Abstract
Background
Red blood cell (RBC) transfusion is commonly used to increase oxygen transport in patients with sepsis. However it does not consistently increase oxygen uptake at either the whole-body level, as calculated by the Fick method, or within individual organs, as assessed by gastric intra-mucosal pH.
Aim
This study evaluates the hemodynamic and oxygen utilization effects of hemoglobin infusion on critically ill septic patients.
Methods
Fifteen septic patients undergoing mechanical ventilation whose hemoglobin was <10 g% were eligible. Ten patients (APACHE II: 25.5 ± 7.6) received an infusion of 1 unit of packed RBC over 1 h while sedated and paralyzed. The remaining five control patients (APACHE II: 24.3 ± 6.0) received a 5% albumin solution (500 ml) over 1 h. Hemodynamic data, gastric tonometry and calorimetry were obtained prior to and immediately after RBC transfusion or 5% albumin infusion.
Results
Transfusion of RBC was associated with an improvement in left ventricular systolic work index (38.6 ± 12.6 to 41.1 ± 13.0 g/min/m2; P = 0.04). In the control group there was no significant change in the left ventricular systolic work index (37.2 ± 14.3 to 42.2 ± 18.9 g/min/m2). An increase in pulmonary vascular resistance index (203 ± 58 to 238 ± 49 dyne/cm5/m2; P = 0.04) was also observed, while no change was produced by colloid infusion (237 ± 87.8 to 226.4 ± 57.8 dyne/cm5/m2). Oxygen utilization did not increase either by Fick equation or by indirect calorimetry in either group. Gastric intramucosal pH increased only in the control group but did not reach statistical significance.
Conclusion
Hemoglobin increase does not improve either global or regional oxygen utilization in anemic septic patients. Furthermore, RBC transfusion may hamper right ventricular ejection by increasing the pulmonary vascular resistance index.
Keywords: albumin, blood transfusion, fluid therapy, oxygen consumption, septic patients
Introduction
The treatment of septic patients emphasizes the optimization of oxygen utilization by tissues through maintenance of an adequate oxygen supply, minimizing the cellular dysfunction progression [1]. Blood cell transfusion is frequently used with the intention of augmenting arterial oxygen content and its utilization by the tissues [2]. Blood cell transfusion efficacy in septic patients is still not convincingly demonstrated and previous studies report conflicting results. When oxygen consumption is calculated by Fick's method [3], it is demonstrated to have increased following red blood cell (RBC) transfusion. However, such increase is not always corroborated by indirect calorimetry [4]. Discrepancies of results may be explained by the mathematical coupling of data used to calculate both oxygen transport and consumption [5]. Blood cell transfusion has been implicated with a deterioration in gastric oxygenation when it was measured by gastric intramucosal pH (pHi), determined by tonometry [6]. This deterioration has been attributed to prolonged storage time of transfused red blood cells which may lead to cellular stiffening, decreasing their deformation capacity and exacerbating the rheological abnormalities observed in sepsis. We conducted an interventional, prospective, randomized, controlled study to evaluate the immediate consequences of RBC transfusion on hemodynamic parameters and on systemic and gastric oxygen consumption, using indirect calorimetry and gastric tonometry.
Materials and methods
This study was performed in a general intensive care unit, in a private tertiary hospital, from January to December 1996 after approval by the Internal Ethics Committee. As a result of the clinical condition of patients, consent was obtained from the next of kin.
Subjects
Septic patients [7] with hemoglobin concentration values less than 10 g%, who were being mechanically ventilated, with an oxygen inspired fraction (FIO2) less than 60% and with pulmonary artery catheter monitoring (Swan-Ganz CCOmbo CCO/SVO2: Baxter, Edwards Critical Care Division, Irvine, USA) were included. They were all studied for a period of 48 h after sepsis was diagnosed. Exclusion criteria included: age <18 or >80 years, pregnant women, patients on dialysis, and patients who had recently undergone a gastrointestinal surgical procedure and established septic shock [7].
Intervention
A tonometry probe device (Tonometrics: Datex, Helsinki, Finland) was inserted and its position was confirmed by X-ray. All patients received gastric protection with antacids and enteral diet was interrupted during the study period. A calorimeter (Deltatrac II Metabolic Monitor: Datex, Helsinki, Finland) was installed in the mechanical ventilator circuit to measure carbon dioxide and oxygen concentrations in the inspired and expired air and to calculate systemic oxygen consumption and carbon dioxide production. A radial artery catheter (Radial artery catheter: Arrow, Reading, USA) was introduced to measure mean arterial pressure. Mechanical ventilation (Servo 900 C: Siemens Elema, Lünd, Sweden) parameters were adjusted in order to maintain carbon dioxide arterial tension within the normal range and an oxygen arterial saturation of more than 92%. All patients were sedated with midazolam and paralyzed with pancuro-nium. After initial measurements, patients were randomized 2:1 in order to receive one packed RBC unit or 5% albumin, 500 ml (5% Albumin: Baxter AG, Vienna, Austria), respectively. Storage time of the transfused RBC packages was 12.8 ± 8.1 days. The infusion time was 60 min. Immediately after this period, all measurements were repeated and the study was finished. No alterations in cardiovascular and ventilatory support were performed during the study. Patients were not aspirated, decubitus changes were not allowed nor any other physiotherapeutic procedures. Hyperthermia was treated with dipirone.
Measurements
Arterial lactate measurements, hemodynamic, tonometric and calorimetric parameters were calculated immediately before initiation of the RBC infusion and repeated immediately after the infusion was completed.
Hemodynamic parameters included measurement of systemic arterial pressure, pulmonary arterial pressure, central venous pressure and pulmonary capillary pressure. Cardiac output was assessed by continuous thermodilution (Vigilance Monitor: Baxter, Edwards Critical-care Division, Santa Ana, USA).
All flow and volume measurements were indexed to body surface area. Blood specimens were collected from the radial artery catheter and from the distal extremity of the pulmonary artery catheter in order to determine blood gas values (ABL-725: Radiometer, Copenhagen, Denmark), oxygen saturation (ABL-725), lactate (Vitrus 500, Johnson & Johnson Clinical Diagnosis) and hemoglobin (STKS: Coulter Electronics, Hialeah, Florida, USA) concentrations. Oxygen consumption, oxygen transport, left ventricular systolic work index, systemic vascular resistance index and pulmonary vascular resistance index were calculated by the standard methods. Gastric oxygen consumption was indirectly measured by gastric tonometry and pHi determination [8]. A tonometry catheter is a nasogastric tube with a silicon balloon in its tip, which is extremely sensitive to carbon dioxide. This balloon was filled with 2.5 ml of saline solution until an equilibrium with the gastric mucosa was achieved (60 min before each measurement). Once equilibrium was reached, the balloon was aspirated and the carbon dioxide level was determined. The pHi was calculated from the carbon dioxide value by applying the Henderson-Hasselbach equation. The normal pHi value was 7.39 ± 0.03. Systemic oxygen consumption was measured by indirect calorimetry [9]. The calorimeter measures carbon dioxide and oxygen concentrations in the inspired and expired air in each minute and calculates carbon dioxide production as well as the oxygen consumption in each minute. The calorimeter was calibrated before each measurement. Thirty minutes after stabilization of the patient, oxygen consumption was measured for a period of 15 min. The APACHE II index was used to evaluate the severity of the patient's condition [10].
Statistical analysis
Data were expressed by mean values ± standard deviation. Comparisons taken before versus after intervention were evaluated using the Wilcoxon Signed Rank test in each group. In order to study the relationship between the age of the RBCs and the effect of this on pHi we used Pearson's correlation coefficient. We resubmitted the data for variance analysis with repeated measures and related designs with the intention to compare hemodynamic response to the interventions between study groups. For statistical significance we considered P < 0.05.
Results
Fifteen male patients were studied. Ten patients received RBC transfusion. Their APACHE II score was 25.5 ± 7.6. Five patients received 5% albumin and their APACHE II score was 24.3 ± 6.0. Table 1 shows baseline patient characteristics and Table 2 shows hemodynamic data, oxygen utilization parameters and hemoglobin concentration in the group that received RBC transfusion.
Table 1.
Patient baseline characteristics
| Variable | RBC group | Albumin group |
| Gender M/F | 10/0 | 5/0 |
| APACHE II* | 25.5 ± 7.67 | 24.3 ± 6.0 |
| Diagnosis % | ||
| Clinical | 50 | 40 |
| Surgical | 50 | 60 |
| Source of infection % | ||
| Intra-abdominal | 50 | 40 |
| Lung | 30 | 40 |
| Heart | 10 | 0 |
| Unknown origin | 10 | 20 |
| ICU mortality | 70 | 60 |
*Mean ± SD
Table 2.
Results from the group receiving red blood cell transfusion
| Variable | Baseline | After infusion | P |
| HR (bpm) | 109.4 ± 20.3 | 108.9 ± 21.7 | NS |
| RAP (mmHg) | 10.4 ± 3.7 | 10.7 ± 4.9 | NS |
| MPAP (mmHg) | 25.2 ± 5.0 | 26.9 ± 5.5 | NS |
| WP (mmHg) | 13.4 ± 3.8 | 13.2 ± 4.5 | NS |
| MAP (mmHg) | 76.8 ± 15.8 | 82.9 ± 17.2 | NS |
| CI (L/min/m2) | 4.7 ± 0.7 | 4.7 ± 1.1 | NS |
| LVSWI (g/min/m2) | 38.6 ± 12.6 | 41.1 ± 13.0 | <0.05 |
| SVRI (dyne/s/cm5/m2) | 1050.3 ± 336.0 | 1148.3 ± 398.0 | NS |
| PVRI (dyne/s/cm5/m2) | 203.7 ± 58.0 | 238.8 ± 49.8 | <0.05 |
| DO2 (ml/min/m2) | 607.3 ± 123.5 | 647.5 ± 167.7 | NS |
| VO2 (ml/min/m2) | |||
| Calorimetry | 168.9 ± 63.1 | 162.5 ± 67.7 | NS |
| Fick | 142.2 ± 44.9 | 149.6 ± 41.9 | NS |
| Hb (g%) | 9.4 ± 0.5 | 10.1 ± 0.8 | <0.05 |
| Ht (%) | 27.8 ± 1.7 | 29.8 ± 1.8 | <0.05 |
| pHi | 7.19 ± 0.07 | 7.21 ± 0.16 | NS |
| Lactate (mmol/l) | 1.8 ± 0.5 | 1.7 ± 0.5 | NS |
*Mean ± SD. CI, cardiac index; DO2, systemic oxygen delivery index; Hb, hemoglobin; HR, heart rate; Ht, hematocrit; LVSWI, left ventricular stroke work index; MAP, mean arterial pressure; MPAP, mean pulmonary artery pressure; pHi, gastric intramucosal pH; PVRI, pulmonary vascular resistance index; RAP, right atrial pressure; SVRI, systemic vascular resistance index; VO2, systemic oxygen consumption index; WP, wedge pressure.
Blood transfusion increased hemoglobin concentration from 9.4 ± 0.5 to 10.1 ± 0.8 g% (P < 0.001). This was associated with an increase in the left ventricular systolic work index (38.6 ± 12.6 to 41.1 ± 13.0 g/min/m2; P < 0.05). Although systemic vascular resistance was unchanged, pulmonary vascular resistance was increased (203.7 ± 58.0 to 238.0 ± 49.8 dyne/s/cm5/m2; P < 0.05). Filling pressures, and systemic and regional oxygenation indices were not significantly changed.
Table 3 depicts hemodynamic data and oxygen utilization parameters for the colloid (control) group. The administration of 5% albumin had no effect on hemodynamic parameters. Although global oxygen consumption did not change, regional gastric mucosa oxygenation was improved, with an increase in pHi from 7.14 ± 0.2 to 7.24 ± 0.1 but did not reach statistical significance (P = 0.1). Lactate levels were unchanged.
Table 3.
Results in the albumin group
| Variable | Baseline | After infusion | P |
| HR (bpm) | 118.2 ± 33.9 | 120.2 ± 35.6 | NS |
| RAP (mmHg) | 11.4 ± 4.9 | 14.0 ± 3.3 | NS |
| MPAP (mmHg) | 24.2 ± 4.3 | 28.49 ± 3.5 | NS |
| WP (mmHg) | 11.6 ± 4.5 | 14.2 ± 3.6 | NS |
| MAP (mmHg) | 80.4 ± 22.0 | 81.6 ± 19.1 | NS |
| CI (L/min/m2) | 4.94 ± 1.05 | 5.20 ± 1.26 | NS |
| LVSWI (g/min/m2) | 37.2 ± 14.3 | 42.2 ± 18.9 | NS |
| SVRI (dyne/s/cm5/m2) | 1052.8 ± 261.0 | 1049.8 ± 236.5 | NS |
| PVRI (dyne/s/cm5/m2) | 237.0 ± 87.8 | 226.4 ± 57.8 | NS |
| DO2 (ml/min/m2) | 699.6 ± 183.0 | 732.0 ± 194.8 | NS |
| VO2 (ml/min/m2) | |||
| Calorimetry | 144.0 ± 30.5 | 147.2 ± 30.2 | NS |
| Fick | 110.8 ± 13.8 | 127.8 ± 30.9 | NS |
| Hb (g%) | 9.19 ± 0.49 | 9.12 ± 0.75 | NS |
| Ht (%) | 27.1 ± 2.69 | 27.4 ± 2.6 | NS |
| pHi | 7.14 ± 0.2 | 7.24 ± 0.1 | NS |
| Lactate (mmol/l) | 1.8 ± 0.5 | 1.7 ± 0.5 | NS |
*Mean ± SD. CI, cardiac index; DO2, systemic oxygen delivery index; Hb, hemoglobin; HR, heart rate; Ht, hematocrit; LVSWI, left ventricular stroke work index; MAP, mean arterial pressure; MPAP, mean pulmonary artery pressure; pHi, gastric intramucosal pH; PVRI, pulmonary vascular resistance index; RAP, right atrial pressure; SVRI, systemic vascular resistance index; VO2, systemic oxygen consumption index; WP, wedge pressure.
We were unable to find a correlation between the age of the RBCs and the effects on pHi (r = 0.257, P = 0.473). Figure 1 shows the relationship between the age of RBCs and the change in pHi. Figure 2 shows that the systemic oxygen delivery index (DO2) response to the interventions was effective in both groups. Analysis of variance with repeated measurements showed that DO2 increased (Group 1: from 607.3 ± 123.5 to 647.5 ± 167.7 ml/min/m2 and Group 2 from 699.6 ± 183.0 to 732.0 ± 194.8 ml/min/m2, P < 0.05).
Figure 1.
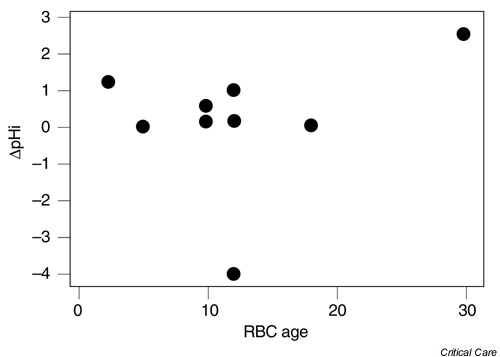
Change in intramucosal pH (pHi) versus red blood cell (RBC) age.
Figure 2.
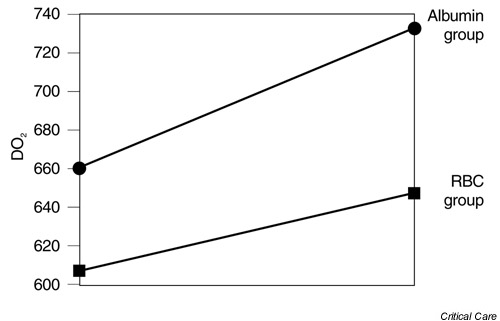
Systemic oxygen delivery index (DO2) response after intervention in both groups. Repeated measures ANOVA (P < 0.05). RBC, red blood cell.
Discussion
In this group of septic patients, red blood cell transfusion did not improve systemic oxygen consumption, whether indirectly calculated by the Fick method or actually measured by calorimetry, despite increased arterial oxygen content. This could be as result of cellular changes in stored RBC prior to transfusion. Red blood cells stocked for transfusions become rigid. This decreases 2,3-diphosphoglycerate levels and renders red cells less capable of offering oxygen to tissues [6]. These changes in stocked red blood cells are reversible in vivo within 24 h [11,12]. The fact that we were unable to detect an acute increase in the oxygen consumption could be because we did not allow sufficient time for the above-mentioned cellular changes to revert. When oxygen consumption is calculated by the Fick method, the formula couples oxygen consumption (VO2) to oxygen delivery (DO2):
DO2 = CI × CaO2
VO2 = CI × (CaO2 - CvO2)
where CI = cardiac index (l/min/m2), CaO2 = oxygen content of arterial blood (ml/l) and CvO2 = oxygen content of venous blood (ml/l).
Thus, an increase in oxygen supply could result in a false increase in the oxygen consumption. On the other hand, indirect calorimetry offers the possibility of directly measuring oxygen consumption and is probably a more reliable method [13]. Another disadvantage with the Fick method is that it could potentially underestimate systemic oxygen consumption because of its inability to measure data from bronchial circulation and Thebesian's circulations [14]. In the study reported here, we did not observe the effect of mathematical coupling of supply to consumption; RBC transfusion did not result in increased O2 consumption, whether calculated by the Fick method or measured by indirect calorimetry methods.
In this study another possible explanation for our findings is the fact that our patients would not be in a delivery-dependent state as we might speculate observing the baseline lactate values. Gastric tonometry with pHi calculation has been utilized as an indicator of regional gastric perfusion. It is a good prognostic indicator in critically ill patients [15,16]. Regional gastric oxygenation behavior in this study was similar to that of systemic oxygenation and did not change in response to RBC transfusion. Marik and Sibbald reported worsening of gastric oxygenation when transfused blood cells were stored for more than 15 days and they postulated that prolonged storage time could make the cells rigid and this could, in turn, lead to capillary obstruction and consequent decrease in local blood flow [6]. Worsening of gastric mucosal and splanchnic oxygenation could be associated with increased mucosal permeability and bacteria translocation [17,18,19]. Blood viscosity is increased by RBC transfusion and this may decrease oxygen transport to tissues [6,20,21]. In the study reported here, pulmonary vascular resistance increased significantly after RBC transfusion and there was a nonsignificant trend towards an increase in systemic vascular resistance. These changes can be deleterious in septic patients, who often present with associated myocardial dysfunction. An infusion of 500 ml of 5% albumin was used in this study with the intention of promoting a plasma expansion varying from 250 to 500 ml [22,23] to serve as a control group for the 10 patients that received RBC transfusion.
Use of albumin as a plasma expander is controversial. The Cochrane Injuries Group has reviewed the use of albumin in critically ill patients and has concluded that it could increase mortality [24], but this review has been severely criticized [25] and albumin is widely used as a colloid expander. Colloid expansion did not change filling pressures, cardiac index or systemic oxygen consumption but caused a nonsignificant increasing in gastric pHi, denoting improved oxygenation of the gastric mucosa. The trend towards an increase in gastric oxygenation could be related to some degree of hemodilution and amelioration of gastric mucosal rheology, although neither of these factors was demonstrated in our study, perhaps because of the small number of patients involved.
The effects of colloid infusion on gastric mucosal perfusion are still controversial. Boldt's group randomized septic patients to receive hydroxyethyl starch or albumin (both groups also received saline infusion) for a period of 5 days. The pHi was low in both groups and was increased by hydroxyethyl starch but not by albumin, but this effect was only evident after 48 h [26,27,28]. Forrest reported that 10% pentastarch infusion did not change pHi in hypovolemic septic patients [29], but measurements were only taken for 2 h after infusion and it is known that the effects of colloidal infusion are not immediately evident [30]. Indication for RBC transfusion should be always a carefully judged clinical decision. A recent study failed to demonstrate reduced mortality in critically ill patients with hemoglobin levels between 7 and 10 g% who received RBC transfusion [31]. Anemia does not aggravate gastric mucosal acidosis [32], whereas RBC transfusion can worsen it [6].
In this study we found that albumin (and probably other colloid expansions) may improve gastric pHi. It seems clear that restoration of circulating blood to normal levels is capable of restoring gastric perfusion and it should precede blood cell transfusion in septic patients with hemoglobin levels above 8 g%, who are not suffering from associated coronary heart disease.
Conclusions
Blood transfusion does not lead to acute improvement in systemic or regional oxygen utilization and can hamper right ventricular ejection by increasing pulmonary vascular resistance, whereas restoration of circulating blood with colloidal solutions may improve gastric perfusion in septic patients.
Competing interests
None declared
Abbreviations
pHi = intramucosal pH; RBC = red blood cell.
Acknowledgments
Acknowledgement
We are indebted to Frederico Rafael Moreira, Gianni Yanaguibashi and Professor Clovis de Araujo Peres for their statistical analysis.
References
- Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine Practice parameters for hemodynamic support of sepsis in adult patients in sepsis. Crit Care Med. 1999;27:639–660. doi: 10.1097/00003246-199903000-00049. [DOI] [PubMed] [Google Scholar]
- Rashkin MC, Bosken C, Baughman RP. Oxygen delivery in critically ill patients: relationship to blood lactate and survival. Chest. 1985;87:580–584. doi: 10.1378/chest.87.5.580. [DOI] [PubMed] [Google Scholar]
- Weg JG. Oxygen transport in adult respiratory distress syndrome and other acute circulatory problems: relationship of oxygen delivery and oxygen consumption. Crit Care Med. 1991;19:650–657. doi: 10.1097/00003246-199105000-00011. [DOI] [PubMed] [Google Scholar]
- Ronco JJ, Phang PT, Walley KR, Wiggs B, Fenwick JC, Russel JA. Oxygen consumption is independent of changes in oxygen delivery in severe adult respiratory distress syndrome. Am Rev Respir Dis. 1991;143:1267–1273. doi: 10.1164/ajrccm/143.6.1267. [DOI] [PubMed] [Google Scholar]
- Bartlett RH, Dechert RE. Oxygen kinetics: pitfalls in clinical research. J Crit Care. 1990;5:77–80. [Google Scholar]
- Marik P, Sibbald W. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024–3029. [PubMed] [Google Scholar]
- Society of Critical Care Medicine Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- Creteur J, De Backer D, Vincent JL. Monitoring gastric mucosal carbon dioxide pressure using gas tonometry: in vitro and in vivo validation studies. Anesthesiology. 1997;87:504–510. doi: 10.1097/00000542-199709000-00008. [DOI] [PubMed] [Google Scholar]
- Ronco JJ, Phang PT. Validation of an indirect calorimeter to measure oxygen consumption in critically ill patients. J Crit Care. 1991;6:36–41. [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- Sugerman HJ, Davidson DT, Vibul S, Delivoria-Papadopoulos M, Miller LD, Oski FA. The basis of defective oxygen delivery from stored blood. Surg Gynecol Obstet. 1970;131:733–741. [PubMed] [Google Scholar]
- Apstein CS, Dennis RC, Briggs L, Vogeli WM, Fracer J, Valeri CR. Effect of erythrocyte storage and oxyhemoglobin affinity changes on cardiac function. Am J Physiol. 1985;285(suppl):H508–H515. doi: 10.1152/ajpheart.1985.248.4.H508. [DOI] [PubMed] [Google Scholar]
- Epstein CD, Peerless JR, Martin JE, Malangoni MA. Comparison of methods of measurements of oxygen consumption in mechanically ventilated patients with multiple trauma: the Fick method versus indirect calorimetry. Crit Care Med. 2000;28:1363–1369. doi: 10.1097/00003246-200005000-00017. [DOI] [PubMed] [Google Scholar]
- Nunn JF. Pulmonary oxygen consumption. Intensive Care Med. 1996;22:275–276. doi: 10.1007/BF01700446. [DOI] [PubMed] [Google Scholar]
- Marik PE. Gastric intramucosal pH: a better predictor of multi-organ dysfunction syndrome than oxygen-derived variables in patients with sepsis. Chest. 1993;104:225–229. doi: 10.1378/chest.104.1.225. [DOI] [PubMed] [Google Scholar]
- Doglio GR, Pusajo JF, Egurrola A, Bonfigli GC, Parra C, Vetere L, Hernandez MS, Palizas F, Gutierrez G. Gastric mucosal pH as a prognostic index of mortality in critically ill patients. Crit Care Med. 1992;19:1037–1040. doi: 10.1097/00003246-199108000-00011. [DOI] [PubMed] [Google Scholar]
- Fink MP. Gastrointestinal mucosal injury in experimental models of shock, trauma and sepsis. Crit Care Med. 1991;19:627–641. doi: 10.1097/00003246-199105000-00009. [DOI] [PubMed] [Google Scholar]
- Mythen MG, Webb AR. The role of gut mucosal hypoperfusion in the pathogenesis of post-operative organ dysfunction. Intensive Care Med. 1994;20:203–209. doi: 10.1007/BF01704701. [DOI] [PubMed] [Google Scholar]
- Nelson D, Beyer C, Samsel R, Wood LD, Schumacker PT. Pathologic supply dependence of systemic and intestinal O2 uptake during bacteremia in the dog. J Appl Physiol. 1987;63:1487–1489. doi: 10.1152/jappl.1987.63.4.1487. [DOI] [PubMed] [Google Scholar]
- Dietrich KA, Contrad SA, Herbert CA, Levy GL, Romero MD. Cardiovascular and metabolic response to red blood cell transfusion in critically ill volume-resuscitated nonsurgical patients. Crit Care Med. 1990;18:940–944. doi: 10.1097/00003246-199009000-00007. [DOI] [PubMed] [Google Scholar]
- Argwal JB, Paltoo R, Palmer WH. Relative viscosity of blood at varying hematocrits in pulmonary circulation. J Appl Physiol. 1970;29:866–871. doi: 10.1152/jappl.1970.29.6.866. [DOI] [PubMed] [Google Scholar]
- Lamke LO, Liljedahl SO. Plasma volume changes after infusion of various plasma expanders. Resuscitation. 1976;5:85–92. doi: 10.1016/0300-9572(76)90029-0. [DOI] [PubMed] [Google Scholar]
- Shoemaker WC. Comparisons of the relative effectiveness of whole blood transfusions and various types of fluid therapy in resuscitation. Crit Care Med. 1976;4:71–78. doi: 10.1097/00003246-197603000-00006. [DOI] [PubMed] [Google Scholar]
- Cochrane Injuries Group Albumin Reviewers Human albumin administration in critically ill patients: systematic review of randomized controlled trials. Br Med J. 1998;317:235–240. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison SP, Lobo DN. Debate: albumin administration should not be avoided. Crit Care. 2000;4:147–150. doi: 10.1186/cc687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt J, Mueller M, Menges T, Papsdorf M, Hempelmann G. Influence of different volume therapy regimens on regulators of circulation in the critically ill. Br J Anaesthiol. 1996;77:480–487. doi: 10.1093/bja/77.4.480. [DOI] [PubMed] [Google Scholar]
- Boldt J, Mueller M, Heesen M. Influence of different volume therapies and pentoxifyline infusion on circulating soluble adhesion molecules in critically ill patients. Crit Care Med. 1996;24:385–391. doi: 10.1097/00003246-199603000-00005. [DOI] [PubMed] [Google Scholar]
- Boldt J, Heesen M, Mueller M, Papsdorf M, Hempelmann G. The effects of albumin versus hydroxyethyl starch solution on cardiorespiratory and circulatory variables in critically ill patients. Anesth Analg. 1996;83:254–261. doi: 10.1097/00000539-199608000-00010. [DOI] [PubMed] [Google Scholar]
- Forrest DM, Baigorri F, Chittock DR, Spinelli JJ, Russell JA. Volume expansion using pentastarch does not change gastric arterial CO2 gradient or gastric intramucosal pH in patients who have sepsis syndrome. Crit Care Med. 2000;28:2254–2258. doi: 10.1097/00003246-200007000-00012. [DOI] [PubMed] [Google Scholar]
- Marik PE, Iglesias J. Would the colloid detractors please sit down! Crit Care Med. 2000;28:2652–2654. doi: 10.1097/00003246-200007000-00083. [DOI] [PubMed] [Google Scholar]
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E, The Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- Bacher A, Mayer N, Rajek AM, Haider W. Acute normovolaemic haemodilution does not aggravate gastric mucosal acidosis during cardiac surgery. Intensive Care Med. 1998;24:313–321. doi: 10.1007/s001340050573. [DOI] [PubMed] [Google Scholar]


