [PubMed] [Google Scholar]
- Alonso F. O. M., Sabino B. D., Guimarães M. A. A. M., Varella R. B. (2020). Recurrence of SARS-CoV-2 infection with a more severe case after mild COVID-19, reversion of RT-qPCR for positive and late antibody response: case report. J. Med. Virol. 14:10.1002/jmv.26432. 10.1002/jmv.26432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J., Liao X., Xiao T., Qian S., Yuan J., Ye H., et al. (2020). Clinical characteristics of recovered COVID-19 patients with re-detectable positive RNA test. Ann. Transl. Med. 8:1084. 10.21037/atm-20-5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi-Pooya A. A., Simani L. (2020). Central nervous system manifestations of COVID-19: a systematic review. J. Neurol. Sci. 413:116832. 10.1016/j.jns.2020.116832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam M., Sulistiana R., Ratnawati M., Fibriana A. I., Bahrudin U., Widyaningrum D., et al. (2020). Recurrent SARS-CoV-2 RNA positivity after COVID-19: a systematic review and meta-analysis. Sci. Rep. 10:20692. 10.1038/s41598-020-77739-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A. M. (2020). Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci. Ther. 7, 499–501. 10.1111/cns.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentivegna E., Sentimentale A., Luciani M., Speranza M. L., Guerritore L., Martelletti P. (2020). New IgM seroconversion and positive RT-PCR test after exposure to the virus in recovered COVID-19 patient. J. Med. Virol. 2020:10.1002/jmv.26160. 10.1002/jmv.26160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmwald K., Galvez N., Ríos M., Kalergis A. M. (2018). Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 12:386. 10.3389/fncel.2018.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongiovanni M., Vignati M., Giuliani G., Manes G., Arienti S., Pelucchi L., et al. (2020). The dilemma of COVID-19 recurrence after clinical recovery. J. Infect. 81, 979–997. 10.1016/j.jinf.2020.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt T., Dieterich M. (2017). The dizzy patient: don't forget disorders of the central vestibular system. Nat. Rev. Neurol. 13, 352–362. 10.1038/nrneurol.2017.58 [DOI] [PubMed] [Google Scholar]
- Cao H., Ruan L., Liu J., Liao W. (2020). The clinical characteristic of eight patients of COVID-19 with positive RT-PCR test after discharge. J. Med. Virol. 92, 2159–2164. 10.1002/jmv.26017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cento V., Colagrossi L., Nava A., Lamberti A., Senatore S., Travi G., et al. (2020). Persistent positivity and fluctuations of SARS-CoV-2 RNA in clinically-recovered COVID-19 patients. J. Infect. 81, e90–e92. 10.1016/j.jinf.2020.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K. J., Jin G. Y., Lee C. S., Lee H. B., Lee J. H., Kwon K. S. (2020). Positive conversion of COVID-19 after two consecutive negative RT-PCR results: a role of low-dose CT. Eur. J. Radiol. 129:109122. 10.1016/j.ejrad.2020.109122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Xu W., Lei Z., Huang Z., Liu J., Gao Z., et al. (2020). Recurrence of positive SARS-CoV-2 RNA in COVID-19: a case report. Int. J. Infect. Dis. 93, 297–299. 10.1016/j.ijid.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Xu X., Hu J., Chen Q., Xu F., Liang H., et al. (2020). Clinical course and risk factors for recurrence of positive SARS-CoV-2 RNA: a retrospective cohort study from Wuhan, China. Aging (Albany NY). 12, 16675–16689. 10.18632/aging.103795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., An W., Xia F., Yang P., Li K., Zhou Q., et al. (2020). Clinical characteristics of rehospitalized patients with COVID-19 in China. J. Med. Virol. 92, 2146–2151. 10.1002/jmv.26002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. L., Xu H., Feng H. Y., Sun J. F., Li X., Zhou L., et al. (2020). Epidemiological and clinical findings of short-term recurrence of severe acute respiratory syndrome coronavirus 2 ribonucleic acid polymerase chain reaction positivity in 1282 discharged coronavirus disease 2019 cases: a multicenter, retrospective, observational study. Open Forum Infect. Dis. 7:ofaa432. 10.1093/ofid/ofaa432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. (2020). Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368:m1091. 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Laurent S., Onur O. A., Kleineberg N. N., Fink G. R., Schweitzer F., et al. (2020). A systematic review of neurological symptoms and complications of COVID-19. J. Neurol. 20, 1–11. 10.1007/s00415-020-10067-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Bai W., Liu B., Huang J., Laurent I., Chen F., et al. (2020). Re-evaluation of retested nucleic acid-positive cases in recovered COVID-19 patients: Report from a designated transfer hospital in Chongqing, China. J. Infect. Public Health. 13, 932–934. 10.1016/j.jiph.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chern A., Famuyide A. O., Moonis G., Lalwani A. K. (2021). Bilateral sudden sensorineural hearing loss and intralabyrinthine hemorrhage in a patient with COVID-19. Otol. Neurotol. 42, e10–e14. 10.1097/MAO.0000000000003233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigr F., Merzouki M., Najimi M. (2020). Autonomic brain centers and pathophysiology of COVID-19. ACS Chem. Neurosci. 11, 1520–1522. 10.1021/acschemneuro.0c00265 [DOI] [PubMed] [Google Scholar]
- Chirakkal P., Hail A. N. A., Zada N., Vijayakumar D. S. (2020). COVID-19 and tinnitus. Ear Nose Throat J. 4:145561320974849. 10.1177/0145561320974849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. W., Brann D. H., Farruggia M. C., Bhutani S., Pellegrino R., Tsukahara T., et al. (2020). COVID-19 and the chemical senses: supporting players take center stage. Neuron 107, 219–233. 10.1016/j.neuron.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia A. O., Feitosa P. W. G., Moreira J. L. S., Nogueira S. Á. R., Fonseca R. B., Nobre M. E. P. (2020). Neurological manifestations of COVID-19 and other coronaviruses: a systematic review. Neurol. Psychiatry Brain Res. 37, 27–32. 10.1016/j.npbr.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouwel F., Waaijenberg-Warmenhoven P., Buiter H. J. C., de Boer N. K. (2020). Recurrent COVID-19 in a patient with ulcerative colitis on vedolizumab therapy. J. Crohns Colitis. 22:jjaa259. 10.1093/ecco-jcc/jjaa259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao T. L., Hoang V. T., Gautret P. (2021). Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: a narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 40, 13–25. 10.1007/s10096-020-04088-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen C., Lenarz T., Willenborg K. (2020). Acute profound sensorineural hearing loss after COVID-19 pneumonia. Mayo Clin. Proc. 95, 1801–1803. 10.1016/j.mayocp.2020.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Sole F., Farcomeni A., Loffredo L., Carnevale R., Menichelli D., Vicario T., et al. (2020). Features of severe COVID-19: a systematic review and meta-analysis. Eur. J. Clin. Invest. 50:e13378. 10.1111/eci.13378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Guang T. W., Yang M., Li J. R., Jiang D. P., Li C. Y., et al. (2020). Positive results for patients with COVID-19 discharged form hospital in Chongqing, China. BMC Infect. Dis. 20:429. 10.1186/s12879-020-05151-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo D. T., Montemurro N., Petrella G., Siciliano G., Ceravolo R., Perrini P. (2020). Exploring the clinical association between neurological symptoms and COVID-19 pandemic outbreak: a systematic review of current literature. J. Neurol. 1, 1–9. 10.21203/rs.3.rs-27152/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou C., Xie X., Peng Z., Tang H., Jiang Z., Zhong Z., et al. (2020). A case presentation for positive SARS-CoV-2 RNA recurrence in a patient with a history of type 2 diabetes that had recovered from severe COVID-19. Diabetes Res. Clin. Pract. 166:108300. 10.1016/j.diabres.2020.108300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou P., Zhang S., Wang C., Cai L., Liu Z., Xu Q., et al. (2020). Serial CT features in discharged COVID-19 patients with positive RT-PCR re-test. Eur. J. Radiol. 127:109010. 10.1016/j.ejrad.2020.109010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H. W., Chen J.-N., Pan X. B., Chen X.-L., Yixian-Zhang Fang S. F., et al. (2020). Prevalence and outcomes of re-positive nucleic acid tests in discharged COVID-19 patients. Eur. J. Clin. Microbiol. Infect. Dis. 31, 1–5. 10.1007/s10096-020-04024-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan N. M., Ludy S. M., Shannon B. C., Reisner A. T., Wilcox S. R. (2021). Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results. Am. J. Emerg. Med. 39, 256.e1–256.e3. 10.1016/j.ajem.2020.06.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul M. A., Benjamin L., Singh B., Lant S., Michael B. D., Easton A., et al. (2020). Neurological associations of COVID-19. Lancet Neurol. 19, 767–783. 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadakar N., Ghaemmaghami S., Masoompour S. M., Shirazi Yeganeh B., Akbari A., Hooshmandi S., et al. (2020). A first case of acute cerebellitis associated with coronavirus disease (COVID-19): a case report and literature review. Cerebellum 31, 1–4. 10.1007/s12311-020-01177-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falahi S., Kenarkoohi A. (2020). COVID-19 reinfection: prolonged shedding or true reinfection? New Microbes New Infect. 38:100812. 10.1016/j.nmni.2020.100812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Valente Takeda C., Moura de Almeida M., Gonçalves de Aguiar Gomes R., Cisne Souza T., Alves de Lima Mota M., Pamplona de Góes Cavalcanti L., et al. (2020). Case report: recurrent clinical symptoms of COVID-19 in healthcare professionals: a series of cases from Brazil. Am. J. Trop. Med. Hyg. 103, 1993–1996. 10.4269/ajtmh.20-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidan V. (2020). New type of corona virus induced acute otitis media in adult. Am. J. Otolaryngol. 41:102487. 10.1016/j.amjoto.2020.102487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W., Chen Q., Wang T. (2020). Letter to the Editor: three cases of redetectable positive SARS-CoV-2 RNA in recovered COVID-19 patients with antibodies. J. Med. Virol. 92, 2298–2301. 10.1002/jmv.25968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Zhu Z., Fan L., Ye S., Huang Z., Shi Q., et al. (2020). Absent immune response to SARS-CoV-2 in a 3-month recurrence of coronavirus disease 2019 (COVID-19) case. Infection 28, 1–5. 10.1007/s15010-020-01485-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geling T., Huaizheng G., Ying C., Hua H. (2020). Recurrent positive nucleic acid detection in a recovered COVID-19 patient: a case report and literature review. Respir. Med. Case Rep. 31:101152. 10.1016/j.rmcr.2020.101152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidari A., Nofri M., Saccarelli L., Bastianelli S., Sabbatini S., Bozza S., et al. (2021). Is recurrence possible in coronavirus disease 2019 (COVID-19)? Case series and systematic review of literature. Eur. J. Clin. Microbiol. Infect. Dis. 40, 1–12. 10.1007/s10096-020-04057-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousseff M., Penot P., Gallay L., Batisse D., Benech N., Bouiller K., et al. (2020). Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J. Infect. 81, 816–846. 10.1016/j.jinf.2020.06.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W. J., Ni Z. Y., Hu Y., Liang W. H., Ou C. Q., He J. X., et al. (2020). China Medical Treatment Expert Group for Covid-19. clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Zeng L., Huang Z., He Y., Zhang Z., Zhong Z. (2020). Longer duration of SARS-CoV-2 infection in a case of mild COVID-19 with weak production of the specific IgM and IgG antibodies. Front. Immunol. 11:1936. 10.3389/fimmu.2020.01936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibzadeh P., Sajadi M. M., Emami A., Karimi M. H., Yadollahie M., Kucheki M., et al. (2020). Rate of re-positive RT-PCR test among patients recovered from COVID-19. Biochem. Med. 30:030401. 10.11613/BM.2020.030401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S., Lian J., Lu Y., Jia H., Hu J., Yu G., et al. (2020). Decreased B cells on admission associated with prolonged viral RNA shedding from the respiratory tract in coronavirus disease 2019: a case-control study. J. Infect. Dis. 222, 367–371. 10.1093/infdis/jiaa311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Luo Q., Lei M., Fan L., Shao X., Hu K., et al. (2020). Successful recovery of recurrence of positive SARS-CoV-2 RNA in COVID-19 patient with systemic lupus erythematosus: a case report and review. Clin. Rheumatol. 39, 2803–2810. 10.1007/s10067-020-05230-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., et al. (2020). Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 382, 2268–2270. 10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T. (2020). Systematic review and meta-analysis of factors associated with re-positive viral RNA after recovery from COVID-19. J. Med. Virol. 93, 2234–2242. 10.1002/jmv.26648 [DOI] [PubMed] [Google Scholar]
- Hu R., Jiang Z., Gao H., Huang D., Jiang D., Chen F., et al. (2020). Recurrent positive reverse transcriptase-polymerase chain reaction results for coronavirus disease 2019 in patients discharged from a hospital in China. JAMA Netw. Open 3:e2010475. 10.1001/jamanetworkopen.2020.10475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Song C., Xu C., Jin G., Chen Y., Yu X., et al. (2020). Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. Chi. Life Sci. 63, 706–711. 10.1007/s11427-020-1661-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zheng L., Li Z., Hao S., Ye F., Chen J., et al. (2020). Recurrence of SARSCoV-2 PCR positivity in COVID-19 patients: a single center experience and potential implications. MedRxiv 2020.05.06.20089573. 10.1101/2020.05.06.20089573 [DOI] [Google Scholar]
- Ibrahim W. (2020). Neurological manifestations in coronavirus disease 2019 (COVID-19) patients: a systematic review of literature. CNS Spectr. 21, 1–12. 10.1017/S1092852920001935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iltaf S., Sr., Fatima M., Salman S., Sr., Salam J. U., Abbas S. (2020). Frequency of neurological presentations of coronavirus disease in patients presenting to a tertiary care hospital during the 2019 coronavirus disease pandemic. Cureus 12:e9846. 10.7759/cureus.9846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISARIC Clinical Characterisation Group (2021). COVID-19 symptoms at hospital admission vary with age and sex: results from the ISARIC prospective multinational observational study. Infection 1–17. 10.1007/s15010-021-01599-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Li Y., Han M., Wang Z., Zhang Y., Du X. (2020). Recurrent PCR positivity after hospital discharge of people with coronavirus disease 2019 (COVID-19). J. Infect. 81, 147–178. 10.1016/j.jinf.2020.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbani N., Olds J. L. (2020). Does COVID19 infect the brain? Mol. Pharmacol. 97, 351–353. 10.1124/molpharm.120.000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y.-J. (2020). South Korea's COVID-19 infection status: from the perspective of re-positive test results after viral clearance evidenced by negative test results. Disaster Med. Public Health Prep. 2020, 1–3. 10.1017/dmp.2020.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadaş Ö., Öztürk B., Sonkaya A. R. (2020). A prospective clinical study of detailed neurological manifestations in patients with COVID-19. Neurol. Sci. 41, 1991–1995. 10.1007/s10072-020-04547-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Galougahi M., Naeini A. S., Raad N., Mikaniki N., Ghorbani J. (2020). Vertigo and hearing loss during the COVID-19 pandemic - is there an association? Acta Otorhinolaryngol. Ital. 40, 463–465. 10.14639/0392-100X-N0820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KCDA. (2020). Findings From Investigation and Analysis of Re-Positive Cases. Available online at: https://www.cdc.go.kr/board/board.es?mid=a30402000000andbid=0030. (accessed September 7, 2020).
- Kilic O., Kalcioglu M. T., Cag Y., et al. (2020). Could sudden sensorineural hearing loss be the sole manifestation of COVID-19? An investigation into SARS-COV-2 in the etiology of sudden sensorineural hearing loss. Int. J. Infect. Dis. 97, 208–211 10.1016/j.ijid.2020.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klironomos S., Tzortzakakis A., Kits A., Öhberg C., Kollia E., Ahoromazdae A., et al. (2020). Nervous system involvement in coronavirus disease 2019: results from a retrospective consecutive neuroimaging cohort. Radiology 297, E324–E334. 10.1148/radiol.2020202791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamounier P., Franco Gonçalves V., Ramos H. V. L., Gobbo D. A., Teixeira R. P., Dos Reis P. C., et al. (2020). A 67-year-old woman with sudden hearing loss associated with SARS-CoV-2 infection. Am. J. Case Rep. 21:e927519. 10.12659/AJCR.927519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L., Xu D., Ye G., Xia C., Wang S., Li Y., et al. (2020). Positive RT-PCR test results in patients recovered from COVID-19. JAMA 323, 1502–1503. 10.1001/jama.2020.2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi F., Gremese E., Rota E., Carfi A., Benvenuto F., Ciciarello F., et al. (2020). Positive RT-PCR nasopharyngeal swab in patients recovered from COVID-19 disease: when does quarantine really end? J. Infect. 81, e1–e3. 10.1016/j.jinf.2020.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi M., Padovani A., McArthur J. C. (2020). Neurological manifestations associated with COVID-19: a review and a call for action. J. Neurol. 267, 1573–1576. 10.1007/s00415-020-09896-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Luo F., Xie L., Gao Y., Zhang N., Wu B. (2020). Chest CT study of fifteen COVID-19 patients with positive RT-PCR retest results after discharge. Quant. Imaging Med. Surg. 10, 1318–1324. 10.21037/qims-20-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Long X., Fang X., Zhang Q., Hu S., Lin Z., et al. (2020). SARS-CoV-2 positivity in a discharged COVID-19 patient: a case report. Clin. Microbiol. Infect. 26, 1115–1117. 10.1016/j.cmi.2020.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. Y., Li L., Zhang Y., Wang X. S. (2020). Expression of the SARSCoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 9:45. 10.1186/s40249-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Hu Y., Yu Y., Zhang X., Li B., Wu J., et al. (2020). Positive result of Sars-Cov-2 in faeces and sputum from discharged patients with COVID-19 in Yiwu, China. J. Med. Virol. 92, 1938–1947. 10.1002/jmv.25905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. C., Bai W. Z., Hashikawa T. (2020). The neuroinvasive potential of SARSCoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J. Med. Virol. 92, 552–555. 10.1002/jmv.25824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Xu S. B., Lin Y. X., Tian D., Zhu Z. Q., Dai F. H., et al. (2020). Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J. (Engl.). 133, 1039–1043. 10.1097/CM9.0000000000000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti F. M., Menchinelli G., Marchetti S., Morandotti G. A., Sanguinetti M., Posteraro B., et al. (2021). Evaluation of three commercial assays for SARS-CoV-2 molecular detection in upper respiratory tract samples. Eur. J. Clin. Microbiol. Infect. Dis. 40, 269–277. 10.1007/s10096-020-04025-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Shi Y., Zhang W., Li R., He Z., Yang X., et al. (2020). Recovered COVID-19 patients with recurrent viral RNA exhibit lower levels of anti-RBD antibodies. Cell. Mol. Immunol. 17, 1098–1100. 10.1038/s41423-020-00528-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Ye L., Xia R., Zheng X., Yuan C., Wang Z., et al. (2020a). Chest CT and clinical follow-up of discharged patients with COVID-19 in Wenzhou City, Zhejiang, China. Ann. Am. Thorac. Soc. 17, 1231–1237. 10.1513/AnnalsATS.202004-324OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Zhou J., Xia L., Cheng X., Lu D. (2020b). 18F-FDG PET/CT and serial chest CT findings in a COVID-19 patient with dynamic clinical characteristics in different period. Clin. Nucl. Med. 45, 495–496. 10.1097/RLU.0000000000003068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Cai Z. B., Huang J. S., Yu W. Y., Niu H. Y., Zhang Y., et al. (2020). Positive SARS-CoV-2 RNA recurs repeatedly in a case recovered from COVID-19: dynamic results from 108 days of follow-up. Pathog Dis. 78:ftaa031. 10.1093/femspd/ftaa031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Lian R., Zhang G., Hou B., Wang C., Dong J., et al. (2020). Changes in serum virus-specific IgM/IgG antibody in asymptomatic and discharged patients with reoccurring positive COVID-19 nucleic acid test (RPNAT). Ann. Med. 53, 34–42. 10.1080/07853890.2020.1811887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Wu S., Zeng G., Zhou F., Li Y., Guo F., et al. (2020). Recurrent positive SARS-CoV-2: immune certificate may not be valid. J. Med. Virol. 29:10.1002/jmv.26074. 10.1002/jmv.26074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loconsole D., Passerini F., Palmieri V. O., Centrone F., Sallustio A., Pugliese S., et al. (2020). Recurrence of COVID-19 after recovery: a case report from Italy. Infection 16, 1–3. 10.1007/s15010-020-01444-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lon I. L., Lio C. F., Cheong H. H., Lei C. I., Cheong T. H., Zhong X., et al. (2020). Evolution of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int. J. Biol. Sci. 16, 1698–1707. 10.7150/ijbs.45357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Peng J., Xiong Q., Liu Z., Lin H., Tan X., et al. (2020). Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR. EBio Med. 59:102960. 10.1016/j.ebiom.2020.102960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani M., Bentivegna E., Spuntarelli V., Lamberti P. A., Cacioli G., Del Porto F., et al. (2020). Recurrent COVID-19 pneumonia in the course of chemotherapy: consequence of a weakened immune system? J. Med. Virol. 28:10.1002/jmv.26701. 10.1002/jmv.26701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw W. J., Pogue A., Hill J. M. (2020). SARS-CoV-2 infectivity and neurological targets in the brain. Cell Mol. Neurobiol. 2020, 1–8. 10.1007/s10571-020-00947-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley S. F., O'Donnell D., Stoesser N. E., Matthews P. C., Howarth A., Hatch S. B., et al. (2020). Antibody status and incidence of SARS-CoV-2 infection in health care workers. N. Engl. J. Med. 384, 533–540. 10.1056/NEJMoa2034545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnavita N., Tripepi G., Di Prinzio R. R. (2020). Symptoms in health care workers during the COVID-19 epidemic. a cross-sectional survey. Int. J. Environ. Res. Public Health. 17:5218. 10.3390/ijerph17145218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharaj S., Bello Alvarez M., Mungul S., Hari K. (2020). Otologic dysfunction in patients with COVID-19: a systematic review. Laryngos. Investig. Otolaryngol. 5, 1192–1196. 10.1002/lio2.498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharaj S., Hari K. (2020). Congenital inner ear abnormalities and COVID-19-related ear infections. Ear Nose Throat J. 23:145561320968934. 10.1177/0145561320968934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malayala S. V., Raza A. (2020). A case of COVID-19-induced vestibular neuritis. Cureus 12:e8918. 10.7759/cureus.8918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Wang M., Chen S., He Q., Chang J., Hong C., et al. (2020). Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. medRxiv 2020.02.22.20026500. 10.1101/2020.02.22.20026500 [DOI] [Google Scholar]
- Mardani M., Nadji S. A., Sarhangipor K. A., Sharifi-Razavi A., Baziboroun M. (2020). COVID-19 infection recurrence presenting with meningoencephalitis. New Microbes New Infect. 37:100732. 10.1016/j.nmni.2020.100732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y., Shimizu T., Noguchi T., Nakano S., Yamamoto M., Nagao M. (2020). Comparison of 12 molecular detection assays for SARS-CoV-2. bioRxiv 2020: 06.24.170332. 10.1101/2020.06.24.170332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Q., Li J., Du R., Yuan X., Li M., Li J. (2020). Assessment of patients who tested positive for COVID-19 after recovery. Lancet Infect. Dis. 20, P1004–P1005. 10.1016/S1473-3099(20)30433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi B., Chen L., Xiong Y., Xue H., Zhou W., Liu G., et al. (2020). Characteristics and early prognosis of COVID-19 infection in fracture patients. J. Bone Joint Surg. 102, 750–758. 10.2106/JBJS.20.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepal G., Rehrig J. H., Shrestha G. S., Shing Y. K., Yadav J. K., Ojha R., et al. (2020). Neurological manifestations of COVID-19: a systematic review. Crit. Care. 24:421. 10.1186/s13054-020-03121-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özçelik Korkmaz M., Egilmez O. K., Özçelik M. A., Güven M. (2020). Otolaryngological manifestations of hospitalised patients with confirmed COVID-19 infection. Eur. Arch. Otorhinolaryngol. 3, 1–11. 10.1007/s00405-020-06396-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz-Mondolfi C., Bryce Z., Grimes R. E., Gordon J., Reidy J., Lednicky E., et al. (2020). Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med. Virol. 92, 699–702. 10.1002/jmv.25915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panupattanapong S., Brooks E. B. (2020). New spectrum of COVID-19 manifestations in children: Kawasaki-like syndrome and hyperinflammatory response. Cleve Clin. J. Med. 1–7 10.3949/ccjm.87a.ccc039 [DOI] [PubMed] [Google Scholar]
- Peng J., Wang M., Zhang G., Lu E. (2020). Seven discharged patients turning positive again for SARS-CoV-2 on quantitative RT-PCR. Am. J. Infect. Control. 48, 725–726. 10.1016/j.ajic.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X.-M., Xu X.-F., Zi H., Liu G.-X., Li B.-H., Du X., et al. (2020). Re- positive cases of nucleic acid tests in discharged patients with COVID-19: a follow-up study. Front. Med. 7:349. 10.3389/fmed.2020.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. (2020). Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 71, 762–768. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravioli S., Ochsner H., Lindner G. (2020). Reactivation of COVID-19 pneumonia: a report of two cases. J. Inf. Secur. 81, e72–e73. 10.1016/j.jinf.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez C. M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., et al. (2020). Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology 95, e1060–e1070. 10.1212/WNL.0000000000009937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcin S., Fontem F. (2020). Recurrent Sars-Cov-2 infection resulting in acute respiratory distress syndrome and development of pulmonary hypertension: a case report. Respir. Med. Case Rep. 5:101314. 10.1016/j.rmcr.2020.101314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M. K., Gupta N., Yadav S. R., Kumar R., Singh B., Ish P. (2020). Contentious issue in recurrent COVID-19 infection: reactivation or reinfection. Turk Thorac. J. 21, 463–466. 10.5152/TurkThoracJ.2020.20164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahriarirad R., Khodamoradi Z., Erfani A., Hosseinpour H., Ranjbar K., Emami Y., et al. (2020). Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID-19) in the South of Iran. BMC Infect. Dis. 20:427. 10.1186/s12879-020-05128-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Sardar S., Mohammad Arshad A., Ata F., Zara S., Munir W. (2020). A patient with asymptomatic SARS-CoV-2 infection who presented 86 days later with COVID-19 pneumonia possibly due to reinfection with SARS-CoV-2. Am. J. Case Rep. 21:e927154. 10.12659/AJCR.927154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia J. (2020). Dizziness can be an early sole clinical manifestation for COVID-19 infection: a case report. J. Am. Coll. Emerg. Physicians Open. 23:10.1002/emp2.12185. 10.1002/emp2.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriwijitalai W., Wiwanitkit V. (2020). Hearing loss and COVID-19: a note. Am. J. Otolaryngol. 41:102473. 10.1016/j.amjoto.2020.102473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R., Liu H., Wang X. (2020). Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J. Radiol. 21, 541–544. 10.3348/kjr.2020.0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M., Long Y., Hong Y., Zhang X., Zha Y. (2020). The treatment and follow-up of 'recurrence' with discharged COVID-19 patients: data from Guizhou, China. Environ. Microbiol. 22, 3588–3592. 10.1111/1462-2920.15156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K. K.-W., Hung I. F.-N., Ip J. D., Chu A. W.-H., Chan W.-M., Tam A. R., et al. (2020). COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin. Infect. Dis. 2020:ciaa1275. 10.1093/cid/ciaa1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavougios G. D. (2020). SARS-CoV-2 dysregulation of PTBP1 and YWHAE/Z gene expression: a primer of neurodegeneration. Med. Hypotheses. 144:110212. 10.1016/j.mehy.2020.110212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola P., Ralli M., Pisani D., Malanga D., Sculco D., Messina L., et al. (2020). Tinnitus and equilibrium disorders in COVID-19 patients: preliminary results. Eur. Arch. Otorhinolaryngol. 23, 1–6. 10.1007/s00405-020-06440-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus – infected pneumonia in Wuhan – China. J. Am. Med. Assoc. 323, 1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Li Y., Wang F., Du H., Lu X. (2020). Rehospitalization of a recovered coronavirus disease 19 (COVID-19) child with positive nucleic acid detection. Pediatr. Infect. Dis. J. 39:e69. 10.1097/INF.0000000000002690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Chen D., Wu W., Tang H., Kan L., Zong Z., et al. (2021). Analytical performance evaluation of five RT-PCR kits for severe acute respiratory syndrome coronavirus 2. J. Clin. Lab. Anal. 2021:e23643. 10.1002/jcla.23643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Xu H., Jiang H., Wang L., Lu C., Wei X., et al. (2020). Clinical features and outcomes of discharged coronavirus disease 2019 patients: a prospective cohort study. QJM. 113, 657–665. 10.1093/qjmed/hcaa178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker A., Anson M., Harky A. (2020). Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol. Scand. 142, 14–22. 10.1111/ane.13266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J., Koh W. C., Momin R. N., Alikhan M. F., Fadillah N., Naing L. (2020). Probable causes and risk factors for positive SARS-CoV-2 test in recovered patients: evidence from Brunei Darussalam. J. Med. Virol. 92, 2847–2851. 10.1002/jmv.26199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhang W., Zhang L., Wang D., Wan Y. (2020). Discontinuation of antiviral drugs may be the reason for recovered COVID-19 patients testing positive again. Br. J. Hosp. Med. 81, 1–2. 10.12968/hmed.2020.0156 [DOI] [PubMed] [Google Scholar]
- Wu J., Liu X., Liu J., Liao H., Long S., Zhou N., et al. (2020). Coronavirus disease 2019 test results after clinical recovery and hospital discharge among patients in China. JAMA Netw. Open. 3:e209759. 10.1001/jamanetworkopen.2020.9759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., et al. (2020). Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 87, 18–22. 10.1016/j.bbi.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao A. T., Tong Y. X., Zhang S. (2020). False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J. Med. Virol. 9:10.1002/jmv.25855. 10.1002/jmv.25855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Shi X., She Q., Chen Q., Pan H., Zhang J., et al. (2020). Exploration of turn-positive RT-PCR results and factors related to treatment outcome in COVID-19: a retrospective cohort study. Virulence 11, 1250–1256. 10.1080/21505594.2020.1816076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Lu J., Wu D., Zhang L., Zhao H., Rao B., et al. (2020). False negative rate of COVID-19 is eliminated by using nasal swab test. Travel Med. Infect. Dis. 37:101668. 10.1016/j.tmaid.2020.101668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Mo P., Xiao Y., Zhao O., Zhang Y., Wang F. (2020). Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China, January to February 2020. Euro Surveill. 25:2000191. 10.2807/1560-7917.ES.2020.25.10.2000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Mu J., Guo J., Lu L., Liu D., Luo J., et al. (2020). New onset neurologic events in people with COVID-19 in 3 regions in China. Neurology 95, e1479–e1487. 10.1212/WNL.0000000000010034 [DOI] [PubMed] [Google Scholar]
- Yachou Y., El Idrissi A., Belapasov V., Ait Benali S. (2020). Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2, understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. 41, 2657–2669. 10.1007/s10072-020-04575-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahav D., Yelin D., Eckerle I., et al. (2021). Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin. Microbiol. Infect. 27, 315–318. 10.1016/j.cmi.2020.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Jiang M., Wang X., Tang X., Fang S., Li H., et al. (2020). Viral RNA level, serum antibody responses, and transmission risk in recovered COVID-19 patients with recurrent positive SARS-CoV-2 RNA test results: a population-based observational cohort study. Emerg. Microbes Infect. 9, 2368–2378. 10.1080/22221751.2020.1837018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G., Pan Z., Pan Y., Deng Q., Chen L., Li J., et al. (2020). Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J. Infect. 80, e14–e17. 10.1016/j.jinf.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S. Y., Lee Y., Lee G. H., Kim D. H. (2020). Reactivation of SARSCoV-2 after recovery. Pediatr. Int. 62, 879–881. 10.1111/ped.14312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B., Liu H. Q., Yang Z. R., Chen Y. X., Liu Z. Y., Zhang K., et al. (2020). Recurrence of positive SARS-CoV-2 viral RNA in recovered COVID-19 patients during medical isolation observation. Sci. Rep. 10:11887. 10.1038/s41598-020-68782-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Kou S., Liang Y., Zeng J., Pan Y., Liu L. (2020). PCR assays turned positive in 25 discharged COVID-19 patients. Clin. Infect. Dis. 8:ciaa398. 10.1093/cid/ciaa398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Liu S., Dong Y., Zhang L., Zhong Q., Zou Y., et al. (2020). Positive rectal swabs in young patients recovered from coronavirus disease 2019 (COVID-19). J. Infect. 81:e49–e52. 10.1016/j.jinf.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-F., Yan K., Ye H.-H., Lin J., Zheng J.-J., Cai T. (2020). SARS-CoV-2 turned positive in a discharged patient with COVID-19 arouses concern regarding the present standards for discharge. Int. J. Infect. Dis. 97, 212–214. 10.1016/j.ijid.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Zhou L., Wang J., Wang K., Wang Y., Pan X., et al. (2020). The nervous system-A new territory being explored of SARS-CoV-2. J. Clin. Neurosci. 82(Pt A):87–92. 10.1016/j.jocn.2020.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R. Z., Deng W., He J., Song Y. Y., Qian C. F., Yu Q., et al. (2020). Case Report: recurrence of positive SARS-CoV-2 results in patients recovered from COVID-19. Front. Med. 7:585485. 10.3389/fmed.2020.585485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Wang Y., Tang Y., Zhao W., Fan Y., Liu G., et al. (2020). Characteristics of children with reactivation of SARS-CoV-2 infection after hospital discharge. Clin. Pediatr. (Phila). 59, 929–932. 10.1177/0009922820928057 [DOI] [PubMed] [Google Scholar]
- Zheng K. I., Wang X. B., Jin X. H., Liu W. Y., Gao F., Chen Y. P., et al. (2020). A case series of recurrent viral RNA positivity in recovered COVID-19 Chinese patients. J. Gen. Intern. Med. 35, 2205–2206. 10.1007/s11606-020-05822-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z. F., Huang J., Yang X., Peng J. L., Zhang X. Y., Hu Y., et al. (2020). Epidemiological and clinical characteristics of COVID-19 patients in Hengyang, Hunan Province, China. World J. Clin. Cases 8, 2554–2565. 10.12998/wjcc.v8.i12.2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Fu L., Jin Y., Shao J., Zhang S., Zheng N., et al. (2020). Clinical features of COVID-19 convalescent patients with re-positive nucleic acid detection. J. Clin. Lab. Anal. 34:e23392. 10.1002/jcla.23392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Xu Z., Castiglione G. M., Soiberman U. S., Eberhart C. G., Duh E. J. (2020). ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 18, 537–544. 10.1016/j.jtos.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Zhou J., Zhao J. (2020). Recurrent pneumonia in a patient with new coronavirus infection after discharge from hospital for insufficient antibody production: a case report. BMC Infect. Dis. 20:500. 10.1186/s12879-020-05231-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Fu B., Zheng X., et al. (2020). Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl. Sci. Rev. 13:nwaa041. 10.1093/nsr/nwaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair A. S., McAlpine L. S., Gardin T., Farhadian S., Kuruvilla D. E., Spudich S. (2019). Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 77, 1018–1027. 10.1001/jamaneurol.2020.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.