Abstract
The proliferating cell nuclear antigen (PCNA) is a highly conserved cellular protein that functions both in DNA replication and in DNA repair. Exposure of a rat embryo fibroblast cell line (CREF cells) to γ radiation induced simultaneous expression of PCNA with the p53 tumor suppressor protein and the cyclin-dependent kinase inhibitor p21WAF1/Cip1. PCNA mRNA levels transiently increased in serum-starved cells exposed to ionizing radiation, an observation suggesting that the radiation-associated increase in PCNA expression could be dissociated from cell cycle progression. Irradiation of CREF cells activated a transiently expressed PCNA promoter chloramphenicol acetyltransferase construct through p53 binding sequences via a mechanism blocked by a dominant negative mutant p53. Electrophoretic mobility shift assays with nuclear extracts prepared from irradiated CREF cells produced four p53-specific DNA-protein complexes with the PCNA p53 binding site. Addition of monoclonal antibody PAb421 (p53-specific) or AC238 (specific to the transcriptional coactivator p300/CREB binding protein) to the mobility shift assay distinguished different forms of p53 that changed in relative abundance with time after irradiation. These findings suggest a complex cellular response to DNA damage in which p53 transiently activates expression of PCNA for the purpose of limited DNA repair. In a population of nongrowing cells with diminished PCNA levels, this pathway may be crucial to survival following DNA damage.
The cellular response to genotoxic agents includes an increase in the level and the activity of p53 tumor suppressor protein (references 28, 53, and 58 and references therein). Upon activation, p53 inhibits replication of the genome under unfavorable conditions by regulating cell cycle progression and cell viability, thereby preventing proliferation of cells with damaged genes. The high incidence of p53 mutations in human tumors suggests that these activities are central to tumor suppression.
The functions of p53 largely depend on its ability to both activate and repress transcription (28, 53, 58). Identification of target genes transcriptionally activated by p53 provides understanding of the biological effects of p53. Among p53-inducible genes, the p21WAF1/cip1 gene encodes a protein that inhibits cyclin-dependent kinase activity and leads to G1 growth arrest (23, 36), and the mdm-2 gene encodes a protein that prevents transcriptional activation by p53 (102) and accelerates p53 degradation (37, 55). In some cells, p53-mediated transcriptional activation of bax gene expression correlates with induction of programmed cell death (69). Alternatively, p53 promotes apoptosis by activating genes that alter the cellular redox status (83).
Transcriptional activation by p53 requires the N-terminal transactivation domain and the core sequence-specific DNA binding domain (28, 53, 58). p53 specifically binds DNA at a pair of 10-nucleotide repeats with the consensus sequence 5′-PuPuPuC(A/TA/T)GPyPyPy-3′ (24), and mutations in the core DNA binding domain (amino acids 100 to 300) of p53 prevent transcription activation (28, 53, 58). The C terminus of p53 negatively regulates specific DNA binding, but perturbation of this negative effect can be achieved by a variety of means, including C-terminal phosphorylation, acetylation, and interactions with other proteins (28, 29, 53). Wild-type p53 generally represses transcription of genes that do not harbor a specific p53 binding site (28), and some evidence suggests that the interaction between p53 and TATA box binding protein mediates transcriptional repression (28). p53 overexpression correlates with transcriptional repression (17, 62, 95), which may contribute to the process of apoptosis (11). Consistent with this view, proteins that block apoptosis, such as E1B 19K and bcl-2 (85, 88), also prevent transcriptional repression by p53.
Interaction with the transcriptional coactivator p300/CBP (CREB binding protein) participates in transcriptional activation and repression by p53 (2, 30, 60, 86). Activation of transcription via p300/CBP appears to be mediated, in part, through a histone acetyltransferase activity that can remodel chromatin to increase the accessibility of genes to the transcription machinery (3, 79). The viral oncoproteins adenovirus E1A and simian virus 40 large T antigen both interact with p300/CBP (21, 22, 61, 106) and through that interaction repress the transcriptional activation by p53 (84, 93, 94). The acetyltransferase activity of p300/CBP also acetylates the C-terminal regulatory region of p53 and thereby enhances specific DNA binding (29).
Proliferating cell nuclear antigen (PCNA) is a highly conserved processivity factor for DNA polymerases δ and ɛ (52). In addition, PCNA interacts with several other DNA replication and repair factors, such as the primer recognition complex replication factor C (76), endonuclease Fen-1 (103), DNA ligase I (57), and the xeroderma pigmentosum group G protein (27). In DNA repair assays in vitro, PCNA participates in both nucleotide excision repair and mismatch repair (47). These observations suggest that PCNA serves as a docking site for many proteins that participate in DNA replication and repair, in addition to increasing the efficiency of DNA synthesis. PCNA also directly binds two p53-inducible proteins, GADD45 and p21 (92, 105, 107), and these interactions may regulate PCNA-dependent DNA replication (59, 99). These observations indicate that PCNA may integrate the cellular processes that regulate DNA replication and repair. The pattern of PCNA expression in response to mitogens or genotoxic stress is consistent with this view. Agents that stimulate DNA synthesis activate PCNA expression via sequences near the site of transcription initiation (38, 56, 72, 73). PCNA is also readily detected simultaneously with p53 expression after genotoxic insult (15, 34). However, no mechanism for activation of PCNA expression in response to genotoxic stress has been elucidated.
Initial investigations of PCNA regulation by p53 demonstrated that p53, when expressed in transient cotransfection experiments (17, 44, 62, 95) or induced with a hormone-controlled system (65), had no effect or repressed expression from the PCNA promoter. Later observations from this lab and others demonstrated that wild-type p53 binds the human PCNA promoter and transactivates PCNA promoter-directed gene expression in a concentration-dependent manner; lower levels activate, whereas higher levels do not (70, 90). This concentration-dependent response of the PCNA promoter to p53 appears to reconcile previous observations of p53-mediated repression with the later results demonstrating activation. However, the effects of the levels and activities of p53 on PCNA expression during a normal biological response to DNA damage remain unclear.
p53 may directly control DNA replication and repair by modulating the levels of PCNA. In addition to the essential roles of PCNA in DNA metabolism, the aforementioned interactions of PCNA with cellular regulatory proteins establish a pathway through which p53 influences multiple cellular activities. Since previous studies examined the transcriptional regulation of PCNA by exogenously expressed p53, a more physiologically relevant study is required to establish p53-mediated transcriptional regulation of PCNA expression. The induction of a p53-dependent cellular response to ionizing radiation (IR) is well documented (28, 53, 58). In the work presented here, we exposed a rat fibroblast cell line to IR to induce p53 and examined regulation of PCNA expression postexposure.
MATERIALS AND METHODS
Plasmids.
The PCNA-chloramphenicol transferase (CAT) reporter constructs contained human PCNA promoter sequence −249 to +62 or −213 to +62 relative to the initiation site (+1) fused to the CAT reporter sequences in pBACAT as previously described (70, 73). Plasmid pCMV-DMp53 expresses a mutant p53 protein from the cytomegalovirus early promoter in the pCG expression vector (97). In comparison to the wild type, the mutant protein possesses a cysteine-to-arginine change at residue 141 and serine-to-aspartate change at residue 392 and displays dominant negative activity (5).
Cell culture.
CREF cells (26) (obtained from P. Fisher, Columbia University) were grown in Dulbecco’s modified Eagle medium (DMEM; Sigma) with 5% (vol/vol) fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 μg/ml) (Gibco BRL) in a humidified incubator with 5% CO2.
Irradiation of cells.
Cells were exposed to IR from a 137Cs source in a Gammacell 40 low-dose-rate research irradiator (Nordian International). The indicated doses, usually 12 Gy, were achieved at a rate of 1.25 Gy per min.
Transient transfection assays.
CREF cells were seeded into 6-cm-diameter dishes to be 50 to 60% confluent and transfected by the calcium phosphate-mediated method as previously described (72). Each transfection mixture contained 5 μg of the reporter plasmid. For the experiments represented in Fig. 4C, pCMV-DMp53 (5 μg) was cotransfected with the reporter plasmids. Salmon sperm DNA was added to bring the total DNA amount for each transfection to 20 μg. Six hours after transfection, the DNA precipitate was removed. The cells were subjected to a glycerol shock, and fresh medium was applied. Twenty-four hours posttransfection, the cells were irradiated with the indicated dose of IR. Twenty-four hours postirradiation, the transfected cells were harvested and CAT activity was determined as previously described (72). The CAT results were normalized to the amount of recovered protein (Bio-Rad protein assay kit). By convention, 1 CAT unit equals 1% conversion of chloramphenicol to its acetylated form in a 100-μl reaction volume by 50 μl of extract in 1 h at 37°C (71, 72).
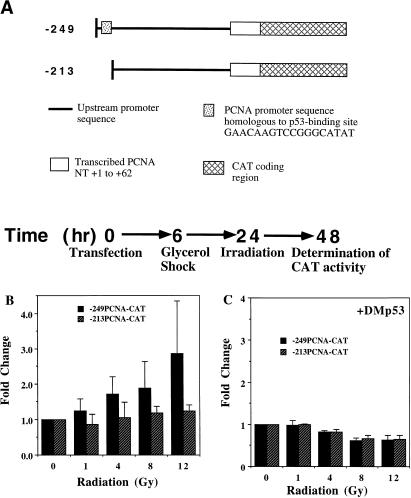
FIG. 4.
Transient transfection and irradiation in CREF cells. (A) Diagram of PCNA-CAT reporter constructs and experimental protocol. The position of the p53 binding site is indicated by the stippled box in the −249 construct. NT, nucleotides. (B) γ irradiation activates the PCNA promoter via the wild-type p53 binding site. Twenty-four hours postirradiation, the cells transfected with the −249 or −213 construct (A) were harvested, and CAT activity was determined for equal amounts of protein from each lysate. The graph shows the fold change (average ± standard error for three different experiments performed in duplicate) in CAT activity versus the indicated dose of IR (relative to unirradiated cells, 0 Gy) for transfected cells. (C) A dominant negative mutant p53 (DMp53) prevents IR-induced activation of the PCNA promoter. The protocol was the same as for panel B except that the CREF cells were cotransfected with pCMV-DMp53 and the −249PCNA-CAT or −213PCNA-CAT construct prior to irradiation. The results shown are averages of two experiments performed in duplicate.
Western blot analysis.
Cell extracts were prepared from mock-irradiated or irradiated cells at 1, 3, 8, and 24 h after exposure. Briefly, the cells were washed with cold phosphate-buffered saline and lysed in radioimmunoprecipitation assay buffer with protease inhibitors (150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 50 mM Tris [pH 8.0], aprotonin [1 μg/ml], leupeptin [1 μg/ml], E64 [1 μg/ml], phenylmethylsulfonyl fluoride [0.5 mM]). Equal amounts of protein (Bio-Rad) from each extract were fractionated in a SDS-polyacrylamide gel and then transferred to nitrocellulose (1). Equal protein transfer per lane was confirmed by reversible Ponceau S (0.2% Ponceau S, 3% trichloroacetate, 3% sulfosalicylic acid) staining of the membrane (1) prior to immunodetection. Generally, nitrocellulose membranes were preincubated in blocking solution (5% nonfat dry milk in 1× TTBS ([0.1% Tween 20 in 100 mM Tris Cl {pH 7.5}, 0.9% NaCl]) for at least 1 h. All antibodies (primary, secondary, and streptavidin-conjugated horseradish peroxidase) were diluted in blocking buffer and were incubated for 1 h with the membranes. Nitrocellulose membranes were washed at least three times, each for 15 min with fresh washing solution, after each incubation. To assess PCNA levels, the immunoblot was preblocked and then probed with a PCNA-specific mouse monoclonal antibody (19F4 [78] at 1:4,000 dilution). Detection of the bound antibody was with 125I-labeled goat anti-mouse immunoglobulin G (ICN), followed by exposure to X-ray film. Sheep polyclonal antibody Ab-7 (1:2,500 dilution) and rabbit polyclonal antibody Ab-5 (1:100 dilution) (both from Oncogene Science) were used as the primary antibodies to detect p53 and p21, respectively. After incubation with a biotinylated secondary antibody (rabbit anti-sheep for p53 or goat-anti-rabbit for p21; Jackson Immunoresearch), followed by streptavidin-conjugated horseradish peroxidase, the immunoconjugate was detected with enhanced chemiluminescence (ECL kit; Amersham) upon exposure to X-ray film. Changes were quantified by densitometric scanning of the X-ray film.
Northern blot analysis.
CREF cells were serum starved for 48 h in DMEM medium with 0.1% FBS before irradiation and maintained in low serum thereafter. At various times postirradiation, total RNA was prepared by the guanidinium-phenol method (1). Each RNA sample of 30 μg was separated in a 1% agarose-formaldehyde gel, which was then blotted to a polyvinylidene difluoride membrane (Millipore). The RNA blot was probed with a gel-purified 700-bp PstI fragment of pCR-1 containing rat PCNA cDNA (64) and a 2-kb BamHI fragment of the human β-actin cDNA (31), radiolabeled by random priming (25). Hybridization was carried out at 62°C overnight for both β-actin and PCNA mRNAs with labeled probes (approximately 4 × 106 cpm/ml) in a mixture of 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 50 mM KH2PO4 (pH 7), 5× Denhardt’s solution, and herring sperm DNA (20 μg/ml) as a carrier. Washing conditions were twice for 15 min each time in 2× SSC–0.5% SDS at room temperature; once for 15 min in 2× SSC–0.5% SDS at 62°C, and then twice for 20 min each time in 0.2× SSC–0.5% SDS at 62°C.
Nuclear extracts and electrophoretic mobility shift assay (EMSA).
Nuclear extracts from CREF cells were prepared at various times postirradiation by a method described by Osborn et al. (81). Double-stranded PCNA or p21 oligonucleotides were end labeled with [32P]dCTP for use as probes in the gel mobility shift assays. For the binding reactions, the labeled probe (104 cpm) was incubated with 15 μg of nuclear extract in the presence of 0.3 μg of poly(dI-dC) at 20°C for 30 min as previously described (70). In some experiments, 1 μl of PAb421 (Oncogene Science), 2 μl of AC238 (60), 4 μl of 2A10 monoclonal supernatant (10), or 2 μl of 19F4 (78) was preincubated with nuclear extract for 5 to 10 min before addition of the probe. The binding reaction mixtures were resolved in a nondenaturing 5% polyacrylamide gel containing (22.5 mM Tris-borate) at 100 to 160 V for 1.5 to 3 h as previously described (70). After the bromophenol blue had reached the bottom, the gel was placed on 3MM Whatman paper and dried in a vacuum. The dried gel was exposed to X-ray film, and the retarded bands were quantified by phosphorimage analysis.
RESULTS
Radiation activates PCNA expression.
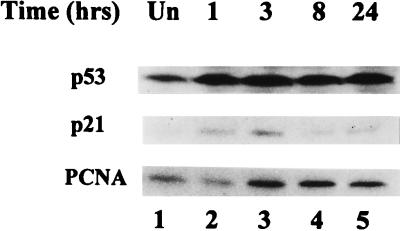
To determine the biological significance of p53-mediated regulation of PCNA expression, we used γ radiation to activate p53 in cultured cloned rat embryo fibroblasts (CREF cells). These cells are a clonal derivative of REF52 which resist gene amplification in the presence of selective drugs but become permissive for gene amplification upon inactivation of p53 function by transformation (43). Exposure of CREF cells to increasing doses of γ radiation produced a corresponding decrease in the number of viable cells (data not shown). The decline in cell number appeared to reflect reduced cell cycling, as microscopic analyses of the irradiated cells did not reveal an obvious increase in the number of dead or apoptotic cells through 72 h postirradiation (not shown). Immunoblotting of extracts from asynchronously proliferating CREF cells revealed that p53 increased upon exposure to γ radiation (Fig. 1). Cellular levels of p53 increased rapidly twofold by 24 h postirradiation. Furthermore, irradiation of CREF cells enhanced expression of p21WAF1/Cip1, a p53-inducible inhibitor of cyclin-dependent kinases (23, 36) that also directly binds PCNA and thereby inhibits DNA replication (59, 99, 105). The quantity of p21WAF1/Cip1 increased within 1 h from an undetectable amount in unirradiated cells to a maximum at 3 h postirradiation; protein production waned thereafter but was still detectable 24 h postirradiation (Fig. 1). This radiation-associated increase in levels of p21WAF1/Cip1 was consistent with previous reports indicating that exposure of cells to IR-induced transcriptional activation of p21WAF1/Cip1 by wild-type p53 (19). IR also caused an approximately twofold increase in cellular PCNA protein levels, although the change was slightly delayed relative to the radiation-induced increase in p53 and p21WAF1/Cip1 (Fig. 1). PCNA levels remained at the higher level through 24 h postirradiation.
FIG. 1.
Levels of p53, p21, and PCNA in CREF cells at various times postirradiation. Asynchronously growing subconfluent cells were exposed to 12 Gy of IR, and whole-cell lysates were prepared at the indicated times postexposure. Equal amounts of protein from each lysate were fractionated in polyacrylamide gels, which were probed by immunoblotting with specific antibodies to p53 (top), p21 (middle), and PCNA (bottom). Levels of these proteins in unirradiated CREF cells (Un; lane 1) and at 1, 3, 8, and 24 h postirradiation (lanes 2 to 5) are shown. The slight decrease in PCNA expression at 1 h postirradiation was not reproducible.
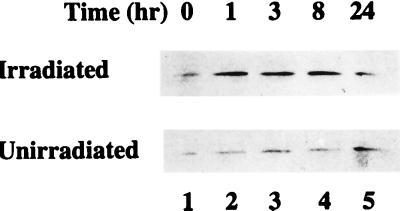
Activation of p53 blocks cell cycle progression at specific checkpoints during the G1 and G2 phases of the cell cycle (references 28 and 53 and the references therein). Consistent with this observation, exposure of human fibroblasts to IR shortly after release from contact inhibition activates the G1 checkpoint and blocks progression to S phase (20). Although failure to progress to S phase in irradiated cells correlates with reduced cyclin-dependent kinase activity, cyclins D1 and E accumulate in irradiated cells to the levels observed in unirradiated cells, whereas cyclin A, which is more specific for the transition through S phase (82), does not (20). To correlate regulation of PCNA levels in irradiated cells with these previous findings, we evaluated PCNA expression in CREF cells with a similar experimental protocol. Confluent cell cultures released from contact inhibition by replating were exposed to IR 6 h later, and cellular PCNA levels were assessed by immunoblotting at various times thereafter. The amount of PCNA increased within 1 h after exposure of the cells to IR, remained at this elevated level through 8 h, and then declined by 24 h postirradiation (Fig. 2). In comparison, PCNA protein remained at a constant low level through 8 h in mock-irradiated CREF cells similarly released from contact inhibition (Fig. 2). In the unirradiated cells, PCNA levels appear higher at 24 h, an observation consistent with progression to S phase by these cells. These observations suggest that IR provides another mechanism leading to increased PCNA expression.
FIG. 2.
IR increases cellular levels of PCNA. Confluent cultures of CREF cells were released from growth arrest by replating at 70% confluence. Six hours after replating, the cells were mock irradiated or exposed to 12 Gys of γ radiation. Cell lysates were prepared in radioimmunoprecipitation assay buffer from the unirradiated and irradiated cells, and equal amounts of protein from each lysate were assessed for PCNA levels by immunoblotting. Top and bottom panels show immunoblots indicating PCNA levels in irradiated and unirradiated CREF cells, respectively, at the indicated times postexposure.
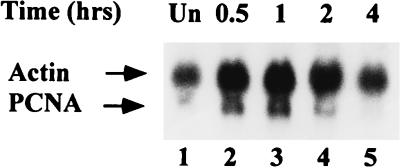
IR can enhance translation of p53 mRNA (75). By analogy, the IR-associated increase in PCNA shown in Fig. 1 and 2 might be achieved through a translational mechanism. PCNA mRNA is abundant in continuously cycling cells, with only slight fluctuations as the cells progress through the cell cycle (8, 74). Nevertheless, cellular levels of PCNA mRNA correlate with growth, and nongrowing cells contain little detectable PCNA mRNA (8). To deplete PCNA mRNA and thereby highlight its elevation associated with radiation, CREF cells were maintained in low serum for 2 days prior to exposure to γ radiation. Total RNA was prepared from the serum-starved cells before and 0.5, 1, 2, and 4 h after exposure to IR at 12 Gy. Equal amounts of RNA from each preparation were probed on a Northern blot for PCNA mRNA as well as for β-actin mRNA (Fig. 3). Cells maintained in low serum possessed very low levels of PCNA mRNA (lane 1). After irradiation, the amount of PCNA mRNA increased by 30 min (lane 2), peaked at 1 h (lane 3), and declined to the level observed in unirradiated cells by 4 h postirradiation (lane 5). Although we observed some similar variations in the amount of the β-actin mRNA, the PCNA mRNA levels increased postirradiation approximately two- to threefold relative to the β-actin mRNA control at the 1-h time point. The rapid radiation-associated increase in PCNA mRNA reflects the similarly rapid elevation in PCNA protein described above.
FIG. 3.
PCNA mRNA levels in irradiated CREF cells. Total cell RNA was prepared from unirradiated (lane 1) or irradiated (lanes 2 to 5) CREF cells kept in DMEM containing 0.1% FBS at the indicated times postexposure to 12 Gys of IR. Each sample (30 μg) was fractionated in a formaldehyde-agarose gel that was subsequently transferred to a polyvinylidene difluoride membrane. The blot was probed by hybridization to radioactive cDNA probes specific for β-actin and PCNA. The hybridized filter was exposed to X-ray film with an intensifying screen for 48 h.
IR activates PCNA expression via p53 binding sequences.
Previous observations that p53 bound and activated expression from the PCNA promoter (70, 90) suggested that the enhanced PCNA mRNA and protein expression in irradiated cells could be p53 dependent. To test whether radiation can activate expression from the PCNA promoter via p53, we performed transient expression assays with PCNA-CAT constructs with and without an intact p53 binding site (Fig. 4A). As shown schematically in Fig. 4A, 1 day posttransfection, the cells were exposed to increasing amounts of IR and harvested for determination of CAT activity after an additional day. CAT expression from the construct with the p53 binding site intact (−249PCNA-CAT) increased about threefold relative to the level expressed from the same construct in mock-irradiated cells (Fig. 4B). Higher doses of IR did not further increase CAT expression from the −249PCNA-CAT construct (not shown). In contrast, IR did not alter CAT expression from the PCNA-CAT construct with the p53 binding site deleted (−213PCNA-CAT) relative to that observed in unirradiated cells (Fig. 4B). These data indicate that sequences in the PCNA promoter that correlate with a p53 binding site mediate transcriptional activation in irradiated cells.
Some mutant p53 proteins may overcome the activity of the wild-type protein in a dominant negative manner by driving the wild-type protein into the mutant conformation (67). To confirm that IR activates PCNA-CAT expression via a p53-dependent mechanism, we cotransfected a dominant negative mutant p53 expression construct with the PCNA-CAT reporter constructs diagrammed in Fig. 4A. As specified by the protocol depicted in Fig. 4A, the cells were exposed to increasing doses of IR at 24 h posttransfection. As indicated in Fig. 4C, levels of CAT expression from the −249PCNA-CAT construct in the presence of the dominant negative mutant p53 remained similar postirradiation to the levels observed in unirradiated cells. Again, the construct lacking a p53 binding site, −213PCNA-CAT, did not respond to increasing amounts of IR (Fig. 4C). These findings indicate that expression of the dominant negative mutant p53 blocked p53 binding-site-mediated activation of the PCNA promoter in irradiated cells.
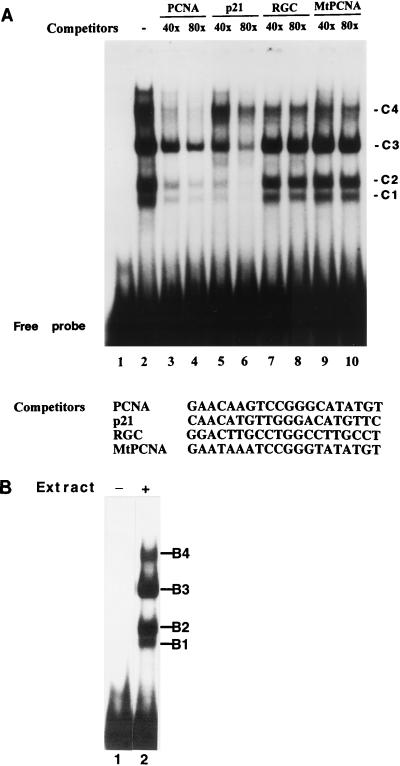
The results described above suggest a cellular response to ionizing radiation in which p53-mediated activation of the PCNA promoter contributes to increased levels of PCNA. To assess p53 binding to PCNA promoter sequences in vitro, EMSAs were performed with a double-stranded oligonucleotide corresponding to the p53 binding site of the PCNA promoter and nuclear extracts from CREF cells exposed to IR at 12 Gy, a dose that induced maximal PCNA-CAT activity in the experiment represented in Fig. 4B. Four major complexes formed in the EMSA with the PCNA p53 binding site and an extract from irradiated CREF cells (Fig. 5A, lane 2). Unlabeled competitor DNAs were added to the binding assay at a 40- or 80-fold excess over the radiolabeled target to determine the specificity of binding. The unlabeled PCNA competitor (self-competition) reduced the abundance of all four complexes formed with the PCNA p53 binding site (lanes 3 and 4). Similarly, a high-affinity p53 binding site from the p21WAF1/cip1 gene (23, 46) reduced formation of all four complexes (lanes 5 and 6). A low-affinity p53 binding site from the ribosomal gene cluster (24, 46, 70) disrupted the complexes formed with the PCNA p53 binding site with reduced efficiency (lanes 7 and 8). A mutant PCNA oligonucleotide that we showed previously does not bind p53 (70) did not compete effectively for binding with the wild-type PCNA probe (lanes 9 and 10). Thus, mutations in the PCNA sequence that target nucleotides that are conserved among p53 binding sequences (fourth C and seventh G) prevented competition in this assay. These observations indicate that the DNA-protein complexes exhibited a sequence specificity consistent with binding by wild-type p53. Moreover, the radiolabeled p21WAF1/Cip1 oligonucleotide incubated with same nuclear extract produced four complexes with mobilities identical to those observed with the PCNA probe (Fig. 5B), although the specificity for p53 binding of the p21-specific complexes remains to be determined. The multiple complexes with p53 binding specificity shown in Fig. 5 suggest that p53 exists in multiple forms in CREF cells, but an immunoblot of the nuclear extract revealed only a single species of p53 (data not shown). The relevance of multiple DNA-protein complexes to transcriptional regulation of PCNA expression by p53 remains to be demonstrated.
FIG. 5.
Binding specificity of complexes formed in EMSAs with nuclear extracts prepared from irradiated CREF cells. (A) Binding to the PCNA p53 binding site. A double-stranded oligonucleotide corresponding to the PCNA p53 binding site (PCNA) was used as the radiolabeled probe. Double-stranded oligonucleotides corresponding to the p53 binding site in the WAF1 gene (p21), the ribosomal gene cluster (RGC), or a mutated version of the PCNA site (MtPCNA) that fails to bind p53 (70) were used as unlabeled competitors in the EMSA. Lanes 1 and 2 show the radiolabeled probe without extract and with extract prepared from CREF cells 3 h postirradiation, respectively. Experimental conditions for lanes 3 to 10 were identical to those for lane 2 except that the indicated competitors were included in the binding mix at either 40- or 80-fold excess compared to labeled probe. The four specific complexes that form in the assay are designated C1 to C4. The gel was overexposed (more than 48 h) to reveal the oligonucleotide competition for all four complexes. The detection of the relatively minor C4 complex varied between experiments. (B) Binding to the p53 binding site of the WAF1 gene. Details are as for panel A except that a double-stranded oligonucleotide corresponding to the p21WAF1 p53 binding site was used as the radiolabeled probe. Complexes B1 through B4 comigrate with complexes C1 through C4 formed with the PCNA probe in panel A (not shown). Lane 1, WAF1 probe without extract; lane 2, WAF1 probe with nuclear extract prepared from CREF cells at 3 h postirradiation.
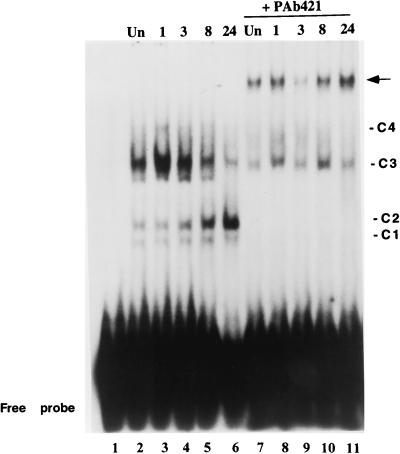
To reveal the effect of IR on the pattern of complexes formed with the PCNA p53 binding site, we prepared nuclear extracts from CREF cells before and 1, 3, 8, and 24 h after exposure to 12-Gy IR. The experimental protocol included release from contact inhibition 6 h prior to irradiation and extract preparation; therefore, binding to PCNA promoter sequences in Fig. 6 could be related to protein expression shown in Fig. 2. Although immunoblotting of the nuclear extracts from irradiated cells indicated a single species of p53 that steadily increased (data not shown), the relative abundance of multiple p53-related complexes that form on the PCNA promoter sequence changed differently with time postirradiation (Fig. 6). The two most abundant complexes, C2 and C3, displayed quite distinct responses to IR. C2 gradually increased with time to a maximal amount at 24 h post-IR, while the abundance of C3 increased shortly after radiation exposure and decreased thereafter (lanes 2 to 6). The ratio of C3 to C2 at the 1-h time point increased approximately 50% from that observed in unirradiated cells and declined about 100-fold from this maximum at 24 h postirradiation. In general, radiation-induced changes in C1 and C4 agreed with those observed for C2 and C3, respectively.
FIG. 6.
DNA-protein complex formation with the PCNA p53 binding site varies with time postirradiation. A radiolabeled oligonucleotide corresponding to the PCNA p53 binding site was used as the probe with nuclear extracts prepared from uniradiated cells (lane 2) or irradiated cells at the indicated times (hours) postirradiation (lanes 3 to 6). The pattern of the probe without extract is shown in lane 1. Lanes 7 to 11 are identical to lanes 2 to 6 except that 1 μl of p53-specific monoclonal antibody PAb421 was added in each binding mix. Complexes C1 to C4 are designated as in Fig. 5. The arrow indicates the new, slower-migrating complex formed in the presence of PAb421. Of a variety of antibodies tested, no other antibody produced a band with the mobility of the arrow. The faint band below C3 is not reproducible between experiments. The gel was exposed to X-ray film for 15 to 20 h.
p53 is subject to extensive posttranslational modifications which contribute to conversion between latent and active forms (references 28 and 53 and references therein). A p53-specific monoclonal antibody, PAb421, recognizes C-terminal residues that are differentially modified in different forms of p53 (42). To evaluate the various p53-specific complexes in Fig. 6, PAb421 was added to the EMSA with each time point (Fig. 6, lanes 7 to 11). Addition of PAb421 completely depleted complexes C1, C2, and C4, reduced the formation of C3, and produced a new, slower-migrating complex (arrow). The abundance of the complex formed upon addition of the antibody appeared to increase in cell extracts at later times postirradiation, with the highest amount detected at 24 h after irradiation. These observations corroborate the presence of p53 in the complexes and indicate that the two most prominent complexes, C3 and C2, differ in reactivity to PAb421. The relative abundance of the PAb421-sensitive C2 complex increases at later times postirradiation, while the PAb421-resistant C3 complex declines.
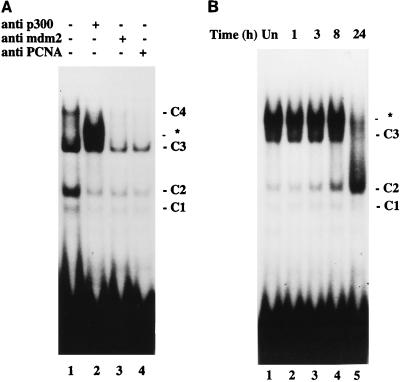
Recently, several groups reported that the transcriptional adapter protein p300/CBP binds p53 and mediates both transcriptional activation and repression (2, 30, 60, 86). To test whether p300/CBP associates with p53 bound by PCNA promoter sequences, we added several p300/CBP-specific antibodies to the EMSA. Since MDM-2 is inducible by p53 and negatively regulates p53 (102) by interacting with the N-terminal activation domain, the presence of MDM-2 in the p53-PCNA promoter complexes could provide the means to negatively regulate the PCNA promoter. In addition, an MDM-2-related protein that is recognized by monoclonal antibody 2A10 (10) has been identified in a specific p53-DNA complex (32). Therefore, in the same experiment we used 2A10 to test whether MDM-2 or an MDM-related protein participates in p53-specific binding to the PCNA promoter. Addition of a monoclonal antibody AC238 (60) to the binding mix produced a new complex that migrated just above C3 (Fig. 7A, lane 2), whereas monoclonal antibodies to MDM-2 (lane 3) or to PCNA (lane 4) reduced the signal overall but did not alter the binding pattern. Two other p300/CBP-specific antibodies, RW128 (60) and NM1 (16), also generated the new complex, and none of the p300/CBP-specific antibodies produced this complex without the addition of the nuclear extract (data not shown). This observation suggests an association between p300/CBP and p53 bound to PCNA promoter sequences. To evaluate the effect of IR on the p53-p300/CBP association, monoclonal antibody AC238 was added to the gel shift assay with the extracts prepared from irradiated cells shown in Fig. 6. A new, intense band appeared in each assay except with the extract from cells 24 h postirradiation (Fig. 7B). Immunoblotting of the nuclear extracts revealed roughly equivalent or even greater amounts of p300 in the extract from irradiated cells at 24 h postirradiation, thereby indicating that lack of p53 binding was not due to the radiation-induced loss of p300 (data not shown). These results suggest that at later times after irradiation, the association between PCNA promoter-bound p53 and p300/CBP weakens.
FIG. 7.
Binding of p300/CBP to p53 in irradiated CREF cells. (A) Specificity of anti-p300/CBP antibody. Binding to an oligonucleotide corresponding to the radiolabeled PCNA p53 binding site was assessed by EMSA with equal amounts of nuclear extract prepared from CREF cells 3 h after irradiation. The assays were without antibody (lane 1), with 2 μl of anti-p300/CBP AC238 ascites fluid (lane 2), with 4 μl of anti-MDM-2 2A10 monoclonal supernatant (lane 3), or with 2 μl of anti-PCNA 19F4 (lane 4). ∗, a new complex generated by adding the p300/CBP-specific antibody. (B) Binding to p300/CBP varies with time postirradiation. Nuclear extracts were isolated at the indicated times (h) postirradiation. Equal amounts of protein from each extract were evaluated by EMSA with an oligonucleotide corresponding to the PCNA p53 binding site. The extracts from unirradiated (lane 1) and irradiated (lane 2 to 5) cells were preincubated with 2 μl of AC238 (anti-p300/CBP) before the addition of probe.
DISCUSSION
Most nongrowing cells contain little PCNA mRNA and protein (reference 52 and references therein). The DNA repair function of PCNA necessitates activation of PCNA synthesis for the purpose of DNA repair after genotoxic insult in a population of nongrowing cells. We show here that exposure of cells to IR enhances both PCNA mRNA and protein expression. Since p53 binds to human PCNA DNA sequences in vitro and modulates expression from the PCNA promoter in transient expression assays (70, 90), we explored p53-mediated regulation of PCNA expression in irradiated cells. Our data indicate that exposure of cells to IR induces p53-mediated transcriptional activation of PCNA expression. This finding agrees with previous observations that exposure of cells to genotoxic insults promotes simultaneous expression of both p53 and PCNA (15, 34, 68). IR promotes conversion of p53 to different forms that bind PCNA promoter sequences in vitro and a corresponding alteration in the interaction of p53 with the transcriptional coactivator p300/CBP. The radiation-induced alterations in the pattern of PCNA protein and mRNA expression and the analyses of p53 binding to PCNA promoter sequences in vitro suggests a model in which radiation modestly activates PCNA expression via a p53-dependent mechanism that is self-limiting.
CREF cells respond to IR with an increase in cellular p53 levels and a coincident increase in p21 (Fig. 1). This observation agrees with p53-dependent cell cycle arrest executed by p21 in irradiated cells (19) and suggests that the radiation-induced signal transduction pathway leading to p53-mediated activation of downstream target genes is functional in this cell line. Thus, a similar rapid increase in PCNA (Fig. 1) could be rendered by p53 in irradiated cells. That the increase in PCNA levels can precede progression to S phase (Fig. 2) is also consistent with activation by p53 and distinct from the observed elevation in PCNA levels associated with progression into S phase (6, 74). p53 transcriptionally activates its downstream target genes upon receiving an appropriate signal by accumulating and converting from a latent to an active form (35, 39–41, 46). Cellular p53 levels increase primarily through an increase in the half-life of the protein (50). The mechanism of p53 stabilization in cells exposed to IR appears to require the function of ATM (ataxia telangiectasia mutated) (7, 51), which is distinct from the mechanism leading to p53 accumulation in UV-irradiated cells (63). In cells with DNA damage, phosphorylation of N-terminal residues of p53 enhances transcription of downstream target genes (91). Inhibition of interactions between p53 and its inhibitor, MDM-2, by N-terminal phosphorylation (66, 89) may account, in part, for this greater activity. In the absence of stress, p53 exists in primarily a latent state stabilized by the inhibitory effects of C-terminal sequences. Conversion from the latent form to a form active for DNA binding can be achieved by a variety of means, including phosphorylation, glycosylation, acetylation, and interactions with other proteins (references 28, 29, 53, and 87 and references therein).
In growth-arrested cells, induction of PCNA mRNA is transient (Fig. 3). Rapid activation of transcription from the PCNA promoter potentiated by p53 in irradiated cells could account for the rapid increase, though IR-induced posttranscriptional regulation may contribute to PCNA activation. The results presented in Fig. 4 are consistent with radiation-induced and p53-mediated activation of the PCNA promoter. Furthermore, the rapid increase in the relative abundance of complex C3 in nuclear extracts from irradiated cells shown in Fig. 6 coincides with rapid activation of PCNA expression. Whether the decline in PCNA mRNA in growth-arrested cells (Fig. 3) stems from transcriptional inactivation of the PCNA promoter is unclear. The level of p53 expression can be an important determinant of the protein’s biological effects (11, 12), and in transient expression assays, higher levels of p53 reduce expression from a PCNA promoter-CAT construct (70, 95). Similarly, prolonged elevation of p53 expression in irradiated cells may lead to repression of the PCNA promoter. A transient expression assay as represented in Fig. 4B may not reveal radiation-induced and p53-mediated transcriptional repression because CAT protein, which is stable, would accumulate before p53 achieved levels sufficient to repress transcription. Consistent with this view, irradiation of transfected CREF cells at earlier times posttransfection led to less activation of the PCNA promoter (unpublished data). In irradiated cells released from contact inhibition, there is a prolonged interval of elevated PCNA protein levels that declines by 24 h postirradiation (Fig. 2). Since an identical experimental protocol was used, the decrease in PCNA protein levels at 24 h shown in Fig. 2 appears to correlate with a decrease in the abundance of the C3 complex and an increase in the C2 complex shown in Fig. 6. This correlation suggests a model in which the PCNA promoter is activated by a mechanism governed by the abundance of the C3 complex and repressed by a pathway associated with accumulation of the C2 complex. Moreover, the reduced association between p53 and p300/CBP at the 24-h time point observed in Fig. 7B is consistent with this model. Thus, at later times postirradiation, the predominant form of p53 is one that binds the PCNA promoter but does not activate transcription due to an inability to interact with the essential coactivator p300/CBP. Although DNA bound p53 generally activates transcription (references 28, 53, and 58 and references therein), a form of p53 that binds DNA sequence specifically but fails to activate transcription appears in cells treated with inhibitors of protein kinase C PKC (13). Although p53 does not appear to be a direct target of PKC in vivo (67), phosphorylation of the p53 C terminus by PKC in vitro both activates sequence-specific DNA binding by p53 and disrupts interaction with PAb421 (18, 42, 77, 96). Phosphorylation within the C-terminal epitope recognized by PAb421 may account for the differences in antibody reactivity between C3 and C2 shown in Fig. 6. Other factors likely account for the different mobilities of C3 and C2, as phosphorylation of bacterially expressed p53 by PKC in vitro within the PAb421 epitope does not affect the gel mobility of p53-DNA complexes (96).
The alternative forms of p53 and p53-related proteins described in other systems might account for the dissimilar mobilities of complexes C3 and C2 in the gel shift assays shown here (Fig. 6). Similar to the results described here, two forms of p53 appear in X-irradiated mouse cells with differential kinetics (104). A full-length form with an intact C terminus appears transiently with a rapid peak and decline postirradiation. A constitutively active alternatively spliced form, p53as, appears with a slightly delayed response, and the levels remain elevated for an extended period. The expression pattern of p53as coincides with that of p21 in irradiated mouse cells. In contrast to the findings with mouse cells, the results described here indicate that the p53 form with a delayed and sustained pattern of expression (C2) possesses an intact C terminus since this form reacts with the C-terminus-specific antibody PAb421. Moreover, the pattern of p21 expression in irradiated CREF cells corresponds to the more rapidly appearing C3 complex, which fails to interact with PAb421. Whether alternative splicing could account for different forms of p53 in irradiated CREF cells remains to be determined, but so far this form of p53 regulation has been described only for mice, not for rats (101). Another putative member of the p53 gene family, p53 competing protein (p53CP) (4), a 40-kDa nuclear protein found in mouse and human cells, could account for the similar binding specificities and disparate mobilities of complexes C2 and C3. Binding of p53CP to specific sites appears to correlate inversely with p53 binding. Consequently, p53CP might also bind to PCNA promoter sequences and thereby account for the greater mobility of complex C2 and repression of PCNA expression at later times postirradiation. However, C2 binds PAb421, and this antibody appears to be specific for p53 (4). Furthermore, the conserved C and G nucleotides at positions 4, 7, 14, and 17 of the p53 consensus binding site are critical for binding by p53CP, and the p53 binding site of the PCNA promoter possesses an A rather than a G at one of these conserved positions (4, 70). Another p53-related protein, p73 (48, 49), activates transcription of p53-responsive genes and interacts with p53 in the yeast two-hybrid system (49). These observations suggest that p73 could be a component of the C2 or C3 complexes, but there is no evidence that p73 mediates cellular responses to DNA damage (49), and an interaction between p53 and p73 at physiological levels of the proteins has not been described.
Here we describe p53-mediated transcriptional regulation of PCNA expression in irradiated cells. In contrast to these findings, exposure of p53+ and p53− human lymphoblastoid cell lines to 1.5 to 3 Gy of IR does not alter PCNA protein levels (100). Instead, in lymphoblastoid cells, the association of PCNA with a tightly bound nuclear fraction is regulated posttranslationally in a manner that depends on p53. Whether the higher dose of IR (12 Gy) used here will induce p53-dependent PCNA expression in lymphoblastoid cells remains unknown, but IR at the 1- to 4-Gy range does not effectively activate the PCNA promoter via the p53 binding site in CREF cells (Fig. 4B). Differences in the relative levels of PCNA in the cell might also account for differences in the response to IR. Continuously cycling cells maintain cellular PCNA levels with little fluctuation (8, 74). Therefore, in cycling lymphoblastoid cells the levels of PCNA may be sufficient for DNA repair, and the existing cellular pool of the protein may simply be diverted for that purpose. In addition, the accumulation of p53 in the nucleus and enhanced expression of p21WAF1/cip1 in irradiated cells occurs more readily in the G1 and early S phases of the cell cycle (54). Consequently, it seems likely that the regulation of PCNA levels in irradiated cells becomes obvious in the experiments described here because a high radiation dose is used and the cells are synchronized by contact inhibition or serum deprivation, which also serves to reduce the cellular PCNA levels.
The data presented here suggest a model in which PCNA expression is tightly regulated by p53 in irradiated cells. This tight regulation of PCNA expression may provide p53 the basis for altering a number of aspects of cellular metabolism. In addition to critical functions in DNA replication and repair, PCNA forms complexes with p21, cyclins, and cyclin-dependent kinases (105, 107), and through that association PCNA levels may influence regulation of the cell cycle. Indeed, enhanced PCNA expression in yeast blocks cell cycle progression (98). Although human osteosarcoma cell lines continue to cycle upon PCNA overexpression (80), these transformed cells may lack the signal transduction pathways affected by cellular PCNA levels. In mouse fibroblasts, reduction of cellular PCNA levels also correlates with a halt in cell cycling (45). In addition to altering cell cycle activities, the ratio of PCNA levels with p21WAF1/cip1 can affect association of PCNA with DNA methyltransferases and thereby influence the extent of DNA methylation (14). Since most PCNA-interacting proteins bind within an overlapping region in the interdomain connector loop of PCNA (9, 27, 33, 57, 103), the interactions of DNA replication and repair proteins with PCNA may occur sequentially during DNA synthesis and p21WAF1/cip1 could alter this process by competing for binding.
ACKNOWLEDGMENTS
We thank Steve Grossman, Elizabeth Moran, and Jiandong Chen for providing antibodies AC238, RW128, NM11, and 2A10. We also thank Krishna Agrawal for the access to the Gammacell 40 irradiator and Cindy Morris for helpful discussion and critical reading of the manuscript. We express our gratitude to Weihong Lei for technical assistance.
This work was supported by research grants from the Department of Defense and Tulane/Xavier Center for Bioenvironmental Research and grant ES07856 from the National Institute of Environmental Health Sciences. J.X. is a recipient of matching funds from the Tulane Cancer Center and a Research Scholar of the Tulane/Xavier Center for Bioenvironmental Research.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 2.Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 3.Bannister A, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 4.Bian J, Sun Y. p53CP, a putative p53 competing protein that specifically binds to the consensus p53 DNA binding sites: a third member of the p53 family? Proc Natl Acad Sci USA. 1997;94:14753–14758. doi: 10.1073/pnas.94.26.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bischoff J R, Casso D, Beach D. Human p53 inhibits growth in Schizosaccharomyces pombe. Mol Cell Biol. 1992;12:1405–1411. doi: 10.1128/mcb.12.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bravo R, Celis J E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980;84:795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canman C, Chen C Y, Lee M H, Kastan M B. DNA damage responses: p53 induction, cell cycle perturbations, and apoptosis. Cold Spring Harbor Symp Quant Biol. 1994;59:277–286. doi: 10.1101/sqb.1994.059.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Celis J, Madsen P, Celis A, Nielsen H V, Gesser B. Cyclin (PCNA, auxiliary protein of DNA polymerase delta) is a central component of the pathway(s) leading to DNA replication and cell division. FEBS Lett. 1987;220:1–7. doi: 10.1016/0014-5793(87)80865-7. [DOI] [PubMed] [Google Scholar]
- 9.Chen I T, Smith M L, O’Connor P M, Fornace A J., Jr Direct interaction of Gadd45 with PCNA and evidence for competitive interaction of Gadd45 and p21Waf1/Cip1 with PCNA. Oncogene. 1995;11:1931–1937. [PubMed] [Google Scholar]
- 10.Chen J, Marechal V, Levine A J. Mapping of the p53 and mdm-2 interaction domains. Mol Cell Biol. 1993;13:4107–4114. doi: 10.1128/mcb.13.7.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Ko L J, Jayaraman L, Prives C. p53 levels, functional domains and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10:2438–2451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Bargonetti J, Prives C. p53, through p21 (WAF1/CIP1), induces cyclin D1 synthesis. Cancer Res. 1995;55:4257–4263. [PubMed] [Google Scholar]
- 13.Chernov M, Ramana C V, Adler V V, Stark G R. Stabilization and activation of p53 are regulated independently by different phosphorylation events. Proc Natl Acad Sci USA. 1998;95:2284–2289. doi: 10.1073/pnas.95.5.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuang L S, Ian H I, Koh T W, Ng H H, Xu G, Li B F. Human DNA-(cytosine-5) methyltransferase-PCNA complex as a target for p21WAF1. Science. 1997;277:1996–2000. doi: 10.1126/science.277.5334.1996. [DOI] [PubMed] [Google Scholar]
- 15.Coates P, Save V, Ansari B, Hall P A. Demonstration of DNA damage/repair in individual cells using in situ end labelling: association of p53 with sites of DNA damage. J Pathol. 1995;176:19–26. doi: 10.1002/path.1711760105. [DOI] [PubMed] [Google Scholar]
- 16.Dallas P, Yaciuk P, Moran E. Characterization of monoclonal antibodies raised against p300: both p300 and CBP are present in intracellular TBP complexes. J Virol. 1997;71:1726–1731. doi: 10.1128/jvi.71.2.1726-1731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deb S, Jackson C T, Subler M A, Martin D W. Modulation of cellular and viral promoters by mutant human p53 proteins found in tumor cells. J Virol. 1992;66:6164–6170. doi: 10.1128/jvi.66.10.6164-6170.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delphin C, Huang K P, Scotto C, Chapel A, Vincon M, Chambaz E, Garin J, Baudier J. The in vitro phosphorylation of p53 by calcium-dependent protein kinase C--characterization of a protein-kinase-C-binding site on p53. Eur J Biochem. 1997;245:684–692. doi: 10.1111/j.1432-1033.1997.t01-1-00684.x. [DOI] [PubMed] [Google Scholar]
- 19.Di Leonardo A, Linke S P, Clarkin K, Wahl G M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 20.Dulic V, Kaufmann W K, Wilson S J, Tlsty T D, Lees E, Harper J W, Elledge S J, Reed S I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 21.Eckner R, Ludlow J W, Lill N L, Oldread E, Arany Z, Modjtahedi N, DeCaprio J A, Livingston D M, Morgan J A. Association of p300 and CBP with simian virus 40 large T antigen. Mol Cell Biol. 1996;16:3454–3464. doi: 10.1128/mcb.16.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckner R, Ewen M E, Newsome D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 23.El-Deiry W, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 24.El-Deiry W S, Kern S E, Pietenpol J A, Kinzler K W, Vogelstein B. Human genomic DNA sequences define a consensus binding site for p53. Nat Genet. 1992;1:44–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- 25.Feinberg A, Vogelstein B. Addendum. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 26.Fisher P B, Babiss L E, Weinstein B, Ginsberg H S. Analysis of type 5 adenovirus transformation with a cloned rat embryo cell line (CREF) Proc Natl Acad Sci USA. 1982;79:3527–3531. doi: 10.1073/pnas.79.11.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gary R, Ludwig D L, Cornelius H L, MacInnes M A, Park M S. The DNA repair endonuclease XPG binds to proliferating cell nuclear antigen (PCNA) and shares sequence elements with the PCNA-binding regions of FEN-1 and cyclin-dependent kinase inhibitor p21. J Biol Chem. 1997;272:24522–24529. doi: 10.1074/jbc.272.39.24522. [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb T M, Oren M. p53 in growth control and neoplasia. Biochim Biophys Acta. 1996;1287:77–102. doi: 10.1016/0304-419x(95)00019-c. [DOI] [PubMed] [Google Scholar]
- 29.Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 30.Gu W, Shi X L, Roeder R G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 31.Gunning P, Ponte P, Okayama H, Engel J, Blau H, Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983;3:787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall A, Milner J. Specific p53-DNA complexes contain an mdm2-related protein. Oncogene. 1997;14:1371–1376. doi: 10.1038/sj.onc.1200962. [DOI] [PubMed] [Google Scholar]
- 33.Hall P A, Kearsey J M, Coates P J, Norman D G, Warbrick E, Cox L S. Characterisation of the interaction between PCNA and Gadd45. Oncogene. 1995;10:2427–2433. [PubMed] [Google Scholar]
- 34.Hall P A, McKee P H, Menage H D, Dover R, Lane D P. High levels of p53 protein in UV-irradiated normal human skin. Oncogene. 1993;8:203–207. [PubMed] [Google Scholar]
- 35.Hansen S, Hupp T R, Lane D P. Allosteric regulation of the thermostability and DNA binding activity of human p53 by specific interacting proteins. J Biol Chem. 1996;271:3917–3924. doi: 10.1074/jbc.271.7.3917. [DOI] [PubMed] [Google Scholar]
- 36.Harper J W, Adami G R, Wei N, Keyomars K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 37.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 38.Huang D, Shipman-Appasamy P M, Orten D J, Hinrichs S H, Prystowsky M B. Promoter activity of the proliferating-cell nuclear antigen gene is associated with inducible CRE-binding proteins in interleukin 2-stimulated T lymphocytes. Mol Cell Biol. 1994;14:4233–4243. doi: 10.1128/mcb.14.6.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hupp T, Lane D P. Allosteric activation of latent p53 tetramers. Curr Biol. 1994;4:865–875. doi: 10.1016/s0960-9822(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 40.Hupp T R, Meek D W, Midgley C A, Lane D P. Regulation of the specific DNA binding function of p53. Cell. 1992;71:875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- 41.Hupp T R, Sparks A, Lane D P. Small peptides activate the latent sequence-specific DNA binding function of p53. Cell. 1995;83:237–245. doi: 10.1016/0092-8674(95)90165-5. [DOI] [PubMed] [Google Scholar]
- 42.Hupp T R, Lane D P. Two distinct signaling pathways activate the latent DNA binding function of p53 in a casein kinase II-independent manner. J Biol Chem. 1995;270:18165–18174. doi: 10.1074/jbc.270.30.18165. [DOI] [PubMed] [Google Scholar]
- 43.Ishizaka Y, Chernov M V, Burns C M, Stark G R. p53-dependent growth arrest of REF52 cells containing newly amplified DNA. Proc Natl Acad Sci USA. 1995;92:3224–3228. doi: 10.1073/pnas.92.8.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson P, Ridgway P, Rayner J, Noble J, Braithwaite A. Transcriptional regulation of the PCNA promoter by p53. Biochem Biophys Res Commun. 1994;203:133–140. doi: 10.1006/bbrc.1994.2159. [DOI] [PubMed] [Google Scholar]
- 45.Jaskulski D, deRiel J K, Mercer W E, Calabretta B, Baserga R. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988;240:1544–1546. doi: 10.1126/science.2897717. [DOI] [PubMed] [Google Scholar]
- 46.Jayaraman L, Prives C. Activation of p53 sequence-specific DNA binding by short single strands of DNA requires the p53 C-terminus. Cell. 1995;81:1021–1029. doi: 10.1016/s0092-8674(05)80007-8. [DOI] [PubMed] [Google Scholar]
- 47.Johnson R E, Kovvali G K, Guzder S N, Amin N S, Holm C, Habraken Y, Sung P, Prakash L, Prakash S. Evidence for involvement of yeast proliferating cell nuclear antigen in DNA mismatch repair. J Biol Chem. 1996;271:27987–27990. doi: 10.1074/jbc.271.45.27987. [DOI] [PubMed] [Google Scholar]
- 48.Jost C, Marin M C, Kaelin W G., Jr p73 is a human p53-related protein that can induce apoptosis. Nature. 1997;389:191–194. doi: 10.1038/38298. [DOI] [PubMed] [Google Scholar]
- 49.Kaghad M, Bonnet H, Yang A, Creancier L, Biscan J C, Valent A, Minty A, Chalon P, Lelias J M, Dumont X, Ferrara P, McKeon F, Caput D. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 50.Kastan M, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 51.Kastan M B, Zhan Q, El-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 52.Kelman Z. PCNA: structure, functions and interactions. Oncogene. 1997;14:629–640. doi: 10.1038/sj.onc.1200886. [DOI] [PubMed] [Google Scholar]
- 53.Ko L J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 54.Komarova E, Zelnick C R, Chin D, Zeremski M, Gleiberman A S, Bacus S S, Gudkov A V. Intracellular localization of p53 tumor suppressor protein in gamma-irradiated cells is cell cycle regulated and determined by the nucleus. Cancer Res. 1997;57:5217–5220. [PubMed] [Google Scholar]
- 55.Kubbutat M, Jones S N, Vousden K H. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 56.Labrie C, Morris G F, Mathews M B. A complex promoter element mediates transactivation of the human proliferating cell nuclear antigen promoter by the 243-residue adenovirus E1A oncoprotein. Mol Cell Biol. 1993;13:1697–1707. doi: 10.1128/mcb.13.3.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levin D S, Bai W, Yao N, O’Donnell M, Tomkinson A E. An interaction between DNA ligase I and proliferating cell nuclear antigen: implications for Okazaki fragment synthesis and joining. Proc Natl Acad Sci USA. 1997;94:12863–12868. doi: 10.1073/pnas.94.24.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 59.Li R, Waga S, Hannon G, Beach D, Stillman B. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature. 1994;371:534–537. doi: 10.1038/371534a0. [DOI] [PubMed] [Google Scholar]
- 60.Lill N L, Grossman S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 61.Lill N L, Tevethia M J, Eckner R, Livingston D M, Modjtahedi N. p300 family members associate with the carboxyl terminus of simian virus 40 large tumor antigen. J Virol. 1997;71:129–137. doi: 10.1128/jvi.71.1.129-137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mack D H, Vartikar J, Pipas J M, Laimins L A. Specific repression of TATA-mediated but not initiator-mediated transcription by wild-type p53. Nature. 1993;363:281–283. doi: 10.1038/363281a0. [DOI] [PubMed] [Google Scholar]
- 63.Maki C, Howley P M. Ubiquitination of p53 and p21 is differentially affected by ionizing and UV radiation. Mol Cell Biol. 1997;17:355–363. doi: 10.1128/mcb.17.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsumoto K, Moriuchi T, Koji T, Nakane P. Molecular cloning of cDNA coding for rat proliferating cell nuclear antigen (PCNA)/cyclin. EMBO J. 1987;6:637–642. doi: 10.1002/j.1460-2075.1987.tb04802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mercer W E, Shields M T, Lin D, Appella E, Ullrich S J. Growth suppression induced by wild-type p53 protein is accompanied by selective down-regulation of proliferating-cell nuclear antigen expression. Proc Natl Acad Sci USA. 1991;88:1958–1962. doi: 10.1073/pnas.88.5.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milczarek G J, Martinez J, Bowden G T. p53 phosphorylation: biochemical and functional consequences. Life Sci. 1997;60:1–11. doi: 10.1016/s0024-3205(96)00479-1. [DOI] [PubMed] [Google Scholar]
- 67.Milne D, McKendrick L, Jardine L J, Deacon E, Lord J M, Meek D W. Murine p53 is phosphorylated within the PAb421 epitope by protein kinase C in vitro, but not in vivo, even after stimulation with the phorbol ester o-tetradecanoylphorbol 13-acetate. Oncogene. 1996;13:205–211. [PubMed] [Google Scholar]
- 68.Mishra A, Liu J Y, Brody A R, Morris G F. Inhaled asbestos fibers induce p53 expression in the rat lung. Am J Respir Cell Mol Biol. 1997;16:479–485. doi: 10.1165/ajrcmb.16.4.9115760. [DOI] [PubMed] [Google Scholar]
- 69.Miyashita T, Reed J C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 70.Morris G F, Bischoff J R, Mathews M B. Transcriptional activation of the human proliferating cell nuclear antigen promoter by p53. Proc Natl Acad Sci USA. 1996;93:895–899. doi: 10.1073/pnas.93.2.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morris G F, Labrie C, Mathews M B. Modulation of transcriptional activation of the proliferating cell nuclear antigen promoter by the adenovirus E1A 243-residue oncoprotein depends on proximal activators. Mol Cell Biol. 1994;14:543–553. doi: 10.1128/mcb.14.1.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morris G F, Mathews M B. The adenovirus E1A transforming protein activates the proliferating cell nuclear antigen promoter via an activating transcription factor site. J Virol. 1991;65:6397–6406. doi: 10.1128/jvi.65.12.6397-6406.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morris G F, Mathews M B. Analysis of the proliferating cell nuclear antigen promoter and its response to adenovirus early region 1. J Biol Chem. 1990;265:16116–16125. [PubMed] [Google Scholar]
- 74.Morris G F, Mathews M B. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem. 1989;264:13856–13864. [PubMed] [Google Scholar]
- 75.Mosner J, Mummenbrauer T, Bauer C, Sczakiel G, Grosse F, Deppert W. Negative feedback regulation of wild-type p53 biosynthesis. EMBO J. 1995;14:4442–4449. doi: 10.1002/j.1460-2075.1995.tb00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mossi R, Jonsson Z O, Allen B L, Hardin S H, Hubscher U. Replication factor C interacts with the C-terminal side of proliferating cell nuclear antigen. J Biol Chem. 1997;272:1769–1776. doi: 10.1074/jbc.272.3.1769. [DOI] [PubMed] [Google Scholar]
- 77.Mundt M, Hupp T, Fritsche M, Merkle C, Hansen S, Lane D, Groner B. Protein interactions at the carboxyl terminus of p53 result in the induction of its in vitro transactivation potential. Oncogene. 1997;15:237–244. doi: 10.1038/sj.onc.1201174. [DOI] [PubMed] [Google Scholar]
- 78.Ogata K, Kurki P, Celis J E, Nakamura R M, Tan E M. Monoclonal antibodies to a nuclear protein (PCNA/cyclin) associated with DNA replication. Exp Cell Res. 1987;168:475–486. doi: 10.1016/0014-4827(87)90020-6. [DOI] [PubMed] [Google Scholar]
- 79.Ogryzko V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 80.Ogryzko V V, Wong P, Howard B H. WAF1 retards S-phase progression primarily by inhibition of cyclin-dependent kinases. Mol Cell Biol. 1997;17:4877–4882. doi: 10.1128/mcb.17.8.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Osborn L, Kunkel S, Nabel G J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci USA. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Polyak K, Xia Y, Zweier J L, Kinzler K W, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 84.Rushton J, Jiang D, Srinivasan A, Pipas J M, Robbins P D. Simian virus 40 T antigen can regulate p53-mediated transcription independent of binding p53. J Virol. 1997;71:5620–5623. doi: 10.1128/jvi.71.7.5620-5623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sabbatini P, Chiou S K, Rao L, White E. Modulation of p53-mediated transcriptional repression and apoptosis by the adenovirus E1B 19K protein. Mol Cell Biol. 1995;15:1060–1070. doi: 10.1128/mcb.15.2.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scolnick D M, Chehab N H, Stavridi E S, Lien M C, Caruso L, Moran E, Berger S L, Halazonetis T D. CREB-binding protein and p300/CBP-associated factor are transcriptional coactivators of the p53 tumor suppressor protein. Cancer Res. 1997;57:3693–3696. [PubMed] [Google Scholar]
- 87.Shaw P, Freeman J, Bovey R, Iggo R. Regulation of specific DNA binding by p53: evidence for a role for O-glycosylation and charged residues at the carboxy-terminus. Oncogene. 1996;12:921–930. [PubMed] [Google Scholar]
- 88.Shen Y, Shenk T. Relief of p53-mediate transcriptional repression by the adenovirus E1B 19-kDa protein or the Bcl-2 protein. Proc Natl Acad Sci USA. 1994;91:8940–8944. doi: 10.1073/pnas.91.19.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shieh S-Y, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM-2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 90.Shivakumar C V, Brown D R, Deb S, Deb S P. Wild-type human p53 transactivates the human proliferating cell nuclear antigen promoter. Mol Cell Biol. 1995;15:6785–6793. doi: 10.1128/mcb.15.12.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Siliciano J D, Canman C E, Taya Y, Sakaguchi K, Appella E, Kastan M B. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith M L, Chen I T, Zhan Q, Bae I, Chen C Y, Gilmer T M, Kastan M B, O’Connor P M, Fornace A J., Jr Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994;266:1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- 93.Somasundaram K, El-Deiry W S. Inhibition of p53-mediated transactivation and cell cycle arrest by E1A through its p300/CBP-interacting region. Oncogene. 1997;14:1047–1057. doi: 10.1038/sj.onc.1201002. [DOI] [PubMed] [Google Scholar]
- 94.Steegenga W, van Laar T, Riteco N, Mandarino A, Shvarts A, van der Eb A J, Jochemsen A G. Adenovirus E1A proteins inhibit activation of transcription by p53. Mol Cell Biol. 1996;16:2101–2109. doi: 10.1128/mcb.16.5.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Subler M A, Martin D W, Deb S. Inhibition of viral and cellular promoters by human wild-type p53. J Virol. 1992;66:4757–4762. doi: 10.1128/jvi.66.8.4757-4762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takenaka I, Morin F, Seizinger B R, Kley N. Regulation of the sequence-specific DNA binding function of p53 by protein kinase C and protein phosphatases. J Biol Chem. 1995;270:5405–5411. doi: 10.1074/jbc.270.10.5405. [DOI] [PubMed] [Google Scholar]
- 97.Tanaka M, Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990;60:375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- 98.Tournier S, Leroy D, Goubin F, Ducommun B, Hyams J S. Heterologous expression of the human cyclin-dependent kinase inhibitor p21Cip1 in the fission yeast, Schizosaccharomyces pombe reveals a role for PCNA in the chk1+ cell cycle checkpoint pathway. Mol Biol Cell. 1996;7:651–662. doi: 10.1091/mbc.7.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Waga S, Hannon G, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369:574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- 100.Wenz F, Azzam E I, Little J B. The response of proliferating cell nuclear antigen to ionizing radiation in human lymphoblastoid cell lines is dependent on p53. Radiat Res. 1998;149:32–40. [PubMed] [Google Scholar]
- 101.Will K, Warnecke G, Bergmann S, Deppert W. Species- and tissue-specific expression of the C-terminal alternatively spliced form of the tumor suppressor p53. Nucleic Acids Res. 1995;23:4023–4028. doi: 10.1093/nar/23.20.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu X, Bayle J H, Olson D, Levine A J. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 103.Wu X, Li J, Li X, Hsieh C L, Burgers P M, Lieber M R. Processing of branched DNA intermediates by a complex of human FEN-1 and PCNA. Nucleic Acids Res. 1996;24:2036–2043. doi: 10.1093/nar/24.11.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu Y, Huang H, Miner Z, Kulesz-Martin M. Activities and response to DNA damage of latent and active sequence-specific DNA binding forms of mouse p53. Proc Natl Acad Sci USA. 1997;94:8982–8987. doi: 10.1073/pnas.94.17.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xiong Y, Zhang H, Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992;71:505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- 106.Yaciuk P, Moran E. Analysis with specific polyclonal antiserum indicates that the E1A-associated 300-kDa product is a stable nuclear phosphoprotein that undergoes cell cycle phase-specific modification. Mol Cell Biol. 1991;11:5389–5397. doi: 10.1128/mcb.11.11.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang H, Xiong Y, Beach D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol Biol Cell. 1993;4:897–906. doi: 10.1091/mbc.4.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]