Abstract
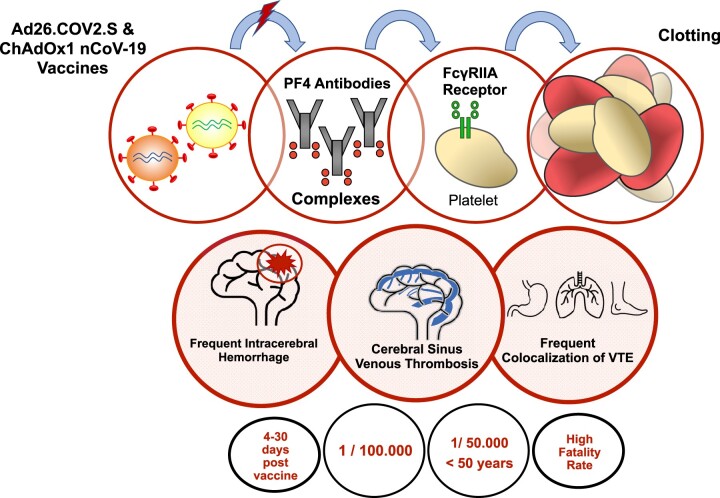
Vaccine-induced immune thrombotic thrombocytopenia (VITT) (also termed thrombosis with thrombocytopenia syndrome or vaccine-induced thrombotic thrombocytopenia or vaccine-induced immune thrombocytopenia) is characterized by (i) venous or arterial thrombosis; (ii) mild-to-severe thrombocytopenia; (iii) positive antiplatelet factor 4 (PF4)–polyanion antibodies or anti-PF4–heparin antibodies detected by the HIT (heparin-induced thrombocytopenia) ELISA; (iv) occurring 5–30 days after ChAdOx1 nCoV-19 (AstraZeneca) or Ad26.COV2.S (Johnson & Johnson/Janssen) vaccination. VITT’s incidence is 1 per 100 000 vaccinated people irrespective of age and up to 1 in 50 000 for people <50 years of age with the AstraZeneca COVID-19 vaccine. The exact mechanism by which adenovirus-vectored COVID-19 vaccines trigger this syndrome is still unclear, as for the increased risk for acute cerebral sinus venous thrombosis and splanchnic vein thrombosis as compared to other locations of venous thrombotic events. VITT is associated with the detection of anti-PF4 antibodies, unrelated to previous use of heparin therapy. PF4 antibodies are thought to activate platelets via the platelet FcγRIIA receptors leading to further platelet activation that causes thrombosis and thrombocytopenia.
Keywords: COVID-19, Vaccine, Thrombosis, Venous thromboembolism, Cerebral sinus venous thrombosis, Thrombocytopenia vaccine-induced immune thrombotic thrombocytopenia, PF4
Graphical Abstract
Graphical Abstract.
More than a year after the onset of the COVID-19 pandemic, most of countries worldwide are still struggling with a surge of infections and thigh restrictions, as vaccination campaigns have been slower than in other countries such as Israel, the UK, and the USA. Making matters worse, doubts and scepticism about AstraZeneca and Johnson & Johnson/Janssen COVID-19 vaccines have been voiced following reports of unusual thrombotic events that amplified and reinforced vaccine hesitancy. Both transatlantic drug regulators and expert panels promptly encouraged patients to proceed with vaccination as the incidence was extremely rare and the vaccine’s benefits outweighed the risks. However, major discrepancies among European government and vaccine policies have emerged over the past few weeks. French heath authority restricted Oxford/AstraZeneca's COVID-19 vaccine for people aged 55 years and over; the UK advised on 7 May 2021 that under 40 should be offered an alternative when possible and finally Germany, that first banned AstraZeneca for the elderly due to a lack of trial data, relaxed on 6 May 2021 previous age restriction, and said it would give the shot to anyone who wants it.
Many questions remain unanswered as more evidence is needed to assess phenotypes, risk factors, natural history, and both early detection and management of the vaccine-induced immune thrombotic thrombocytopenia (VITT) syndrome. Gaps in knowledge include the exact mechanisms by which adenovirus-vectored COVID-19 vaccines trigger VITT, the mechanisms responsible for an increased risk of thrombosis in unusual locations such as acute cerebral sinus venous thrombosis (CSVT) and splanchnic vein thrombosis, length of treatment and specific risk factors other than previous adenoviral vaccine exposure. The median age in Africa is 19.7, Asia is 32, and Latin America 31, while it is 42.5 and 38.6 in Europe and North America, respectively.1 In the race to control the pandemic and reach herd immunity, are age restrictions based on policies in Western countries applicable to other areas of the world that suffer from deadly new surges?
Vaccine-induced immune thrombotic thrombocytopenia: definition
COVID-19 vaccine-related thrombotic and haemorragic adverse events include two distinct entities: (i) VITT with antiplatelet factor 4 (PF4)–polyanion antibodies and (ii) vaccine-induced immune thrombocytopenia (VIITP) or immune thrombocytopenia (ITP) defined as a secondary post vaccine immune thrombocytopenia and usually without antibodies to PF4.2,3 An algorithm for the management of this syndrome was proposed on the basis of immunoassays detecting anti-PF4–heparin antibodies.4
VITT (also termed thrombosis with thrombocytopenia syndrome by the CDC and FDA or vaccine-induced thrombotic thrombocytopenia or vaccine-induced immune thrombocytopenia) is characterized by (i) venous or arterial thrombosis; (ii) mild-to-severe thrombocytopenia; (iii) positive anti-PF4–polyanion antibodies or anti-PF4–heparin antibodies detected by the HIT (heparin-induced thrombocytopenia) ELISA; (iv) occurring 5–30 days after ChAdOx1 nCoV-19 (AstraZeneca) or Ad26.COV2.S (Johnson & Johnson/Janssen) vaccination.5 This definition stands for the classical and typical VITT syndrome described in initial reports6–13 (Table 1). Apart from the ‘typical VITT syndrome’ that associates CSVT and low platelet count, healthcare professionals should be aware of the high frequency of other thrombosis sites such as jugular vein thrombosis and splanchnic vein thrombosis (Table 1). Concomitant or secondary bleeding and intracerebral haemorrhage in particular, is a frequent feature observed in VITT patients.
Table 1.
Summary of the reported cases of vaccine-induced immune thrombotic thrombocytopenia
| Reference | First published | Vaccine | Country | Number of cases | Sex | Age, mean (range) | Days after vaccination, mean (range) | Thrombotic events | Concomitant and/or secondary haemorrhage | Platelet count ×109 L, mean (range) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wolf11 | 9 April 2021 | AZ | Germany | 3 | 3 F | 34.7 (22–36) | 12.3 (7–17) | 3 CVST | No | 75 (60–92) | Fatal 0% |
| Schultz6 | 9 April 2021 | AZ | Norway | 5 |
|
40.8 (32–54) | 8.4 (7–10) |
|
Yes (4 intracerebral haemorrhages) | 27 (10–70)b | Fatal 60% |
| Greinacher7 | 9 April 2021 | AZ | Germany & Austria | 11 |
|
36 (22–49) | 9.3 (5–16) |
|
Yes (1 gastrointestinal bleeding, 1 intracranial haemorrhage) | 20 (9–107)b | Fatal 55% |
| Muir13 | 14 April 2021 | JJ | USA | 1 | 1F | 48 | 14 | CVST + SVT | Yes (intracerebral haemorrhage) | 13a | Critically ill at the time of this report |
| Scully8 | 16 April 2021 | AZ | UK | 23 |
|
46 (21–77) | 12.4 (6–24) |
|
Yes (1 patient with haemorragic symptoms only, 1 adrenal haemorrhage) | 45.2 (7–113) b | Fatal 30% |
| Blauenfeldt10 | 20 April 2021 | AZ | Denmark | 1 | 1F | 60 | 7 | Right middle cerebral artery infarct | Yes (adrenal haemorrhage and a subcapsular renal hematoma) | 118a/5 b | Fatal |
| Tiede12 | 28 April 2021 | AZ | Germany | 5 | 5F | 58.6 (41–63) | 8.4 (5–11) |
|
Yes (2 intracerebral haemorrhages) | 49.2 (12–105)a | Fatal 0% |
| See9 | 30 April 2021 | JJ | USA | 12 | 12F | (18–60) | (6–15) |
|
Yes (7 intracerebral haemorrhages) | (9–127)b | Fatal 25% |
At the time of admission.
Nadir values.
AZ, ChAdOx1 nCoV-19 (AstraZeneca); CSVT, cerebral sinus venous thrombosis; DVT, deep vein thrombosis; JJ, Ad26.COV2.S (Johnson & Johnson/Janssen); PE, pulmonary embolism; PF4, platelet factor 4; SVT, splanchnic vein thrombosis; VITT, vaccine-induced immune thrombotic thrombocytopenia.
The whole spectrum of the VITT syndrome is not yet fully elucidated as this may include haemorragic events only,8 thrombotic microangiopathy,12 thrombocytopenic purpura,14 thrombocytopenia only, mildly elevated D-dimer, >30 days post vaccine events, specific second dose phenotypes, etc. The pathognomonic factor in VITT is positive anti-PF4–polyanion antibodies and functional assays confirming a PF4-dependent platelet activation should be performed in case of thrombotic and/or bleeding events after COVID-19 vaccination. Comparing 43 samples tested for VITT using 10 different assays Platton et al.15 showed that a HIT ELISA should be used in the diagnostic testing of VITT. The authors emphasized the fact that (i) ELISA are not widely available in diagnostic laboratories; (ii) no single ELISA method detected all possible/probable VITT cases; and (iii) if a single ELISA test is negative, a second ELISA or a platelet activation assay should be considered in case of strong clinical suspicion. As these assays may not be specific for VITT-related antibodies,16,17 Handtke et al.18 proposed a widely applicable and rapid functional whole-blood flow cytometry test for the detection of platelet-activating anti-PF4 associated with VITT after ChAdOx1 nCov-19 vaccination.
The CDC COVID-19 Vaccine Task Force released on 12 May 2021, its own definition of thrombosis with thrombocytopenia syndrome (TTS) that better depicts the typical/atypical sites of thrombosis.19 Tier 1 TTS case include (i) thrombosis in an unusual location including cerebral venous sinuses, portal vein, splenic vein, and other rare venous and arterial thromboses, (ii) thrombocytopenia (platelet count <150 000 per microliter), and (iii) while positive heparin–PF4 ELISA HIT antibody is only supportive, but not required. Tier 2 TTS case includes thrombosis in a common location only (e.g. venous thromboembolism, axillary vein thrombosis, deep vein thrombosis, and pulmonary embolism) and excludes isolated acute myocardial infarction or ischaemic stroke, (ii) thrombocytopenia (platelet count <150 000 per microliter), and (iii) positive heparin–PF4 ELISA HIT antibody.
Finally, The Platelet Immunology Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis launched on June 2021, an online registry collecting data for the purposes of understanding the range of presentations of patients suspected of COVID vaccine-related thrombosis and/or thrombocytopenia.20
Vaccine-induced immune thrombotic thrombocytopenia: incidence
VITT’s incidence rate has been a matter of ongoing updates with fluctuating estimates since the number of events is small. Furthermore the number of cases may be underreported, varies between countries, adenovirus-vectored vaccine type and age groups and finally increasing volumes of data being released from drug-safety regulators. Smadja et al.21 reported 639 thrombotic events [334 venous thrombotic events (VTE), 308 arterial thrombotic events (ATE), and 4 concomitants ATE, and VTE] for the Oxford/AstraZeneca's COVID-19 vaccine until 16 March 2021, using the VigiBase databank. Six patients experienced unexpected CSVT with further five cases associated with thrombocytopenia. On 13 April 2021, the FDA and CDC reported six cases of rare and severe VTE with thrombocytopenia out of more than 6.8 million J&J doses administered with a further press release on 23 April 2021, announcing 15 cases of VITT on top of the original six reported cases.22 All these cases occurred in women between the ages of 18 and 59 years, with a median age of 37 years. Data from Denmark and Norway evidenced 59 VTE and 7 cerebral venous thrombosis among 281 264 people in the first 28 days after a first vaccination with ChAdOx1-S from 9 February 2021 to 11 March 2021.23 Altogether, 11 excess VTE per 100 000 vaccinations and 2.5 excess cerebral venous thrombosis events per 100 000 vaccinations were evidenced. This translates to one excess case of VTE every 9090 vaccinations and one excess case of CSVT in every 40 000 people vaccinated. Data from the MHRA in the UK on 9 July 2021 reported 405 cases (208 women, 195 men, and 2 unknown) of major thromboembolic events and the overall case fatality rate was 18% following vaccination with COVID-19 Vaccine AstraZeneca.24 The MHRA reported on 9 July 2021, an overall incidence of VITT after first or unknown doses of 1.48 per 100 000 doses and emphasizes a higher reported incidence rate in the younger adult age groups compared to the older groups. As of 7 July 2021, the risk of VITT in Australia is estimated at around 2.6 per 100 000 in those <60 years; and 1.6 per 100 000 in those ≥60 years.25 Published estimate of the incidence of VITT is 1 per 100 000 vaccinated with the AstraZeneca COVID-19 vaccine in Canada.26 The European Medicines Agency confirmed that VITT occurs in 1 in 100 000 AstraZeneca-vaccinated people irrespective of age27 and up to 1 in 50 000 for those under 50 years of age.28
Using the data from the MHRA (UK) and the US CDC, Bikdeli et al.29 reported the rate of CVST associated with AstraZeneca and Johnson & Johnson vaccines vs. those occurring after COVID-19, and the estimated incidence rates in the US population. As of 14 April 2021, the weighted average rate of CVST in the US population for the months of March and April 2018 was 2.4 per million (99% CI: 2.1–2.6 per million), 207.1 per million in COVID-19 patients (99% CI: 23.3–757.7 per million), and 3.6 per million (99% CI: 2.7–4.8 per million) for vaccine recipients.
Vaccine-induced immune thrombotic thrombocytopenia: pathophysiology
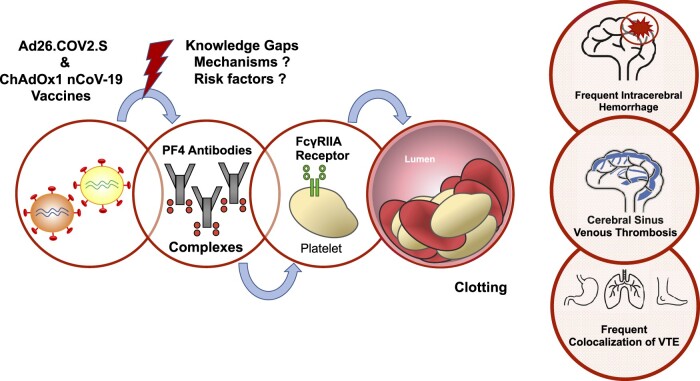
The exact mechanism by which adenovirus-vectored COVID-19 vaccines trigger VITT is still unclear, as for the increased risk for acute CSVT as compared to other VTE topography (Figure 1). VITT is associated with the detection of anti-PF4 antibodies, unrelated to previous use of heparin therapy and face high similarities with autoimmune heparin-induced thrombocytopenia (aHIT). AHIT is a heparin-independent platelet activation process previously described in patients with positive anti-PF4–polyanion antibodies.30 The hallmark of aHIT is antibodies to the PF4 complex that cause thrombocytopenia and thrombosis through platelet activation. The pathogenesis of VITT is thought to involve a FcγRIIA receptors pathway with circulating PF4 antibodies complexes binding platelets but also monocytic cells.31 Huynh et al. published on July 2021 further evidence regarding VITT-mediated platelet activation. VITT patients had anti-PF4 antibodies that bounded to a highly restricted site on PF4 (eight surface amino acids) corresponding to the heparin-binding site.32 These data confirmed that VITT antibodies can mimic the effect of heparin by binding to a similar site on PF4, allowing PF4 tetramers to cluster and form immune complexes, which in turn cause FcγRIIa-dependent platelet activation. Activation of FcγRIIA receptors is known to cause cell monocytic activation, platelet activation, plasma membrane remodelling, phosphatidylserine exposure, P-selectin’s platelet expression, secretion of alpha granules containing PF4 and release of procoagulant microparticles (MPs), leading to further platelet activation that causes thrombosis and thrombocytopenia.31 Increased levels of platelet-leukocyte aggregates were observed during COVID-19 infection, and this emphasized the key role of activated platelets in the direct stimulation of inflammatory cell function.33 Platelet-leukocyte aggregates form via platelet surface expression of P-selectin, contained within α-granules that fuse with the cell membrane following platelet stimulation, binding to leukocyte P-selectin glycoprotein ligand-1. Previous works have demonstrated that the swift accumulation of tissue factor (TF) into developing thrombi in vivo is dependent upon MP P-selectin glycoprotein ligand 1 and platelet P-selectin.34 Moreover, FcγRIIA could also contribute to endothelial cell activation and the acquisition of prothrombotic, proadhesive, and proinflammatory properties by the endothelium layer.
Figure 1.
Model for VITT. We postulate a simplified model for the pathogenesis of VITT according to current evidence. One, adenovirus-vectored COVID-19 vaccines trigger the production of antiPF4–polyanion antibodies. The precise pathogenesis for the immune response and which components (adenoviral sequence, spike protein, other component) of the Ad26.COV2.S and ChAdOx1 nCoV-19 may be held responsible for the production of anti-PF4 antibodies remain unknown. Two, circulating PF4 antibodies complexes bind platelets and monocytic cells. Three, activation of FcγRIIA receptors causes cell monocytic activation, platelet activation, plasma membrane remodelling, phosphatidylserine exposure, P-selectin’s platelet expression, secretion of alpha granules containing PF4 and release of procoagulant microparticles, leading to further platelet activation that causes thrombosis and thrombocytopenia. Healthcare professionals should be aware of the high frequency of other thrombosis sites most likely to include jugular vein thrombosis, pulmonary embolism, deep vein thrombosis and splanchnic vein thrombosis. Concomitant or secondary bleeding and/or intracerebral haemorrhage is a frequent feature observed in VITT patients. PF4, platelet factor 4; VITT, vaccine-induced immune thrombotic thrombocytopenia; VTE, venous thrombotic events.
McGonagle et al.35 proposed that local tissue microtrauma, local microbleeding and immune cell activity at the site of adenovirus injection may bring viral DNA and PF4 together. Antigen-presenting cells uptake, then memory B-cell engagement in the regional lymphnodes, with substantially increased PF4 autoantibody production may lead in rare cases to autoimmune disease, especially in younger subjects. Greinacher et al. recently advocated the following sequence of events to mediate VITT: (i) ChAdOx1 nCoV-19 vaccine constituents form antigenic complexes with PF4, (ii) EDTA increases microvascular permeability, and (iii) vaccine components cause acute inflammatory reactions. Antigen formation in a proinflammatory milieu offers an explanation for anti-PF4 antibody production. High-titer anti-PF4 antibodies activate platelets and induce neutrophil activation and NETs’ formation, fuelling the VITT prothrombotic response.36
The potential for multimodality pathways in VITT pathogenesis remains to be addressed. Furthermore, the reason for an increased risk for acute CSVT as compared to other VTE topography has not been yet elucidated.
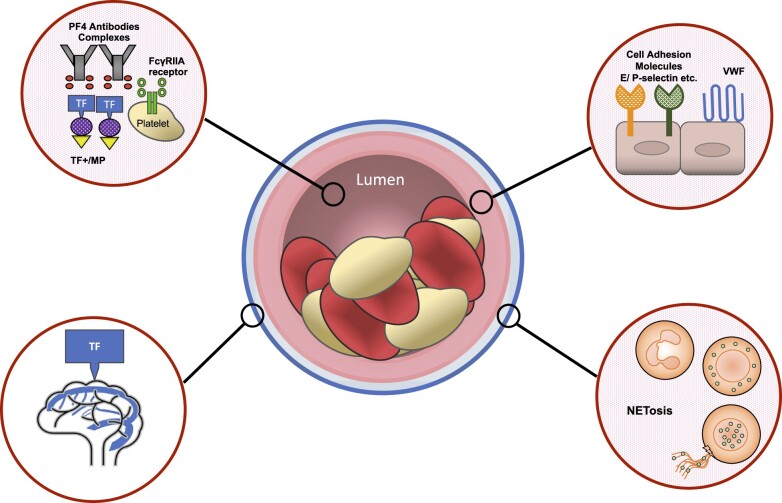
Several hypotheses deserve further investigations (Figure 2):
Figure 2.
Future research in VITT. The molecular basis and multimodality pathways in VITT pathogenesis have not yet been fully elucidated. Future studies should specifically address the following points. (i) Which components (adenoviral sequence, spike protein, other component) of the Ad26.COV2.S and ChAdOx1 nCoV-19 sre responsible for the production of anti-PF4 antibodies. (ii) The role for microparticles in VITT. (iii) The role for a TF-dependent pathway. (iv) The precise mechanisms of a likely pancellular activation including neutrophil activation and NETosis burst, endothelial activation, platelet activation, and monocyte activation. PF4, platelet factor 4; TF, tissue factor); VITT, vaccine-induced immune thrombotic thrombocytopenia.
The precise pathogenesis for the immune response and which components (adenoviral sequence, spike protein, other components) of the Ad26.COV2.S and ChAdOx1 nCoV-19 may be held responsible for the production of anti-PF4 antibodies remain largely unclear.
VITT is an age-related process with predisposing risk factors still to be determined.
The role for MPs in VITT. We recently formulated the hypothesis that the pathogenesis of VITT and high incidence of CSVT may be explained by the key role of platelet and leukocyte-derived MPs37 released upon drastic cell stimulation as witnessed during SARS-Cov2 infection.33,38 Indeed, previous reports acknowledged an increased level of procoagulant circulating MPs and a distinct phenotype of circulating PF4-bearing MPs and TF-bearing MPs in HIT.39–41 Such hypothesis deserves to be tested by measuring MPs in the plasma of VITT patients.
The role for a TF-dependant pathway. Indeed, TF exhibits a nonuniform tissue distribution and is highly expressed in the brain.42 Cerebral microvascular thrombogenesis was further evidenced as an endothelial cell-associated TF response in venules, but not arterioles,43 and platelet–neutrophil interaction triggered by HIT antibodies is known to activate vascular endothelium.44 TF activates factor VII and therefore the extrinsic pathway of the coagulation cascade, resulting in the generation of thrombin that cleaves soluble fibrinogen to insoluble fibrin and activates platelets via protease-associated receptors 1 and 4.45 Altogether, these data support a close interplay between a TF-dependant pathway and VITT-induced CSVT.
VITT is likely to result in a pancellular activation with platelets’ activation, monocyte activation via Fc receptors, endothelial activation with TF expression, and neutrophil activation (NETs) and burst.36
Anti-PF4/heparin antibodies are detected in 3.1–4.4% of healthy subjects, in 8–17% of medical and surgical patients treated with heparin, and up 27–61% after cardiac surgery.31 It is estimated that only 5–30% of patients with anti-PF4/heparin antibodies develop HIT.46 To date, the precise incidence of post vaccine anti-PF4 antibodies remains unknown as for the incidence of patients with positive aHit complex that will develop VITT. Pre-existing antibodies may be part of the foundation, but not enough to determine who will and who will not experience VITT.
Based on the current evidence, VITT events are a rare condition. What about post-vaccine thrombocytopenia and/or anti-PF4/polyanion antibodies? Sørvoll et al. reported low prevalence of both thrombocytopenia and antibodies to PF4/polyanion complexes among 492 health care workers recently vaccinated with the first dose of AstraZeneca COVID-19 vaccine.47 Anti-PF4/polyanion antibodies without platelet-activating properties were only detected in 6 individuals, all with normal platelet counts. While recent guidelines addressed both diagnostic and therapeutic algorithm in patients with thrombocytopenia/thrombosis following vaccination,4,48 there is no recommendation of specific testing in case of mild-to-moderate general post-vaccine symptoms. Even with 60% of the subjects reporting side effects (e.g. fever, headache, and fatigue) and up to >40% reporting moderate to severe symptoms, the report by Sørvoll et al. contests extensive laboratory testing for thrombocytopenia and/or anti-PF4 antibodies in case of inflammatory symptoms with regard to the low probability for anti-PF4 antibody detection and the occurrence of VITT. Thiele et al. determined the frequency of anti-PF4/polyanion antibodies in healthy vaccinees and platelet-activating properties after vaccination with ChAdOx1 nCoV-19 (AstraZeneca) or BNT162b2 (BioNTech/Pfizer).49 Only 19 of 281 participants tested positive for anti-PF4/polyanion antibodies and none had platelet activation. Positive PF4/polyanion can occur with both mRNA- and adenoviral vector-based vaccines, but the majority of these antibodies have either no or minor clinical relevance.
Vaccine-induced immune thrombotic thrombocytopenia: management
Given its similarities with HIT, the current management of VITT include (i) avoidance of platelet transfusions and heparin products, (ii) intravenous immunoglobulin (IVIg) 1 g/kg body weight for 2 days,48,51–53 and (iii) anticoagulation with non-heparin anticoagulants such as argatroban, fondaparinux, or direct oral anticoagulants. Mimicking HIT management,54 current guidelines regarding platelet transfusion in VITT recommend that prophylactic platelet transfusions should be avoided due to the risk of progression of thrombotic symptoms.5,55 In case of intracerebral haemorrhage, perioperative transfusion to correct hypofibrinogenemia and thrombocytopenia are recommended. Despite the lack of data, platelet transfusion may preferably be administered after IVIg. Anticoagulation should involve a multidisciplinary team discussion (haematologist, vascular neurologist, radiologist, and neurosurgeon) and repeated cerebral imaging performed in case of CSVT and/or marked thrombocytopenia as this condition is associated with increased risk of intracerebral haemorrhage. Other therapeutic option may rely on the use of inhibitors of Bruton tyrosine kinase (Btk) approved for B-cell malignancies as they target multiple pathways downstream FcyRIIA-mediated Btk activation such as platelet aggregation, dense granule secretion P-selectin expression and the formation of leuko-platelet aggregates.56 There is limited evidence regarding the additive value and benefit of steroids whereas plasma exchange may be effective for the treatment of refractory.57,58
Conclusion
VITT is a rare complication that should be interpreted in the context of a global pandemic that has caused more than 4.1 million deaths on July 2021. Future research must address the precise mechanisms and molecular pathways triggering the production of anti-PF4 antibodies and the risk-benefit assessments for specific groups of age and sex.
Lead author biography

Dr. Benjamin Marchandot is a general cardiologist in the Cardiac Care Unit at Strasbourg University Hospital, France. He is a member of GERCA (Groupe pour l’Enseignement et la Recherche Cardiovasculaire en Alsace), a multidisciplinary research group that is involved in the field of cardiovascular disease, thrombosis, and haemostasis.
Authors’ contributions
B.M. and O.M. conceived and designed the research. B.M., A.C., and O.M drafted the manuscript. A.T., L.S., and L.G made critical revision of the manuscript for key intellectual content.
Conflict of interest: The authors declare that there is no conflict of interest regarding the publication of this article. O.M. received institutional research grants from Fondation Coeur et Vaisseaux, AstraZeneca, and Boehringher Ingelheim. The manuscript has been read and approved for submission by all authors. All the authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. All the authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Data availability: No new data were generated or analysed in support of this research.
Contributor Information
Benjamin Marchandot, Division of Cardiovascular Medicine, Nouvel Hôpital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, Strasbourg 67000, France.
Anais Curtiaud, Division of Cardiovascular Medicine, Nouvel Hôpital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, Strasbourg 67000, France.
Antonin Trimaille, Division of Cardiovascular Medicine, Nouvel Hôpital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, Strasbourg 67000, France; INSERM (French National Institute of Health and Medical Research), UMR 1260, Regenerative Nanomedicine, FMTS, Strasbourg 67000, France.
Laurent Sattler, Haematology and Haemostasis Laboratory, Centre for Thrombosis and Haemostasis, Nouvel Hôpital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, Strasbourg 67000, France.
Lelia Grunebaum, Haematology and Haemostasis Laboratory, Centre for Thrombosis and Haemostasis, Nouvel Hôpital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, Strasbourg 67000, France.
Olivier Morel, Division of Cardiovascular Medicine, Nouvel Hôpital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, Strasbourg 67000, France; INSERM (French National Institute of Health and Medical Research), UMR 1260, Regenerative Nanomedicine, FMTS, Strasbourg 67000, France.
References
- 1. United Nations Department of Economic and Social Affairs Population Dynamics. Population data. https://population.un.org/wpp/Download/Standard/Population/ (8 May 2021).
- 2. Pishko AM, Bussel JB, Cines DB. COVID-19 vaccination and immune thrombocytopenia. Nat Med 2021;27:1145–1146. [DOI] [PubMed] [Google Scholar]
- 3. Pishko AM, Cuker A. Thrombosis after vaccination with messenger RNA-1273: is this vaccine-induced thrombosis and thrombocytopenia or thrombosis with thrombocytopenia syndrome? Ann Intern Med 2021;M21–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nazy I, Sachs UJ, Arnold DM, McKenzie SE, Choi P, Althaus K, Ahlen MT, Sharma R, Grace RF, Bakchoul T. Recommendations for the clinical and laboratory diagnosis of vaccine-induced immune thrombotic thrombocytopenia (VITT) for SARS-CoV-2 infections: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost 2021;19:1585–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Makris M, Pavord S, Lester W, Scully M, Hunt BJ. Vaccine-induced immune thrombocytopenia and thrombosis (VITT). Res Pract Thromb Haemost 2021;5. doi:10.1002/rth2.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, Wiedmann M, Aamodt AH, Skattør TH, Tjønnfjord GE, Holme PA. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, Goldblatt D, Kotoucek P, Thomas W, Lester W. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, Streiff MB, Rao AK, Wheeler AP, Beavers SF, Durbin AP, Edwards K, Miller E, Harrington TA, Mba-Jonas A, Nair N, Nguyen DT, Talaat KR, Urrutia VC, Walker SC, Creech CB, Clark TA, DeStefano F, Broder KR. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA 2021;325:e217517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost 2021. doi:10.1111/jth.15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after "COVID-19 Vaccine AstraZeneca" exposure. J Clin Med 2021;10:1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tiede A, Sachs UJ, Czwalinna A, Werwitzke S, Bikker R, Krauss JK, Donnerstag FG, Weißenborn K, Höglinger GU, Maasoumy B, Wedemeyer H, Ganser A. Prothrombotic immune thrombocytopenia after COVID-19 vaccine. Blood 2021;138:350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med 2021;384:1964–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Candelli M, Rossi E, Valletta F, De Stefano V, Franceschi F. Immune thrombocytopenic purpura after SARS-CoV-2 vaccine. Br J Haematol 2021. doi:10.1111/bjh.17508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Platton S, Bartlett A, MacCallum P, Makris M, McDonald V, Singh D, Scully M, Pavord S. Evaluation of laboratory assays for anti-Platelet Factor 4 antibodies after ChAdOx1 nCOV-19 vaccination. J Thromb Haemost 2021. doi:10.1111/jth.15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vayne C, Rollin J, Gruel Y, Pouplard C, Galinat H, Huet O, Mémier V, Geeraerts T, Marlu R, Pernod G, Mourey G, Fournel A, Cordonnier C, Susen S. PF4 immunoassays in vaccine-induced thrombotic thrombocytopenia. N Engl J Med 2021;385:376–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reilly-Stitt C, Kitchen S, Jennings I, Horner K, Jones R, Makris M, Walker ID. Anti-PF4 testing for vaccine-induced immune thrombocytopenia and thrombosis and heparin induced thrombocytopenia: results from a UK National External Quality Assessment Scheme exercise April 2021. J Thromb Haemost 2021. doi:10.1111/jth.15423. [DOI] [PubMed] [Google Scholar]
- 18. Handtke S, Wolff M, Zaninetti C, Wesche J, Schönborn L, Aurich K, Ulm L, Hübner NO, Becker K, Thiele T, Greinacher A. A flow cytometric assay to detect platelet-activating antibodies in VITT after ChAdOx1 nCov-19 vaccination. Blood 2021;137:3656–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shimabukuro T. CDC COVID-19 Vaccine Task Force Vaccine Safety Team. Update: Thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 vaccination Advisory Committee on Immunization Practices (ACIP), 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/07-COVID-Shimabukuro-508.pdf (13 May 2021).
- 20. Choi PY, Grace RF, Therese Ahlen M, Nazy I, Sachs UJ, Arnold DM, McKenzie SE, Althaus K, Sharma R, Bakchoul T. The SSC platelet immunology register of VITT and VIITP: Toward standardization of laboratory and clinical parameters. J Thromb Haemost 2021. doi:10.1111/jth.15402. [DOI] [PubMed] [Google Scholar]
- 21. Smadja DM, Yue QY, Chocron R, Sanchez O, Lillo-Le Louet A. Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur Respir J 2021;58:2100956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. FDA and CDC Lift Recommended Pause on Johnson & Johnson (Janssen) COVID-19 Vaccine Use Following Thorough Safety Review. https://www.cdc.gov/media/releases/2021/fda-cdc-lift-vaccine-use.html (8 May 2021).
- 23. Pottegård A, Lund LC, Karlstad Ø, Dahl J, Andersen M, Hallas J, Lidegaard Ø, Tapia G, Gulseth HL, Ruiz PL, Watle SV, Mikkelsen AP, Pedersen L, Sørensen HT, Thomsen RW, Hviid A. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ 2021;373:n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Research and analysis Coronavirus vaccine—weekly summary of Yellow Card reporting, updated 9 July 2021. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting (17 July 2021).
- 25. An update from the Australian Technical Advisory Group on Immunisation (ATAGI) following their weekly meeting on 7 July 202. https://www.health.gov.au/news/atagi-update-following-weekly-covid-19-meeting-7-july-2021 (17 July 2021).
- 26. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Recommendations on the use of COVID-19 vaccines. https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/recommendations-use-covid-19-vaccines-en.pdf (8 May 2021).
- 27. European Medicines Agency. AstraZeneca’s COVID-19 vaccine: benefits and risks in context. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-benefits-risks-context (8 May 2021).
- 28. European Medicines Agency. Annex to Vaxzevria Art.5.3—visual risk contextualisation. https://www.ema.europa.eu/en/documents/chmp-annex/annex-vaxzevria-art53-visual-risk-contextualisation_en.pdf (8 May 2021).
- 29. Bikdeli B, Chatterjee S, Arora S, Monreal M, Jimenez D, Krumholz HM, Goldhaber SZ, Elkind MSV, Piazza G. Cerebral venous sinus thrombosis in the US population, after adenovirus-based SARS-CoV-2 vaccination, and after COVID-19. J Am Coll Cardiol 2021. doi:10.1016/j.jacc.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J ThrombHaemost 2017;15:2099–2114. [DOI] [PubMed] [Google Scholar]
- 31. Nevzorova TA, Mordakhanova ER, Daminova AG, Ponomareva AA, Andrianova IA, Le Minh G, Rauova L, Litvinov RI, Weisel JW. Platelet factor 4-containing immune complexes induce platelet activation followed by calpain-dependent platelet death. Cell Death Discov 2019;5:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopenia. Nature 2021. doi:10.1038/s41586-021-03744-4. [DOI] [PubMed] [Google Scholar]
- 33. Canzano P, Brambilla M, Porro B, Cosentino N, Tortorici E, Vicini S, Poggio P, Cascella A, Pengo MF, Veglia F, Fiorelli S, Bonomi A, Cavalca V, Trabattoni D, Andreini D, Omodeo Salè E, Parati G, Tremoli E, Camera M. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic Transl Sci 2021;6:202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med 2003;197:1585–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McGonagle D, De Marco G, Bridgewood C. Mechanisms of immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection. J Autoimmun 2021;121:102662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greinacher A, Selleng K, Wesche J, Handtke S, Palankar R, Aurich K, Lalk Karen Methling M, Völker U, Hentschker C, Michalik S, Steil L, Schönborn L, Beer M, Franzke K, Rangaswamy C, Mailer RK, Thiele T, Kochanek S, Krutzke L, Siegerist F, Endlich N, Warkentin TE, Renné T. Towards understanding ChAdOx1 nCov-19 vaccine-induced immune thrombotic thrombocytopenia (VITT). 10.21203/rs.3.rs-440461/v1. [DOI] [PMC free article] [PubMed]
- 37. Marchandot B, Carmona A, Trimaille A, Curtiaud A, Morel O. Procoagulant microparticles: a possible link between vaccine-induced immune thrombocytopenia (VITT) and cerebral sinus venous thrombosis. J Thromb Thrombolysis 2021;1–3. doi:10.1007/s11239-021-02505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosell A, Havervall S, von Meijenfeldt F, Hisada Y, Aguilera K, Grover SP, Lisman T, Mackman N, Thålin C. Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality-brief report. Arterioscler Thromb Vasc Biol 2021;41:878–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Warkentin TE, Hayward CP, Boshkov LK, Santos AV, Sheppard JA, Bode AP, Kelton JG. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: an explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood 1994;84:3691–3699. [PubMed] [Google Scholar]
- 40. Hughes M, Hayward CP, Warkentin TE, Horsewood P, Chorneyko KA, Kelton JG. Morphological analysis of microparticle generation in heparin-induced thrombocytopenia. Blood 2000;96:188–194. [PubMed] [Google Scholar]
- 41. Campello E, Radu CM, Duner E, Lombardi AM, Spiezia L, Bendo R, Ferrari S, Simioni P, Fabris F. Activated platelet-derived and leukocyte-derived circulating microparticles and the risk of thrombosis in heparin-induced thrombocytopenia: a role for pf4-bearing microparticles? Cytometry B Clin Cytom 2018;94:334–341. [DOI] [PubMed] [Google Scholar]
- 42. Østerud B, Bjørklid E. Sources of tissue factor. Semin Thromb Hemost 2006;32:11–23. [DOI] [PubMed] [Google Scholar]
- 43. Nagai M, Yilmaz CE, Kirchhofer D, Esmon CT, Mackman N, Granger DN. Role of coagulation factors in cerebral venous sinus and cerebral microvascular thrombosis. Neurosurgery 2010;66:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walenga JM, Jeske WP, Prechel MM, Bakhos M. Newer insights on the mechanism of heparin-induced thrombocytopenia. Semin Thromb Hemost 2004;30(Suppl 1):57–67. [DOI] [PubMed] [Google Scholar]
- 45. Parker WAE, Storey RF. Platelets and the endothelium: active participants in severe COVID-19 infection. JACC Basic Transl Sci 2021;6:219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Warkentin TE, Sheppard JA, Moore JC, Cook RJ, Kelton JG. Studies of the immune response in heparin-induced thrombocytopenia. Blood 2009;113:4963–4969. [DOI] [PubMed] [Google Scholar]
- 47. Sørvoll IH, Horvei KD, Ernstsen SL, Laegreid IJ, Lund S, Grønli RH, Olsen MK, Jacobsen HK, Eriksson A, Halstensen AM, Tjønnfjord E, Ghanima W, Ahlen MT. An observational study to identify the prevalence of thrombocytopenia and anti-PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination. J Thromb Haemost 2021. doi:10.1111/jth.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oldenburg J, Klamroth R, Langer F, Albisetti M, von Auer C, Ay C, Korte W, Scharf RE, Pötzsch B, Greinacher A. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie 2021;41:e1. [DOI] [PubMed] [Google Scholar]
- 49. Thiele T, Ulm L, Holtfreter S, Schönborn L, Kuhn SO, Scheer C, Warkentin TE, Bröker B, Becker K, Aurich K, Selleng K, Hübner NO, Greinacher A. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood 2021;138:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thaler J, Ay C, Gleixner KV, Hauswirth AW, Cacioppo F, Grafeneder J, Quehenberger P, Pabinger I, Knöbl P. Successful treatment of vaccine-induced prothrombotic immune thrombocytopenia (VIPIT). J Thromb Haemost 2021. doi:10.1111/jth.15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karnam A, Lacroix-Desmazes S, Kaveri SV, Bayry J. Vaccine-induced prothrombotic immune thrombocytopenia (VIPIT): consider IVIG batch in the treatment. J Thromb Haemost 2021. doi:10.1111/jth.15361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Elalamy I, Gerotziafas G, Alamowitch S, Laroche JP, van Dreden P, Ageno W, Beyer-Westendorf J, Cohen AT, Jiménez D, Brenner B, Middeldorp S, Cacoub P; Scientific Reviewer Committee. SARS-CoV-2 vaccine and thrombosis: expert opinions. Thromb Haemost 2021;121:982–991. doi: 10.1055/a-1499-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Warkentin TE. High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: a review. Expert Rev Hematol 2019;12:685–698. [DOI] [PubMed] [Google Scholar]
- 54. Watson H, Davidson S, Keeling D; Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol 2012;159:528–540. [DOI] [PubMed] [Google Scholar]
- 55. American Heart Association/American Stroke Association Stroke Council Leadership. Diagnosis and management of cerebral venous sinus thrombosis with vaccine-induced thrombotic thrombocytopenia. Stroke 2021. doi:10.1161/STROKEAHA.121.035564. [DOI] [PubMed] [Google Scholar]
- 56. von Hundelshausen P, Lorenz R, Siess W, Weber C. Vaccine-induced immune thrombotic thrombocytopenia (VITT): targeting pathomechanisms with Bruton tyrosine kinase inhibitors. Thromb Haemost 2021. doi:10.1055/a-1481-3039. [DOI] [PubMed] [Google Scholar]
- 57. Patriquin CJ, Laroche V, Selby R, Pendergrast J, Barth D, Côté B, Gagnon N, Roberge G, Carrier M, Castellucci LA, Scarvelis D, Mack JP. Therapeutic plasma exchange in vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med 2021. doi:10.1056/NEJMc2109465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rock G, Weber V, Stegmayr B. Therapeutic plasma exchange (TPE) as a plausible rescue therapy in severe vaccine-induced immune thrombotic thrombocytopenia. Transfus Apher Sci 2021;103174. [DOI] [PubMed] [Google Scholar]