Abstract
STUDY QUESTION
Does the immune response to coronavirus disease 2019 (COVID-19) infection or the BNT162b2 mRNA vaccine involve the ovarian follicle, and does it affect its function?
SUMMARY ANSWER
We were able to demonstrate anti-severe acute respiratory syndrome coronavirus 2 (SARS–CoV-2) IgG in follicular fluid (FF) from both infected and vaccinated IVF patients, with no evidence for compromised follicular function.
WHAT IS KNOWN ALREADY
No research data are available yet.
STUDY DESIGN, SIZE, DURATION
This is a cohort study, composed of 32 consecutive IVF patients, either infected with COVID-19, vaccinated or non-exposed, conducted between 1 February and 10 March 2021 in a single university hospital-based IVF clinic.
PARTICIPANTS/MATERIALS, SETTING, METHODS
A consecutive sample of female consenting patients undergoing oocyte retrieval was recruited and assigned to one of the three study groups: recovering from confirmed COVID-19 (n = 9); vaccinated (n = 9); and uninfected, non-vaccinated controls (n = 14). Serum and FF samples were taken and analyzed for anti-COVID IgG as well as estrogen, progesterone and heparan sulfate proteoglycan 2 concentration, as well as the number and maturity of aspirated oocytes and day of trigger estrogen and progesterone measurements. Main outcome measures were follicular function, including steroidogenesis, follicular response to the LH/hCG trigger, and oocyte quality biomarkers.
MAIN RESULTS AND THE ROLE OF CHANCE
Both COVID-19 and the vaccine elicited anti-COVID IgG antibodies that were detected in the FF at levels proportional to the IgG serum concentration. No differences between the three groups were detected in any of the surrogate parameters for ovarian follicle quality.
LIMITATIONS, REASONS FOR CAUTION
This is a small study, comprising a mixed fertile and infertile population, and its conclusions should be supported and validated by larger studies.
WIDER IMPLICATIONS OF THE FINDINGS
This is the first study to examine the impact of SARS–Cov-2 infection and COVID-19 vaccination on ovarian function and these early findings suggest no measurable detrimental effect on function of the ovarian follicle.
STUDY FUNDING/COMPETING INTEREST(S)
The study was funded out of an internal budget. There are no conflicts of interest for any of the authors.
TRIAL REGISTRATION NUMBER
CinicalTrials.gov registry number NCT04822012.
Keywords: COVID-19 / SARS–CoV-2 / BNT162b2 mRNA vaccine / IgG / follicle function / IVF / immune response / coronavirus disease 2019 / severe acute respiratory syndrome coronavirus 2
Introduction
Since emerging in the last months of 2019, over 127 000 000 individuals have contracted confirmed severe acute respiratory syndrome Coronavirus 2 (SARS–CoV-2) infection, and the documented death toll from coronavirus disease 2019 (COVID-19) had reached 2 788 639 individuals globally by the end of March 2021 (CDC, 2021). Jerusalem, the setting of the present study, has presented one of the highest incidence rates of COVID-19 in Israel (Abu Jabal et al., 2021).
Nationwide anti-COVID-19 vaccination began in Israel in December 2020, using the Pfizer—BioNtech vaccine (BNT162b2 mRNA; BioNTech SE, Rhineland-Palatinate, Germany). By the end of March 2021, 5 216 801 and 4 700 957 individuals received the first and second doses of the vaccine, representing 56.1% and 50.55% of Israel’s population, respectively. Vaccination for individuals of reproductive age began in January 2021; by the end of March 2021, 72.3% and 61.3% of 20–29 year olds, 77.1% and 67.8% of 30–39 year olds, and 82% and 74.5% of 40–49 year olds had received the first and second doses of the vaccine, respectively: the highest proportions in the world (Abu Jabal et al., 2021).
Both the rapidly spreading disease and the vaccination campaign were associated with concerns regarding potential detrimental effects on future fertility (Jing et al., 2020; Blake Evans et al., 2021; Flynn et al., 2021). There is still a lack of real-world data to assist clinicians in counseling their IVF patients regarding the possible impact of recent recovery from COVID infection, or vaccination against it, on the potential for success of ART. While it has been suggested that COVID-19 might impact fertility, no studies, to the best of our knowledge, have proven a direct gonadal effect of either the disease or the vaccine (Frendo et al., 2003; Chen et al., 2008; Jing et al., 2020; Blake Evans et al., 2021).
We aimed to determine the impact of confirmed COVID-19 disease and/or immunization on human follicular function, by comparing follicular steroidogenesis, response to the LH/hCG trigger, and oocyte quality biomarker (heparan sulfate proteoglycan 2: HSPG2), in the aspirated follicular fluid (FF) of patients undergoing oocyte retrieval.
Materials and methods
The study was approved by the Hadassah Medical Center IRB (Permit 0053-21-HMO). The study was registered at the clinical trial registry and assigned the registration number NCT04822012 (Protocol # 0053-21-HMO). Consecutive patients undergoing oocyte retrieval for either IVF, ICSI, or oocyte cryopreservation were approached to participate in the study on the day of oocyte retrieval. Eligibility criteria were age older than 18 years plus willingness to participate and provide informed consent. Patients younger than 18 or older than 44 years, as well as patients with poor ovarian response (less than 3 mature follicles), were excluded.
After providing informed consent, the patients were questioned about their confirmed past SARS–Cov-2 infection/vaccination status. For recovering patients, the date of the recovery (negative nasopharyngeal COVID PCR test) was recorded. Vaccinated patients were asked about the date of the first and second vaccines. None of the recovering patients were vaccinated with anti-COVID vaccine. The clinic’s strict policy is to screen all patients using a nasopharyngeal swab for COVID-19 a week prior to the procedure, except those patients who were less than 3 months following recovery from SARS–Cov-2 infection, and those more than 2 weeks after the second COVID-19 vaccine. As a result, together with the negative anti-COVID serum antibody test, we could safely presume that all control patients in this study were indeed COVID-19 negative. During the oocyte retrieval the first follicle/s were aspirated into an empty tube. If the sample was contaminated with blood another clean sample was taken, until a total volume of 5 ml of a clean sample was achieved. A 5 ml blood sample was also taken during the procedure. Following the isolation of the oocyte, the FF was centrifuged at 1500g and the blood sample at 3000g for 7 min. The supernatant fraction of the FF and the serum fraction of the blood sample were each aliquoted and later within 1 h of aspiration snap frozen, and stored at −80°C until analysis.
Data including the patient age, IVF indication, antral follicle count (AFC), serum estradiol and progesterone on the day of ovulation trigger (36 h before oocyte retrieval), type of trigger, the number of oocytes, and mature oocytes, were recorded.
Once the target date was reached, the samples were thawed and analyzed using the assays described below. The analysis of the blood and FF samples for all outcome parameters was conducted with blinding of the COVID/vaccine status of the participant.
Serum and FF anti-COVID IgG measurement
The levels of specific anti-SARS–CoV-2 spike protein receptor binding domain (RBD) IgG were assessed in serum and FF specimens, using the Architect SARS–CoV-2 IgG II Quant assay (Abbott Diagnostics, Chicago, IL, USA), according to the manufacturer's specifications.
Briefly, the SARS–Cov2 IgG II Quant assay is an automated two-step immunoassay for the qualitative and quantitative determination of IgG antibodies to SARS–CoV2 S-RBD, using a chemiluminescent microparticle immunoassay on the ARCHITECT I System. Sample (200 µl), SARS–CoV2 antigen-coated paramagnetic microparticles, and assay diluent are combined and incubated. Following a wash cycle, incubation with anti-human IgG acridinium-labeled conjugate, and repeated wash cycle, with the addition of trigger solutions, the resulting chemiluminescent reaction is measured as a relative light unit. The final result is expressed as arbitrary units (AU)/ml. IgG levels ≥ 50 AU/ml were considered positive.
Assessment of ovarian follicle functions
Steroidogenesis
We examined the ability of the theca-granulosa cells that form the wall of the follicles to produce steroids, namely estradiol and progesterone, by measuring their concentration both in the serum and FF.
The measurement was conducted using the Atellica IM Siemens Healthineers system (Siemens Healthcare GmbH, Henkestr. 127, 91052 Erlangen, Germany). Estradiol concentration was measured using the Enhanced Estradiol Kit (# 10995561), an ELISA based on an acridinium-labeled sheep monoclonal anti-estradiol antibody with a measuring range of 43.31–11 010.0 pmol/l. For measuring FF estradiol, the typical concentration of which exceeds the measuring range of the kit, the sample was diluted using the Atellica IM eE2 diluent (10995563) according to a protocol described elsewhere (Andersen et al., 2006). The measurement of serum and FF progesterone was conducted using the PRGE kit (10995660). The kit is based on direct chemiluminescent technology using an acridinium-labeled mouse monoclonal anti-progesterone antibody with a measuring interval of 0.67–190.80 nmol/l. For FF progesterone measurement, the sample was diluted with the Atellica IM Multi-diluent (10995645) according to the protocol (Andersen et al., 2006).
To standardize estradiol production, we used the ratio of serum estradiol on the day of the trigger per retrieved oocyte. This ratio was found in a large study to be an age independent predictor of IVF treatment outcome. In that study an estradiol/oocyte ratio of > 1000 pmol/l/oocyte was associated with a reduction in pregnancy and clinical pregnancy rate (Vaughan et al., 2016).
Follicular response to the LH/hCG trigger assessment
To assess the adequacy of response of the follicle to the LH/hCG trigger, we compared three parameters.
First, oocyte yield, was calculated as the ratio between the number of retrieved oocytes and the number of mature follicles measured on the day of the trigger injection. This ratio represents the ability of the granulosa cells to secrete enzymes that release the cumulus oocyte complex from the wall of the follicle in response to the LH surge, thereby allowing its aspiration. An oocyte yield of 45% or less was defined as sub-optimal (Popovic-Todorovic et al., 2019).
Second, we assessed the ratio of mature oocytes to total aspirated oocytes, representing another aspect of the adequacy of response to the LH/hCG trigger—promoting the completion of the first meiotic division.
The third parameter was the ratio of the number of retrieved oocytes to the number of follicles that began luteinization, obtained by dividing the number of retrieved oocytes by the serum concentration of progesterone on the day of oocyte retrieval.
Assessment of oocyte quality FF biomarkers
HSPG2 (pearlecan) is a ubiquitously expressed member of the heparan sulfate glycoprotein family. It is also produced by granulosa cells and secreted to the FF where it serves as the major estrogen binding protein. It has also been recognized as a key factor in proper follicular function and implantation. We chose to measure its concentration in FF following a proteomic analysis of over 500 FF proteins that identified the concentration of HSPG2 in FF as the strongest biomarker to predict oocyte fertilization and IVF success (Bayasula et al., 2013).
We analyzed the concentration of HSPG2 in FF using the Human HSPG2 SimpleStep ELISA kit (Abcam, Discovery Drive, Cambridge Biomedical Campus, Cambridge, UK). This ELISA system uses an affinity tag labeled capture antibody and a reporter conjugated detector antibody read at 450–600 nm. The test range of measurement is 47–7000 pg/ml. Dilution of the samples with up to 1:50k was with the sample diluent provided. The analysis was conducted according to the manufacturer’s instructions.
Statistical analysis
All analyses were performed using SPSS 23.0 (SPSS Inc., Chicago, IL, USA). Continuous intervals and ratios that were not normally distributed were transformed to a logarithmic scale for normality correction. Normally, distributed data were compared across study groups by univariate ANOVA. Rates and proportions were compared with the Chi-square or Fisher's exact tests as appropriate in case of small numbers. Correlations were presented by scatter plots and the goodness of fit calculated by R2. All P-values were tested as two tailed and considered significant at <0.05.
Results
The raw data including all the results of the study are included in Supplementary Table SI.
Patient characteristics
Overall, 32 patients consented to participate in the study, of which 9 reported vaccination (Group 1—Vaccine); 7 to have recovered from COVID (Group 2—disease); and 16 denied both and were allocated to the non-exposed group (Group 3). However, serum analysis for the presence of anti-COVID-19 IgG identified two of the patients in the control group as recoverees from a likely asymptomatic SARS–Cov-2 infection and therefore were re-allocated to Group 2. Mean time periods from vaccine or recovery to recruitment and sampling were as follows: 98.14 days from recovery to sampling (range 48–169 days); 11.7 days from the first vaccine dose to sampling in the four patients who were recruited after completion of a single vaccine dose (range 8–18 days) and 48.6 days from the first vaccine dose to sampling in five patients recruited that completed two doses (range 25–67 days). Patient demographics are described in Table I.
Table I.
Demographics of patients in a study of the effects of immune response to COVID-2019 or an mRNA vaccine on the ovarian follicle.
| Vaccine | COVID | Control** | Total | |||
|---|---|---|---|---|---|---|
| Number | 9 | 9 | 14 | 32 | P-value | |
| Age (years) | Mean ± (SD) | 35.3 ± 3.97 | 34.1 ± 4.7 | 32.5 ± 5.3 | 33.75 (± 4.8) | 0.383 |
| Median | 35 | 34 | 33 | 34 | ||
| AFC | Mean (SD) | 13.3 ± 4.7 | 13.6 ± 4.1 | 15.6 ± 6.7 | 14.4 ± 5.7 | 0.592 |
| Median | 14 | 13 | 17.5 | 14.5 | ||
| Indication | Male (%) | 2 (22.2%) | 1 (11.1%) | 4 (28.6%) | 7 (21.9%) | 0.113 |
| Non male (%) | 2 (22.2%) | 5 (55.6%) | 9 (64.3%) | 16 (50%) | ||
| Egg freeze (%) | 5 (55.6%) | 3 (33.3%) | 1 (7.1%) | 9 (28.1%) | ||
| Non-infertile | 7 (77.8%) | 4 (44.4%) | 5 (35.7%) | 16 (50%) | ||
| Time interval * | Mean | 32.2 | 98.14 | N/A | 0.003 | |
| SD | 22.1 | 45.5 | N/A |
Univariate ANOVA was used for analysis of age, AFC and time interval and Chi-square for analysis of indication.
Time interval (days) from recovery or first vaccine to the day of oocyte retrieval.
Non-vaccinated-non infected patients.
COVID-19, coronavirus disease 2019; AFC, antral follicle count.
The mean age of participants in the study was 33.7 years. Age was found to be normally distributed and did not vary significantly among the three groups (P-value 0.383). Similarly, measurement of AFC, as a reflection of ovarian reserve, did not differ among the groups (P-value 0.592). Indications for treatment were grouped into non-male related infertility, male related infertility, and oocyte cryopreservation: the latter two groups, therefore, included women with no proven infertility. Only women in the non-male-related infertility, male-related infertility groups had their oocytes fertilized following the oocyte retrieval. There were more women undergoing oocyte retrieval for oocyte freezing in the vaccine group (55.6%) compared to the other groups (33.3% and 7.1%): this difference was close to but did not reach statistical significance (P-value 0.07). In all groups, a large proportion of women had oocyte retrieval for non-female infertility related indications (50%, range 35.7–77.8%). As most patients participating in the study were treated with an antagonist protocol, the use of hCG only trigger was limited (6.3%). The use of GnRH agonist trigger reflected the distribution of oocyte retrieval for oocyte cryopreservation in the different groups (44%, 44%, and 28.6% in the vaccinated, COVID, and control groups, respectively, P-value 0.766).
Anti-COVID IgG antibodies
Table II describes the presence of anti-COVID IgG induced by either SARS–COV-2 infection or secondary to vaccination with the Pfizer—BioNtech vaccine (BNT162b2 mRNA). All the patients who received two doses of the BNT162b2 mRNA vaccine had a high concentration of COVID IgG in their serum and FF (range 850–19 672 and 463–15 883 in the serum and FF, respectively). IgG concentrations were positively correlated with the time interval from the date of the vaccine: two of the patients that had only received the first dose, 13 and 18 days before oocyte retrieval, were found to harbor anti-COVID IgG in their serum and FF (range 194–228 and 64–198 in the serum and FF respectively), while the two who received the first dose only 8 days prior to oocyte retrieval had no measurable anti-COVID IgG either in their serum or FF.
Table II.
Anti-SARS–CoV-2 IgG levels in serum and FF.
| IgG presence | Vaccine | COVID | Control | Total | ||
|---|---|---|---|---|---|---|
| Number | 9 | 9 | 14 | 32 | P-value | |
| Serum | Positive | 7 | 8 | 0 | 15 | <0.001 |
| Negative | 2 | 1 | 14 | 17 | ||
| Positive | 77.8% | 88.9% | 0% | 53.1% | ||
| IgG* mean | 4877.4 | 351.2 | 0 | 0.096 | ||
| IgG SD | 7219 | 333.3 | 0 | |||
| FF | Positive | 7 | 8 | 0 | 15 | <0.001 |
| Negative | 2 | 1 | 14 | 17 | ||
| Positive | 77.8% | 88.9% | 0% | 53.1% | ||
| IgG mean | 3720.6 | 266.8 | 0 | 0.1256 | ||
| IgG SD | 6038.8 | 242.8 | 0 | |||
| Ratio FF/serum | Mean ± SD | 0.62 ± 0.21 | 0.79 ± 0.08 | N/A | 0.0665 |
The presence of IgG was analyzed with Chi-square and its quantity with univariate ANOVA.
Arbitrary units/ml.
SARS–CoV-2, severe acute respiratory syndrome Coronavirus 2; FF, follicular fluid.
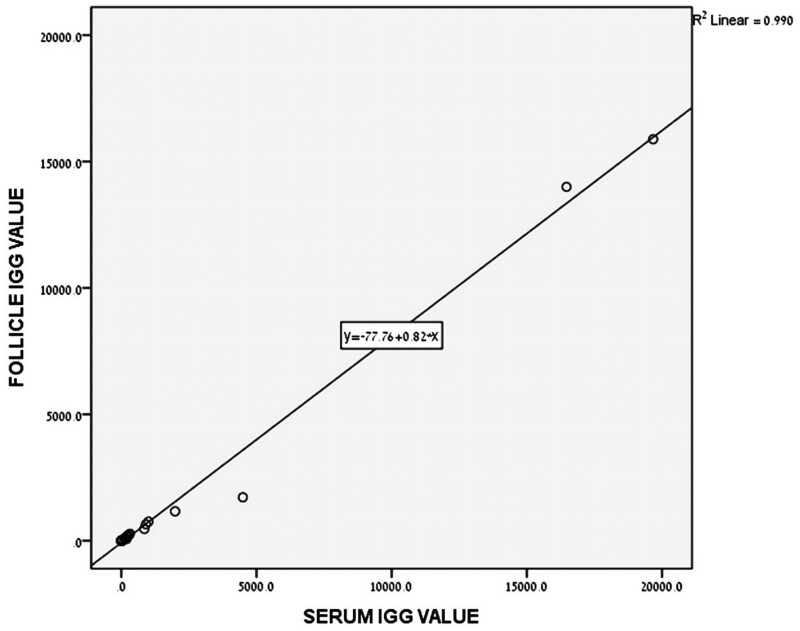
All the patients with positive serum anti-COVID IgG had detectable levels of anti-COVID IgG in their FF. Figure 1 shows a linear association between the serum and FF concentrations of anti-COVID IgG (R2 =0.99), suggesting a non-regulated passage of the anti-COVID IgG across the follicular membrane.
Figure 1.
Association between follicular fluid (FF) and serum anti-SARS–CoV-2 IgG concentration. All the women undergoing IVF with positive serum anti-COVID IgG had detectable levels of anti-COVID IgG in their FF. The linear association between the serum and FF concentrations (arbitrary units) of anti-COVID IgG (R2 =0.99) suggests a non-regulated passage of anti-COVID IgG across the follicular membrane. SARS–CoV-2, severe acute respiratory syndrome Coronavirus 2; COVID, coronavirus disease.
No difference was found between women acquiring anti-COVID antibodies following exposure or vaccination in the ratio of FF to serum anti-COVID IgG.
Assessment of ovarian follicle functions
Table III describes treatment and outcome parameters. These parameters provide data on the steroidogenic function of the follicle (trigger day serum and FF estradiol and progesterone concentrations) and the magnitude of the ova recruitment, as reflected by the number of aspirated eggs.
Table III.
IVF treatment outcome parameters.
| Parameter | Group | Vaccine | COVID | Control | Total | |
|---|---|---|---|---|---|---|
| Number | 9 | 9 | 14 | 32 | P-value | |
| Trigger day estradiol (pmol/l) | Mean (SD) | 8874 ± 2555 | 10 810 ± 5867 | 6354 ± 2657 | 8379 ± 4167 | 0.351 |
| Median | 8507 | 9913 | 6155 | 8506 | ||
| Trigger day progesterone (nmol/l) | Mean (SD) | 3.29 ± 2.09 | 3.31 ± 1.14 | 1.64 ± 0.67 | 2.6 ± 1.54 | 0.008 |
| Median | 2.63 | 3.26 | 1.35 | 2.46 | ||
| Type of trigger | hCG only (%) | 1 (11.1%) | 0 | 1 (7.1%) | 2 (6.3%) | 0.766 |
| GnRHa (%) | 4 (44.4%) | 4 (44.4%) | 4 (28.6%) | 12 (37.5%) | ||
| Dual (%) | 4 (44.4%) | 5 (55.6%) | 9 (64.3%) | 18 (56.3%) | ||
| Oocyte retrieval serum estradiol (pmol/ml) | Mean (SD) | 4133 ± 1212 | 5321 ± 1884 | 3535 ± 1855 | 4206 ± 1891 | 0.082 |
| Median | 3880 | 4982 | 3070 | 3995 | ||
| Oocyte retrieval serum progesterone (nmol/l) | Mean (SD) | 22.6 ± 16.4 | 26.5 ± 12.1 | 18.6 ± 10.5 | 22 ± 13.5 | 0.40 |
| Median | 15.8 | 23.5 | 13.5 | 17.85 | ||
| FF estradiol (nmol/ml) | Mean (SD) | 2160 ± 1105 | 2890 ± 1510 | 2217 ± 1355 | 2390 ± 1332 | 0.426 |
| Median | 2077 | 3029 | 1927 | 2005 | ||
| FF progesterone | Mean (SD) | 34 569 ± 13 925 | 32 886 ± 10 504 | 32 811 ± 15 234 | 33 327 ± 13 284 | 0.950 |
| Median | 31 620 | 36 249 | 27 821 | 30 632 | ||
| Number of oocytes | Mean (SD) | 12.4 ± 8.7 | 10.89 ± 4.8 | 11.2 ± 6.7 | 11.5 ± 6.7 | 0.877 |
| Median | 8 | 10 | 10 | 9 | ||
| Number of mature oocytes | Mean (SD) | 7.25 ± 2.77 | 8.37 ± 4.1 | 7.75 ± 4.7 | 7.8 ± 4.1 | 0.870 |
| Median | 6.5 | 7 | 6.5 | 7 | ||
| Estradiol @ trigger/oocyte | Mean (SD) | 874.1 ± 302.6 | 1127.75 ± 605 | 812.4 ± 534 | 921.9 ± 528.5 | 0.3812 |
| Median | 813 | 787.6 | 652.3 | 777.92 | ||
| Oocyte/oocyte retrieval day serum progesterone | Mean (SD) | 0.63 ± 0.31 | 0.45 ± 0.2 | 0.71 ± 0.51 | 0.61 ± 0.41 | 0.372 |
| Median | 0.49 | 0.41 | 0.50 | 0.49 | ||
| Oocyte yield (%) | Mean (SD) | 139.7 ± 59 | 153.1 ± 68.7 | 163.3 ± 47.2 | 152.6 ± 61.05 | 0.772 |
| Median | 128.6 | 125.4 | 169.05 | 140 | ||
| Mature/total oocyte ratio | Mean (SD) | 0.72 ± 0.2 | 0.77 ± 0.12 | 0.69 ± 0.14 | 0.72 ± 0.16 | 0.554 |
| Median | 0.73 | 0.79 | 0.69 | 0.71 | ||
| FF HSPG2 (ng/ml) | Mean (SD) | 9953 ± 9620 | 5305 ± 3694 | 4610 ± 2771 | 6340 ± 7102 | 0.385 |
| Median | 3916 | 5025 | 5741 | 3250 | ||
| GQ Day 3/2PNs | Mean (SD) | 0.43 ± 0.05 | 0.55 ± 0.14 | 0.72 ± 0.34 | 0.63 ± 0.3 | 0.314 |
| Median | 0.43 | 0.48 | 0.86 | 0.5 |
Univariate ANOVA was used for analysis of all the parameters included in this table.
GnRHa, GnRH agonist; HSPG2, heparan sulfate proteoglycan 2; PN, pronuclei; GQ, good quality embryos.
Mean serum estradiol on the day in which the trigger injection was administered (36 h prior to oocyte retrieval) did not differ among the groups (P-value 0.351). The normalized ratio of serum estradiol on trigger day/oocyte was similar and within the optimal range for most of the patients in all groups. Serum progesterone on the same day was lower in the non-exposed group (3.29, 3.31, and 1.64 nmol/l in the vaccine, COVID, and non-exposed, respectively, P-value 0.008); however, no difference was observed in serum progesterone on the day of oocyte retrieval (22.6, 26.5, and 18.6 nmol/l in the vaccine, COVID, and control groups, respectively, P-value 0.40).
Although both FF estradiol and progesterone concentrations were hundreds fold higher compared to serum concentrations on the same day, no difference was found among the groups (2160, 2890, and 2217 nmol/l for estradiol and 34.569, 32.886, and 32.811 nmol/l for progesterone in the vaccine, COVID, and non-exposed groups, respectively, P-values 0.426 and 0.950).
The adequacy of the follicular response to ovulation triggering was assessed by serum and FF progesterone as well as by the oocyte yield (oocytes retrieved/trigger day mature follicle count, oocytes retrieved/oocyte retrieval day serum progesterone, ratio of mature/total number of aspirated oocytes).
Oocyte yield was high in all groups (mean 152%, P-value 0.772), well above the 45% threshold defining a sub-optimal response. Similarly, the rate of mature oocytes was normal (mean 0.72; P-value 0.554). The ratio between the number of aspirated oocytes and oocyte retrieval day progesterone was 0.63, 0.45, and 0.71 in the vaccine, COVID, and non-exposed groups, respectively, P-value 0.372.
Assessment of oocyte quality with FF HSPG2
None of the above follicle quality reporter surrogates suggested any meaningful differences among the three groups. The mean FF HSPG2 for all samples was 6340 ng/ml (median 3250, range 302–43 603). Comparison of HSPG2 between the groups showed similar concentrations (P-value 0.385).
Discussion
Recent publications have indicated a potential for severe morbidity and mortality among parturients affected by the emerging variants of the SARS–COV-2 virus (Zambrano et al., 2020). Despite the low incidence of severe morbidity among parturients affected by COVID-19, the Center for Disease Control (CDC) added pregnancy to the list of high-risk conditions to prioritize vaccination, and the American College of Obstetricians and Gynecologists (ACOG) recommends not withholding vaccination from pregnant women at any stage of the pregnancy (ACOG Expert Work Group in collaboration with Riley, 2020). The enthusiasm surrounding the vaccine rollout was accompanied by unsubstantiated rumors, spread via social media, suggesting that the vaccine may lead to female sterility (Blake Evans et al., 2021). The risk for severe COVID-related illness on one hand and the lack of knowledge about the potential effects of anti-COVID vaccination, led to much apprehension among patients planning to conceive, resulting in couples postponing their plans to conceive (Zambrano et al., 2020; Flynn et al., 2021). To the best of our knowledge, our study is the first to determine ovarian involvement with the COVID immune response, as reflected by the presence of anti-SARS–COV-2 IgG in recently vaccinated versus infected and non-vaccinated non-infected IVF patients, all of whom were PCR negative at the time of oocyte retrieval.
Our results show that all the patients reported as COVID-19 recoverees had measurable anti-SARS-COV-2 IgG both in their serum and FF. The presence of anti-COVID IgG following vaccination was shown as early as 13 days following the first dose, both in the serum and FF. The concentration of anti-COVID IgG correlated with the time interval from the vaccine. The anti-COVID IgG in the FF reflects its serum concentration in a linear association, as previously described for other vertically transmitted viruses (Ardizzoni et al., 2011).
Our assessment of a potential effect of either infection with SARS–COV-2 or vaccination on ovarian function included several aspects related to the egg-follicle performance. Assessment of follicular steroidogenesis showed similar and normal rates of estrogen and progesterone production among groups. The only significant difference we detected was a lower trigger day serum progesterone in the non-exposed group, a difference that was not shown on the day of oocyte retrieval, either in the serum or FF, and, therefore, is unlikely to represent a real biological difference. Otherwise, none of the synthetic parameters studied showed any differences among the three groups. Assessment of the response of the follicles to the LH/hCG trigger showed a normal and similar response in all three groups.
Oocyte quality assessment
We compared the concentration of FF HSPG2, a validated biomarker of oocyte quality, among groups. HSPG2 has been recognized as key element in follicular function. It is a product of granulosa cells and its secretion from granulosa cells to the FF increases with gonadotrophin stimulation and mirrors the follicular estrogen production. Furthermore, it was identified as the main estradiol binding protein in FF and the most predictive FF solute of oocyte quality and IVF outcome (Bayasula et al., 2013; Bentov et al., 2016). In our analysis, FF samples showed a high concentration of HSPG2 that was not significantly different between the groups.
Hence, despite the clear evidence of intimate follicular immune exposure post infection with SARS–COV-2 or following BNT162b2 mRNA vaccine, the steroidogenic machinery of the follicle, controlling the ultimate maturation of the oocyte and its hormonal milieu, did not show any measurable difference as compared to non-exposed women. This evidence joins other data on IVF treatment outcomes among recoverees from COVID-19 infection observed in our center, which showed similar outcomes (data not shown).
Strengths and limitations
To the best of our knowledge, this is the first study to examine the presence of a natural or vaccine-elicited humoral immune response in the ovarian follicle. In addition, we also investigated the possible association between its presence and several aspects of follicular function and oocyte yield.
The eligibility of the FF sample for analysis was based on a visual determination of its cleanliness, thus, potentially allowing for minor blood contamination. Yet, our findings with regards to the presence of anti-viral IgG are similar to previous publications that described the local immune response to other viral infections, making this an unlikely source of bias.
This is, however, a small study, comprising a mixed fertile and infertile population, and its conclusions should be supported and validated by larger studies. Owing to its timing, our study was able to assess short-term effects of vaccine and disease but cannot rule out later sequelae.
Conclusion
Exposure to either the SARS–CoV-2 virus or the BNT162b2 mRNA vaccine results in a rapid formation of anti-COVID IgG that can be detected in the FF, correlating with serum concentrations. In our study, neither infection or the BNT162b2 mRNA vaccine nor the immune response to them resulted in any measurable detrimental effect on the function of the ovarian follicle.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Authors’ roles
Y.B.: study design, patient recruitment, data analysis, and writing; O.B.: study design, patient recruitment, data analysis, writing, and test performance; A.M.Z.: study design, patient recruitment, and test performance; M.K.: study design, patient recruitment, and test performance; M.G.: patient recruitment; C.S.G.: study design and test performance; M.K.G.: study design and test performance; E.H.B.: study design and patient recruitment; H.E.G.H.: study design and patient recruitment; D.W.: study design, data analysis, writing, and test performance; E.O.D.: study design and test performance; O.B.: study design, patient recruitment, data analysis, writing, and test performance; I.B.: study design and test performance; D.G.W.: study design, data analysis, writing, and test performance; S.Y.: study design; A.W.: study design; A.H.K.: study design, patient recruitment, data analysis, and writing.
Funding
The study was funded internally.
Conflict of interest
None of the authors declares a conflict of interest.
Supplementary Material
References
- ACOG Expert Work Group in collaboration with Laura E. Riley MRB, Jamieson DJ, Hughes BL, Swamy G, O’Neal Eckert L, Turrentine M, Carroll S.. Vaccinating pregnant and lactating patients against COVID-19. In ACOG (ed). ACOGORG. Washington, DC: ACOG, Obstetrics and Gynecology, 2020. [Google Scholar]
- Andersen CY, Humaidan P, Ejdrup HB, Bungum L, Grondahl ML, Westergaard LG.. Hormonal characteristics of follicular fluid from women receiving either GnRH agonist or hCG for ovulation induction. Hum Reprod 2006;21:2126–2130. [DOI] [PubMed] [Google Scholar]
- Ardizzoni A, Manca L, Capodanno F, Baschieri MC, Rondini I, Peppoloni S, Righi E, La Sala GB, Blasi E.. Detection of follicular fluid and serum antibodies by protein microarrays in women undergoing in vitro fertilization treatment. J Reprod Immunol 2011;89:62–69. [DOI] [PubMed] [Google Scholar]
- Bayasula IA, Kobayashi H, Goto M, Nakahara T, Nakamura T, Kondo M, Nagatomo Y, Kotani T, Kikkawa F.. A proteomic analysis of human follicular fluid: comparison between fertilized oocytes and non-fertilized oocytes in the same patient. J Assist Reprod Genet 2013;30:1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentov Y, Jurisicova A, Kenigsberg S, Casper RF.. What maintains the high intra-follicular estradiol concentration in pre-ovulatory follicles? J Assist Reprod Genet 2016;33:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake Evans M, Alexander C, Barnard E, Max Ezzati M, Hill MJ, Hoyos E, Hariton E, Mikhael S, Penzias A. COVID-19 vaccine and infertility: baseless claims and unfounded social media panic. In ASRM, Fertility and Sterility. 2021; https://www.fertstertdialog.com/posts/covid-19-vaccine-and-infertility-baseless-claims-and-unfounded-social-media-panic.
- CDC. COVID-19 Mortality Overview. 2021. Centers for Disease Control & Prevention, CDC.gov, https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days.
- Chen CP, Chen LF, Yang SR, Chen CY, Ko CC, Chang GD, Chen H.. Functional characterization of the human placental fusogenic membrane protein syncytin 2. Biol Reprod 2008;79:815–823. [DOI] [PubMed] [Google Scholar]
- Flynn AC, Kavanagh K, Smith AD, Poston L, White SL.. The impact of the COVID-19 pandemic on pregnancy planning behaviors. Womens Health Rep (New Rochelle) 2021;2:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frendo JL, Olivier D, Cheynet V, Blond JL, Bouton O, Vidaud M, Rabreau M, Evain-Brion D, Mallet F.. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol Cell Biol 2003;23:3566–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabal Ben-Amram AK, Beiruti H, Batheesh K, Sussan Y, Zarka C, Edelstein SM.. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill 2021;26: 2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Run-Qian L, Hao-Ran W, Hao-Ran C, Ya-Bin L, Yang G, Fei C.. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod 2020;26:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic-Todorovic B, Santos-Ribeiro S, Drakopoulos P, De Vos M, Racca A, Mackens S, Thorrez Y, Verheyen G, Tournaye H, Quintero L.. et al. Predicting suboptimal oocyte yield following GnRH agonist trigger by measuring serum LH at the start of ovarian stimulation. Hum Reprod 2019;34:2027–2035. [DOI] [PubMed] [Google Scholar]
- Vaughan DA, Harrity C, Sills ES, Mocanu EV.. Serum estradiol:oocyte ratio as a predictor of reproductive outcome: an analysis of data from >9000 IVF cycles in the Republic of Ireland. J Assist Reprod Genet 2016;33:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, Woodworth KR, Nahabedian JF 3rd, Azziz-Baumgartner E, Gilboa SM, CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team. et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status – United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1641–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.