Abstract
Background
There is limited information on the risk of hospital-acquired coronavirus disease 2019 (COVID-19) among high-risk hospitalized patients after exposure to an infected patient or healthcare worker (HCW) in a nonoutbreak setting.
Methods
This study was conducted at a tertiary care cancer center in New York City from 10 March 2020 until 28 February 2021. In early April 2020, the study institution implemented universal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing at admission and retesting every 3 days through the hospital stay. Contact tracing records were reviewed for all exposures to SARS-CoV-2 positive patients and HCWs.
Results
From 10 March 2020 to 28 February 2021, 11 348 unique patients who were SARS-CoV-2 polymerase chain reaction (PCR) negative at the time of admission underwent 31 662 postadmission tests during their hospitalization, and 112 tested positive (0.98%). Among these, 49 patients housed in semiprivate rooms during admission resulted in 74 close contacts and 14 secondary infections within 14 days, for an overall attack rate of 18.9%. Among those exposed to a roommate undergoing an aerosol-generating procedure (AGP), the attack rate was 35.7%. Whole genome sequencing (WGS) corroborated transmission in 6/8 evaluated pairs. In addition, three transmission events occurred in 214 patients with significant exposure to 105 COVID-19 positive healthcare workers (1.4%).
Conclusions
The overall risk of hospital-acquired COVID-19 is low for hospitalized cancer patients, even during periods of high community prevalence. However, shared occupancy with an unrecognized case is associated with a high secondary attack rate in exposed roommates.
Keywords: SARS-CoV-2, COVID-19, hospital-acquired, cancer
There was low risk of hospital-acquired severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients after direct contact with infected patients or healthcare workers (HCW). There was greater transmission risk during shared room occupancy with exposure to aerosol-generating procedures. Routine testing is not necessary when community rates are low.
Since severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was first reported in the United States, several coronavirus disease 2019 (COVID-19) outbreaks have been reported in acute care settings [1, 2]. Universal testing for SARS-CoV-2 at the time of hospital admission is a recommended strategy to prevent nosocomial SARS-CoV-2 infection when the community prevalence is high. However, individuals early in the infection course may not have a detectable virus, and false-negative results may occur due to analytic reasons or poor sampling technique [3]. Late detection of SARS-CoV-2 in hospitalized patients has been described as the source of several reported outbreaks. Overall, for patients admitted to an acute care setting, the risk of SARS-CoV-2 transmission from other patients or infected healthcare workers is rarely described in a nonoutbreak setting [4–7].
New York City confirmed its first case of COVID-19 on 1 March 2020. The first wave of the epidemic peaked in mid-April, followed by sustained low case counts until 1 October 2020. A second surge soon followed with a peak in January 2021 [8]. As part of the nosocomial risk mitigation strategy, the study institution implemented serial testing of all hospitalized patients every 3 days until discharge and regardless of COVID-19 symptoms. We also recommended biweekly asymptomatic healthcare worker (HCW) testing in inpatient and procedural settings.
This report characterizes the risk of SARS-CoV-2 acquisition in hospitalized cancer patients after direct contact with an infected patient or healthcare worker.
METHODS
Study Methods
Memorial Sloan Kettering Cancer Center (MSKCC) is a 514-bed tertiary care cancer center with 22 417 annual admissions and 160 298 inpatient days in 2020. In total, 57% of beds are in shared occupancy. On 10 March 2020, polymerase chain reaction (PCR) testing for SARS-CoV-2 was made available for patients. Starting on 6 April, all patients admitted to MSKCC were tested on admission, and starting on 13 April all hospitalized patients with an initial negative test were routinely retested every 3 days through their hospital stay.
Patients with confirmed SARS-CoV-2 were transferred to designated COVID-19 units and placed in private rooms under airborne-contact isolation. In the event of an exposure to a confirmed case of SARS-CoV-2 in a patient or HCW, contact tracing was done for all patients. As per Centers for Disease Control and Prevention (CDC) guidelines, significant exposure was defined as being within 6 feet of an infected person for at least 15 minutes [9]. The exposure period was defined as 2 days before symptom onset or 2 days before positive PCR specimen collection if index case was asymptomatic. Exposed roommates of a positive case were placed on droplet precautions for 14 days from last exposure, with the last follow-up date on 28 February 2021. Prior to routine retesting of all hospitalized patients, postexposure testing was done 5 and 12 days after last contact.
All inpatients exposed to an infected HCW were also placed on droplet precautions and post-discharge quarantine for 14 days. Duration of interaction, healthcare worker role, and personal protective equipment (PPE) were assessed for all other staff exposures. Quarantined patients followed the same testing schedule outlined above.
Universal masking for all healthcare workers was introduced on 20 March 2020, followed by universal face shields on 22 April 2020. Starting 11 April, all patients were required to wear a face mask during their visit at the study institution. Masking for inpatients was implemented on 21 December 2020. N95 respirators and face shields were required for healthcare workers for all aerosol-generating procedures (AGPs). For AGPs in shared rooms, the curtains are drawn, all shared equipment is moved from the immediate vicinity, and the roommate dons a surgical mask.
SARS-CoV-2 Reverse Transcription Polymerase Chain Reaction
Testing for SARS-CoV-2 RNA was performed on nasopharyngeal swabs (NPS) or saliva samples. SARS-CoV-2 RNA was detected using either a laboratory-developed test based on the CDC protocol, the Xpert Xpress SARS-CoV-2 test (Cepheid, Sunnyvale, California, USA), the Cobas SARS-CoV-2 test (Roche Molecular Diagnostics, Indianapolis, Indiana, USA) or the TaqPath COVID-19 combo test (Thermo Fischer, Waltham, Massachusetts, USA). Samples were reported as positive per the manufacturers’ instructions or laboratory protocols for the laboratory developed test (LDT). Lab turnaround time from receipt of specimen was 12–24 hours. The cycle threshold (Ct) value for each positive sample was retrieved from each instrument records. Ct values by groups are reported as means with standard deviation. Means between groups were compared using a 2-tailed t test.
SARS-CoV-2 Whole Genome Sequencing
Whole genome sequencing (WGS) was performed on all available samples with a Ct value <30. Total viral nucleic acids was extracted from 200 µL of NPS or saliva samples on the KingFisher Flex Magnetic Particle Processor using the MagMAX Viral/Pathogen Nucleic Acid Isolation Kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Amplicon sequencing was performed following the Artic protocol with version 3 primers (Integrated DNA Technologies [IDT], Coralville, Iowa, USA). Following cDNA synthesis and multiplexed PCR, libraries were prepared for the 2 amplicons pools using the Nextera XT DNA kit and sequencing done on an Illumina Miseq (Illumina, San Diego, California, USA) as paired end (2 × 150 base pair reads).
Bioinformatic Analysis
SARS-CoV-2 lineage assignments was performed using the Pangolin COVID-19 lineage assigner web application (https://pangolin.cog-uk.io/) (ref: PMID: 32669681). Whole genome, single-nucleotide polymorphisms (wgSNPs) analysis was performed using the Bionumerics version 7.6 software (bioMérieux Inc, Austin, Texas, USA). SARS-CoV-2 FASTQ files were imported into the Bionumerics software and mapped to the Wuhan-Hu-1 SARS-CoV-2 reference genome (NC_045512). SNP analysis was performed using the default SNP filtering function with cluster analysis performed using the categorical (SNPs) similarity coefficient and the complete linkage method.
MSKCC Institutional Review Board reviewed the study and granted a Health Insurance Portability and Accountability Act waiver of authorization.
RESULTS
Between 10 March 2020 until 28 February 2021, there were 21 243 admissions, with 13 885 placements in a shared room. Because admission testing was implemented, 441/11 789 patients tested positive at the time of the admission (3.7%). For 11 348 unique patients without a previous positive test, 31 662 post admission tests were done during their hospitalization, and 112 among these tested positive (0.98%). The median time to positive test was 4 days (range 0–70 days).
Secondary Transmission in Roommates After Contact With an Infected Patient
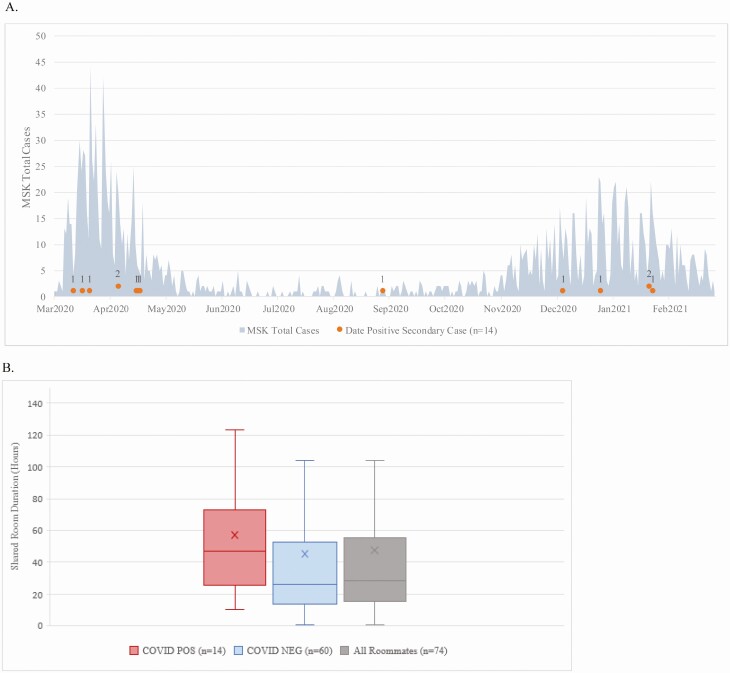
Among 112 patients with first positive test on postadmission testing, 49 had been housed in semi-private rooms before the diagnosis (median = 3 days, 1–34). This resulted in 74 close contact exposures in their roommates, 14 of whom eventually tested positive within two weeks. As shown in Figure 1A, secondary transmission after exposure to newly positive inpatients was primarily identified during NYC surge periods (93%), and the overall secondary attack rate among exposed roommates was 18.9% (Table 1, Figure 1B and 2). 4 secondary transmissions occurred in patients with hematologic malignancies, and 10 in patients with solid tumors. None of the 74 identified close contact encounters had a history of lab-confirmed COVID-19. No intermediary healthcare workers with COVID-19 were an alternate potential source during the roommate overlap period, ruling out healthcare worker-to-patient transmission. Visitors were not permitted during this time.
Figure 1.
A, Timing of 14 secondary cases (gray dots) among exposed roommates depicted against all laboratory confirmed patient cases diagnosed at the study institution. B, Shared room duration by hours with the index case. Abbreviation: MSK, Memorial Sloan Kettering. Color figures available online.
Table 1.
Post-exposure Secondary Attack Rate in Patients After Exposure to an Infected Healthcare Worker (HCW) or Roommate
| HCW | Roommate | |
|---|---|---|
| No transmission | 528 (98.88%) | 60 (81.08%) |
| Secondary infection | 6 (1.12%) | 14 (18.92%) |
| Total exposed | 534 | 74 |
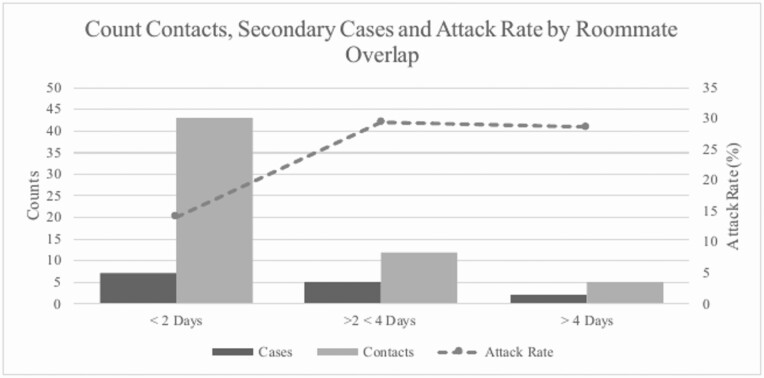
Figure 2.
Attack rate among close contact exposures examined by shared room occupancy duration in days.
Among 49 index cases, the mean age was 68 (range 17–91). There were 11 patients with hematologic malignancies and 38 with solid tumors; 29 patients were male (59.18%). For 74 exposed roommates, most were solid tumor patients (n = 56, 75.68%). There were 40 male and 34 female contacts with average age of 67 years (range 4–89). In 49 index cases, 14 were identified on symptom triggered COVID-19 test, and 35 tested positive on a surveillance test. For the latter, 13 remained asymptomatic, 16 deemed as presymptomatic, and 6 reported symptoms on date of surveillance test. In total, 60 roommates exposed to 41 unique COVID-19 positive index cases did not develop an infection. Collectively, 152 tests were done on the 74 exposed roommates in the 14-day monitoring period.
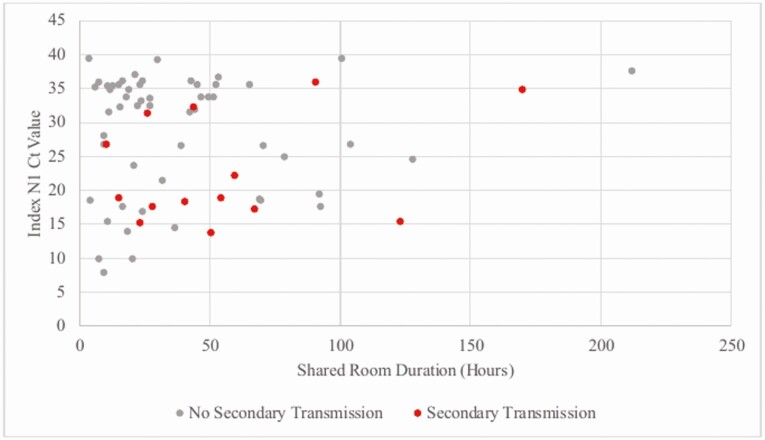
The median time to infection from index case diagnosis in exposed persons was 3 days (range 1–12 days). The exposures for these cases were from 9 symptomatic and 4 asymptomatic index cases. The mean Ct value for target 1 in index patients with no secondary transmission and those with transmission was 28.62 ± 9.20 (SD) and 23.12 ± 8.09 (SD), respectively (t = 1.88, P = .07). There were 4 index cases with Ct value > 30 with secondary transmission to roommates (Figure 3). Secondary cases had similar room overlap with index cases (interquartile range [IQR] 39 hours) compared to close contact who did not convert (IQR 37 hours) (Figure 1B).
Figure 3.
COVID-19 transmission among 74 close contacts by shared room duration and N1 target Ct value of index patient. Abbreviations: COVID-19, coronavirus disease 2019; Ct, cycle threshold.
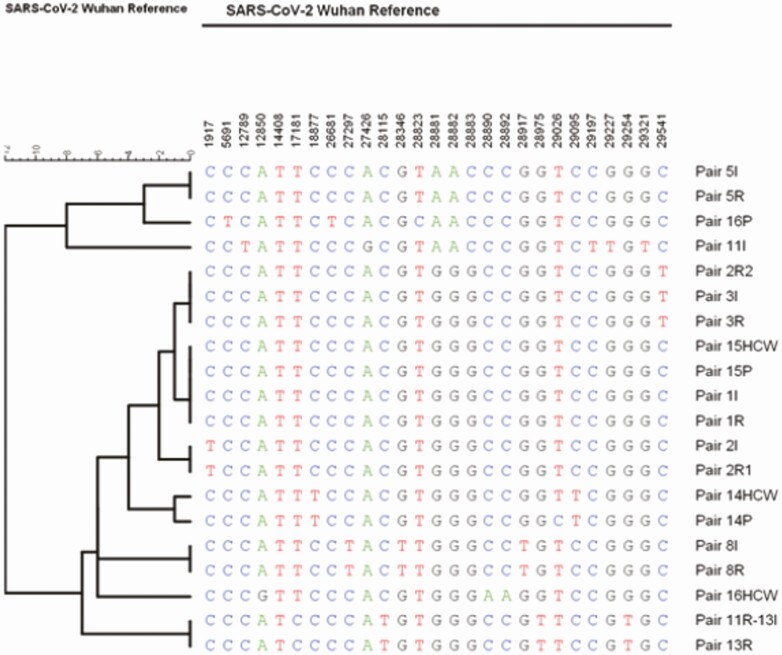
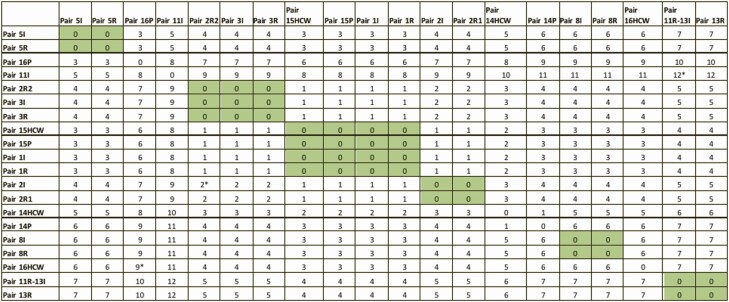
Of 74 total roommates, 14 were exposed to AGPs performed prior to the index case testing positive for COVID-19. Five of 14 patients exposed to AGPs developed infection during the 14-day monitoring period (35.71%) (Table 2). Out of 27 index and secondary cases, whole genome sequencing was done for 22 stored samples that evaluated 8 complete epidemiologically linked pairs (index and roommate). WGS corroborated transmission from the index case in 6/8 infections diagnosed among exposed roommates as shown in Table 3, and Figures 4 and 5. For 2 pairs with SNP differences ≥2, 1 was possibly community acquired 10 days after discharge, and 11 days from last exposure to the index case (pair 2I, 2R2). The probable alternate source of transmission for the other case remains unrecognized (pair 11I, 11R).
Table 2.
Aerosol Generating Procedures (AGP) Performed on Index Patients During Shared Room Occupancy
| AGP | Close Contact Present During AGP | Close Contact Converted to Positive | % |
|---|---|---|---|
| Endotracheal intubation and extubation | 0 | 0 | |
| Bronchoscopy | 0 | 0 | |
| Positive pressure ventilation (BiPAP and CPAP) | 6 | 2 | 33.33% |
| Sputum induction | 0 | 0 | |
| High-flow oxygen | 0 | 0 | |
| Tracheostomy care | 0 | 0 | |
| Nebulizer | 7 | 2 | 28.57% |
| Chest physiotherapy | 1 | 1 | 100% |
| Any AGP | 14 | 5 | 35.71% |
Fourteen exposed close contacts with 5 probable transmission events.
Abbreviations: BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure.
Table 3.
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Pango Lineages of Patient Index (I) cases and Roommates (RM) or Healthcare Worker (HCW) and Patient (P) Pairs
| Patient | Type | Pairs | Lineage |
|---|---|---|---|
| 1I | Index | 1 | B.1 |
| 1R | RM | 1 | B.1 |
| 2I | Index | 2- | B.1.332 |
| 2R1 | RM | 2 | B.1 |
| 2R2 | RM | 2 | B.1 |
| 3I | Index | 3 | B.1 |
| 3R | RM | 3 | B.1 |
| 4I | Index | 4 | B.1 |
| 5I | Index | 5 | B.1.1 |
| 5R | RM | 5 | B.1.1 |
| 6I | Index | 6 | B.1.332 |
| 7I | Index | 7 | B.1.332 |
| 8I | Index | 8 | B.1 |
| 8R | RM | 8 | B.1.115 |
| 9I | Index | 9 | B.1.1.119 |
| 9R | RM | 9 | B.1.1 |
| 10I | Index | 10 | B.1.517 |
| 11I | Index | 11 | B.1.1.222 |
| 11R | RM | 11 | B.1.2 |
| 12I | Index | 12 | B.1.1.222 |
| 13I | Index | 13 | B.1.2 |
| 13R | RM | 13 | B.1.2 |
| 14P | Patient | 14 | B.1 |
| 14HCW | HCW | 14 | B.1 |
| 15P | Patient | 15 | B.1.314 |
| 15HCW | HCW | 15 | B.1 |
| 16P | Patient | 16 | B.1.1.294 |
| 16HCW | HCW | 16 | B.1.517 |
Figure 4.
Phylogenetic tree of available SARS-CoV-2 sequences for each pair of index (I) cases, their roommates (R) or the healthcare worker (HCW) and patient (P). Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 5.
Similarity matrix of all pairs with available sequences. Gray: No Single Nucleotide Polymorphism Differences between pairs.*Pairs with SNP ≥2. Pair 11R-13I represents one patient. Positive after exposure to Index 11 but was briefly housed in a semi-private room prior to Index 11’s detection. Patient was relabeled as Index 13 with roommate exposure. Abbreviations: HCW, healthcare worker; I, index; P, patient; R, roommates; SNP, single nucleotide polymorphism. Color figures available online.
Secondary Transmission in Hospitalized Patients After Contact With an Infected HCW
During the study period, more than 17 000 employees across the study institution were tested, totaling over 100 000 tests. In sum, 105 COVID-19 positive HCWs in the inpatient setting were identified, with 534 patient exposures; 214 exposures were considered high risk and quarantined. The index cases included 62 nurses, 18 physicians or advanced providers, and 25 nursing support staff. Overall, 3 quarantined patients (1.4%) tested positive during the 14 day follow-up period. WGS established transmission in two HCW-patient pairs (Figure 5, Table 3, pair 14, 15); the third did not have adequate viral load for sequencing. Among 320 low risk exposures (<15 minute cumulative interaction), 3 patients tested positive (0.94%). The index cases included 1 nurse and 2 nursing support staff; all 3 were symptomatic. In 1 event the patient was exposed the day before the nurse’s symptom onset during 2 vitals assessments lasting <10 minutes. Exposures to the 2 nursing support staff occurred within 2 days after their symptom onset and included brief interactions to assess patient intake and output during the day, lasting <10 minutes. Two pairs were excluded from WGS due to low viral load, while pair 16 (Figure 5, Table 3) was typed as different lineages with a SNP difference of 9 between the HCW-patient strain. Overall, among the 6 suspected transmission events (1.12%), the median time to detection in patients after last HCW interaction was 5 days (range 1–11 days) and WGS corroborated 2/3 evaluated patient conversions.
Discussion
This study describes an overall low SARS-CoV-2 nosocomial risk during hospitalization despite two distinct regional surges of widespread SARS-CoV-2 community transmission. Most cases of nosocomial infection occurred when community prevalence rates were high [10–12]. High secondary attack rates were observed among those in shared occupancy with an unrecognized case, especially after exposure to AGPs. This clinical scenario merits strong consideration for the routine application of post-exposure prophylactic use of monoclonal antibodies among highly vulnerable patients.
Of 49 infected index cases, 45 tested positive within 14 days of admission with a median of 3 days. Given the median incubation period of COVID-19 is 4–5 days, index cases primarily represented patients exposed in the community. False negative results are possible with SARS-CoV-2 PCR testing due to early undetectable viral loads or sampling technique [3].
Among 74 close contact exposures in the present study, 14 secondary cases were detected, 75% of evaluated pairs were corroborated by WGS. The mean Ct of all index cases was 27.06 ± 9.16, with a median shared room duration of fewer than 2 days with the index case, and was not significantly different between source patients with and without secondary transmission. One potential reason for this observation is that contact tracing was based on the first detection of PCR confirmed COVID-19; however, the overall risk of hospital-acquired COVID-19 might have been influenced by the transmissibility of the source case. The likelihood of intermittent RNA shedding post-acute infection that is incidentally detected on routine surveillance is one potential explanation for the lack of observed transmission. Studies have found a lower secondary clinical attack rate >5 days from symptom onset [13, 14], and prolonged PCR positivity is common in cancer patients [15, 16].
Regarding high secondary attack with AGPs, prior literature on aerosol transmission comes from the 2003 SARS-CoV pandemic [17, 18], and the description of risk in COVID-19 transmission remains limited to case studies [7, 19]. Our study also found a low attack rate from HCWs to patients in the inpatient setting even during close and prolonged contact (1.12%), consistent with observations from other facilities [20].
The risk of acquiring COVID-19 increases with the amount of contact with infected individuals. The most intense exposure between patients or between patients and providers occurs during hospitalization for acute care. Despite the universal adoption of comprehensive testing and PPE practices, several hospital-based COVID-19 outbreaks were reported last year. The pandemic has also caused uncertainty and fear in cancer patients with frequent healthcare-related exposure, with several interrupting their care due to the fear of acquiring COVID-19 and devastating consequences on the overall prognosis. The general secondary attack of SARS-CoV-2 in published studies from healthcare settings is estimated to be around 0.7% [13]. However, this risk is not stratified by the type of exposure within the acute care setting, with most studies describing secondary attack rates in HCW exposed to infected patients.
The availability of highly effective vaccines dramatically reduces the risk of COVID-19 infection and will likely have a considerable impact on nosocomial risk. However, there is still limited experience with vaccine effectiveness in real-world conditions, specifically in cancer patients in whom the vaccine-induced protection may be blunted. The practice of surveillance testing as a risk mitigation strategy is still being followed at many places as community rates decline, and especially among specific high-risk populations with limited protection from the vaccine. Our data shows risk of secondary transmission to be low, and the small number of cases occurred only during surge periods, with 13 of 14 secondary infections occurring from March to May 2020 or December 2020 to February 2021. This suggests that enhanced test-based surveillance of asymptomatic patients is unnecessary even in highly susceptible populations when the overall community risk is low, conserving testing resources and reducing patient burden from repeat testing.
Although collectively our study findings provide reassurance on the low risk of nosocomial acquisition, additional strategies may be needed further to reduce the risk to patients in shared occupancy. Postexposure prophylaxis with monoclonal antibodies is a promising strategy that has proven beneficial among exposed household contacts or nursing home residents [21, 22], and the Food and Drug Administration (FDA) should strongly consider authorizing its use in the hospital setting with comparable attack rates to those observed after household exposures.
Our study has several limitations. First, patients discharged during the monitoring period were only tested if they reported new symptoms, potentially missing asymptomatic infections diagnosed in the outpatient or other setting. Second, contact investigations were based on documented symptom onset or first positive test. Third, not all paired samples were available for WGS to confirm transmission events although the majority that were evaluated revealed no SNP differences. Finally, the impact of COVID-19 vaccination in patients and healthcare workers is not measured as the study dates preceded broad dissemination of vaccines.
In summary, this report demonstrates an overall low risk of hospital-acquired COVID-19 among immunocompromised patients even during high SARS-CoV-2 community prevalence. However, postexposure prophylaxis should be considered for patients in shared occupancy after exposure to an infected hospital roommate. In addition, despite the uncertain vaccine effectiveness in cancer patients, routine testing of asymptomatic patients in oncology settings is unnecessary when the community rates are low.
Notes
Financial support. This work was supported by a National Institutes of Health (NIH)/National Cancer Institute (NCI) Cancer Center Support Grant (P30 CA 008748).
Philanthropic support from Jack and Dorothy Byrne Foundation (to M. K. and N. E. B.).
Potential conflicts of interest. N. E. B. reports grants from GenMark for Clinical Trial Research Funding and personal fees from Roche and Karius for serving on Scientific Advisory Board, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Klompas M, Baker MA, Rhee C, et al. . A SARS-CoV-2 cluster in an acute care hospital. Ann Intern Med. 2021; 174:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ariza-Heredia EJ, Frenzel E, Cantu S, et al. . Surveillance and identification of clusters of healthcare workers with coronavirus disease 2019 (COVID-19): multidimensional interventions at a comprehensive cancer center. Infect Control Hosp Epidemiol 2021; 42:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . Overview of testing for SARS-CoV-2. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. Accessed 21 April 2021.
- 4. Rivett L, Sridhar S, Sparkes D, et al. . Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020; 9: e58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lombardi A, Consonni D, Carugno M, et al. . Characteristics of 1573 healthcare workers who underwent nasopharyngeal swab testing for SARS-CoV-2 in Milan, Lombardy, Italy. Clin Microbiol Infect 2020; 26:1413.e9–1413.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhee C, Baker M, Vaidya V, et al. ; CDC Prevention Epicenters Program. . Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw Open 2020; 3:e2020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong SCY, Kwong RT, Wu TC, et al. . Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect 2020; 105:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. New York City Department of Health COVID-19 Data. 2021. Available at: https://www1.nyc.gov/site/doh/covid/covid-19-data.page. Accessed 14 May 2021.
- 9. Centers for Disease Control and Prevention . Quarantine and Isolation.2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/quarantine.html. Accessed 21 April 2021.
- 10. Asch DA, Sheils NE, Islam MN, et al. . Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021; 181:471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rickman HM, Rampling T, Shaw K, et al. . Nosocomial transmission of coronavirus disease 2019: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin Infect Dis 2021; 72:690–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kluytmans-van den Bergh MFQ, Buiting AGM, Pas SD, et al. . Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open. 2020; 3:e209673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng HY, Jian SW, Liu DP, Ng TC, Huang WT, Lin HH; Taiwan COVID-19 Outbreak Investigation Team. . Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med 2020; 180: 1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishiura H, Linton NM, Akhmetzhanov AR. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis 2020; 93:284–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li N, Wang X, Lv T. Prolonged SARS-CoV-2 RNA shedding: not a rare phenomenon. J Med Virol 2020; 92:2286–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou B, She J, Wang Y, Ma X. Duration of viral shedding of discharged patients with severe COVID-19. Clin Infect Dis 2020; 71:2240–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu I, Li Y, Wong TW, et al. . Evidence of airborne transmission of severe acute respiratory syndrome virus. 2004; 350:1731–9. [DOI] [PubMed] [Google Scholar]
- 18. Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012; 7:e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heinzerling A, Stuckey MJ, Scheuer T, et al. . Transmission of COVID-19 to Health Care Personnel During Exposures to a Hospitalized Patient — Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep 2020; 69:472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baker M, Fiumara K, Rhee C, et al. . Low risk of coronavirus disease 2019 (COVID-19) among patients exposed to infected healthcare workers. Clin Infect Dis 2021; 73:e1878–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Regeneron Pharmaceuticals . Phase 3 prevention trial showed 81% reduced risk of symptomatic SARS-CoV-2 infections with subcutaneous administration of REGEN-COV™ (casirivimab with imdevimab).2021. Available at: https://www.prnewswire.com/news-releases/phase-3-prevention-trial-showed-81-reduced-risk-of-symptomatic-sars-cov-2-infections-with-subcutaneous-administration-of-regen-cov-casirivimab-with-imdevimab-301266366.html. Accessed 21 April 2021.
- 22. Cohen MS, Nirula A, Mulligan M, et al. . Bamlanivimab prevents COVID-19 morbidity and mortality in nursing-home setting. Conference on Retroviruses and Opportunistic Infections webinar. 2021. Available at: https://www.croiconference.org/abstract/bamlanivimab-prevents-covid-19-morbidity-and-mortality-in-nursing-home-setting/. Accessed 25 June 2021.