Abstract
Aims
Immune checkpoint inhibitors (ICI) improve survival across a range of malignancies but are also associated with a spectrum of gastrointestinal immune-related adverse events (GI-irAE). This study aims to explore the diagnostic value of gastric and duodenal biopsies and address considerations in the differential diagnosis.
Methods and Results
We identified 39 patients who were treated with ICIs and had a subsequent upper GI biopsy. We recorded clinical data and endoscopic findings, and reviewed their gastric, duodenal, and colonic biopsies.
21 (54%) patients were treated with an anti-PD1/anti-PD-L1 antibody alone and 17 (44%) with combination anti-CTLA-4 and anti-PD-1 antibodies. 32 (82%) patients presented with diarrhea. Gastric alterations included peri-gland inflammation and granulomas while the duodenal changes included villous blunting, intraepithelial lymphocytosis, granulomas, and neutrophilic activity. We recognized four patterns of colonic injury: 1) acute self-limiting colitis, 2) lymphocytic colitis, 3) collagenous colitis, and 4) apoptosis only. 29 (74%) and 10 (26%) patients were diagnosed clinically as positive and negative for GI-irAE, respectively. Gastric peri-gland inflammation (p=0.004) and increased colonic lamina propria mononuclear cells (p=0.04) correlated with the clinical diagnosis of GI-irAE. Histologic alterations associated with ICI injury were more often identified in upper GI (71%) than colonic biopsies (65%).
Conclusions
The morphologic spectrum of ICI-related GI disease is broad and mimics a range of infectious and inflammatory diseases. Gastric peri-gland inflammation represents one of the more characteristic histologic features of GI-irAE. The study underscores the importance of a comprehensive review of upper and lower GI biopsies for the diagnosis of irAE.
Keywords: checkpoint proteins, PD-1, CTLA-4, gastrointestinal, immune-mediated adverse events
INTRODUCTION
Immune checkpoint inhibitors (ICI) improve survival across a range of malignancies. Current FDA-approved ICIs include monoclonal antibodies that block the immune regulatory proteins cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed cell death ligand 1 (PD-L1). Both the CTLA-4 and PD-(L)1 pathways play central roles in inhibiting T-cell responses to cancer and prevent productive antitumor responses, even in settings where tumor-specific T cells are generated. While the inhibition of these proteins reinvigorates host anti-tumor immune response, broad inhibition of these central immune regulators leads to a unique spectrum of immune-related adverse events (irAE). IrAEs can affect any organ system, including the gastrointestinal tract, endocrine glands, skin, and liver.1,2,3 These irAEs are presumed to result from activation of T cells that recognize self-proteins or commensal microorganisms, though neither the mechanisms driving these toxicities nor the immunologic targets are currently understood.4
Gastrointestinal (GI)-irAEs are among the most common toxicities of current ICIs, though severe GI-irAEs are considerably more common with anti-CTLA-4 compared to treatments that target PD-(L)1 alone.2 In a study of 44 patients referred to a digestive care unit for suspected anti-PD-1-induced GI-irAE, half had alternative etiologies identified, including intestinal metastases,6 reinforcing the need for histological confirmation. Diarrhea is the most common clinical presentation for a GI-irAE, with most patients showing histologic evidence of enterocolitis.2 The incidence of diarrhea is reported to be up to 13% for anti-PD1/PD-L1 and 35% for anti-CTLA-4 therapy.2, 5–7 Severe enterocolitis, one of the most frequent severe irAEs, is principally caused by anti-CTLA-4 and in rare cases, leads to perforation and death.2,8 Patients may also experience abdominal pain, nausea, and/or vomiting; hematochezia and fever are less frequent symptoms.9 In contrast to the side effects of chemotherapy, irAE typically have a delayed onset and prolonged duration of disease, sometimes months or even years after the discontinuation of treatment, making diagnosis and management more challenging.10 The management of irAE is based on clinical experience and calls for corticosteroid immunosuppression as first-line therapy.11–13 Although the impact of immunosuppression on the overall survival of patients is unclear, the possibility that steroids may adversely impact antitumor response and/or overall survival remains a consideration.1,4,14,15,16
The distinction of GI-irAE from infections and de novo primary diseases of the GI tract represents a significant diagnostic challenge for the surgical pathologist. An accurate diagnosis of a GI-irAE is paramount, both to alleviate ICI-associated symptoms and to limit the use of immunosuppressive agents to patients in whom such medications are strictly necessary.
While the histologic features of ICI-related colitis have been reported, there is little data on the upper GI manifestations of irAE. This study aims to describe the histologic findings of upper GI-irAE, explore the diagnostic value of gastric and duodenal biopsies, and address considerations in the differential diagnosis. Our findings suggest that the upper GI tract biopsies represent an important tool in the diagnosis of GI-irAE.
MATERIALS AND METHODS
Study Population
This study was approved by the Massachusetts General Hospital Institutional Review Board. We retrospectively evaluated 39 patients with upper GI biopsies, many of whom also had concurrent colonic biopsies, obtained between 2015 to 2019 from unique adult patients with suspected GI-irAE.
Clinical Criteria
Patient data, including demographic information, clinical notes, endoscopy reports, and laboratory results, were collected from medical records and reviewed by a multidisciplinary team consisting of gastroenterologists, radiologists, oncologists, and pathologists in order to assess the association/causality between ICI therapy and GI-irAEs. The diagnosis of a GI-irAE was based on the following criteria: 1) onset of diarrhea or upper GI symptoms while on ICIs or after cessation of ICI therapy, 2) exclusion of other causes of these symptoms, both clinical and/or histological, and 3) improvement in symptoms following immunosuppressive therapy. Based on fulfillment of these criteria, patients were classified as positive or negative for GI-irAE. The histology review (see below) was blinded to the clinical outcome, although the reviewers were aware that all patients received ICI therapy. None of these patients reported prior evidence of celiac disease or chronic colitis.
Infectious etiologies were excluded through evaluation of microbiological studies (stool culture, stool ova and parasite exam, Clostridium difficile toxin and CMV DNA testing). Immunohistochemical studies for Helicobacter pylori and CMV were performed on selected cases at the time of the original evaluation. Special stains for acid fast bacilli (Ziehl-Neelsen) and fungi (Grocott-Gomori's methenamine silver stain) were performed on selected cases. Serological tests for celiac disease (i.e. antibodies against tissue transglutaminase [TTG] and/or gliadin) were reviewed, if available.
Histologic Criteria
For the purposes of this study, histologic changes of ICI-associated gastritis include: 1) inflammation surrounding and infiltrating more than 5 gastric glands (i.e. greater than 5 foci of peri-gland inflammation), and/or 2) granuloma unrelated to crypt rupture. Duodenal changes associated with ICIs, as assessed on a non-duodenal bulb biopsy, included: 1) villous blunting, and/or 2) diffuse increase in lamina propria lymphocytes and plasma cells, and/or 3) granuloma unrelated to ruptured crypts. Colonic changes associated with GI-irAE included: 1) increased lamina propria lymphocytes and plasma cells compared to normal for the site, and/or 2) active colitis involving greater than 5 crypts, and/or 3) greater than 5 apoptotic cells per 10 high-power fields (HPF), and/or 4) histologic evidence of lymphocytic or collagenous colitis. We acknowledge the relatively uncertainty with regard to the specificity of these findings; however, these criteria were primarily used to compare the sensitivity of upper GI with colon biopsies for ICI-associated GI injury, and the diagnosis of GI-irAE is not implied.
In addition, we evaluated diffuse gastric lymphoplasmacytic infiltration, graded 0, 1, 2, and 3, per the Sydney classification.17 Gastric, duodenal, and colonic biopsies were also assessed for intraepithelial lymphocytosis, which was defined as diffuse increase in intraepithelial lymphocytes, and >20 lymphocytes per 100 epithelial cells. The colonic biopsies were independently assessed for histologic features of chronicity. In addition, we assessed apoptotic activity at all 3 sites, recorded per 10 HPF.
Statistical Analysis
Statistical analyses for the data was performed using SPSS statistical software (version 21). Categorial variables were analyzed using the chi square test. P values of <0.05 were considered statistically significant.
RESULTS
Twenty-one patients (54%) received anti-PD-1 or anti-PD-L1 antibody monotherapy, 17 (44%) received a combination of anti-CTLA-4 and anti-PD-1 antibodies, and a single patient received anti-CTLA-4 antibody monotherapy.
Demographic and Clinical Features
The 39 patients included 15 males and 24 females with a median age of 60 years (range 33 to 81). In this cohort, ICI(s) were used to treat a range of malignant neoplasms, including melanoma (n=21), lung carcinoma (n=6), colon carcinoma (n=3), carcinoma of Mullerian origin (n=3), breast carcinoma (n=1), undifferentiated thyroid carcinoma (n=1), glioblastoma (n=1), plasma cell myeloma (n=2), and renal cell carcinoma (n=1).
Patients treated with anti-PD1/PD-L1 antibody were biopsied on average after 8 cycles of therapy (range 1–40) and an average of 73 days (range 5 to 210) following the last cycle of therapy. Patients treated with combination anti-PD1 and anti-CTLA-4 antibodies were biopsied on average after the start of cycle number 3 (range 1–4) and an average of 41 days (range 7 to 193) following the last cycle of therapy.
Clinical Symptoms
32/39 (82%) patients presented with diarrhea, among which 15 (38%) patients also complained of nausea and vomiting; one patient complained exclusively of nausea and vomiting. Notably, we found no correlation between the presenting symptom and location of histologic involvement (p=0.58); 14 (44%) patients presenting with diarrhea showed histologic involvement of both the upper and the lower GI tract. 10 of 32 (31%) patients presenting with diarrhea lacked histologic evidence of colitis; notably, 6 of these 10 patients showed histologic evidence of upper GI involvement.
Clinical diagnosis of GI-irAE
Based on our criteria for association/causality, we characterized 29 patients as positive and 10 as negative for GI-irAE; the latter group showed improvement in their symptoms without immunosuppression. We present a global view of the histologic findings, followed by a comparison of patients clinically positive and negative for GI-irAE, and finally we contrast patients treated with monotherapy versus dual ICI therapy.
Gastric Involvement
Endoscopic features
The endoscopic changes included erythema alone (n=20) and erythema with erosions (n=5). One case each revealed polyps, ulcer, and prominent folds. In 11 patients, the endoscopic appearance was unremarkable.
We did not identify a correlation between gastric peri-gland inflammation (see below) and the endoscopic appearance (p=0.28). Eight of 20 (40%) patients with erythematous gastric mucosa showed peri-gland inflammation, while 6 of 11 (55%) patients with endoscopically unremarkable stomachs also showed this histologic feature.
Histologic findings
We identified histologic changes in both compartments of the stomach (gastric body/fundus and antrum) without predilection for either zone. Fifteen cases (39%) showed inflammation in the gastric pit/isthmus/neck region, termed peri-gland disease (Table 1 and Table 2) (Figure 1). The lymphocytic aggregates, devoid of plasma cells and histiocytes, were typically localized to gastric pits/isthmus/neck, although occasional involvement of the gastric base was also noted. A diffuse mild (n=14) to moderate (n=5) lymphoplasmacytic infiltrate was also noted. In 12 (31%) patients, we noted non-necrotizing granulomas. Multiple tissue levels were examined to ensure that these granulomas were unrelated to ruptured gastric glands. Special stains for acid fast bacilli and fungi, performed on 4 of 12 cases, were negative. Five patients showed active gastritis with neutrophilic abscesses. Five patients showed a prominent reactive gastropathy pattern of injury, and in two patients, these changes could be ascribed to iron pill gastritis with mucosal iron encrustations confirmed on an iron stain.
Table 1:
Morphologic changes associated with ICI in the gastrointestinal tract
| Biopsy site | Cases positive for ICI injury* | Diffuse increase in mucosal lymphocytes and plasma cells | Granuloma | Giant cells | Active gastritis Neutrophils and/or abscesses |
Duodenal blunting/colon architectural alteration | Increased intraepithelial lymphocytes | Increased apoptosis (>5 per 10 HPF) |
|---|---|---|---|---|---|---|---|---|
|
Stomach
**
N=39 |
22/39 (56%) | Mild = 14 Moderate = 5 15 (39%) patients showed peri-gland inflammation |
12/39 (31%) | 2/39 (5%) | 5/39 (13%) | NA | 1/39 (3%) | 1/39 (3%) |
|
Duodenum
N=33 |
14/33 (42%) | 12/33 (36%) | 6/33 (18%) | 2/33 (6%) | 12/33 (36%) | None = 21 (64%) Mild = 5 (15%) Moderate = 4 (12%) Severe = 3 (9%) |
13/33 (39%) | 1/33 (3%) |
|
Colon
N=31 |
20/31 (65%) | 11/31 (35%) | 0/31 (0%) | 0/31 (0%) | 13/31 (42%) | Chronic colitis =0 | 10/31(32%) (2 collagenous colitis) |
7/31 (23%) |
Cases positive for disease are defined in the methods section
Cases positive for disease in the stomach and/or duodenum = 71%
ICI=immune checkpoint inhibitor
Table 2:
Comparison of patients diagnosed as positive and negative for GI-irAE
| Histological parameters | All patients | Negative for GI-irAE | Positive for GI-irAE | p value |
|---|---|---|---|---|
| Stomach (n= 39) | ||||
| Diffuse inflammation (lymphocytes and plasma cells), Moderate or severe | 5/39 (13%) | 0/10 (0%) | 5/29 (17%) | 0.160 |
| Peri-gland inflammation * | 15/39 (39%) | 0/10 (0%) | 15/29 (52%) | 0.004 |
| Granuloma/giant cells * | 12/39 (31%) | 2/10 (20%) | 10/29 (35%) | 0.392 |
| Duodenum (n= 33) | ||||
| Villous blunting * | 12/33 (36%) | 0/5 (0%) | 12/28 (43%) | 0.067 |
| Increased intraepithelial lymphocytes | 13/33 (39%) | 1/5 (20%) | 12/28 (43%) | 0.335 |
| Increased lamina propria lymphocytes and plasma cells * | 12/33 (36%) | 0/5 (0%) | 12/28 (43%) | 0.067 |
| Active neutrophilic duodenitis | 12/33 (36%) | 1/5 (20%) | 11/28 (39%) | 0.67 |
| Colon (n= 31) | ||||
| Increased lamina propria lymphocytes and plasma cells * | 11/31 (36%) | 0/6 (0%) | 11/25 (44%) | 0.04 |
| Increased intraepithelial lymphocytes | 10/31 (32%) | 1/6 (17%) | 9/25 (36%) | 0.36 |
| Neutrophilic activity * | 13/31 (42%) | 0/6 (0%) | 13/25 (52%) | 0.07 |
| Increased apoptotic activity * | 7/31 (23%) | 0/6 (0%) | 7/25 (28%) | 0.147 |
Criteria used to define ICI-associated injury
GI-irAE=gastrointestinal immune-related adverse events
Figure 1:
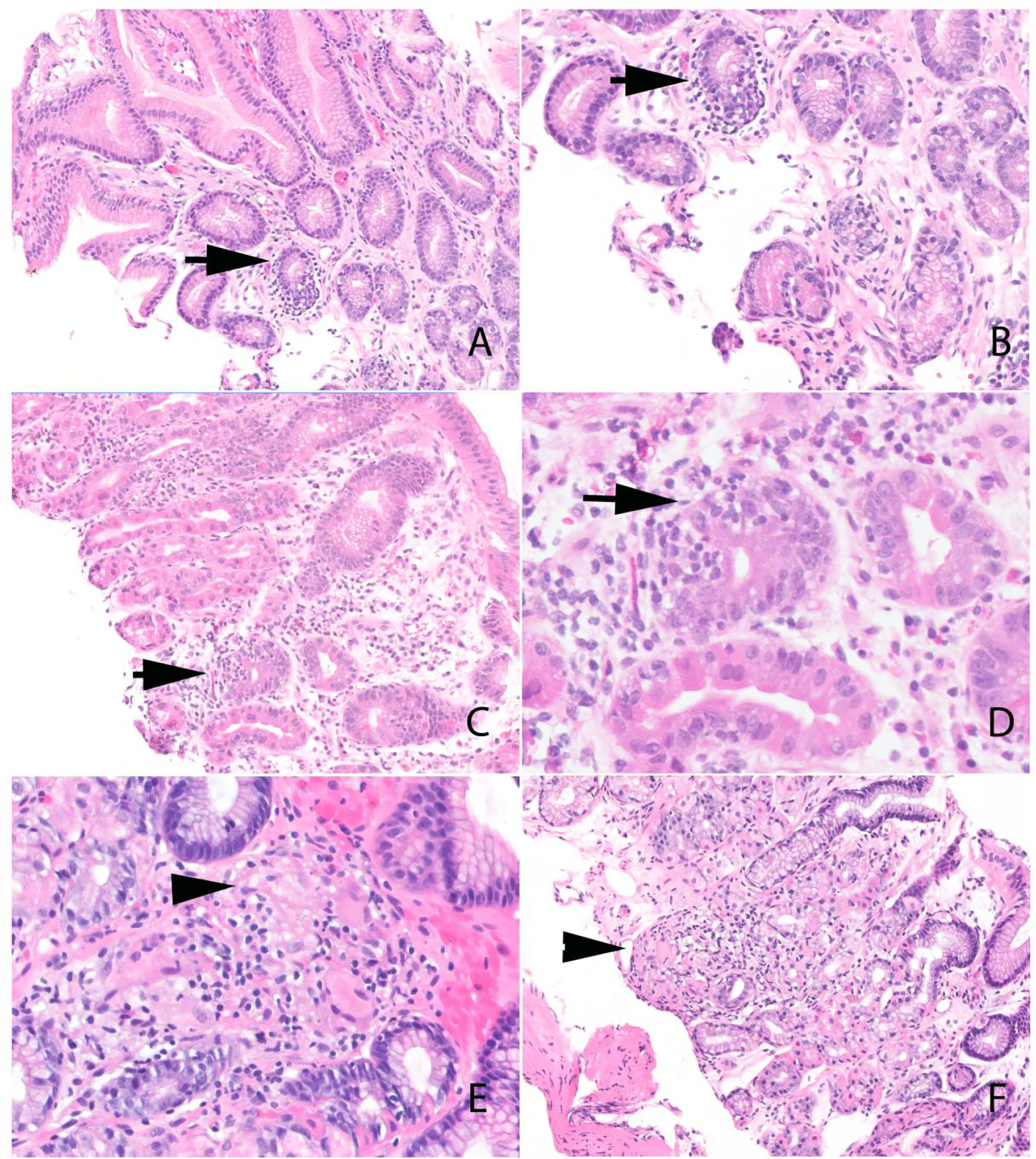
Histologic features of ICI-associated gastritis. A-D) Gastric peri-gland inflammation (arrows). The inflammation is localized to the gastric pit/isthmus/neck region. A mild diffuse lymphoplasmacytic infiltrate is also present in the lamina propria. E and F) Gastric non-necrotizing granulomas (arrowhead). Note the absence of ruptured crypts.
Helicobacter pylori organisms were not identified on hematoxylin and eosin stain in any of the cases; an immunohistochemical stain was also performed and negative in 14 cases.
Duodenal Involvement
Endoscopic findings
The mucosal abnormalities included erythematous mucosa (n=17) and erythema and erosions (n=1); other alterations included benign stricture (n=1), mucosal flattening (n=1), ulcer (n=2), and white exudate (n=1). Thirteen patients showed no endoscopic abnormalities. Like the gastric findings, there was no correlation between the endoscopic abnormalities and ICI-associated histologic changes (p=0.58); 9 of 17 (53%) patients with mucosal erythema showed ICI-associated microscopic disease, while 3 of 13 (23%) patients with endoscopically normal duodenal mucosa also showed these changes.
Histologic findings
Twelve of 33 cases (36%) showed villous blunting with moderate to severe blunting in 7 patients (Table 1 and 2) (Figure 2); the blunting was accompanied by a patchy increase in intraepithelial lymphocytes in 6 cases. Twelve (36%) patients showed an unequivocal expansion in lamina propria mononuclear cells, although only 6 of these cases showed an intraepithelial lymphocytosis. The intraepithelial lymphocytosis was typically patchy.
Figure 2:
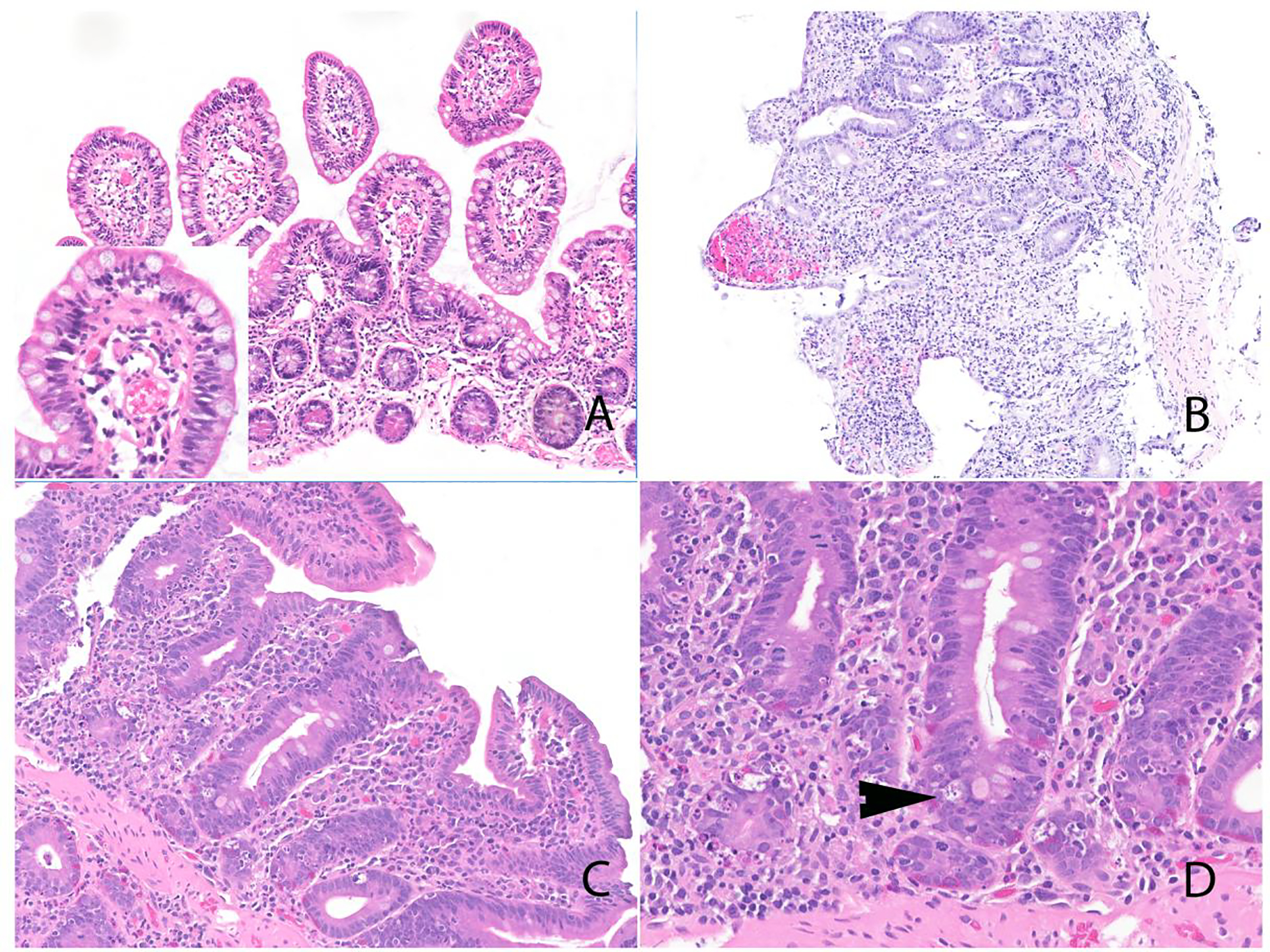
Histologic features of ICI-associated duodenitis. A) Duodenal mucosa with intact villus:crypt ratio and intraepithelial lymphocytosis; inset shows high-power image. B) Duodenum with villous blunting, diffuse epithelial injury, and increase in mucosal lymphocytes and plasma cells. Notably, there was no increase in intraepithelial lymphocytes. C) Duodenal mucosa with villous blunting and expansion of the mucosa by lymphoid infiltrate. D) Prominent increase in apoptotic activity (arrowhead).
Non-necrotizing granulomas were observed in 6 patients (18%); all 6 also showed gastric granulomas. Notably, on follow-up, the patients with gastric and duodenal granulomas did not show evidence of a systemic granulomatous disease such as sarcoidosis, and there was no evidence of infectious disease (mean follow-up (39 patients) 22 months).
Colonic Involvement
Endoscopy
On endoscopy, 14 cases showed erythema/loss of vascular pattern/mucosal granularity; two of these patients also showed erosions. One patient each was noted to show a mass (on histology no malignancy was identified), ulcer, and flattening of mucosa. The endoscopic evaluation was unremarkable in 14 cases (Table 3). Of note, 50% of patients with lymphocytic-pattern colitis showed erythematous mucosa.
Table 3:
Correlation of colonic histologic changes with endoscopic appearance
| Histologic pattern | Erythema/loss of vascular pattern and/or erosions | Normal endoscopy |
|---|---|---|
| Normal | 2 (22%) | 7 (78%) |
| Acute self-limiting colitis | 7 (100%) | 0 |
| Lymphocytic colitis | 4 (50%) | 4 (50%) |
| Collagenous colitis | 1 (50%) | 1 (50%) |
| Increased apoptosis only pattern | 0 | 2 (100%) |
Others not included in table: one patient each was noted to show a mass (on histology no malignancy was identified), ulcer, and flattening of mucosa on endoscopy.
Histologic changes
We identified four patterns of colitis: 1) acute self-limiting colitis (n=8), 2) lymphocytic colitis (n=8), 3) collagenous colitis (n=2), and 4) apoptosis only (n=2), defined as >5 apoptoses per 10 HPFs (Figure 3). In 11 patients, the colonic mucosa was histologically unremarkable; 5 of these patients presented with diarrhea.
Figure 3:
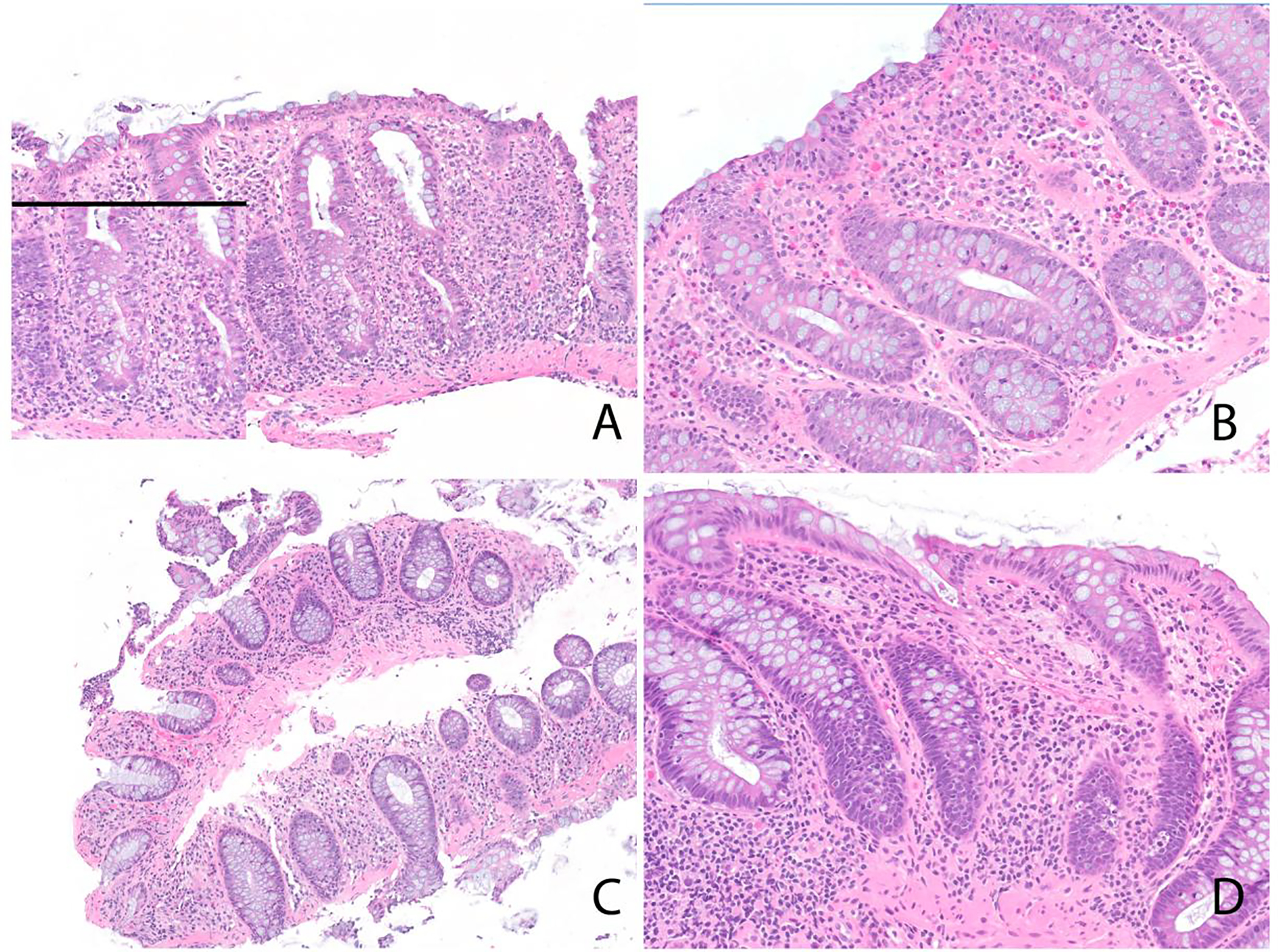
Histologic features of ICI-associated colitis. A) Acute self-limiting colitis pattern. Inset highlights neutrophilic cryptitis. B) Lymphocytic colitis pattern. C) Collagenous colitis pattern. D) Apoptosis only pattern.
The acute self-limiting pattern of colitis was accompanied by an increase in lamina propria mononuclear cells in 4 cases and neutrophilic cryptitis or crypt abscesses in 7 cases. Three patients with the acute self-limiting colitis pattern of injury also showed more than 5 apoptotic cells per 10 HPF. In patients with the lymphocytic colitis pattern of injury, the intraepithelial lymphocytosis was accompanied by an expansion of the lamina propria by mononuclear cells and neutrophilic cryptitis in 5 cases each. Patients with the collagenous colitis pattern injury showed a thickened subepithelial collagen layer as well as intraepithelial lymphocytosis; neither case showed neutrophilic activity. In the two patients with apoptosis only pattern, the apoptotic activity ranged from 6–10 per 10 HPF.
Viral cytopathic effect was not seen. Immunohistochemical stains performed for CMV (n=12) and adenovirus (n=3) were negative.
Histological features that distinguished patients clinically positive and negative for GI-irAE
Based on this grouping, the histological features that best correlated with patients positive for GI-irAE (Table 2) were peri-gland gastric inflammation (p=0.004) and increased colonic mononuclear cells (p=0.04).
Comparison of histologic features in stomach/duodenum and colon
The sensitivity of gastric, duodenal, and colonic biopsies for ICI-associated injury was 56%, 42% and 65%, respectively. Collectively, the sensitivity of upper GI biopsies (stomach and duodenum) was 71%.
Some notable differences with regards to the location of specific microscopic findings (Table 1) included those involving the duodenal mucosa which showed architectural changes (villous blunting); the colon and stomach lacked these alterations. Duodenal and colonic mucosal biopsies frequently showed diffuse intraepithelial lymphocytosis while gastric intraepithelial lymphocytes were confined to the zones of peri-gland inflammation. Prominent apoptotic activity was generally confined to the colon. Conversely, granulomas were localized to the upper GI tract, predominantly the stomach.
Comparison of anti-PD-1/PD-L1 with combination anti-PD-1/anti-CTLA-4 antibodies
Patients on combination anti-CTLA-4 and anti-PD-1/PD-L1 therapy were more likely to show ICI-associated gastric and duodenal injury (Table 4). Gastric peri-gland inflammation and granulomas as well as increased duodenal lamina propria mononuclear cells were more often seen with combination therapy; notably, there were no differences in the colonic findings between the two groups.
Table 4:
Comparison of all patients and GI-irAE positive cases treated with anti-PD-1/PD-L1 monotherapy and combination anti-PD1/PD-L1 and anti-CTLA-4
| Histological Parameters | Total cases | Positive for GI-irAE cases | ||||
|---|---|---|---|---|---|---|
| PD-1 inhibitor (n = 21) | PD-1 and CTLA-4 inhibitor (n = 17) | p value | PD-1 inhibitor N=14 | PD-1 and CTLA-4 inhibitor N=14 | p value | |
| Stomach | ||||||
| ICI associated gastric injury * | 7/21 (33%) | 14/17 (82%) | 0.003 | 6/14 (43%) | 13/14 (92%) | 0.005 |
| Peri-gland inflammation | 4/21 (19%) | 10/21 (59%) | 0.01 | 4/14 (29%) | 10/14 (71%) | 0.02 |
| Gastric or duodenal granulomas | 3/21 (14%) | 8/17 (47%) | 0.03 | 2/14 (14%) | 7/14 (50%) | 0.04 |
| Increased apoptotic activity | 0/21 (0%) | 1/17 (6%) | 0.26 | 0/14 (0%) | 1/14 (7%) | 0.31 |
| Stomach or duodenum abnormal | 10/18 (56%) | 14/16 (88%) | 0.04 | 9/14 (64%) | 13/14 (93%) | 0.07 |
| Duodenum | ||||||
| ICI associated duodenal injury * | 4/17 (24%) | 10/15 (67%) | 0.02 | 4/14 (29%) | 10/13 (77%) | 0.01 |
| Increased lamina propria mononuclear cells | 3/17 (18%) | 9/15 (60%) | 0.02 | 3/14 (21%) | 9/13 (69%) | 0.01 |
| Increased intraepithelial lymphocytes | 7/17 (41%) | 5/15 (33%) | 0.65 | 7/14 (50%) | 4/13 (31%) | 0.31 |
| Moderate to severe villous blunting | 2/17 (12%) | 5/15 (33%) | 0.14 | 2/14 (14%) | 5/13 (38%) | 0.15 |
| Duodenal neutrophilic activity | 5/17 (29%) | 7/15 (47%) | 0.58 | 4/14 (29%) | 7/13 (54%) | 0.37 |
| Increased apoptotic activity | 0/17 (0%) | 1/15 (7%) | 0.28 | 0/14 (0%) | 1/13 (8%) | 0.29 |
| Colon | ||||||
| ICI associated colonic injury * | 13/17 (77%) | 6/13 (46%) | 0.09 | 12/13 (92%) | 6/11 (55%) | 0.03 |
| Increased lamina propria mononuclear cells | 6/17 (35%) | 5/13 (39%) | 0.86 | 6/13 (46%) | 5/11 (46%) | .97 |
| Increased intraepithelial lymphocytes | 8/17 (47%) | 2/13 (15%) | 0.07 | 7/13 (54%) | 2/11 (18%) | 0.07 |
| Neutrophilic activity | 8/17 (47%) | 4/13 (31%) | 0.47 | 8/13 (62%) | 4/11 (36%) | .41 |
| Increased apoptotic activity | 5/17 (29%) | 2/13 (15%) | 0.37 | 5/13 (39%) | 2/11 (18%) | 0.28 |
Based on criteria used to define immune checkpoint inhibitor-associated injury.
Treatment
Patients negative for GI- irAE (n=10)
These patients did not require steroids and their symptoms were not considered to represent GI-irAE. Within this group, 7 patients presented with diarrhea, of which 3 also presented with nausea and vomiting.
Patients positive for GI- irAE (n=29)
Twenty-six of these 29 patients were treated with steroids. Two cases with lymphocytic colitis and one case with collagenous colitis received budesonide. Thirteen patients also required infliximab to control symptoms.
DISCUSSION
Herein, we report the largest series of upper gastrointestinal biopsies from patients with suspected GI-irAE and reach the following conclusions: 1) histologic abnormalities are often found in endoscopically normal mucosa, 2) the sensitivity for ICI-associated injury of upper GI biopsies appears to be equivalent to that for colon biopsies, and concurrent upper and lower GI biopsies could aid in the distinction of GI-irAE from its mimics, 3) patients treated with ICIs often show non-necrotizing granulomas, although almost exclusively in the upper GI tract, 4) gastric peri-gland inflammation may represent one of the more characteristic features of ICI-associated injury, although the finding may mimic the “focal enhancing gastritis” of Crohn’s disease, 5) ICI-associated histologic changes mimic a broad range of primary GI diseases including Crohn’s disease, celiac disease, infectious enterocolitis, lymphocytic colitis and collagenous colitis, and 6) although the histologic appearance of mono and dual antibody therapy overlap, granulomas and gastric peri-gland inflammation are more frequently identified in patients receiving dual therapy while intraepithelial lymphocytosis is typically seen in patients on anti-PD1 monotherapy.
ICI-associated gastritis is characterized by lymphocytes surrounding and infiltrating gastric pits/isthmus/neck region, herein termed peri-gland inflammation. The presence of peri-gland inflammation in conjunction with non-necrotizing granulomas would logically raise concern for Crohn's disease. However, the peri-gland inflammation in Crohn’s disease, often referred to as "focal enhancing gastritis,” comprises of an admixture of lymphocytes and histiocytes—the mixed inflammation is an uncommon finding in ICI-associated gastritis. Although reported in gastric irAE, we found that a diffuse increase in intraepithelial lymphocytes is uncommon.18 Other more common forms of gastritis, including Helicobacter pylori gastritis and autoimmune gastritis, typically show a diffuse mononuclear infiltrate. Although a diffuse mononuclear infiltrate is occasionally noted with ICI-associated gastritis, an accentuation in the peri-gland area is more readily observed (Figures 1C and 1D).
Celiac disease is virtually indistinguishable from duodenal ICI-associated injury: both diseases feature villous blunting, expansion of the lamina propria by mononuclear cells, and intraepithelial lymphocytosis. Subtle changes such as patchy intraepithelial lymphocytosis may suggest a diagnosis of ICI-related duodenitis. Furthermore, granulomas are not a feature of celiac disease. Nevertheless, we acknowledge the subtlety and relative lack of specificity of these differences. Additionally, it is theoretically possible for ICI-associated injury to unmask latent celiac disease and thus, the definitive distinction between celiac disease and ICI-associated duodenitis would require correlation with serological studies. We note that the 23 patients tested in this series lacked serological markers of celiac disease. Given the presence of granulomas and patchy distribution of the disease, Crohn’s duodenitis should also be considered in the differential diagnosis. Other potential etiologies to consider include non-steroidal anti-inflammatory drugs (NSAID)-related duodenitis and infections, and in this context clinical information and a detailed drug history are highly relevant.
While the findings in the stomach and duodenum fall within a narrow histologic spectrum, ICI-associated colitis is linked to a broad range of morphologic alterations.19, 20 Of note, one-quarter of all patients presenting with diarrhea lacked detectable colonic histological abnormalities, and the majority of these cases showed ICI-associated histologic changes in the upper GI biopsies, an observation that reinforces the need to evaluate gastric and duodenal biopsies. ICI-associated colonic injury mimics acute self-limiting colitis, and in these instances, it would be virtually impossible to exclude infectious colitis; however, involvement of the upper gastrointestinal tract, particularly gastric peri-gland inflammation, granulomas, and celiac disease-like appearance in the duodenum would support GI-irAE. Similarly, lymphocytic and collagenous colitis pattern injuries are virtually indistinguishable from the corresponding de novo diseases, although we note that half of all patients with lymphocytic colitis pattern injury showed evidence of activity, creating a mixed picture—a relatively uncommon appearance for de novo idiopathic lymphocytic colitis.
Other potential drugs that may be associated with colitis include idelalisib, a PI3K delta inhibitor. Although this drug is distinct from anti-PD-1/PD-L1 and anti-CTLA-4, the agent disrupts immune system homeostasis and is associated with diarrhea.21 In the colon, idelalisib-associated colitis shows a spectrum of changes including prominent apoptosis with acute cryptitis, crypt abscesses, increased intraepithelial lymphocytes, and lamina propria expansion.21 In the context of lymphocytic and collagenous colitis, other considerations include NSAIDs, proton pump inhibitors, and histamine receptor inhibitors. Finally, although increased colonic apoptotic activity is often identified, it is uncommon to encounter an apoptosis-only pattern of colitis; in conjunction with the clinical picture, this observation makes diseases such as graft-versus-host disease,22 autoimmune enteropathy,23 and drugs such as mycophenolate mofetil, for the most part, only a theoretical possibility.
Of note, changes of chronic colitis were not observed in this cohort. Patients with pre-existing autoimmune and inflammatory disorders including ulcerative colitis and Crohn’s disease are generally excluded from trials evaluating ICIs. Recent data suggests that the risk of flare in patients with inflammatory bowel disease is about 30% with anti-CTLA-4 therapy and possibly lower with anti-PD-1 therapy.5,24 Collectively, although ulcerative colitis is an unlikely histologic consideration, it may be virtually impossible to distinguish active Crohn’s disease from GI-irAE.
The histologic findings reported in this study are generally similar to those illustrated in prior studies.20, 23, 25 One notable difference is the lack of association between granulomas and ruptured crypts25 and their localization to the upper GI tract, an uncommon site of crypt rupture-associated granulomas. Although the presence of granulomas is a known ICI-associated event, the possibility of an alternative trigger should be investigated. Of note, on follow-up, we did not identify other etiologies for granulomas in this series.
To define ICI-associated gastrointestinal injury, we chose histologic features (see Materials and Methods) based largely on prior literature,20,25 but we also attempted to limit the use of relatively common alterations such as gastric lamina propria inflammation. However, the goal of this exercise was not to define individual histologic parameters of GI-irAE, but instead to compare the sensitivity of the biopsies from the upper and lower GI tract. Notably, the sensitivity of upper and lower GI biopsies for GI-irAE appear equivalent. The relatively small number of cases negative for GI-irAE makes it difficult to precisely define the diagnostic features with the highest yield and specificity. With these caveats in mind, the histologic features identified in this study that best correlated with a clinical diagnosis of GI-irAE included gastric peri-gland inflammation (p=0.004) and increased colonic mononuclear cells (p=0.04).
In summary, while the individual manifestations of ICI-related gastroenterocolitis are relatively non-specific and raise a broad differential diagnosis, the findings in our cohort underscore the importance of a global review of upper and lower gastrointestinal biopsies in patients with clinically-suspected GI-irAE. Patients presenting with diarrhea may lack histologic evidence of colitis, but often show ICI-associated histologic changes in the upper GI tract. ICI-associated histologic changes may be observed in endoscopically normal mucosa, and biopsies from macroscopically unremarkable mucosa may have diagnostic value. The collective presence of gastric peri-gland inflammation, upper GI granulomas, and the acute self-limiting colitis/lymphocytic/collagenous patterns of injury may assist in arriving at a single unifying diagnosis of GI-irAE. Awareness of the histologic spectrum of anti-PD-1/PD-L1 and anti-CTLA-4-associated gastroenterocolitis is important to distinguish it from potential mimics, particularly Crohn’s disease, celiac disease, as well as infectious and other types of immune-mediated colitis.
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
REFERENCES
- 1.Weber JS, Hodi FS, Wolchok JD et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35;785–792. [DOI] [PubMed] [Google Scholar]
- 2.Dougan M. Checkpoint blockade toxicity and immune homeostasis in the gastrointestinal tract. Front Immunol 2017;8;1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds K, Thomas M, Dougan M. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist 2018;23;991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauken KE, Dougan M, Rose NR, Lichtman AH, Sharpe AH. Adverse events following cancer immunotherapy: Obstacles and opportunities. Trends Immunol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soularue E, Lepage P, Colombel JF et al. Enterocolitis due to immune checkpoint inhibitors: A systematic review. Gut 2018;67;2056–2067. [DOI] [PubMed] [Google Scholar]
- 6.Collins M, Michot JM, Danlos FX et al. Inflammatory gastrointestinal diseases associated with pd-1 blockade antibodies. Ann Oncol 2017;28;2860–2865. [DOI] [PubMed] [Google Scholar]
- 7.Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology 2017;6;e1344805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang DY, Salem JE, Cohen JV et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol 2018;4;1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A, De Felice KM, Loftus EV, Khanna S Systematic review: Colitis associated with anti-ctla-4 therapy. Aliment Pharmacol Ther 2015;42;406–417. [DOI] [PubMed] [Google Scholar]
- 10.Pennock GK, Chow LQ. The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist 2015;20;812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puzanov I, Diab A, Abdallah K et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the society for immunotherapy of cancer (sitc) toxicity management working group. J Immunother Cancer 2017;5;95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36;1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haanen J, Carbonnel F, Robert C et al. Management of toxicities from immunotherapy: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28;iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 14.Horvat TZ, Adel NG, Dang TO et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol 2015;33;3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faje AT, Lawrence D, Flaherty K et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124;3706–3714. [DOI] [PubMed] [Google Scholar]
- 16.Arbour KC, Mezquita L, Long N et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 2018;36;2872–2878. [DOI] [PubMed] [Google Scholar]
- 17.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated sydney system. International workshop on the histopathology of gastritis, houston 1994. Am J Surg Pathol 1996;20;1161–1181. [DOI] [PubMed] [Google Scholar]
- 18.Yip RHL, Lee LH, Schaeffer DF, Horst BA, Yang HM. Lymphocytic gastritis induced by pembrolizumab in a patient with metastatic melanoma. Melanoma Res 2018;28;645–647. [DOI] [PubMed] [Google Scholar]
- 19.Baroudjian B, Lourenco N, Pages C et al. Anti-pd1-induced collagenous colitis in a melanoma patient. Melanoma Res 2016;26;308–311. [DOI] [PubMed] [Google Scholar]
- 20.Oble DA, Mino-Kenudson M, Goldsmith J et al. Alpha-ctla-4mab-associated panenteritis: A histologic and immunohistochemical analysis. Am J Surg Pathol 2008;32;1130–1137. [DOI] [PubMed] [Google Scholar]
- 21.Louie CY, DiMaio MA, Matsukuma KE, Coutre SE, Berry GJ, Longacre TA. Idelalisib-associated enterocolitis: Clinicopathologic features and distinction from other enterocolitides. Am J Surg Pathol 2015;39;1653–1660. [DOI] [PubMed] [Google Scholar]
- 22.Washington K, Jagasia M. Pathology of graft-versus-host disease in the gastrointestinal tract. Hum Pathol 2009;40;909–917. [DOI] [PubMed] [Google Scholar]
- 23.Masia R, Peyton S, Lauwers GY, Brown I. Gastrointestinal biopsy findings of autoimmune enteropathy: A review of 25 cases. Am J Surg Pathol 2014;38;1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menzies AM, Johnson DB, Ramanujam S et al. Anti-pd-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28;368–376. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez RS, Salaria SN, Bohannon CD, Huber AR, Feely MM, Shi C. Pd-1 inhibitor gastroenterocolitis: Case series and appraisal of 'immunomodulatory gastroenterocolitis'. Histopathology 2017;70;558–567. [DOI] [PubMed] [Google Scholar]


