Abstract
Objective
To estimate the mortality and length of stay in the intensive care unit (ICU) attributable to clinically important gastrointestinal bleeding in mechanically ventilated critically ill patients.
Design
Three strategies were used to estimate the mortality attributable to bleeding in two multicentre databases. The first method matched patients who bled with those who did not (matched cohort), using duration of ICU stay prior to the bleed, each of six domains of the Multiple Organ Dysfunction Score (MODS) measured 3 days prior to the bleed, APACHE II score, age, admitting diagnosis, and duration of mechanical ventilation. The second approach employed Cox proportional hazards regression to match bleeding and non-bleeding patients (model-based matched cohort). The third method, instead of matching, derived estimates based on regression modelling using the entire population (regression method). Three parallel analyses were conducted for the length of ICU stay attributable to clinically important bleeding.
Setting
Sixteen Canadian university-affiliated ICUs.
Patients
A total of 1666 critically ill patients receiving mechanical ventilation for at least 48 hours.
Measurements
We prospectively collected data on patient demographics, APACHE II score, admitting diagnosis, daily MODS, clinically important bleeding, length of ICU stay, and mortality. Independent adjudicators determined the occurrence of clinically important gastrointestinal bleeding, defined as overt bleeding in association with haemodynamic compromise or blood transfusion.
Results
Of 1666 patients, 59 developed clinically important gastrointestinal bleeding. The mean APACHE II score was 22.9 ± 8.6 among bleeding patients and 23.3 ± 7.7 among non-bleeding patients. The risk of death was increased in patients with bleeding using all three analytic approaches (matched cohort method: relative risk [RR]= 2.9, 95% confidence interval (CI)= 1.6–5.5; model-based matched cohort method: RR = 1.8, 95% CI = 1.1–2.9; and the regression method: RR = 4.1, 95% CI = 2.6–6.5). However, this was not significant for the adjusted regression method (RR = 1.0, 95% CI = 0.6–1.7). The median length of ICU stay attributable to clinically important bleeding for these three methods, respectively, was 3.8 days (95% CI = -0.01 to 7.6 days), 6.7 days (95% CI = 2.7–10.7 days), and 7.9 days (95% CI = 1.4–14.4 days).
Conclusions
Clinically important upper gastrointestinal bleeding has an important attributable morbidity and mortality, associated with a RR of death of 1–4 and an excess length of ICU stay of approximately 4–8 days.
Keywords: critical care, gastrointestinal bleeding, length of stay, matching mortality, regression analysis, stress ulceration
Introduction
Major upper gastrointestinal bleeding is a well-recognized complication of critical illness, prompting three decades of randomized trials of stress ulcer prophylaxis [1]. Clinically important upper gastrointestinal bleeding is defined as macroscopic bleeding that results in haemodynamic instability or the need for red blood cell transfusion [2,3]. In heterogeneous ICU patients, clinically important bleeding rates are low, but high risk groups are those requiring mechanical ventilation for longer than 48 hours [4] or those who have a coagulopathy [2,4,5,6,7]. Histamine-2-receptor antagonists reduce the incidence of clinically important bleeding from 4 to 2% in high risk mechanically ventilated patients [8].
Although early reports of stress ulcer bleeding demonstrated the frequent need for emergency surgery and indicated a high mortality rate [9], the impact of clinically important gastrointestinal bleeding in recent years appears to be more modest. While randomized trials of prophylaxis have shown that prophylaxis can reduce bleeding rates by up to 50%, they have not shown a decrease in mortality or duration of ICU stay [10]. Nevertheless, when events do occur, they may still be serious. In a small, matched cohort study, we found a trend toward a higher risk of mortality associated with clinically important bleeding (RR = 1.14, 95% CI = 0.7–2.0), and a trend toward an increased length of ICU stay of 6.5 days (95% C = -12.3 to 25.3 days) [11].
The objectives of this study were to more precisely estimate the attributable mortality and length of ICU stay in mechanically ventilated patients who develop clinically important gastrointestinal bleeding. To better understand the influence of different analytic methods on the estimation of burden of illness, we used three statistical approaches. The rationale for using three different methods is that the study objectives involve estimation of bleeding-attributable mortality and length of stay in the ICU, based on observational data. Because the occurrence of bleeding is obviously not a randomized event, comparison of patients with and without bleeds is potentially subject to confounding and interaction with other variables. Modelling the effects of these additional variables is not straightforward, and there is not one 'correct' approach. We selected three methods that involve different assumptions, each with advantages and disadvantages. Consistency of the mortality and length of stay results between methods would be an indication of their robustness to assumptions, and hence would provide more confidence of their validity.
Methods
Two multicentre databases were used for this analysis. The first included 1200 patients mechanically ventilated for ≥ 48 hours enrolled in a double-blind, concealed randomized trial of sucralfate versus ranitidine to determine rates of gastrointestinal bleeding and ventilator-associated pneumonia (VAP) [8]. Exclusion criteria were an admitting diagnosis of gastrointestinal bleeding or pneumonia, gastrectomy, predicted survival <72 hours, or more than two prior doses of stress ulcer prophylaxis. For these analyses, we also excluded patients who died, were discharged or had gastrointestinal bleeding in the first 48 hours; therefore, 1077 patients were included from this study.
The second database was from a prognosis study of 2252 patients who were prospectively followed to determine the incidence and risk factors of clinically important bleeding [4]. Patients were ineligible if they had gastrectomy, facial trauma, epistaxis, brain death, or gastrointestinal bleeding 48 hours prior to admission. A subset of 64 of these patients contributed to our preliminary analysis of attributable morbidity and mortality of clinically important bleeding described earlier [11]. For the current analyses, we excluded patients who were not mechanically ventilated for at least 48 hours, and those who died, were discharged or had gastrointestinal bleeding within 48 hours following ICU admission; therefore, 589 patients were included from this study.
In total, we considered 1666 mechanically ventilated patients from these two databases for analyses of the mortality and length of ICU stay associated with clinically important bleeding. In both studies, signs of overt gastrointestinal bleeding included haematemesis, bloody nasogastric aspirate, melena or haematochezia. Management was at the discretion of the ICU team and endoscopy was performed when clinically indicated. All relevant clinical, laboratory and diagnostic notes and documents were reviewed in duplicate and independently by a Bleeding Adjudication Committee using previously employed reproducible criteria [2,3].
We defined clinically important bleeding as overt bleeding plus one of the following four features in the absence of other causes. The first feature was a spontaneous drop of systolic or diastolic blood pressure of 20 mmHg or more within 24 hours of upper gastrointestinal bleeding. Second, an orthostatic increase in pulse rate of 20 beats per minute and a decrease in systolic blood pressure of 10 mmHg. The third feature was a decrease in haemoglobin of at least 2 g/dl (20 g/l) in 24 hours and transfusion of 2 U packed red blood cells within 24 hours of bleeding. The final feature was failure of the haemoglobin to increase by at least the number of units transfused minus 2 g/dl (20 g/l). That is, if 8 g/dl (80 g/l) haemoglobin and 4 U packed cells were infused, the bleed would be considered important if the haemoglobin did not rise by at least 2 g/dl (20 g/l) to 10 g/dl (20 g/l).
Demographic data collected included patient characteristics, admission diagnosis, and APACHE II score [12] and daily MODS [13]. MODS combines measures of physiologic dysfunction in six domains: the cardiovascular system (heart rate × right atrial pressure/mean arterial pressure), the pulmonary system (PaO2/FiO2 ratio), the renal system (serum creatinine), the hepatic system (serum bilirubin), the haematologic system (platelet count), and the central nervous system (Glasgow Coma Score). These continuous variables comprise six domains; a score of 0 represents normal function, and a score of 4 reflects marked physiologic derangement. The scores in each domain provide a measure of dysfunction in the system of interest, and the composite scores provide a measure of global dysfunction each day in the ICU.
Analysis
We expressed continuous variables by the mean ± standard deviation when they were normally distributed, or the median and interquartile range (IQR) when they were not. Student's t test was used to compare continuous variables and the chi-square test was used to compare proportions. To allow comparisons of attributable length of ICU stay in two databases, we expressed ICU length of stay using medians and 95% CIs [14], estimating the standard deviation as three-quarters of the interquartile range [15]. All statistical tests were two-tailed.
To provide estimates of the mortality and length of ICU stay attributable to clinically important bleeding, three statistical methods (described in the following) were applied to both databases. There were no significant differences in age, sex or baseline APACHE II score between patients in the first and second databases, and the results of the attributable mortality and length of ICU stay associated with bleeding were similar (P > 0.05). We therefore pooled the estimates from these databases and provide overall results.
ICU mortality attributable to clinically important bleeding
Matched cohort method
In the first approach, we matched individual patients who bled with control patients who did not bleed, but were otherwise similar with respect to their risk of death. We chose the variables for matching, and their order, based on prior studies and physiologic rationale. We matched each patient with clinically important bleeding with one non-bleeding patient according to the following hierarchy: duration of ICU stay prior to the bleed, MODS domains measured 3 days prior to the bleed (central nervous system, renal, respiratory, haematologic, cardiovascular, and hepatic components separately) (± 1), baseline APACHE II score (± 4 points), age (± 10 years), admitting diagnosis, and duration of mechanical ventilation prior to the bleed. If there was more than one match for a bleeding patient, the control patient was selected by the best match according to the matching hierarchy. Using this algorithm, we identified matches for all bleeding patients. The RR of death and its associated 95% CI was calculated using a stratified Mantel-Haenszel RR where each stratum was a matched pair [16]. We then estimated the absolute risk of mortality attributed to bleeding as the mortality in patients with bleeding minus the mortality in the matched patients without bleeding. CIs for the absolute attributable mortality and the statistical test determining whether it was significantly different from zero was based on a matched analysis [17].
Model-based matched cohort method
The second approach also relied on matching. However, variables for the match were empirically determined from the databases. We performed a stepwise Cox proportional hazards regression analysis [18] among patients who did not bleed, using risk of death as the dependent variable. We considered variables for matching in the order indicated by the regression analysis. Variables that were significant in the stepwise procedure at the P < 0.10 level were considered for the final model. For the first database, these variables were age, diagnosis, duration of ventilation, and MODS (cardiovascular, pulmonary, renal, haematologic and central nervous system components). For the second database, the variables were age, baseline APACHE II score, diagnosis, duration of ventilation, and MODS (cardiovascular, pulmonary, renal and central nervous system components).
We censored all patients who were alive in the ICU for longer than 28 days. If patients were transferred to another ward or hospital, or discharged home, we assumed that they were alive and also censored them at 28 days. Patients transferred to another ICU or who were withdrawn from the study were censored on their last day of follow-up or at 28 days, whichever came first. The beta coefficients from the final model were used to create a score that was then used to match each bleeding patient with a non-bleeding patient. In the case of a tie, we used the same approach as already described for the matched cohort method. The absolute attributable mortality was also calculated as described for the matched cohort method.
Regression method
The third approach did not use matching. We performed Cox proportional hazards regression on all patients, using risk of death as the dependent variable. We considered the same variables described in the model-based matched cohort analysis, and our approach to censoring was identical. Independent variables were measured at baseline (age, baseline APACHE II score, and admitting diagnosis) and in a time-dependent manner (ventilation status, six MODS domains, and bleeding status). We calculated the RR of mortality associated with bleeding, adjusted for the other independent variables. We evaluated the assumption of proportional hazards of mortality risk that was attributable to bleeding by including the interaction of bleeding with time as an additional predictor.
Length of ICU stay attributable to clinically important bleeding
We used the same three approaches to calculate the attributable length of stay associated with clinically important bleeding as we did for mortality.
Matched cohort method
For the matched cohort approach, we matched on mortality status first, in addition to the variables described in the attributable mortality analysis. We estimated the absolute attributable length of ICU stay as the median difference in the length of ICU stay between each matched pair, with a 95% CI [17]. We then repeated the matching process and estimation of length of ICU stay but without matching on mortality status.
Model-based matched cohort method
In the second approach for estimating attributable length of ICU stay, the dependent variable was rate of discharge or death. We censored all patients who were transferred to another ICU, withdrew from the study or were followed for 99 days. The beta coefficients from the final model were used to create a score that was then used to match each bleeding patient with a non-bleeding patient. In the case of a tie, we used the same approach as described for the matched cohort mortality analysis. This matching procedure was conducted twice: considering and then not considering mortality as a possible predictor in the regression.
Regression method
For the third approach, we performed a Cox proportional hazards regression as previously described, using time to discharge or death as the dependent variable, censoring patients as already indicated. We then adjusted for all other independent variables and report the median attributable length of stay [19].
Results
Among 1666 mechanically ventilated patients, 59 had clinically important gastrointestinal bleeding (3.5%, 95% CI = 2.7–4.6%). Bleeding and non-bleeding patients were similar in age (mean ± SD: 62.5 ± 15.0 years versus 59.0 ± 17.7 years, P = 0.14), sex (32.2% versus 39.8% female, P = 0.24), and APACHE II score (mean ± SD: 22.9 ± 8.6 versus 23.3 ± 7.7, P = 0.67). There was a significant difference in the length of ICU stay (median, 26 days [IQR = 18–38] versus 8 days [IQR = 5–15], P < 0.0001) and mortality (45.8% versus 20.9%, P < 0.0001) between bleeding and non-bleeding patients.
Attributable mortality
The results are reported in Table 1. The crude comparison of bleeding versus non-bleeding patients yielded a RR of 2.2 (95% CI = 1.6–2.9) and an absolute risk of 24.0% (95% CI = 11.3–36.6). Figure 1 shows the survival curve of mechanically ventilated patients who have clinically important bleeding compared with those who do not. Using the matched cohort method, the RR of mortality attributable to clinically important gastrointestinal bleeding was significant (RR = 2.9, 95% CI = 1.6–5.5). The mortality attributable to bleeding was also statistically significant using the model-based matched cohort method (RR = 1.8, 95% CI = 1.1–2.9). The attributable mortality using the regression method was RR = 4.1 (95% CI = 2.6–6.5) without adjustment for covariates, and RR = 1.0 (95% CI = 0.6–1.7) after adjustment.
Table 1.
Intensive care unit (ICU) mortality attributable to clinically important gastrointestinal bleeding
| ICU mortality | Relative risk (95% confidence interval) | Absolute risk (95% confidence interval) |
| Crude comparison | 2.2 (1.6–2.9) | 24.0 (11.3–36.6) |
| Matched cohort method | 2.9 (1.6–5.5) | 30.3 (15.2–45.3) |
| Model-based matched cohort method | 1.8 (1.1–2.9) | 20.3 (4.3–36.4) |
| Regression method | 4.1 (2.6–6.5) | - |
| Adjusted* | 1.0 (0.6–1.7) | - |
The model-based matched cohort probably yields the best estimate of the attributable mortality. See Table 3 for advantages and disadvantages of these methods. * Adjusted for age, APACHE II score, admitting diagnosis, duration of ventilation, Multiple Organ Dysfunction Score, and bleeding status.
Figure 1.
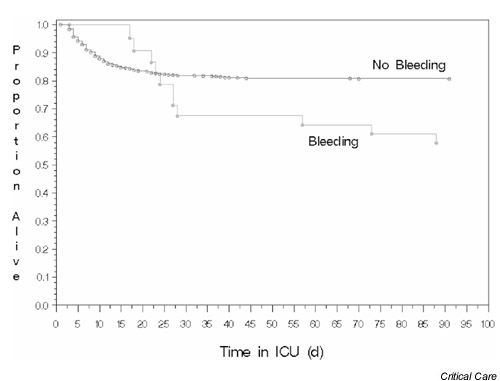
The survival curves for patients with and without clinically important gastrointestinal bleeding. ICU, intensive care unit; d, days.
We also tested whether the risk of mortality attributable to bleeding was constant over time, using the unadjusted regression method. We found that patients who bled earlier in their ICU stay had a lower risk of dying than patients who bled later (P = 0.02): the RR of mortality associated with clinically important bleeding was 0.4 (95% CI = 0.06–2.0) at 2 weeks, 1.6 (95% CI = 0.6–4.0) at 3 weeks, and 7.4 (95% CI = 1.7–32.2) at 4 weeks. One overall estimate of risk of death associated with bleeding in the regression method may therefore be misleading. Note, however, that the other two methods (matched cohort and model-based matched cohort) also calculate overall risk measures of the bleeding effect, but do not take into account or examine potential variation in risk over time. In view of the findings in the regression method, the overall results of the other two methods, which do not take time into account, may also be potentially misleading.
Attributable length of ICU stay
Figure 2 shows the distribution of length of ICU stay among bleeding and non-bleeding patients using all 1666 patients (P < 0.0001). The crude comparison of bleeding and non-bleeding patients yielded an attributable length of ICU stay of 17.2 days (95% CI = 13.2–21.3 days) overall (Table 2). Using the matched cohort method, we found that the attributable length of ICU stay for patients with clinically important bleeding was not significantly increased, at 0.6 days (95% CI = -5.1 to 6.3 days) in non-survivors and 3.8 days (95% CI = -0.01 to 7.6 days) overall. The attributable ICU stay using the model-based matched cohort method was 5.1 days (95% CI = 0.3–9.9 days) incorporating mortality as a possible predictor of length of stay, and 6.7 days (95% CI = 2.7–10.7 days) overall, indicating a significant increase. The regression method showed an increase in ICU length of stay of 7.9 days (95% CI = 1.4–14.4 days) without adjusting for other variables. After adjusting for age, APACHE II score, admitting diagnosis, ventilation status, and six MODS domains, the ICU stay attributable to bleeding by the regression method was 6.2 days (95% CI = 1.0–11.4 days).
Figure 2.
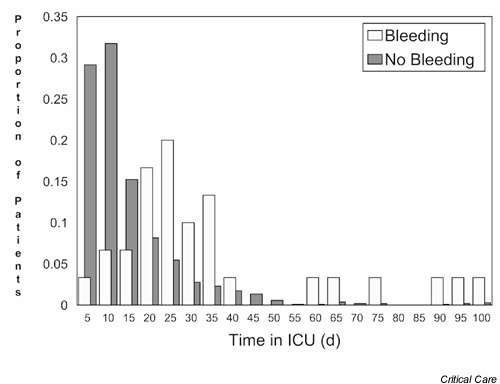
The distributions of the length of intensive care unit (ICU) stay for patients with and without clinically important gastrointestinal bleeding. d, days.
Table 2.
Length of intensive care unit (ICU) stay attributable to clinically important gastrointestinal bleeding
| ICU length of stay | Median difference |
| (days) | (95% confidence interval) |
| Crude comparison | |
| Non-survivors | 14.0 (8.6–19.4) |
| Overall | 17.2 (13.2–21.3) |
| Matched cohort method | |
| Non-survivors | 0.6 (-5.1–6.3) |
| Overall | 3.8 (-0.01–7.6) |
| Model-based matched cohort method | |
| Matching on mortality status | 5.1 (0.3–9.9) |
| Overall | 6.7 (2.7–10.7) |
| Regression method | |
| Unadjusted | 7.9 (1.4–14.4) |
| Adjusted* | 6.2 (1.0–11.4) |
The model-based matched cohort probably yields the best estimate of the length of ICU stay. See Table 3 for advantages and disadvantages of these methods. * Adjusted for age, APACHE II score, admitting diagnosis, duration of ventilation, Multiple Organ Dysfunction Score, and bleeding status.
Discussion
We found that clinically important bleeding was associated with an increase in mortality (absolute risk increase, 20–30%; RR increase, 1–4) and an increased length of ICU stay (approximately 4–8 days) using three different analytic methods. We used two different control groups for matching in the two matched cohort methods to improve the robustness of the results [20]. To control for age, illness severity, admitting diagnosis, ventilation status, bleeding status and organ dysfunction, we used multivariate methods. We considered the potential bias of ICU-acquired confounding factors [21] by controlling for mechanical ventilation status and organ dysfunction in all six MODS domains as time-dependent variables until 3 days prior to the bleed. We used the RR to generate more clinically interpretable and conservative associations than those expressed by odds ratios [22]. We presented median rather than mean durations of ICU stay to avoid inflated estimates of length of stay [23]. We used objective criteria for clinically important upper gastrointestinal bleeding, which were determined in duplicate by blinded adjudicators unaware of this study hypothesis [24].
Other studies have estimated the attributable mortality and length of ICU stay for complications of critical illness such as catheter-related infection [21,25,26,27]. Interpreting such studies requires examination of issues such as population characteristics, case definition, and statistical strategies. In considering statistical strategies to determine the attributable mortality of another ICU outcome (VAP), two basic approaches have been used: logistic regression and comparative methods (with or without matching). The regression method commonly used to determine the attributable mortality of a condition such as VAP considers mortality status as a dependent variable, then selects independent variables (including VAP and other events) to test their association with mortality. Regression analysis can potentially yield distorted associations by selection of only a subset of variables that influence mortality risk, and by omission of key variables that would alter the findings if they were included. Nevertheless, in sufficiently powered studies, VAP was found to be an independent predictor of mortality after adjusting for other characteristics and ICU-acquired events using regression analysis [28,29,30,31].
In addition to regression methods, studies of the attributable morbidity and mortality of VAP have been conducted using comparative methods. In addition to issues of population differences and case definition [32], another explanation for the discordant results is the analytic approaches used. These approaches include unmatched crude comparison of patients with and without VAP [33], simple matching of patients with and without VAP based on baseline characteristics [34], matching of patients based on baseline characteristics and discharge diagnosis [35], and matched cohort analyses that incorporate baseline characteristics and time-dependent variables that could influence outcome [32,36,37,38].
Our analysis of the attributable morbidity and mortality of clinically important gastrointestinal bleeding used both comparison and regression methods. In the absence of a well-accepted single approach or guidelines for an optimal analysis, we summarize the advantages and disadvantages of the methods used to estimate the attributable mortality and length of ICU stay associated with clinically important bleeding (Table 3). The crude comparison method ignores the influence of confounding factors and inevitably yields inaccurate estimates. The three other approaches (the matched cohort method, the model-based matched cohort method, and the regression method) are superior to a crude comparison method because adjustment for potentially important confounding factors is more likely to yield valid estimates.
Table 3.
Advantages and disadvantages of different approaches to estimating attributable mortality and length of intensive care unit stay
| Method | Advantages | Disadvantages |
| Crude comparison | Simple | Ignores influence of confounding factors, possibly |
| yielding biased estimates | ||
| Matched cohort method | Integrates biologic rationale for matching | May fail to adjust for important confounding factors, |
| patients; can be used in multiple databases; | possibly yielding biased estimates; compared with | |
| compared with regression method, avoids bias | regression model, event rate over time is not | |
| if event rate is not constant over time | considered | |
| Model-based matched cohort | Analysis customized to the database; compared | Compared with matched cohort method, chance |
| method | with crude or matched cohort method, more | associations may generate biased estimates due to |
| likely to adjust for important confounding factors; | 'overmatching'; compared with regression model, | |
| compared with regression method, avoids bias | event rate over time is not considered | |
| if event rate is not constant over time | ||
| Regression method | Analysis customized to the database; uses all | Complex; compared with matched cohort method, |
| patient data; considers patterns of events and | chance associations may generate biased estimates | |
| predictors over time and generates most | due to 'overmatching'; biased estimates may also | |
| precise estimates if event rate is constant | result if event rate is not constant over time | |
| over time |
The advantages and disadvantages of several approaches to estimating the attributable mortality and length of intensive care unit stay associated with clinically important bleeding are presented (a crude comparison of bleeding and non-bleeding patients, and the three methods used in these analyses).
Although the matched cohort method is founded on biologic rationale to match patients, clinical judgement may sometimes fail to adjust for all important determinants of outcome. In contrast, both the model-based matched cohort method and the regression method allow for adjustment of additional potentially important confounders. Moreover, they both can be customized to the database in which they are developed. Finally, there is the issue of the effect of bleeding over time. The matched cohort method and model-based matched cohort method both use the Mantel-Haenszel technique, which is founded on an assumption of constant odds over time. The initial regression method used Cox proportional hazards to estimate the risk of mortality, which generates valid estimates if the assumption of proportional hazard holds (e.g. if the risk is stable over time). However, when we tested whether the risk of mortality attributable to bleeding was constant over time using the regression model, we found that it was not. When bleeding occurred in the first 3 weeks of ICU stay, there was a trend toward a decreased risk of death in bleeding patients compared with non-bleeding patients. When bleeding occurred 4 weeks or longer following ICU admission, the risk of death was significantly increased in bleeding patients compared with non-bleeding patients. This interesting finding may reflect the more serious pathophysiology of late onset bleeding due to a longstanding ischaemic gastropathy, which is often considered a manifestation of multiple organ dysfunction [13].
In summary, in this population of 1666 ICU patients ventilated for >48 hours, we have demonstrated that clinically important gastrointestinal bleeding is associated with a significant increase in attributable mortality (full range of RRs, 1–4) and length of ICU stay (approximately 4–8 days). These results build on previous work estimating the clinical and economic consequences of bleeding in which we used a hierarchical matched cohort study of 64 patients. We previously found a trend toward an increased risk of mortality (RR = 1.14, 95% CI = 0.7–2.0), and a trend toward an increased length of ICU stay of 6.5 days (95% CI = -12.3 to 25.3 days) associated with clinically important bleeding [11]. We also found that each episode resulted in a mean of seven additional haema-tology tests, 11 blood product transfusions, and 24 days of treatment, resulting in an overall cost of clinically important bleeding of $12,000. This analysis is limited in that patients who were admitted to ICU with a diagnosis of pneumonia or patients who had two or more doses of prophylaxis were excluded from the first database. Although it possible that the attributable morbidity and mortality of clinically important bleeding in such patients may differ from other ICU patients ventilated for at least 48 hours, this seems very unlikely. Of course, these results do not show that bleeding events themselves directly lead to increased length of stay or death; thus, causation cannot be inferred from these analyses.
In two multicentre studies, the Canadian Critical Care Trials Group has found that clinically important bleeding occurs in 4% of mechanically ventilated patients [4,8]. However, bleeding rates vary; 2.4% of patients had macroscopic bleeding in one study in the Netherlands, in which all patients received selective digestive decontamination, dopamine, nitroglycerin, and ketanserin while mechanically ventilated, and tapering doses of dexamethasone [39]. Prevention may be unnecessary in populations in which clinically important bleeding is documented to be very rare, [40], and thus bleeding prophy-laxis strategies may need to be customized to different settings. A formal cost-effectiveness analysis would best address the impact of alternative stress ulcer prophylaxis policies including targeted prevention for patients at highest risk, which requires accurate estimates and a plausible range of estimates for the target event. The analyses we report for the attributable length of ICU stay and mortality associated with clinically important bleeding can thus be integrated with evidence about incidence, risk factors, and the advantages and disadvantages of various preventive strategies, to better understand the consequences of different approaches to stress ulcer prophylaxis.
Competing interests
None declared.
Abbreviations
CI = confidence interval; ICU = intensive care unit; IQR = interquartile range; MODS = Multiple Organ Dysfunction Score; RR = relative risk; VAP = ventilator-associated pneumonia.
Acknowledgments
Acknowledgements
The authors would like to thank the nurses, pharmacists, research associates and physicians of the Canadian Critical Care Trials Group who supported this study, and Durk Zandstra for interesting discussions on this issue. DJC holds a Chair from the Canadian Institutes for Health Research, MOM is a Scholar of the Medical Research Council of Canada, and DKH is a Career Scientist of the Ontario Ministry of Health. This study was funded by the Medical Research Council of Canada, Hoechst Marion Roussel Incorporated, and Merck Sharpe and Dome.
References
- Hastings PR, Skillman JJ, Bushnell LS, Silen W. Antacid titration in the prevention of acute gastrointestinal bleeding: a controlled randomized trial in 100 critically ill patients. N Engl J Med. 1978;298:1041–1045. doi: 10.1056/NEJM197805112981901. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Pearl RG, Cook RJ, Guyatt GH. Incidence of clinically important bleeding in mechanically ventilated patients. J Intensive Care Med. 1991;6:167–174. [Google Scholar]
- Cook DJ, Witt LG, Cook RJ, Guyatt GH. Stress ulcer prophylaxis in the critically ill: a meta-analysis. Am J Med. 1991;91:519–527. doi: 10.1016/0002-9343(91)90189-5. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Fuller HD, Guyatt GH, Marshall JC, Leasa D, Hall R, Winton TL, Rutledge F, Todd TJR, Roy P, Lacroix J, Griffith L, Willan AH, for the Canadian Critical Care Trials Group Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med. 1994;330:377–381. doi: 10.1056/NEJM199402103300601. [DOI] [PubMed] [Google Scholar]
- Schuster DP, Rowley H, Feinstein S, McGue MK, Zuckerman GR. Prospective evaluation of the risk of upper gastrointestinal bleeding after admission to a medical intensive care unit. Am J Med. 1984;76:623–630. doi: 10.1016/0002-9343(84)90286-9. [DOI] [PubMed] [Google Scholar]
- Kamada T, Fusamoto H, Kawano S, Noguchi M, Hiramatsu K. Gastrointestinal bleeding following head injury: a clinical study of 433 cases. J Trauma. 1977;17:44–47. [PubMed] [Google Scholar]
- Lacroix J, Nadeau D, Laberge S, Gauthier M, Lapierre G, Farrell CA. Frequency of upper gastrointestinal bleeding in a pediatric ICU. Crit Care Med. 1992;20:35–42. doi: 10.1097/00003246-199201000-00013. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Guyatt GH, Marshall J, Leasa D, Fuller H, Hall R, Peters S, Rutledge F, Griffith L, McLellan A, Wood G, Kirby A, Tweeddale M, Pagliarello G, Johnston RH, for the Canadian Critical Care Trials Group A comparison of sucralfate and ranitidine for prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N Engl J Med. 1998;338:791–797. doi: 10.1056/NEJM199803193381203. [DOI] [PubMed] [Google Scholar]
- Skillman JJ, Bushell LS, Goldman H, Silen W. Respiratory failure, hypotension, sepsis and jaundice: a clinical syndrome associated with legal hemorrhage from acute stress ulceration of the stomach. Am J Surg. 1969;117:523–530. doi: 10.1016/0002-9610(69)90011-7. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Reeve BK, Guyatt GH, Griffith LE, Heyland DK, Buckingham L, Tryba M. Stress ulcer prophylaxis in critically ill patients: Resolving discordant meta-analyses. JAMA. 1996;275:308–314. [PubMed] [Google Scholar]
- Heyland D, Gafni A, Griffith L, Cook D, Marshall J, Fuller H, Todd T, Gustlits B, Heule M, Hewson J, Lacroix J, Noseworthy T, Powles P. The clinical and economic consequence of clinically important gastrointestinal bleeding in critically ill patients. Clin Intens Care. 1996;7:121–125. [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–825. [PubMed] [Google Scholar]
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. The multiple organ dysfunction (MOD) score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- Keeping ES. Introduction to Statistical Inference Princeton, NJ: D Van Nostrad; 1962. p. 206.
- Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care. 1990;6:5–30. doi: 10.1017/s0266462300008916. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S. Applications of stratified analysis methods. In Modern Epidemiology Edited by Rothman KJ, Greenland S Philadelphia, PA: Lippincott-Raven Publishers; 1998.
- Gardner MJ, Altman DG. Calculating confidence intervals for proportions and their differences. In Statisticswith Confidence Edited by Gardner MJ, Altman DG London: British Medical Society; 1989. pp. 31–32.
- Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data New York, NY: John Wiley and Sons; 1980.
- Lee ET. StatisticalMethods for Survival Data Analysis New York, NY: Wiley-Interscience Publications; 1992. pp. 88–95.
- Hayden GF, Kramer MS, Horwitz RI. The case-control study: a practical review for the clinician. JAMA. 1982;247:326–331. [PubMed] [Google Scholar]
- Soufir L, Timsit JF, Mahe C, Carlet J, Regnier B, Chevret S. Attributable morbidity and mortality of catheter-related septicemia in critically ill patients: a matched, risk-adjusted, cohort study. Infect Control Hosp Epidemiol. 1999;20:392–394. doi: 10.1086/501639. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- Weissman C. Analyzing intensive care unit length of stay data: problems and possible solutions. Crit Care Med. 1997;25:1594–1600. doi: 10.1097/00003246-199709000-00031. [DOI] [PubMed] [Google Scholar]
- Laupacis A, Sekar N, Stiell I. Clinical prediction rules: a review and suggested modifications of methodologic standards. JAMA. 1997;277:488–494. [PubMed] [Google Scholar]
- DiGiovine B, Chenoweth C, Watts C, Higgins M. The attributable mortality and costs of primary nosocomial bloodstream infections in the intensive care unit. Am J Resp Crit Care. 1999;160:976–981. doi: 10.1164/ajrccm.160.3.9808145. [DOI] [PubMed] [Google Scholar]
- Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients: excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271:1598–1601. doi: 10.1001/jama.271.20.1598. [DOI] [PubMed] [Google Scholar]
- Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital-acquired candidemia: the attributable mortality and excess length of stay. Arch Intern Med. 1998;148:2642–2645. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- Kollef MH. Ventilator-associated pneumonia: a multivariate analysis. JAMA. 1993;270:1965–1970. [PubMed] [Google Scholar]
- Kollef MH, Silver P, Murphy DM, Trovillion E. The effect of late-onset ventilator-associated pneumonia in determining patient mortality. Chest. 1995;108:1655–1662. doi: 10.1378/chest.108.6.1655. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Bihari JD, Suter PM, Bruining HA, White J, Nicolas-Chanion MH, Wolff M, Spencer RC, Hemmer M. The prevalence of nosocomial infection in intensive care units in Europe: results of the European prevalence of infection in intensive care (EPIC) study. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]
- Fagon JY, Chastre J, Vuagnat A, Trouillet JL, Novara A, Gibert C. Nosocomial pneumonia and mortality among patients in intensive care units. JAMA. 1996;275:866–869. [PubMed] [Google Scholar]
- Heyland DK, Cook DJ, Griffith LE, Keenan SP, Brun-Buisson CH, for the Canadian Critical Care Trials Group The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. Am J Respir Crit Care Med. 1999;159:1249–1256. doi: 10.1164/ajrccm.159.4.9807050. [DOI] [PubMed] [Google Scholar]
- Cunnion KM, Weber DJ, Broadhead WE, Hanson LC, Peiper CF, Rutala WA. Risk factors for nosocomial pneumonia: comparing adult critical care populations. Am J Resp Crit Care Med. 1996;153:358–362. doi: 10.1164/ajrccm.153.1.8542110. [DOI] [PubMed] [Google Scholar]
- Craig CP, Connelly S. Effect of intensive care unit nosocomial pneumonia on duration of stay and mortality. Am J Infect Control. 1984;12:233–238. doi: 10.1016/0196-6553(84)90114-7. [DOI] [PubMed] [Google Scholar]
- Baker AM, Meredith JW, Haponik EF. Pneumonia in intubated trauma patients. Am J Resp Crit Care Med. 1996;153:343–349. doi: 10.1164/ajrccm.153.1.8542141. [DOI] [PubMed] [Google Scholar]
- Kappstein I, Schulgen G, Beyer U, Geiger L, Schumacher M, Daschner FD. Prolongation of hospital stay and extra costs due to ventilator-associated pneumonia in an intensive care unit. Eur J Clin Microbiol Infect Dis. 1992;11:504–508. doi: 10.1007/BF01960804. [DOI] [PubMed] [Google Scholar]
- Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94:281–288. doi: 10.1016/0002-9343(93)90060-3. [DOI] [PubMed] [Google Scholar]
- Papazian L, Bregeon F, Thirion X, Gregoire R, Saux P, Denis JP, Perin G, Charrel J, Dumon JF, Affray JP, Gouin F. Effect of ventilator-associated pneumonia on mortality and morbidity. Am J Resp Crit Care Med. 1996;154:91–97. doi: 10.1164/ajrccm.154.1.8680705. [DOI] [PubMed] [Google Scholar]
- Zandstra DF, Stoutenbeek CP. The virtual absence of stress-ulceration related bleeding in ICU patients receiving prolonged mechanical ventilation without any prophylaxis. Int Care Med. 1994;20:335–340. doi: 10.1007/BF01720905. [DOI] [PubMed] [Google Scholar]
- Tryba M. Stress ulcer prophylaxis – quo vadis? Intensive Care Med. 1994;20:311–313. doi: 10.1007/BF01720900. [DOI] [PubMed] [Google Scholar]


