Abstract
Background
Hydroxychloroquine has shown potential to block viral replication of SARS-CoV-2 in some in vitro studies. This randomised, double-blinded, placebo controlled clinical trial evaluated the efficacy of hydroxychloroquine plus azithromycin (HCQ/AZT) in reducing viral loads in patients with early and mild SARS-CoV-2 infection.
Methods
A single-centre randomised placebo-controlled clinical trial was conducted with outpatients with early and mild SARS-CoV-2 infection. Inclusion criteria were: patients aged 18–65 years with symptoms suggestive of COVID-19 for < 5 days, no significant comorbidities, and positive nasopharyngeal/oropharyngeal swab screening tests (POCT-PCR). Randomised patients received either hydroxychloroquine for 7 days plus azithromycin for 5 days or placebo. The primary endpoint was viral clearance within a 9-day period. Secondary endpoints included viral load reduction, clinical evolution, hospitalization rates, chest computed tomography evolution, and adverse effects.
Results
From 107 potential trial participants, 84 were enrolled following predetermined criteria. Statistical analyses were performed on an intention-to-treat (N = 84) and per-protocol (PP) basis (N = 70). On the PP analysis, the treatment (N = 36) and placebo (N = 34) groups displayed similar demographic characteristics. At 95% CI, no statistically significant between-group differences were found in viral clearance rates within 9 days following enrolment (P = 0.26).
Conclusions
This randomised, double-blinded, placebo-controlled clinical trial evaluating outpatients with early and mild COVID-19 showed that viral clearance rates within a 9-day period from enrolment did not change with HCQ/AZT treatment compared with placebo, although no major cardiovascular events were observed in participants without comorbidities. Secondary outcomes were also not significantly improved with HCQ/AZT treatment compared with placebo. These findings do not support use of HCQ/AZT in this setting.
Keywords: COVID-19, SARS-CoV-2, RCT, Hydroxychloroquine, Azithromycin
1. INTRODUCTION
At the time of writing over 173 million confirmed cases of the novel coronavirus disease (COVID-19) have been reported globally, resulting in severe acute respiratory syndrome caused by SARS-CoV-2 infection, leading to over 3.7 million deaths [1]. Although clinical research for potential therapies and vaccines is underway in several countries, there is currently no effective treatment. Many drugs have been proposed as antiviral agents – including chloroquine, hydroxychloroquine (HCQ), arbidol, remdesivir, and favipiravir – with potential to inhibit SARS-CoV-2 replication in vitro [2,3]. Chloroquine and HCQ were proposed as promising candidates for treatment after a small open-label, non-randomised clinical trial conducted in hospitalised patients associated their use with higher and faster virological clearance compared with the placebo group [4]. Those data were supported by in vitro studies showing the antiviral effect of chloroquine analogues against several viruses, including SARS-CoV-2 [3,5]. Some studies have also suggested that azithromycin (AZT) might have in vitro activity against the same virus [6] by a different mechanism, with potential additive effect [7]. Clinically, AZT has been used for acute infections in chronic inflammatory diseases due to its immunomodulatory effects [8]. A Spanish randomised clinical trial using HCQ/AZT in outpatients with mild COVID-19 did not show virological and clinical benefits [9]. Other observational and randomized studies evaluating virological and clinical endpoints have suggested no beneficial effect of HCQ in hospitalised patients with mild to moderate or severe infection by SARS-CoV-2 [9], [10], [11], [12], [13], [14], [15], [16]. Finally, a systematic review concluded that the association of AZT with HCQ did not show any benefit, although all included studies were conducted in hospitals [17].
This paper reports the results of a randomised, double-blinded, placebo-controlled clinical trial of the association of HCQ/AZT in outpatients with early and mild COVID-19, evaluating the viral clearance in nasopharyngeal/oropharyngeal swabs and clinical improvement.
2. METHODS
2.1. Study design and participants
This study was designed as a prospective, double blinded, placebo-controlled, randomised clinical trial in accordance with The Consolidated Standards of Reporting Trials (CONSORT) Statement, approved by the local ethics and research committee, and registered at REBEC (30413020.8.0000.0008). Written informed consent was obtained from all subjects and it followed the 1964 Declaration of Helsinki (amended mostly recently in 2008) of the World Medical Association. It was conducted at a single centre: Hospital Santa Paula located in São Paulo, Brazil. Participants were enrolled after providing written consent in a hospital emergency room setting. Inclusion criteria were: aged 18–65 years, with mild symptoms suggestive of COVID-19, with an interval from symptom onset to enrolment of 2–5 days, and detection of viral RNA in nasopharyngeal/oropharyngeal swabs through a real-time reverse-transcription polymerase chain (RT-PCR) reaction screening point-of-care test (POCT-PCR). The clinical picture suggestive of COVID-19 was defined as two or more of the following: cough, fever, shortness of breath, nausea/vomiting, diarrhoea, body aches, weakness/fatigue, headache, sore throat, runny nose/congestion, and sudden gustatory or olfactory loss. Exclusion criteria included: known hypersensitivity to HCQ or AZT, pre-existing pulmonary disease, history of immunosuppression, active cancer diagnosis, pregnancy or lactation, history of cardiac abnormalities or QTc prolongation (QTc > 480 ms), known glucose-6-phosphate dehydrogenase (G6PD) deficiency, patients requiring hospital admittance, and patients with inadequate haematological parameters, heart, renal, or liver function.
ClinicalTrials.gov definition of serious adverse events was followed, defined as adverse events that result in death, a life-threatening adverse event, inpatient hospitalisation, and persistent or significant incapacity or substantial disruption of the ability to conduct normal functions [18]. Complete information on the inclusion and exclusion criteria is provided in the Supplementary Appendix. All study data were collected and managed using the Research Electronic Data Capture (REDCap) system [19]. All data from the participants were adequately anonymised.
2.2. Sample size calculation
The sample size was calculated using the nSurvival routine from R software [20]. This sample was calculated considering the survival analysis with the Lachin and Foulkes method (1986) [21] based on the follow-up period, recruitment time and hazard ratio, comparing control and intervention groups. The following assumptions were considered: study period of 30 days, recruitment period of 30 days, statistical significance level of 5%, 85% power, and 30% of follow-up losses. For the effect size, viral clearance rates after 4 treatment days were assumed based on Gautret et al. [4]: 83.3% in the treatment group and 25% in the control group. The sample size calculation resulted in 42 participants for each study arm (84 total number of participants).
2.3. Molecular testing
At the enrolment visit, nasopharyngeal/oropharyngeal swabs were collected and combined into 3 mL sterile saline for testing by POCT-PCR. An aliquot of 300 µL was submitted to RNA extraction in the Veri-Q PREP M16 equipment using the Viral DNA/RNA Prep Kit – Airway Clinical Sample (Micobiomed, Korea) with an elution volume of 50 µL. Amplification and detection were performed using the Veri-Q PCR 316 real-time thermocycler using the Coronavirus disease 2019 (COVID-19) Detection Kit (Micobiomed). Both systems and kits work together as a small footprint integrated system, suitable for simultaneously running 1–6 samples in approximately 2 hours. This kit targets two SARS-CoV-2 genomic regions: the N gene and ORF 3a, in addition to an internal control added at the PCR step. Cycle threshold (Ct) values < 35 in any of the two genes were assigned as positive, while Ct values 35–40 were considered ‘indeterminate’, according to the manufacturer's instructions.
An aliquot from the emergency department day 0 sample and all samples from the subsequent home visits at 3, 6 and 9 days were processed at the molecular biology lab from Diagnósticos da América S.A. (Dasa, Brazil). An aliquot of 200 µL was extracted by the DSP Virus/Pathogen kit in the automated platform QIAsymphony and eluted in 60 µL; 5 µL of eluate was submitted to RT-PCR with primers and probe from the viral E gene in duplex to the cellular control RNAseP, as described [22], employing TaqMan Fast Virus 1-Step Master Mix (ThermoFisher, Brazil). A Ct value of 35 was adopted as the cut-off. This system is referred to as RT-PCR to distinguish it from the POCT-PCR platform used in initial screening. The limit of detection was determined as 408 copies/mL by probit analysis using the ACCUPLEX SARS-COV-2 reference material (0505-0126, Seracare, USA).
2.4. Randomisation and double-blinding
Eligible patients were randomly assigned (1:1) to receive either HCQ/AZT or placebo. Patients, treating clinicians, and study personnel were all blinded to study group assignment. An independent pharmacist dispensed all trial medications (or placebo) according to a computer-generated randomisation list. The therapeutics and placebo were provided in capsule form and were identical in appearance. Capsules were pre-packed in envelopes and consecutively numbered for each participant according to the randomisation schedule. Double-blinding was maintained throughout the trial.
2.5. Interventions
Following randomisation, the participants had blood samples collected and were prescribed appropriately. Treatment group participants received two 200 mg HCQ capsules twice a day (bid) for a total course of 7 days (i.e. 28 capsules in total) and one 500 mg AZT capsule taken on day 1, followed by one 250 mg AZT capsule daily for the next 4 days (i.e. six capsules in total). The placebo capsules were formulated to have similar size, shape, colour and taste as HCQ and AZT capsules, and with an identical dosing regimen. The first dose of medication was given orally to all patients in the emergency department under pharmacist supervision and the remaining doses ingested at home. As standard of care, no vitamin or mineral supplements were prescribed. Participants also received instructions regarding social distancing and hygiene to be followed at home and were given 24/7 telephonic access to clinical staff in case of questions, symptom evolution or adverse effects. At their homes, participants received telephonic check-up calls on days 3, 6, 9 and between 14–21 days following enrolment, when they responded to symptom and adverse effect questionnaires. Participants also received visits from diagnostic staff on days 3, 6 and 9 who collected blood samples, performed electrocardiograms, and collected nasopharyngeal/oropharyngeal swabs for RT-PCR testing. Between days 14–21, participants returned to a diagnostic facility where an additional chest computed tomography was performed followed by the telephonic check-up as before.
All of the nasopharyngeal specimens of the included patients that were collected to perform the study screening enrolment POCT-PCR were also submitted for PCR testing in a central lab in order to obtain the day-0 viral load, in order to apply the same molecular method for the primary and secondary virological outcomes. Additionally, enrolment samples were submitted to a molecular assay comprising a panel of 20 respiratory pathogens (FilmArray, BioMerieux, Brazil) to investigate co-infection with other viral and bacterial agents. Complete information of the trial procedures and examinations performed at each key date is provided in the Supplementary Appendix.
2.6. Outcomes
The primary outcome was the time (days) to viral clearance within a 9-day evaluation period following enrolment after the onset of symptoms and the study enrolment dates. Viral clearance was defined as a Ct > 35 by the described RT-PCR assay. Secondary outcomes of interest included: viral load reduction, improvement of symptoms, hospitalisation rates, and adverse effects to the trial medications.
2.7. Statistical analysis
Quantitative variables were described by mean and standard deviation. Categorical variables were described by frequency and proportion. The baseline quantitative variables were described for the total of enrolled patients and the two intervention groups were compared using Student's t test (for variables observed to have a normal distribution) or using Mann-Whitney test (for variables observed not to have a normal distributed). The categorical variables were compared using χ2 test. The primary and secondary outcomes were evaluated both by intention-to-treat (ITT) analyses and per-protocol (PP) analysis. The PP analysis was used as the primary basis to evaluate intervention response. The Ct value variable analysis was made using generalized linear models in order to assess intergroup response. A Kaplan-Meier survival analysis was used to compare the end of symptoms mean time and the mean time until the observation of non-detectable viral load. The proportion of individuals with different clinical symptom intensity was compared between the two groups with the χ2 test in each analysed moment. The adopted level of statistical significance was 5%. SPSS version 23.0 software was used to perform the statistical analysis.
3. RESULTS
3.1. Patients
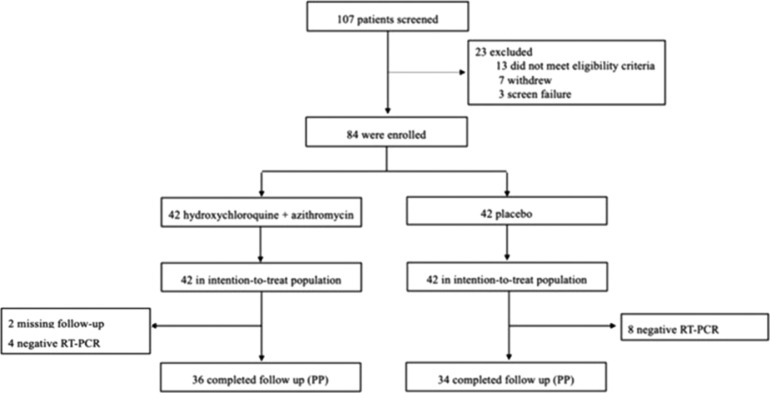
Between 12 April and 13 May 2020, 107 patients were screened, of whom 84 were eligible (Figure 1 ). Thirteen patients were excluded because they did not meet the eligibility criteria, seven withdrew written informed consent, and three had screening failures. Among the enrolled patients, 12 participants (four in the treatment group and eight in the placebo group) were found to have negative confirmatory RT-PCR results on the screening date and all subsequent dates, despite being positive on the POCT-PCR screening test on day 0 and were excluded from the PP analysis. In addition, among the remaining trial participants, two participants from the treatment group failed to continue with trial procedures after day 6 and were also excluded from the PP analysis. The two groups had similar characteristics at baseline, as shown in Table 1 .
Figure 1.
case-selection flowchart.
Note: PP: Per-Protocol
Table 1.
Demographic and Clinical Characteristics at Baseline (Intention-to-treat)
| Characteristic | TreatmentGroup (N=42) | ControlGroup (N=42) | p-value |
|---|---|---|---|
| Age (years), mean ± SD | 36.3 ± 9.8 | 36.8 ± 9.5 | 0.81 |
| Gender (female), n (%) | 16 (38.1) | 18 (42.9) | 0.66 |
| Ethnicity, n (%)WhiteMixedBlackOther | 19 (45.2)17 (40.5)2 (4.8)4 (9.5) | 25 (59.5)13 (31.0)3 (7.1)1 (2.4) | 0.34 |
| Reported prior exposure to COVID-19, n (%) | 17 (40.5) | 17 (40.5) | 1.00 |
| Co-infection with other respiratory pathogens* | 0 (0) | 0 (0) | - |
| Time since onset of symptoms (days), mean ± SD | 3.8 ± 1.0 | 3.6 ± 1.2 | 0.57 |
| Symptoms at baseline, n (%)CoughFeverMyalgiaFatigueAnosmiaDiarrhea | 30 (71.4)24 (57.1)26 (61.9)23 (54.8)25 (59.5)12 (28.6) | 37 (88.1)26 (61.9)34 (80.9)28 (66.7)24 (57.1)9 (21.4) | 0.220.480.160.720.660.71 |
| O2 Saturation, % ± SD | 97.8 ± 1.6 | 97.9 ± 1.2 | 0.84 |
| Heart rate at rest (1/m) | 76.3 ± 11.9 | 74.9 ± 11.8 | 0.59 |
| Respiratory rate | 17.2 + 1.4 | 16.7 + 1.2 | 0.129 |
| Laboratory results, mean ± SDHemoglobin g/dLLymphocytes (/µL)Ferritin, ng/mlLactate dehydrogenase, U/LD-dimer, ng/mlAspartate aminotransferase U/LAlanine aminotransferase U/L | 15,1 ± 1.41,554.0 ± 633.0286.6 ± 206.5186.7 ± 36.10.386 ± 0.10629.4 ± 12.848.5 ± 28.3 | 15.0 ± 1.41,635.3 ± 707.1239.7 ± 207.1185.1 ± 37.90.402 ± 0.30427.4 ± 9.738.9 ± 17.3 | 0.900.740.230.820.190.470.17 |
| Chest CT est. extent of parenchyma involvement, n (%)NormalLess than 25%Greater than 25% | 18 (42.9)24 (57.1)0 (0) | 28 (66.7)13 (31.0)1 (2.4) | 0.05 |
RT-PCR-BioFire®Filmarray: Influenza A/B, respiratory syncytial virus, non–SARS-CoV-2 Coronaviridae, adenovirus, parainfluenza 1-4, human metapneumovirus, rhinovirus/enterovirus, Chlamydophila pneumoniae, Mycoplasma pneumoniae, Bordetella pertussis
3.2. Primary endpoints
The HCQ/AZT and placebo groups viral clearance rates within 9 days following enrolment were similar in ITT analysis (P = 0.85). Although there was a numerical difference in the PP analysis, it was not statistically significant (P = 0.26), as summarised in Table 2 .
Table 2.
SARS- CoV-2 viral clearance according to intervention
 |
3.3. Secondary endpoints
There were no significant differences between the HCQ/AZT and placebo groups in the viral load reduction, as measured by the SARS-CoV-2 gene E Ct values at days 3, 6 or 9 (Table 3 ). Regarding the clinical response to treatment, there were no statistically significant differences in the time to improvement of any symptoms evaluated after 21 days (Table 4 ). However, the proportion of patients with absence of cough symptom on day 6 in the HCQ/AZT group compared with placebo was statistically significant (ITT: 66.7% vs. 38.1%, P = 0.01; PP: 66.7% vs. 32.4%, P = 0.002, respectively). During the follow-up, one patient in the treatment group was hospitalised due to nonspecific symptoms, but no evidence of clinical or laboratory deterioration of COVID-19 was detected. Another patient in the placebo group had bloodstream infection due to Streptococcus parasanguinis on day 16 and was treated with antimicrobial therapy for 7 days (3 of them in a day hospital). Prolongation of the QTc interval on day 9 was more common in patients in the treatment group than the placebo group (ITT: 406.5 ms vs. 398.3 ms, P = 0.024; PP: 406.1 ms vs. 397.5 ms, P = 0.069) but there was no drug withdrawal due to this event in both groups. No serious adverse events related to the use of HCQ/AZT were observed during the study.
Table 3.
Comparison of gene E Ct values between control and treatment groups
| Intention to Treat |
Per Protocol |
||||||
|---|---|---|---|---|---|---|---|
| Analysis | Treatment Group | Control Group | p-value | Treatment Group | Control Group | p-value | |
| N | 42 | 42 | 36 | 34 | |||
| Ct gene E analyses | |||||||
| Day 0 | 21.55 (7.65) | 23.64 (10.33) | 0.586 | 20.00 (5.4) | 19.78 (7.22) | 0.485 | |
| Day 3 | 29.50 (8.03) | 28.99 (8.25) | 28.67 (7.52) | 26.39 (6.94) | |||
| Day 6 | 32.33 (7.36) | 33.72 (6.20) | 31.97 (6.96) | 32.34 (6.09) | |||
| Day 9 | 36.57 (5.67) | 36.16 (5.35) | 36.92 (4.76) | 35.26 (5.58) | |||
Table 4.
Clinical outcomes after 21 days
| ITT |
PP |
|||||
|---|---|---|---|---|---|---|
| Symptom | Treatment Group (N=42) | Control Group (N=42) | p-value | Treatment Group (N=36) | Control Group (N=34) | p-value |
| Average (SD) | ||||||
| Cough | 12.5 (0.8) | 14.2 (0.7) | 0.12 | 12.5 (0.9) | 14.4 (0.8) | 0.09 |
| Sore throat | 10.2 (0.9) | 9.8 (0.8) | 0.77 | 10.7 (1.0) | 10.0 (0.9) | 0.66 |
| Nasal congestion | 12.1 (0.8) | 12.4 (0.7) | 0.86 | 12.6 (0.8) | 11.9 (0.7) | 0.47 |
| Coryza | 12.8 (0.9) | 13.1 (0.8) | 0.63 | 12.8 (1.0) | 12.0 (0.8) | 0.20 |
| Sneezing | 13.1 (0.9) | 12.8 (0.8) | 0.60 | 13.3 (0.9) | 12.2 (0.9) | 0.33 |
| Anosmia | 13.9 (0.7) | 15.5 (0.6) | 0.09 | 14.2 (0.7) | 16.0 (0.6) | 0.06 |
| Loss of appetite | 13.1 (0.7) | 12.1 (0.8) | 0.49 | 13.1 (0.7) | 12.2 (0.9) | 0.65 |
| Headaches | 11.7 (0.7) | 12.6 (0.8) | 0.55 | 11.5 (0.7) | 12.5 (0.9) | 0.41 |
| Fever | 7.8 (0.6) | 8.0 (0.4) | 0.61 | 7.5 (0.5) | 8.0 (0.4) | 0.29 |
| Myalgia | 11.8 (0.7) | 11.7 (0.8) | 0.73 | 11.3 (0.7) | 11.4 (0.9) | 0.67 |
| Fatigue | 14.1 (0.7) | 13.5 (0.6) | 0.26 | 14.4 (0.7) | 12.9 (0.7) | 0.11 |
| Malaise | 11.2 (0.7) | 10.0 (0.6) | 0.19 | 11.0 (0.8) | 9.7 (0.6) | 0.14 |
| Vomiting | 8.8 (1.5) | 9.2 (1.2) | 0.68 | 8.8 (1.5) | 7.3 (1.2) | 0.54 |
| Nausea | 12.8 (0.8) | 11.8 (0.8) | 0.30 | 12.1 (0.7) | 11.0 (0.7) | 0.28 |
| Diarrhea | 12.8 (0.6) | 10.8 (1.4) | 0.28 | 12.3 (0.6) | 10.5 (1.5) | 0.38 |
| Patients Without Symptom (%) | ITT |
PP |
||||
| Treatment Group (N=42) | Control Group (N=42) | p-value | Treatment Group (N=36) | Control Group (N=34) | p-value | |
| Cough D0 | 28.6% | 11.9% | 0.10 | 30.6% | 11.8% | 0.08 |
| D3 | 42.9% | 33.3% | 0.37 | 41.7% | 32.4% | 0.42 |
| D6 | 66.7% | 38.1% | 0.01 | 66.7% | 32.4% | 0.02 |
| D9 | 73.8% | 61.9% | 0.24 | 75.0% | 58.8% | 0.15 |
| D14 | 83.3% | 73.8% | 0.28 | 86.1% | 73.5% | 0.19 |
| Fever D0 | 42.9% | 38.1% | 0.66 | 41.7% | 32.4% | 0.42 |
| D3 | 95.2% | 81.0% | 0.04 | 97.2% | 79.4% | 0.02 |
| D6 | 92.9% | 95.2% | 0.64 | 94.4% | 94.1% | 0.95 |
| D9 | 97.6% | 97.6% | 1.00 | 100.0% | 97.1% | 0.31 |
| D14 | 97.6% | 100.0% | 0.31 | 100.0% | 100.0% | 1.00 |
| Hospitalization rate N (%) | 1 (2.4) | 0 | - | 1(2.8) | 0 | - |
| Serious adverse events* | 1 (2.4) | 0 | - | 1(2.8) | 0 | - |
| Death | 0 | 0 | - | 0 | 0 | - |
The serious adverse event observed was hospitalization due to dyspnea related (with normal respiratory rate and O2 saturation)
4. DISCUSSION
In this randomised, double-blinded, placebo-controlled clinical trial evaluating outpatients with early and mild COVID-19 treated with HCQ/AZT or placebo, there was no benefit in the treatment arm on primary and secondary outcomes. The study population comprised adult patients aged < 65 years, with no comorbidities and mild symptom onset 2–5 days prior to enrolment. Viral clearance rates within a 9-day period from symptom onset were similar in both groups, showing that the HCQ/AZT combination is not effective in reducing viral shedding and thus unlikely to minimise SARS-CoV-2 rate of transmission.
Different HCQ dosing regimens have been used in several studies. This medication is known to have a very long half-life (11–50 days) and with lung concentrations exceeding 400-fold plasma concentrations at steady-state [23]. Although physiologically-based pharmacokinetic models based on in vitro and in vivo data have suggested a high loading dose of 400 mg bid. for the first 4 days followed by a lower maintenance dose of 200 mg bid. for > 4 days [24] after this trial, a wide range of doses were used for patients with different severities and in different setups [17, 25].
No statistically significant differences were found in clinical outcomes (Figure 2) or on viral clearance rates within 9 days following enrolment between the HCQ/AZT and placebo groups in both ITT (P = 0.85) and PP (P = 0.26) analysis (Table 2).
The findings are similar to those observed in a published non-placebo controlled randomised trial from Spain. In this multicentric study, 157 patients with COVID-19 were enrolled to receive HCQ and 136 to receive usual care within 5 days from symptom onset. No significant reduction in viral load of nasopharyngeal swabs between arms at day 3 or 7 or clinical improvement was observed [9]. Also, other observational and randomised studies evaluating virological and clinical endpoints have suggested no beneficial effect of HCQ in hospitalised patients with mild-to-moderate or severe infection by SARS-CoV-2, and a systematic review concluded that the association of AZT with HCQ did not show any benefit. It is believed that this is the first published study corroborating these results in outpatients with early and mild COVID-19.
No major cardiovascular events related to HCQ/AZT use were observed; however, prolongation of the QTc interval was more common in the treatment group than in the placebo group. It is worth mentioning that the patients included in this study were already expected to have a low risk for cardiovascular events, considering their age and lack of comorbidities. Older patients mainly with structural cardiovascular disease might present with higher risk, as observed in the Coalition controlled trial study including 667 hospitalised patients with mild-to-moderate COVID-19, in which there was no clinical improvement in patients receiving HCQ alone or associated with AZT. However, prolongation of QTc interval was more common in patients using HCQ with or without AZT [13].
An unexpected loss of 12 (14.3%) participants due to discrepancies between POCT-PCR and RT-PCR tests in the same enrolment sample may be attributed to variations in sensitivity, fluctuations in samples with very low viral load, amplicon contamination, or other non-identified variables. This POCT-PCR was adopted for the screening of study candidates in order to allow for a faster recruitment, as RT-PCR testing has a much longer turnaround time for results (approximately 72 hours). In the study period, this was the only compact real-time PCR method available in Brazil. Well-known systems like the GenXpert (Cepheid) or ID-Now (Abbott) became available weeks after the end of the study. Since the POCT-PCR is less automated and requires certain manual steps and liquid transfers, it is more susceptible to carry-over, which may have contributed to the discrepancies pointed above, resulting in the exclusion of 12 participants in the PP analysis. However, these exclusions did not change the overall conclusions since the analysis of ITT and PP groups essentially showed the same results.
This study had some limitations. It was performed in a single centre and with loss of 14.3% of the participants. Participants were aged 18–65 years, without comorbidities and in early stage of the disease, which may have limited the generalisation of the findings; therefore, caution should be taken when extrapolating to other populations.
HCQ has garnered a large number of supporters during the pandemic, despite several studies showing its ineffectiveness [9,10,13,16,17,26,27]. These results could help the scientific community to further judge the benefits and risks for drugs with potential viral activity when used in the early stage of COVID-19. Other drugs should be evaluated in combination with other strategies aimed at improving outcomes in these patients.
Acknowledgments
ACKNOWLEDGEMENTS
The authors acknowledge the relevant contributions of Carlo Padovano, João Paulo Juvêncio, Gustavo Riedel, Valdecir Marvulle, Aleocidio S Balzanelo, Sonia Rogeri, Artur Brito Santos, and Carla Fernandes representing the hospital pharmaceutical team in this project.
DECLARATIONS
Funding: This study was funded by Diagnósticos da América S.A. (Dasa), Ímpar Serviços Hospitalares S.A., and DNA Capital Foundation.
Competing Interests: All authors report no conflicts of interest relevant to this article.
Ethical Approval: The present study was designed as a prospective, double blinded, placebo-controlled, randomised clinical trial, in accordance with The Consolidated Standards of Reporting Trials (CONSORT) Statement, approved by the local ethics and research committee, registered at REBEC (30413020.8.0000.0008),
Editor: Dr Jim Gray
Footnotes
Registered at REBEC under the number 30413020.8.0000.0008 (http://www.ensaiosclinicos.gov.br/rg/RBR-95yjmq/)
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2021.106428.
Appendix. Supplementary materials
REFERENCES
- 1.Organization GWH. WHO coronavirus disease (COVID-19) dashboard. Available at: https://covid19.who.int/[accessed 07 June 2021 ].
- 2.Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Cao R, Xu M, Wang X, Zhang X, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Disc. 2020;6(1):1–4. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Touret F, Gilles M, Barral K, Nougairéde A, van Helden J, Decroly E, et al. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci Reports. 2020;10(1):1–8. doi: 10.1038/s41598-020-70143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID-19. Clin Pharmacol Therapeut. 2020;108(2):201–211. doi: 10.1002/cpt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann P, Ziesenitz VC, Curtis N, Ritz N. The immunomodulatory effects of macrolides—a systematic review of the underlying mechanisms. Front Immunol. 2018;9:302. doi: 10.3389/fimmu.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitjà O, Corbacho-Monné M, Ubals M, Tebe C, Penafiel J, Tobias A, et al. Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial. Clin Infect Dis. 2020;2000:ciaa1009. doi: 10.1093/cid/ciaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020:369. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Eng J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VA, Avezum A, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Eng J Med. 2020;383(21):2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahevas M, Tran V-T, Roumier M, Chabrol A, Paule R, Guillaud C, et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. Medrxiv. 2020 [Google Scholar]
- 15.Million M, Lagier J-C, Gautret P, Colson P, Fournier P-E, Amrane S, et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trial R. No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19. Press release from the chief investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial (https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19). 2020 [last accessed 07 Jun 2021].
- 17.Ghazy RM, Almaghraby A, Shaaban R, Kamal A, Beshir H, Moursi A, et al. A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment. Sci Reports. 2020;10(1):1–18. doi: 10.1038/s41598-020-77748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov. ClinicalTrials.gov “Basic Results” Data Element Definitions. 2013. Available at: http://prsinfo.clinicaltrials.gov/results_definitions.html#AdverseEventsDefinition). [accessed 07 June 2021].
- 19.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Informatics. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Team RC. R Foundation for Statistical Computing; Vienna, Austria: 2013. R: A language and environment for statistical computing. 2013; Supplementary Figure S 2015; 2. [Google Scholar]
- 21.Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics. 1986:507–519. [PubMed] [Google Scholar]
- 22.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicol MR, Joshi A, Rizk ML, Sabato PE, Savic RM, Wesche D, et al. Pharmacokinetics and Pharmacological Properties of Chloroquine and Hydroxychloroquine in the Context of COVID-19 Infection. Clin Pharmacol Therapeut. 2020;108(6):1135–1149. doi: 10.1002/cpt.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perinel S, Launay M, Botelho-Nevers É, Diconne É, Louf-Durier A, Lachand R, et al. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients. Clin Infect Dis. 2020;71(16):2227–2229. doi: 10.1093/cid/ciaa394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Eng J Med. 2020;383(6):517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Principi N, Esposito S. Chloroquine or hydroxychloroquine for prophylaxis of COVID-19. Lancet Infect Dis. 2020;20(10):1118. doi: 10.1016/S1473-3099(20)30296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.