Abstract
As current psychosocial and pharmacological interventions show limited efficacy in the treatment of anorexia nervosa (AN), interest in the potential value of neurosurgical intervention and neuromodulation in managing severe and enduring illness has grown. We conducted a systematic review of 20 trials of neurosurgical and neuromodulatory treatments for AN, including neurosurgical ablation, deep brain stimulation (DBS), repetitive transcranial magnetic stimulation (rTMS), and transcranial direct current stimulation (tDCS). Overall, there is evidence to support the role of stereotactic ablation and DBS in the treatment of AN. In contrast, results for rTMS and tDCS have been modest and generally more mixed. Neurosurgical treatment may offer important new avenues for the treatment of AN. Additional randomized clinical trials with comparable patient populations will be needed, in which change in affective, cognitive, and perceptual symptom phenomena, and interrogation of targeted circuits, pre- and post-intervention, are carefully documented.
Anorexia nervosa (AN) is a disabling, and potentially chronic and life-threatening eating disorder, characterized by self-imposed starvation, physical emaciation, an intense fear of weight gain, and a marked disturbance in the way one’s shape and weight is experienced (American Psychiatric Association, 2013). Notably, AN demonstrates the highest mortality rates among all psychiatric disorders (Arcelus et al., 2011) and approximately 60% of those afflicted still meet diagnostic criteria two decades after illness onset (Fichter et al., 2017). At present, no FDA-approved pharmacotherapy exists, and psychosocial treatments typically yield short-term symptom remission in approximately one third of adolescent-age patients, and even less in adults (Watson & Bulik, 2012). In consideration of the challenge of treatment resistance, and managing cases of severe and enduring illness, interest has grown in the potential value of surgical and neuromodulatory strategies.
A range of brain abnormalities have been reported in AN, spanning neural function and structure (Cowdrey et al., 2011; Fonville et al., 2013; Frank et al., 2014; Steinglass et al., 2015; Titova et al., 2013), as well as neurotransmission and neurochemistry (Bailer et al., 2005; Frank et al., 2005). However, the primacy and translational implications of these findings for modeling the causal psychopathology of AN and its treatment remains in question. Notwithstanding, it has been well stated that treatments for AN can no longer “remain brainless” (Schmidt & Campbell, 2013). As emerging psychosocial treatments have highlighted the need to incorporate neurobiological constructs into treatment approaches (W. H. Kaye et al., 2015), so too has interest increased in the possible merits of neurosurgical and neuromodulatory strategies.
Surgical interventions for AN date back some 50 years. Prefrontal leucotomies were performed on 17 patients with AN between 1950 and the early 1970s, with largely inconclusive outcomes (N. Lipsman, Woodside, Giacobbe, Lozano et al., 2013a). Following the introduction of stereotactic head frames in the 1970s, mixed results were reported in three patients who received limbic leucotomy, and in two patients undergoing a dorsal thalamotomy (N. Lipsman, Woodside, Giacobbe, Lozano et al., 2013b).
More recently, however, several important advances have resulted in a relative renaissance of neurosurgical treatments in psychiatry, alongside the emergence of neuromodulatory treatments. First, the rapid evolution of neuroimaging technology has resulted in advances in our understanding of the neurobiology of AN, allowing for more informed hypotheses relating to (i) how specific brain regions are implicated in the psychopathology of AN (G. K. W. Frank et al., 2019), and (ii) preliminary hypotheses as to which brain regions might serve as optimal targets in targeted treatments (W. H. Kaye et al., 2009). Second, the precision and increased safety with which surgical, as well as non-surgical procedures, can modulate brain activity has advanced impressively.
For instance, radiosurgery and, more recently, MRI-guided focused ultra-sound now allow for ablative neurosurgery to be undertaken without a single incision. Notwithstanding, ablative brain procedures are permanent and non-adjustable once administered. Given the potential for nonreversible side effects, this has led for some to contest that a moral obligation exists in prioritizing research into non-ablative treatments over ablative neurosurgical treatments in the context of AN (Pugh et al., 2018). Non-ablative invasive neurosurgical procedures, such as deep brain stimulation (DBS), have recently been advanced in the context of AN, with the distinct advantage of being reversible and adjustable (via modification of device settings). Nonetheless, DBS too is an invasive procedure which carries operative risks (Fenoy & Simpson, 2014); and it remains unclear if irreversible changes can result from long-term implantation and stimulation. In contrast, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) offer a non-invasive means of modulating neuronal activity, albeit with less spatial precision.
Cumulatively, these treatments have been broadly utilized in a range of movement disorders such as Parkinson’s disorder, essential tremor and dystonia (Bronstein et al., 2011; Flora et al., 2010), and have become increasingly utilized in the treatment of psychiatric disorders such as treatment resistant depression, obsessive–compulsive disorder (OCD), and anxiety disorders (Abelson et al., 2005; Greenberg et al., 2006). However, research examining the effects of neurostimulation on eating disorder psychopathology is still limited. Broad reviews have noted some promise for rTMS and tDCS in reducing food cravings in healthy individuals, yet mixed findings have been reported in the treatment of AN, with some reporting promising outcomes (B. Dalton, Bartholdy, Campbell, & Schmidt, 2018a; J. McClelland et al., 2013a) while others have noted consistent trends towards weight loss (Hall et al., 2018). Several narrative reviews have suggested promising preliminary results for DBS in AN (B. Dalton, Bartholdy, McClelland et al., 2018a; Lee et al., 2018) and a narrative review of neurosurgical interventions for AN over the last 60 years suggested limited evidence of treatment efficacy (N. Lipsman, Woodside, Giacobbe, Lozano et al., 2013b). No studies we know of have simultaneously and systematically assessed neurosurgical and neuromodulatory treatments for AN.
Herein, we extend the scope of earlier reviews by (i) systematically assessing all neurosurgical and neuromodulatory treatments for AN, (ii) aggregating novel evidence not included in previous reviews, (iii) offering alternative findings from recent interpretations of the existing data, and (iv) outlining key initiatives for future research. Specifically, we conduct a systematic review of the efficacy of stereotactic ablative neurosurgery, DBS, TMS and tDCS, exclusively in the context of AN, as defined by either physiological (weight normalization) or psychological symptom indices.
Methods
Selection procedures
We performed a systematic review in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Moher, 2009), and the protocol for this review was prospectively registered with PROSPERO (CRD42019141369). In reviewing recent developments in this literature, a comprehensive database search was conducted for abstracts published between January 1st, 2000 to June 1st, 2019, in MEDLINE/PubMed, PsychINFO, ScienceDirect, EMBASE, and Scopus databases. Key search terms included relevant combinations of eating disorders, anorexia nervosa, deep brain stimulation, gamma knife microsurgery, transcranial magnetic stimulation, transcranial direct current stimulation, transcranial alternating current stimulation, cranial electrotherapy stimulation, and brain stimulation, with Boolean operators. Studies in any language were considered, and where needed, web-based translation was undertaken on studies not written in English. Additional search strategies included screening reference lists of eligible studies, screening existing reviews for AN, and a manual journal search of relevant journals.
Inclusion criteria
Studies were included if they (i) reported the effect of either stereotactic ablative neurosurgery, DBS, TMS, or tDCS on AN symptomatology, (ii) in terms of either weight or psychological symptom (i.e., shape or weight concerns, drive for thinness) status, (iii) across at least two time points (pre- and post-intervention). Given the relatively recent upsurge in brain-based treatments for AN, search criteria were restricted to studies between January 1st, 2000 and June 1st, 2019. Moreover, given the nascency of this evidence base, studies of all methodological design were included (i.e., single patient case studies, case series, open trials, randomized controlled trials), and studies assessing treatments primarily aimed at comorbid conditions in those with AN were included.
Study selection
Keyword search results from each database were combined, and duplicates were removed. All titles and abstracts were screened for potential eligibility. The full text of articles relating to brain-based treatments in the context of AN psychopathology were read in full to determine eligibility. Twenty studies met inclusion criteria and were carried forward for systematic review. A flowchart of keyword search results and study selection is outlined in Figure 1.
Figure 1.
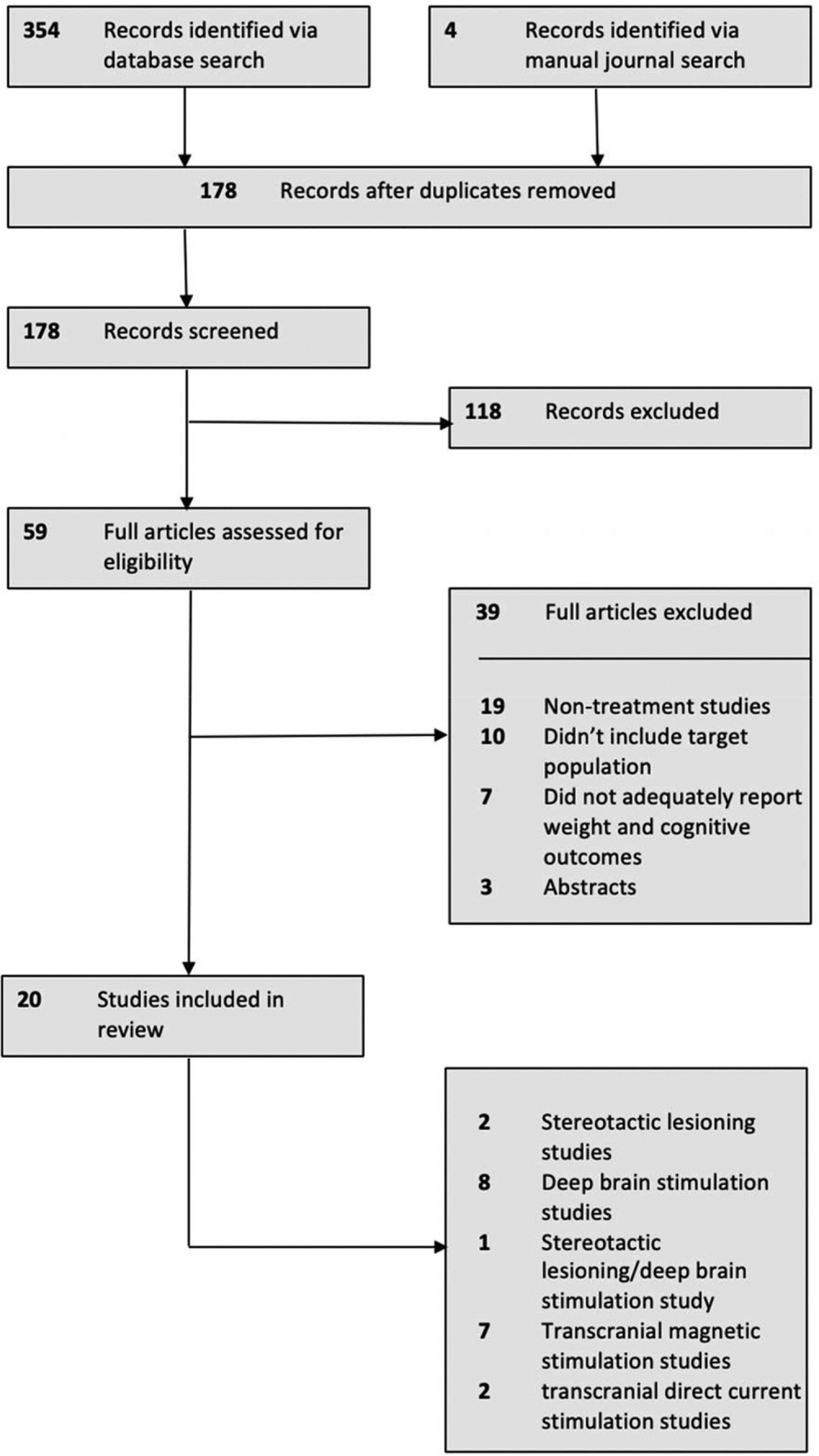
PRISMA flowchart of study selection.
Results
Overall, 20 eligible studies were identified and included for review. Two studies related exclusively to neurosurgical ablation (Barbier et al., 2011; Liu et al., 2017) eight studies related exclusively to DBS (Israël et al., 2010; N. Lipsman, Woodside, Giacobbe, Lozano et al., 2013a; N. Lipsman et al., 2017; Manuelli et al., 2019; McLaughlin et al., 2013; Zhang et al., 2013; Wu et al., 2013; Blomstedt et al., 2017), seven related to rTMS (B. Dalton, Bartholdy, McClelland et al., 2018b; Jaššová et al., 2018; Kamolz et al., 2008; J. McClelland, Bozhilova, Nestler et al., 2013b; J. McClelland et al., 2016a, 2016a; Van den Eynde et al., 2013), two related to tDCS (Costanzo et al., 2018; Khedr et al., 2014), and one study included patients undergoing neurosurgical ablation and DBS (Wang et al., 2013). Tables 1–4 summarize key details for each study, including patient characteristics, illness history, previous treatment, details of the study intervention, the outcome of treatment, and adverse events. The data that support these findings are openly available at https://osf.io/kb4f7/.
Table 1.
Summary of stereotactic lesioning studies for anorexia nervosa.
| Study | N | Patient Details | Procedure | Outcomes | Adverse Events | |
|---|---|---|---|---|---|---|
| Weight Symptoms | Psychological Symptoms | |||||
| Barbier et al., 2011 | 1 | 38-year-old woman with a 24-year history of AN, which was characterized by more than 20 inpatient admissions, and non-successful psychosocial and pharmacological treatment attempts. Patient also had a lifelong history of OCD. | Stereotactic bilateral anterior capsulotomy via radiofrequency ablation, guided by computed tomography and magnetic resonance imaging. | Patient BMI improved from 13.1 pre-operatively, to 23 at 3-month follow-up post-surgery. BMI was maintained at 22.3 at 12-month follow-up. | Eating Disorder Examination - Questionnaire scores improved from a baseline score of 5.7, to a 3-month follow-up score of 3.74, at which time she longer met criteria for AN. | None reported. |
| Wang et al., 2013 | 6 | Adolescents and adults with AN, with a mean age of 20.3 (±2.5) years, and a mean illness duration of 3 (±.63) years. | Stereotactic bilateral radiofrequency ablation of the ventral striatum/nucleus accumbens. | Mean patient BMI improved from a baseline preoperative BMI of 13.38 (±.59), to a 6-month follow-up BMI of 19.15 (±.1.35), and a 12-month follow-up BMI of 20.38 (±1.26). | Psychological symptoms of AN were not reported. | All patients experienced transient headaches of varying severity, lasing 3–4 days. Centric fever was noted in 63% of patients, lasting 3–5 days. |
| Liu et al., 2017 | 74 | Adolescents and adults with AN, with a mean age of 25 (±7.9) years, and a mean illness duration of 5 years (range = 3.7–6 years). | Bilateral stereotactic anterior capsulotomy via radiofrequency ablation. | Patient BMI increased from a pre-surgery mean of 13.63 (±1.57), to a 12-month follow-up BMI of 18.17 (±3.4), and a 3-year follow-up BMI of 19.33 (±3.57). | Psychological symptoms of AN were not reported. | Acute side effects included urinary incontinence (N=7), sleep disorders (N=8), and fatigue (N=6). Long-term side effects included disinhibition (N=6), memory loss (N=3), and lethargy (N=4). |
Table 4.
Summary of transcranial direct current stimulation studies for anorexia nervosa.
| Study | N | Patient Details | Procedure | Outcomes | Adverse Events | ||
|---|---|---|---|---|---|---|---|
| Weight Symptoms | Psychological Symptoms | ||||||
| Khedr et al., 2014 | 7 | Adolescents and adults with AN, with a mean age of 21.75 (±7.8) years, a mean illness duration of 3.42 (±.79) years, and a mean BMI of 14.86 (±1.57). | 25 mins per day of 1mA Anodal tDCS for 10 days, positioned over the left dorsolateral prefrontal cortex. | Patient BMI at EOT not reported. | Mean Eating Attitudes Test score improved from a baseline score of 60.9 (±7.4), to a 1-month follow-up score of 39.4 (±19.1). Overall, a variable response was noted across participants, with improvements reported in 3 patients at 1-month follow-up, and no improvement reported in 4 patients at 1-month follow-up. |
Transient local itching in 2 patients. | |
| Costanzo et al., 2018 | 23 | 3 × 20 mins of 1mA tDCS per week, for 6 weeks (18 sessions). Anodal electrode positioned over the left dorsolateral prefrontal cortex, and the cathodal electrode over the right dorsolateral prefrontal cortex. tDCS occurred in the context of TAU. |
Patient BMI increased significantly in those receiving tDCS from a mean baseline BMI of 14.7, to 16.6 (±2.3) at EOT. However, this was not significantly different from improvements observed in the control condition (Baseline BMI = 15.5, ±1.6; EOT BMI = 16.1, ±1.3). |
Total Eating Disorder inventory-3 scores improved from a baseline score of 62.4 (±21.0), to a score of 48.9 (±24.9) at EOT. However, this was not significantly different from improvements observed in the control condition (Baseline Total EDI-3 = 70.7, ±13.3; EOT Total EDI-3 = 56.2, ±18.3). |
27% of those receiving tDCS reported moderate headaches, 27.7% reported a moderate burning sensation, 27.2% reported moderate itching, and 45.4% reported moderate local redness. | ||
|
tDCS + TAU (N=11) Mean age of 13.9 (±1.8) years, and a mean baseline BMI was 14.7 (±2.2). |
FBT + TAU (N=12) Mean age of 15.1 (±1.5) years, and a mean baseline BMI of 15.5 (±1.6). |
||||||
Stereotactic ablative surgical procedures
In total, three eligible studies examined stereotactic neurosurgical ablative procedures in AN in a total of 81 patients, spanning both adolescents and adults. This consists of (i) one case study of a 38-year-old woman with a 24-year history of AN (Barbier et al., 2011), (ii) one small case series of six female patients ranging from age 18 to 25 years with an illness duration of 2–4 years (Wang et al., 2013), and (iii) one large (N = 74) open label trial of female patients ranging from age 18 to 58 years with an illness duration ranging from 3 to 6 years (Liu et al., 2017). To date, two different neuroanatomical sites have been targeted—the anterior limb of the internal capsule (ALIC) (two studies, total N = 75), and the ventral striatum/nucleus accumbens (one study, total N = 6).
Anterior capsulotomy
The ALIC is a white matter structure, located in the inferomedial part of each cerebral hemisphere, which carries thalamic and brainstem fibers from prefrontal cortical regions, which are thought be to be involved in aspects of emotion, motivation, cognitive processing, and decision making (Safadi et al., 2018). Studies targeting the ALIC reported rapid increases in patient BMI in those with both severe and enduring AN (Barbier et al., 2011) and those with less longstanding illness durations (Liu et al., 2017). In a case study of severe and enduring AN of approximately 24 years, Barbier et al. (2011) reported a dramatic increase in BMI from 13.1 pre-operatively, to a BMI of 23 at just 3-month follow-up. In concert, this patient, who had exhausted multiple outpatient and inpatient treatment options, reported decreases in food related distress and in global eating disorder symptoms following anterior capsulotomy at 3-month follow-up; however, significant eating disorder psychopathology remained.
In an open-label study of 74 patients with a mean illness duration of approximately 5 years, Liu and colleagues (2017) reported an increase in mean BMI from 13.4 pre-operatively, to a mean BMI of 18.17 at 12-month follow-up. Importantly, these patients had yet to exhaust all less invasive psychotherapeutic treatment options prior to neurosurgery. Moreover, this study did not include a measure of psychological AN symptoms; the extent to which weight improvements mirrored improvements in the cognitive symptoms of AN is unknown. Also of note, long-term adverse events were noted in approximately 18% of those undergoing treatment, which included disinhibition (N = 6), memory loss (N = 3), and lethargy (N = 4).
Ventral striatum
The ventral striatum consists of the nucleus accumbens and the olfactory tubercle, and is thought to be critically involved in reward processing, cognition, reinforcement learning, and motivational salience (Daniel & Pollmann, 2014). To date, one case series of six patients with an illness duration of 2–4 years examined stereotactic ablation of the ventral striatum/nucleus accumbens (Wang et al., 2013). Notably, these patients had not exhausted less invasive psychotherapeutic treatments. This case series noted a rapid increase in mean patient BMI from 13.38 (±.59) pre-operatively, to a mean BMI of 19.15 (±.1.35) at 6-month follow-up, and a mean BMI of 20.4 (±1.26) at 12-month follow-up. However, no measure of the psychological symptoms of AN was reported in this study, and it is unclear whether change in patient weight was accompanied by change in the psychological symptoms of AN.
Deep brain stimulation
In total, nine studies have reported treatment outcomes of DBS for AN psychopathology, in a total of 30 patients spanning adolescent and adult populations (Israël et al., 2010; N. Lipsman, Woodside, Giacobbe, Lozano et al., 2013a; N. Lipsman et al., 2017; Manuelli et al., 2019; McLaughlin et al., 2013; Zhang et al., 2013; Wu et al., 2013; Blomstedt et al., 2017; Wang et al., 2013). No sham-controlled trials exist, and these reports comprise case studies, case series and open label trials. Across the existing nine studies, three neuroanatomical sites have been targeted: the ventral striatum/nucleus accumbens (McLaughlin et al., 2013; Wang et al., 2013; Wu et al., 2013; Zhang et al., 2013) (cumulative N = 11 patients), the subcallosal cingulate (Israël et al., 2010; N. Lipsman et al., 2017; N. Lipsman, Woodside, Giacobbe, Lozano et al., 2013b) (cumulative N = 17 patients), and the bed nucleus of the stria terminalis (BNST) (Manuelli et al., 2019; Blomstedt et al., 2017) (cumulative N = 2 patients).
Ventral striatum
Trials targeting the ventral striatum/nucleus accumbens have generally been modest in sample size, ranging from just 1–4 patients, and representing a cumulative total of 11 patients. These trials have included (i) a case study of a 48-year-old woman with primary OCD and a ‘longstanding’ history of AN, whose preoperative AN treatment was unclear (McLaughlin et al., 2013), (ii) a case series of two female patients ranging from age 18–28 years who had an illness duration ranging from 2–3 years (Wang et al., 2013), (iii) a case series of four adolescent females ranging from age 13–17 years, with an illness duration ranging from 1–3 years (Zhang et al., 2013), and (iv) a case series of four adolescent females ranging from age 16–17 years, with an illness duration ranging from 1–2 years (Wu et al., 2013).
These studies have indicated rapid increases in patient weight, which appear evident at just 1-month follow-up (Zhang et al., 2013), are sustained at up to 38-month follow-up (Wu et al., 2013), and may yield as much as a 65% increase in patient body weight (Wu et al., 2013). Further, no adverse events have been noted in studies targeting the ventral striatum/nucleus accumbens. Of note, no measures of the psychological symptoms of AN have been included in any of these studies. Further, several of the case studies targeting the ventral striatum/nucleus accumbens comprised adolescent populations with relatively short illness presentations, ranging from 18 months to 3 years (Khedr et al., 2014; Wu et al., 2013; Zhang et al., 2013).
Subcallosal cingulate
The subcallosal cingulate sits ventral to the corpus callosum, and is thought to be critically involved in affect and emotion regulation (Drevets et al., 2008). Trials targeting the subcallosal cingulate represent a cumulative total sample of 17 patients. These studies have included (i) a case study of a 56-year old woman with a 39-year history of AN (Israël et al., 2010), (ii) an open label study of six women ranging in age from 24–57 years, with an illness duration ranging from 4–37 years (N. Lipsman, Woodside, Giacobbe, Lozano et al., 2013b), and (iii) a larger open label study which included 6 patients from an earlier open label trial, and an additional 10 patients, totaling 16 women aged between 21–57 years, with an illness duration ranging from 9–29 years (N. Lipsman et al., 2017).
A key characteristic of the trials targeting the subcallosal cingulate is the specific inclusion of patients with longstanding illness presentations, ranging from 9–39 years, and extensive histories of unsuccessful specialized treatments. Notwithstanding, while these studies consistently demonstrated marginal weight loss acutely following surgery, a gradual weight gain over the course of these studies was observed, and patient weight at 12-month follow-up was greater than the patients’ historical weight. Moreover, these studies demonstrated that the psychological symptoms of AN, and mood-related symptoms more broadly, generally improved over the course of treatment. However, a series of adverse events was noted in 44–67% of patients in these studies, including localized pain, air embolus, and nausea, which in some instances led to patients (N = 2) requesting that their devices be explanted.
Bed nucleus of the stria terminalis
The BNST is a small gray mater structure located in the stria terminalis—a bundle of axons which connect it to the amygdaloid nuclei, which is thought to be critically involved in stress response, reward processing, and goal-directed behaviors (Dumont, 2009). Two case studies to date have targeted the (BNST). The first study was a case study of a 58-year-old woman with a primary diagnosis of major depressive disorder, comorbid anxiety, and a 44-year history of relapsing and remitting AN, whose pre-operative AN treatment was unclear (Blomstedt et al., 2017). After initially undergoing bilateral DBS to the medial forebrain bundle, the patient noted reduced depressive symptoms, although no effect on AN symptoms. This treatment was terminated (and the device explanted) after 10 months due to blurred vision. At age 58, the patient underwent a second surgery, initiating DBS to the BNST. While formal measures of AN psychopathology were not recorded, the patient noted a complete remission of food-related anxiety, such that tube feeding could be discontinued. However, the authors noted that the patient continued to habitually eat only enough to maintain a minimally stable weight, even in the absence of fear or anxiety (Blomstedt et al., 2017).
A second case study described a 37-year old woman with an 18-year history of severe AN, who underwent extensive outpatient and inpatient psychosocial and pharmacological treatments prior to DBS (Manuelli et al., 2019). During the 6-months following surgery, the patient’s BMI increased from 16.31 to 18.98, global EAT-26 scores improved from 68 to 39, and daily caloric intake increased from 1,489 kCal to 1,781 kCal. Moreover, the patient reported the cessation of compulsive and ritualistic behaviors relating to food preparation and consumption. Importantly, data beyond 6-month follow-up are not reported.
Repetitive transcranial magnetic stimulation
Seven studies have reported effects of rTMS on symptoms of AN in a cumulative total of 64 adolescents and adults with AN (B. Dalton, Bartholdy, McClelland et al., 2018b; Jaššová et al., 2018; Kamolz et al., 2008; J. McClelland, Bozhilova, Nestler et al., 2013b; J. McClelland et al., 2016a; Van den Eynde et al., 2013). Study methodologies have included case studies (Jaššová et al., 2018; Kamolz et al., 2008), case series (J. McClelland, Bozhilova, Nestler et al., 2013b; J. McClelland et al., 2016a; Van den Eynde et al., 2013), open label pilot trials (one study, cumulative N = 10 patients), and randomized controlled trials (B. Dalton, Bartholdy, McClelland et al., 2018b; J. McClelland et al., 2016a). To date, all studies have targeted the left dorsolateral prefrontal cortex (dlPFC), with slight variations in rTMS pulse train duration and intensity. The dlPFC is an area of the prefrontal cortex, which is centrally involved in cognitive control, emotional regulation, working memory, and decision making (Hoshi et al., 2006).
In terms of clinical outcomes, the results are mixed: case series of single-session rTMS reported reduced feelings of fullness, reduced feelings of fatness, and reduced anxiety during a food exposure task (Van den Eynde et al., 2013); however, these results were not replicated in a sham-controlled RCT of single-session rTMS, which reported no significant effects of active treatment upon core AN symptomatology (J. McClelland et al., 2016a). In terms of weight, studies have also yielded mixed results. In assessing the impact of 4–5 week doses of rTMS, several studies reported marginal weight loss over the course of treatment and follow-up (J. McClelland, Bozhilova, Nestler et al., 2013b; J. McClelland et al., 2016a), whereas others reported no significant change in weight (B. Dalton, Bartholdy, McClelland et al., 2018b). This is in keeping with studies of shorter doses of rTMS (Jaššová et al., 2018). Of all the existing studies, only one case study (Kamolz et al., 2008) has reported a marginal increase in patient weight, over the course of 41 sessions of rTMS, although the specific details of this weight gain were not reported.
Similarly, mixed results have been reported in relation to the psychological symptoms of AN over the course of rTMS treatment. Among the smaller studies, reported outcomes have included (i) no effect on AN psychopathology (Jaššová et al., 2018), (ii) a modest improvement in some dimensions of AN psychopathology yet no change in the urge to eat (Van den Eynde et al., 2013), and (iii) significant improvements in AN psychopathology over the course of the study (J. McClelland, Bozhilova, Nestler et al., 2013b; J. McClelland et al., 2016a). However, when utilizing RCT methodology, the two largest studies failed to demonstrate significant effects of rTMS upon the core psychological symptoms of AN over the course of the study (J. McClelland et al., 2016a) or reported small effect sizes (B. Dalton, Bartholdy, McClelland et al., 2018b). Importantly, the durability of these effects remains unknown.
In regard to adverse events, all but two studies noted adverse events and treatment-related side effects, and the two that did not, failed to systematically assess adverse events. Several studies noted patients missing scheduled treatment sessions (one study, N = 1) or discontinuing treatment due to side effects (two studies, N = 3). Other commonly reported side effects included dizziness and light-headedness, headaches and nausea. Yet again, the durability of these effects remains unknown.
Transcranial direct current stimulation
Two studies have investigated tDCS, in a cumulative total of 18 adolescents and adults with AN. These studies utilized open label trial (N = 7) (Khedr et al., 2014), and single-blind controlled trial (N = 11) (Costanzo et al., 2018), and both targeted the left dlPFC. While one study did not systematically report patient weight after 10 sessions of left dlPFC tDCS delivered over two weeks (Khedr et al., 2014), a single-blind controlled trial pairing dlPFC tDCS with treatment as usual over the course of 6 weeks reported significant weight gain over the course of treatment (Costanzo et al., 2018). In keeping, mixed results emerged in regard to the psychological symptoms of AN, with one study noting no main effects of tDCS on AN psychopathology (Costanzo et al., 2018), whereas the other study reported significant improvement in three patients, and no difference in four patients (Khedr et al., 2014). Treatment-related side effects across studies included moderate headaches (27%), moderate burning sensations (28%), local itching (27%), and local redness (45%), although no treatment discontinuation was reported.
Discussion
Several conclusions can be drawn from this review. Overall, findings for neurosurgical interventions such as ablative procedures and DBS show a potential for efficacy, thus presenting promise for future research in the neurosurgical treatment of AN. However, existing studies have been limited by small sample sizes and relatively brief follow-up periods. In contrast, noninvasive neuromodulatory interventions such as tDCS and rTMS have yielded modest and generally mixed results, at least with the dlPFC target and parameters tested thus far. Importantly, methodological issues significantly constrain the generalizability of available data, including the mix of case studies, case series, open label studies, and few blinded, controlled trials. Second, the fact that brain regions targeted across studies are diverse, and that variations exist in the intensity of treatment exposures, a single, specific interpretation of the effects is not possible at this time. Third, the wide differences in patient characteristics having potential prognostic implications—age, illness duration, previous treatment history—obscure any single, meaningful conclusion.
As to the effects on weight, the largest increments in patient weight appeared to result from stereotactic radiofrequency ablation; this was evident even in extremely low weight patients. Across two target regions (anterior limb of internal capsule, the ventral striatum) these ablations resulted in rapid weight gain at both short-term (i.e., 3-month follow-up) and longer-term (i.e., 3-year follow-up) timepoints; however, there are several cautionary notes. One is a single case (Barbier et al., 2011), a second is a case series of six patients (Wang et al., 2013), and the largest trial (Liu et al., 2017) has been criticized for not following standardized guidelines for selecting patients for neurosurgical procedures. As noted by Pugh et al. (2018), several of these studies comprised populations of adolescents and young adults who did not demonstrate severe and enduring AN, and who had not exhausted evidence-based psychosocial treatment options (Liu et al., 2017; Khedr et al., 2014). In short, it is unclear if illness characteristics would have diminished, to a greater or lesser degree over time, with or without less invasive psychological treatments.
The essential importance of documenting psychological symptom outcomes in treatment trials for AN has recently been emphasized (Murray et al., 2018, 2019); yet no existing study of neurosurgery has reported such outcomes, even though rigid pursuit of weight loss in AN occurs in association with a phobic-like anxiety and inhibition of appetitive drive. In this same vein, weight gain alone does not fully mitigate the entire suite of psychological and affective challenges facing those with AN, including the fear of normative body mass and attendant body dissatisfaction, and aversion to calorically dense foods, emotional restraint, and neophobia (Fennig et al., 2015; Murray et al., 2019; Schebendach et al., 2008). In short, weight gain is not the sole outcome in AN.
DBS of the ventral striatum has produced rapid improvements in weight, even in patients with very low pre-surgical BMI, as early as 1-month post-surgery, and is sustained at 38-month follow-up with continued DBS (Blomstedt et al., 2017; Wang et al., 2013; Zhang et al., 2013). However, these studies were in conflict with general neurosurgical eligibility guidelines (Pugh et al., 2018), and here again, these studies failed to report any measure of eating disorder psychopathology, limiting a broader conclusion as to their efficacy. Moreover, the organization of ventral striatal function is complex, and while diminished ventral striatal activity has been reported in AN (W. H. Kaye et al., 2009), evidence of elevated ventral striatal activity upon exposure to images of thin women (Fladung et al., 2010). It therefore remains unclear as to whether the low appetitive motivation characteristic of AN is the result of low hedonic drive, or alternatively, results from the suppression of appetitive motivation by elevated threat sensitivity and the instantiation of avoidance related behaviors and an adaptive metabolic physiology (Watson et al., 2019). In the cases in which ventral striatal DBS induced weight gain, whether the therapeutic processes involved (i) enhancing the hedonic value of food consumption, (ii) directly increasing consummatory behavior, (iii) decreasing the motivational drive to reduce body mass, or (iv) reducing risk-conferring emotional processes—remain unknown.
The ALIC and the ventral striatum have been targeted in DBS for OCD, whose causal pathophysiology is presumed to involve defects in the cortico-striatal-thalamo-cortical circuitry that regulate cognitive and motor habits (Saxena & Rauch, 2000). Given the elevated occurrence of OCD in persons with AN (W. H. Kaye et al., 2004), their increased frequency in first-degree relatives (Strober 2004), genetic correlations between AN and OCD (Watson et al., 2019), and reductions in eating disorder psychopathology following DBS targeting the subcallosal cingulate (N. Lipsman, Woodside, Giacobbe, Lozano et al., 2013b), further mechanistic study of this circuitry in treatment research is justified.
Studies of bilateral subcallosal cingulate DBS have generally targeted those with severe and enduring AN, who have feasibly exhausted all other treatment options. Concerning positive effects of DBS of the subcallosal cingulate on weight gain, eating disorder psychopathology, and metabolic change in the insula and parietal regions (N. Lipsman et al., 2017; N. Lipsman, Woodside, Giacobbe, Lozano et al., 2013b), controlled studies are few. Also needing further investigation is Lipsman’s conjecture (N. Lipsman et al., 2017), intriguing though it is, which postulates that changes in limbic circuitry may well precede symptom change in persons with AN who receive DBS.
While few studies have examined the effects of tDCS in AN, preliminary evidence suggests that moderate weight gain may result from left dlPFC tDCS (Costanzo et al., 2018), although no effect on the psychological symptoms of AN has been noted. Furthermore, in these studies tDCS was administered along with concurrent psychosocial treatment, making it difficult to parse the effects of tDCS versus ongoing psychological treatment. In contrast, studies examining rTMS have not demonstrated consistent weight gain in those with AN. Studies have generally reported marginal weight loss at follow-up, or no effect of treatment on patient weight. Only one study of rTMS, a single-patient case study of a 41-week rTMS of the left dlPFC, reported weight gain, although the actual weight change in this case was not reported (Kamolz et al., 2008). In addition, the larger, controlled studies of rTMS in AN have reported mixed, and generally modest results relating to the psychological symptoms of AN (B. Dalton, Bartholdy, McClelland et al., 2018b; J. McClelland et al., 2016a).
Cumulatively, these preliminary studies raise important questions about the efficacy of noninvasive neuromodulatory interventions in mitigating the symptoms of AN. Alternatively, they may suggest that current methods, dosing, and sites of administration may not be optimal. For instance, rTMS alters the cortical excitability of regions within the coil’s magnetic field, with higher frequency rTMS (i.e., 5 Hz or faster) having an excitatory effect on local cortex, and lower frequency rTMS (i.e., 1 Hz or slower) imparting an inhibitory effect. In AN, aberrant elevations in dlPFC functioning have been associated with elevated cognitive control (Ehrlich et al., 2015)—a phenotypic feature of AN thought to inhibit reward sensitivity (Cowdrey et al., 2011), which in turn dampens typically hedonic urges, including the drive to eat (Wierenga et al., 2015). As such, the rationale for the high-frequency dlPFC rTMS in AN, which may be enhancing excitability in dlPFC regions, is unclear.
It is important to consider adverse events as these treatments as neuromodulatory treatments advance. Across all trials, treatment side effects included local itching (Khedr et al., 2014), burning sensations (Costanzo et al., 2018), headaches (B. Dalton, Bartholdy, McClelland et al., 2018b; Wang et al., 2013), discomfort (Van den Eynde et al., 2013), and painful sensations (Jaššová et al., 2018), which in severe instance resulted in treatment discontinuation. In general, however, the rates of adverse events among these trials appear comparable to those reported in broader studies of neuromodulatory treatments (Anderson et al., 2012; Zis et al., 2020). Notwithstanding the ongoing development of neuromodulatory treatments ought to be considered carefully alongside analysis of the benefit-to-risk ratio.
Importantly, whereas all neurosurgical and neuromodulatory interventions have been predicated on specific hypotheses of causal neurocircuitry, no study to date has examined post-intervention change in these circuits. It is critical to examine whether weight- or cognitive symptom related changes co-occur with altered activity in these hypothesized circuits, in disorder-salient contexts. Moreover, by virtue of small sample sizes, no study has considered the possible impact of trait, environmental, or illness trajectory variations that are intuitively relevant to gene expression on the treatment outcomes observed. Similarly, the majority of studies to date have not documented psychiatric comorbidities. It is critical to understand how the presence of psychiatric comorbidities, each of which are characterized by distinct neural signatures, mediate the outcomes of treatments targeting specific nodes in AN.
In conclusion, neurosurgical procedures in the form of stereotactic ablative procedures and DBS appear to show a potential for efficacy in AN, and further systematic trials are warranted in this often intractable and life-threatening illness. The number of studies and the overall experience with these treatments in AN lag behind that in other disorders such as depression and OCD, despite the high mortality of AN. Encouragingly, however, it is noteworthy that this is a rapidly expanding area of clinical research in AN, with several new trials on the horizon (B. Dalton et al., in press; Knyahnytska et al., 2019). However, further testing to establish optimal targets in the largest proportion of patients, and, ideally, to identify those who may benefit most from which approach, and with what combination of other treatment modalities, is needed.
Table 2.
Summary of deep brain stimulation (DBS) studies for anorexia nervosa.
| Study | N | Patient Details | Procedure | Outcomes | Adverse Events | |
|---|---|---|---|---|---|---|
| Weight Symptoms | Psychological Symptoms | |||||
| Israël et al., 2010 | 1 | 56-year-old woman with childhood onset, treatment-refractory major depression, who also had a history of intermittent bouts of AN since age 17 years. | Bilateral DBS to the subcallosal cingulate. Intended goal of surgery was to reduce major depressive symptoms. | Patient BMI increased from a pre-operative BMI of 18, to a post-operative 2-year follow-up BMI of 19.1. | Eating Attitudes Test score improved from a pre-operative score of 40.56, to a 2-year follow-up score of 1.04, and a 3-year follow-up score of 1. Patient reported feeling “different” about food and was able to complete meals. |
None reported. |
| Zhang et al., 2013 | 4 | Adolescents with AN, with a mean age of 17 (±.8) years, a mean BMI of 12.12 (±.88), and a mean illness duration of 25.2 (±12.8) months. | Bilateral DBS to ventral striatum/nucleus accumbens. | Patient BMI improved from 12.12 (±.88) to 15.65 (±2.24) at 1-month follow-up. | Psychological symptoms of AN were not reported. | None reported. |
| Wu et al., 2013 | 4 | Adolescents with AN, with a mean age of 16.5 years (range 14–16 years), a mean BMI of 11.86 (±1.37), and a mean illness duration of 18.5 (±6.66) months. |
Bilateral DBS to the medial and most ventral part of the ventral striatum/nucleus accumbens. | Mean follow-up was 38 months (range 9–50 months). Patient BMI increased from 11.86 (±1.37) pre-operatively, to 19.6 (range .18.4 – 22.1). | Psychological symptoms of AN were not reported. | None reported. |
| Wang et al., 2013 | 2 | One adolescent with AN, aged 18, who had a 3-year history of AN. One adult with AN, aged 28 years, with a 2-year history of AN. | Bilateral DBS to ventral striatum/nucleus accumbens. | Patient BMI increased from 13.3 and 12.9 (pre-operatively) to 18 and 20.8 (1-year follow-up) respectively. | Psychological symptoms of AN were not reported. | All patients experienced transient headaches of varying severity, lasing 3–4 days. Centric fever was noted in 63% of patients, lasting 3–5 days. |
| Lipsman et al., 2013 | 6 | Adults with severe and enduring AN, with a mean age of 38.3 (±10.76) years, and a mean illness duration of 18.3 (±11.09) years. | Bilateral DBS to subcallosal cingulate. Target site was the white mater bundle immediately below the genu of the corpus callosum. | Patients had a mean BMI over the previous 5 years of 12.48 (±.92), but were renourished pre-operatively to a mean BMI of 16.1 (±1.5). BMI at 9-month follow-up was 16.55 (±3.16), despite an acute minor weight loss across all patients immediately following surgery. | Patient scores on the Yale-Brown-Cornell Eating Disorder Scale significantly improved between baseline (mean YBC-EDS-p = 23.7, ±3.4; mean YBC-EDS-r = 29.3, ±3.7) and 6-month follow-up (mean YBC-EDS-p = 17.7, ±6.9; mean YBC-EDS-r = 19.0, ±9.5) | Serious adverse events were noted in 4 of the 6 patients (67%). |
| McLaughlin et al., 2013 | 1 | 48-year-old woman with childhood onset, intractable OCD, who also had a history of AN. | Bilateral DBS to ventral striatum. Intended goal of surgery was to reduce intractable OCD symptoms. |
Patient BMI increased from 17.4 to a consistent post-surgery BMI range of 18.9 – 19.6. Time period of this follow-up not specified. |
Psychological symptoms of AN were not formally recorded. Patient reported feeling “different” about food, and was able to complete meals. |
None reported. |
| Lipsman et al., 2017 | 16 | Adults with severe and enduring AN, with a mean age of 34.4 (±8.15) years, and a mean AN illness duration of 17.94 (±6.04) years. | Bilateral DBS to subcallosal cingulate. Target site was the white mater bundle immediately below the genu of the corpus callosum. | Patient BMI improved from a mean pre-operative weight of 13.83 (±1.49), to a mean BMI of 17.34 (±3.40) at 12-month follow-up. | Patient scores on the Yale-Brown-Cornell Eating Disorder Scale significantly improved between baseline (mean YBC-EDS-p = 13.68, ±2.36; mean YBC-EDS-r = 13.31, ±1.89) and 12-month follow-up (mean YBC-EDS-p = 9.87, ±3.68; mean YBC-EDS-r = 8.79, ±3.96) | Serious adverse events were noted in 7 of 16 patients (44%), and 2 patients requested that their devices be removed during the course of the study. Other adverse events were reported in an additional 4 patients (69%). |
| Blomstedt et al., 2017 | 1 | 58-year-old woman with childhood onset major depressive disorder, anxiety, and a remitting and relapsing course of AN, whose current episode of AN had lasted 16 years. | Bilateral DBS to medial forebrain bundle, which was terminated after 10 months due to blurred vision. Two years later, patient underwent bilateral DBS to bed nucleus of stria terminalis. Intended goal of both surgeries was to reduce depressive symptoms. |
Patient BMI reduced from 16.2 prior to the first surgery, to 14.3 at 6-month follow-up after the second surgery. | Psychological symptoms of AN were not formally recorded. Patient reported the complete remission of anxiety concerning food and eating. Patient noted eating just enough to keep her weight stable, out of habit. |
Severe blurred vision after the first surgery, leading to the removal of the device. No adverse effects reported after the second surgery. |
| Manuelli et al., 2019 | 1 | 37-year-old woman with a remitting and relapsing course of AN for over 18 years, who had undergone extensive outpatient and inpatient treatment for AN. | Bilateral DBS to bed nucleus of stria terminalis. | Patient BMI improved from 16.31 prior to the first surgery, to 18.98 at 6-month follow-up. | Global EAT-26 scores improved from 68 prior to surgery, to 39 at 6-month follow-up. Patient also reported the cessation of food-related obsessions and rituals. | None reported. |
Table 3.
Summary of transcranial magnetic stimulation studies for anorexia nervosa.
| Study | N | Patient Details | Procedure | Outcomes | Adverse Events | ||
|---|---|---|---|---|---|---|---|
| Weight Symptoms | Psychological Symptoms | ||||||
| Kamolz et al., 2008 | 1 | 24-year-old woman with a 4-year history of AN, and comorbid depressive symptomatology. | 41 sessions (over 20 weeks) of 100 × 2s trains (10s inter-train interval) of rTMS to left dorsolateral prefrontal cortex delivered at 10Hz, and an intensity of 110% of motor threshold. | BMI appeared to improve over the course of treatment, although not formally reported in results. | Psychological symptoms of AN were not reported. | None reported. | |
| McClelland et al., 2013 | 2 | Two patients with AN, aged 23 and 52 years, and with an illness duration of 12 and 35 years, respectively. | 20 sessions (over 5 weeks) of 20 × 5s (55s inter- train interval) of rTMS to left dorsolateral prefrontal cortex delivered at 10Hz, and an intensity of 110% of motor threshold. | At 1-month follow-up, both patients’ BMI had reduced 15.76 to 14.74, and from 16.4 to 15.9, respectively. | Global EDE-Q scores decreased over the course of treatment. | One patient missed one scheduled session of rTMS, as a result of being dizzy/dazed following a previous session. | |
| Van den Eynde et al., 2013 | 10 | Adolescents and adults with AN, with a mean age of 25 years (range = 18–44 years), and an average illness duration of 10 years (range = 3–30 years). Baseline BMI was 15.7 (range = 13.8–17.8), and EDE-Q global score was 4.1 (range = 3.8–5.6). |
1 session of 20 × 5s (55s inter- train interval) of rTMS to left train dorsolateral prefrontal cortex delivered at 10Hz, and an intensity of 110% of motor threshold. | Patient weight were not recorded, given the brevity of the intervention. | rTMS resulted in reduced feeling of fullness, feelings of fatness, and anxiety during a food exposure task. No noted change in urge to restrict eating or changes in mood. | All participants reported significant discomfort during trials. One participant discontinued the trial during rTMS. | |
| McClelland et al., 2016a | 5 | Adults with AN, with a mean age of 35.6 (±11.19) years, a mean BMI of 16.06 (±1.90), and a mean illness duration of 20.4 (±12.60) years. | 20 sessions (over 4 weeks) of 20 × 5s (55s inter- train interval) of rTMS to left dorsolateral prefrontal cortex delivered at 10Hz, and an intensity of 110% of motor threshold. rTMS delivered in the context of treatment-as-usual |
Patient BMI reduced from a mean baseline BMI of 16.06. (±1.90), to a mean BMI of 15.06 (±1.77) at 6-month follow-up, and a mean BMI of 15.04 (±1.77) at 12-month follow-up. | Global EDE-Q score improved over the course of treatment, from a mean baseline score of 4.61 (±.72), to a 6-month follow-up mean score of 3.29 (±1.00), and 12-month follow-up mean score of 3.80 (±1.24). | Non-systematic assessment of adverse events throughout the study. | |
| McClelland et al., 2016b | 60 | Sham controlled RCT of 1 session of 20 × 5s (55s inter- train interval) of rTMS to left dorsolateral prefrontal cortex delivered at 10Hz, and an intensity of 110% of motor threshold. | Patient weight were not recorded, given the brevity of the intervention. | No significant effects of rTMS on (i) urge to restrict. (ii) feeling full, and (iii) feeling fat over the course of the study, relative to sham rTMS. No effect of rTMS on mood-related outcomes. | Two patients discontinued rTMS due to discomfort during trials. | ||
|
rTMS
(N= 28) Mean age of 25.29 (±6.88) years, and an average illness duration of 9.05 (±7.02) years. Baseline BMI was 16.73 (±1.59), and EDE-Q global score was 3.9 (±1.26). |
Sham rTMS (N =32) Mean age of 27.68 (±9.89) years, and an average illness duration of 11.27 (±8.01) years. Baseline BMI was 16.38 (±1.76), and EDE-Q global score was 4.4 (±1.07). |
||||||
| Dalton et al., 2018 | 34 | Double-blind sham- controlled RCT of 20 sessions (on consecutive weekdays) of 20 × 5s (55s inter- train interval) of rTMS to left dorsolateral prefrontal cortex delivered at 10Hz, and an intensity of 110% of motor threshold. rTMS delivered in the context of treatment-as-usual |
By EOT, patient BMI improved by a mean margin of .11 (±.73), and at 4-month follow-up, patient BMI improved by a mean margin of .28. (±1.25). No significant differences between rTMS and sham rTMS were noted, and effect sizes at 4-month follow-up were small (d=0.2). | By EOT, EDE-Q scores improved by a mean margin of .40 (±.79), and at 4-month follow-up by a mean margin of .43. (±.83). No significant differences between rTMS and sham rTMS were noted, and effect sizes at 4-month follow-up were small (d=0.1). | The most commonly reported side effects were headaches (mean frequency of 13 instances in 20 sessions), drowsiness (mean frequency of 10) instances in 20 sessions), discomfort on head (mean frequency of 9 instances in 20 sessions), and nausea (mean frequency of 8 instances in 20 sessions). | ||
|
rTMS (N=17) Mean age of 28.47 (±9.48) years, and an average illness duration of 13.74 (±10.74) years. Baseline BMI was 15.76 (±1.62), and EDE-Q global score was 4.07 (±1.28). |
Sham rTMS (N=17) Mean age of 31 (±11.29) years, and an average illness duration of 14.41 (±11.09) years. Baseline BMI was 16.26 (±1.22), and EDE-Q global score was 4.25 (±.94). |
||||||
| Jaššová et al., 2018 | 1 | 29-year-old woman with a 4-year history of severe AN, requiring multiple inpatient admissions, and a history of comorbid depression and anxiety. | 10 days of 10Hz, 15 trains per day, 100 pulses per train (107s inter-train interval) rTMS to left dorsolateral prefrontal cortex, at an intensity of 100% of motor threshold. | After 10 days, patient improved marginally from 11.98 to 12.13. |
No change was demonstrated in any self-report items assessing eating behaviors. | Patient percieved rTMS to be painful. | |
Clinical implications.
This systematic review examines advances in neuromodulatory and neurosurgical treatments of anorexia nervosa.
Our review included studies of neurosurgical ablation, deep brain stimulation, repetitive transcranial magnetic stimulation, and transcranial direct current stimulation.
Overall, there is promising preliminary evidence to support neurosurgical ablation and deep brain stimulation in the treatment of anorexia nervosa.
Less promising evidence was found for repetitive transcranial magnetic stimulation and transcranial direct current stimulation.
More trials are required, with comparable patient populations, are required in further establishing efficacy, and mechanisms of action.
Acknowledgments
SBM is supported by the National Institute of Mental Health (K23MH115184). MS is supported by the Resnick Endowed Chair in Eating Disorders. RT is supported by the National Institute of Mental Health (K23MH1126117, R01MH121089) and the Brain and Behavior Research Foundation (NARSAD-27111). AAB is supported National Institute of Neurological Disorders and Stroke (UN3NS113661, UH3NS107673) and the Casa Colina Center for Rehabilitation. JDF is supported by the National Institute of Mental Health (R01 MH105662, R21 MH110865), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD087712).
Footnotes
Declaration of interest
SBM reports royalties from Routledge and Oxford University Press.
References
- Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, & Giordani B (2005). Deep brain stimulation for refractory obsessive-compulsive disorder. Biological Psychiatry, 57(5), 510–516. 10.1016/j.biopsych.2004.11.042 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: Diagnostic and statistical manual of mental disorders, fifth edition.
- Anderson RJ, Frye MA, Abulseoud OA, Lee KH, McGillivray JA, Berk M, … Tye SJ (2012). Deep brain stimulation for treatment-resistant depression: Efficacy, safety and mechanisms of action. Neuroscience and Biobehavioral Reviews, 36(8), 1920–1933. 10.1016/j.neubiorev.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Arcelus J, Mitchell AJ, Wales J, & Nielsen S (2011). Mortality rates in patients with anorexia nervosa and other eating disorders: A meta-analysis of 36 studies. Archives of General Psychiatry, 68(7), 724–731. 10.1001/archgenpsychiatry.2011.74 [DOI] [PubMed] [Google Scholar]
- Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, & Kaye WH (2005). Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [carbonyl11C]WAY-100635. Archives of General Psychiatry, 62(9), 1032–1041. 10.1001/archpsyc.62.9.1032 [DOI] [PubMed] [Google Scholar]
- Barbier J, Gabriëls L, van Laere K, & Nuttin B (2011). Successful anterior capsulotomy in comorbid anorexia nervosa and obsessive-compulsive disorder: Case report. Neurosurgery, 69(3), E745–E745. 10.1227/NEU.0b013e31821964d2 [DOI] [PubMed] [Google Scholar]
- Blomstedt P, Naesström M, & Bodlund O (2017). Deep brain stimulation in the bed nucleus of the stria terminalis and medial forebrain bundle in a patient with major depressive disorder and anorexia nervosa. Clinical Case Reports, 5(5), 679–684. 10.1002/ccr3.856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkman J, & DeLong MR (2011). Deep brain stimulation for Parkinson disease: An expert consensus and review of key issues. Archives of Neurology, 68(2), 165. 10.1001/archneurol.2010.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo F, Menghini D, Maritato A, Castiglioni MC, Mereu A, & Vicari S (2018). New treatment perspectives in adolescents with anorexia nervosa: The efficacy of non-invasive brain-directed treatment. Frontiers in Behavioral Neuroscience, 12, 133. 10.3389/fnbeh.2018.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowdrey FA, Park RJ, Harmer CJ, & McCabe C (2011). Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biological Psychiatry, 70 (8), 736–743. 10.1016/j.biopsych.2011.05.028 [DOI] [PubMed] [Google Scholar]
- Dalton B, Bartholdy S, Campbell IC, & Schmidt U (2018a). Neurostimulation in clinical and sub-clinical eating disorders: A systematic update of the literature. Current Neuropharmacology, 16 (8), 1174–1192. 10.2174/1570159X16666180108111532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton B, Bartholdy S, McClelland J, Kekic M, REnnalls SJ, Werthmann J, Carter B, O’Daly OG, Campbell IC, David AS, Glennon D, Kern N, & Schmidt U (2018b). Randomised controlled feasibility trial of real versus sham repetitive transcranial magnetic stimulation treatment in adults with severe and enduring anorexia nervosa: The TIARA study. BMJ Open, 8(7), e021531. 10.1136/bmjopen-2018-021531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton B, Foerde K, Bartholdy S, McClelland J, Kekic M, et al. (In Press). The effect of repetitive transcranial magnetic stimulation on food choice-related self-control in patients with severe, enduring anorexia nervosa. International Journal of Eating Disorders. [DOI] [PubMed] [Google Scholar]
- Daniel R, & Pollmann S (2014). A universal role of the central striatum in reward-based learning: Evidence from human studies. Neurobiology of Learning and Memory, 114, 90–100. 10.1016/j.nlm.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, & Timble M (2008). The subgenual anterior cingulate cortex in mood disorders. SNC Spectrums, 13(8), 663–681. 10.1017/S1092852900013754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC (2009). What is the bed nucleus of the stria terminalis? Progress in Neuropsychopharmacology & Biological Psychiatry, 33(8), 1289–1290. 10.1016/j.pnpbp.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Geisler D, Ritschel F, King JA, Seidel M, & Kroemer N (2015). Elevated cognitive control over reward processing in recovered female patients with anorexia nervosa. Journal of Psychiatry & Neuroscience, 40(5), 307–315. 10.1503/jpn.140249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennig S, Brunstein Klomek A, Shahar B, Sarel-Michnik Z, & Hadas A (2015). Inpatient treatment has no impact on the core thoughts and perceptions in adolescents with anorexia nervosa. Early Intervention in Psychiatry, 11(3), 200–207. 10.1111/eip.12234 [DOI] [PubMed] [Google Scholar]
- Fenoy AJ, & Simpson RK (2014). Risks of common complications in deep brain stimulation surgery: Management and avoidance. Journal of Neurosurgery, 120(1), 132–139. 10.3171/2013.10.JNS131225 [DOI] [PubMed] [Google Scholar]
- Fichter MM, Quadflieg N, Crosby RD, & Koch S (2017). Long-term outcome of anorexia nervosa: Results from a large clinical longitudinal study. International Journal of Eating Disorders, 50(9), 1018–1030. 10.1002/eat.22736 [DOI] [PubMed] [Google Scholar]
- Fladung AK, Grön G, Grammer K, Herrnberger B, Schilly E, & von Wietersheim J (2010). A neural signature of anorexia nervosa in the ventral striatal reward system. American Journal of Psychiatry, 167(2), 206–212. 10.1176/appi.ajp.2009.09010071 [DOI] [PubMed] [Google Scholar]
- Flora ED, Perera CL, Cameron AL, & Maddern GJ (2010). Deep brain stimulation for essential tremor: A systematic review. Movement Disorders, 25(11), 1550–1559. 10.1002/mds.23195 [DOI] [PubMed] [Google Scholar]
- Fonville L, Giampietro V, Williams SCR, Simmons A, & Tchanturia K (2013). Alterations in brain structure in adults with anorexia nervosa and the impact of illness duration. Psychological Medicine, 44(9), 1965–1975. 10.1017/S0033291713002389 [DOI] [PubMed] [Google Scholar]
- Frank GK, Bailer UF, Henry SE, Drevets W, Malttzer CC, & Kaye WH (2005). Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11C]raclopride. Biological Psychiatry, 58(11), 908–912. 10.1016/j.biopsych.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Frank GK, Shott ME, Hagman JO, & Mittal VA (2014). Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. American Journal of Psychiatry, 170(10), 1152–1160. 10.1176/appi.ajp.2013.12101294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GKW, DeGuzman MC, & Shott ME (2019). Motivation to eat and not to eat – The psycho-biological conflict in anorexia nervosa. Physiology & Behavior, 1, 185–190. 10.1016/j.physbeh.2019.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Maline DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, Salloway SP, Okun MS, Goodman WK, & Rasmussen SA (2006). Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology, 31(11), 2384. 10.1038/sj.npp.1301165 [DOI] [PubMed] [Google Scholar]
- Hall PA, Vincent CM, & Burhan AM (2018). Non-invasive brain stimulation for food cravings, consumption, and disorders of eating: A review of methods, findings and controversies. Appetite, 1(124), 78–88. 10.1016/j.appet.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Hoshi E (2006). Functional specialization with the dorsolateral prefrontal cortex: A review of anatomical and physiological studies of non-human primates. Neuroscience Research, 54(2), 73–84. 10.1016/j.neures.2005.10.013 [DOI] [PubMed] [Google Scholar]
- Israël M, Steiger H, Kolivakis T, McGregor L, & Sadikot AF (2010). Deep brain stimulation in the subgenual cingulate cortex for an intractable eating disorder. Biological Psychiatry, 67(9), e53–e54. 10.1016/j.biopsych.2009.11.016 [DOI] [PubMed] [Google Scholar]
- Jaššová K, Albrecht J, Papežová H, & Anders M (2018). Repetitive transcranial magnetic stimulation (rTMS) treatment of depression and anxiety in a patient with anorexia nervosa. Medical Science Monior, 24, 5279–5281. 10.12659/MSM.908250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamolz S, Richter MM, Schmidtke A, & Fallgatter AJ (2008). Transkranielle magnet-stimulation gegen komorbide depression bei anorexie. Der Nervenarzt, 79(9), 1071–1073. 10.1007/s00115-008-2537-8 [DOI] [PubMed] [Google Scholar]
- Kaye WH, Bulik CM, Thornton L, Barbarich N, & Masters K (2004). Comorbidity of anxiety disorders with anorexia and bulimia nervosa. American Journal of Psychiatry, 161 (12), 2215–2221. 10.1176/appi.ajp.161.12.2215 [DOI] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, & Paulus M (2009). New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Reviews. Neuroscience, 10(8), 573–584. 10.1038/nrn2682 [DOI] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Knatz S, Liang J, Boutelle K, Jill L, & Eisler I (2015). Temperament-based treatment for anorexia nervosa. European Eating Disorders Review, 23 (1), 12–18. 10.1002/erv.2330 [DOI] [PubMed] [Google Scholar]
- Khedr EM, Elfetoh NA, Ali AM, & Noamany M (2014). Anodal transcranial direct current stimulation over the dorsolateral prefrontal cortex improves anorexia nervosa: A pilot study. Restorative Neurology and Neuroscience, 32(6), 789–797. 10.3233/RNN-140392 [DOI] [PubMed] [Google Scholar]
- Knyahnytska Y, Blumberger DM, Daskalakis ZJ, Zomorrodi R, & Kaplan AS (2019). Insula H-voil deep transcranial magnetic stimulation in severe and enduring anorexia nervosa (SE-AN): A pilot study. Neuropsychiatric Disease and Treatment, 15, 2247–2256. 10.2147/NDT.S207630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Elias GJB, & Lozano AM (2018). Neuromodulation for the treatment of eating disorders and obesity. Therapeutic Advances in Psychopharmacology, 8(2), 73–92. 10.1177/2045125317743435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsman N, Lam E, Volpini M, Sutandr K, Twose R, Giacobbe P, Sodums DJ, Smith GS, Woodside DB, & Lozano AM (2017). Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1-year follow-up of an open-label trial. The Lancet Psychiatry, 4(4), 285–294. 10.1016/S2215-03661730076-7 [DOI] [PubMed] [Google Scholar]
- Lipsman N, Woodside DB, Giacobbe P, Hamani C, Carter JC, Norwood SJ, Sutandar K, Staab R, Elias G, Lyman CH, Smith GS, & Lozano AM (2013a). Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: A phase 1 pilot trial. The Lancet, 381(9875), 1361–1370. 10.1016/S0140-67361262188-6 [DOI] [PubMed] [Google Scholar]
- Lipsman N, Woodside DB, Giacobbe P, & Lozano AM (2013b). Neurosurgical treatment of anorexia nervosa: Review of the literature from leucotomy to deep brain stimulation. European Eating Disorders Review, 21(6), 428–435. 10.1002/erv.2246 [DOI] [PubMed] [Google Scholar]
- Liu W, Li D, Sun F, Zhang X, Wang T, Zhan S, … Sun B (2017). Long-Term Follow-up Study of MRI-Guided Bilateral Anterior Capsulotomy in Patients With Refractory Anorexia Nervosa. Neurosurgery, 83(1), 86–92 [DOI] [PubMed] [Google Scholar]
- Manuelli M, Franzini A, Galentino R, Bidone R, Dell’Osso B, Porta M, … Cena H (2019). Changes in eating behavior after deep brain stimulation for anorexia nervosa. A case study. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity [DOI] [PubMed] [Google Scholar]
- McClelland J, Bozhilova N, Campbell I, & Schmidt U (2013a). A systematic review of the effects of neuromodulation on eating and body weight: evidence from human and animal studies. European Eating Disorders Review, 21(6), 436–455. 10.1002/erv.2256 [DOI] [PubMed] [Google Scholar]
- McClelland J, Bozhilova N, Nestler S, Campbell IC, Jacob S, Johnson-Sabine E, & Schmidt U (2013b). Improvements in symptoms following neuronavigated repetitive transcranial magnetic stimulation (rTMS) in severe and enduring anorexia nervosa: Findings from two case studies. European Eating Disorders Review, 21(6), 500–506. 10.1002/erv.2266 [DOI] [PubMed] [Google Scholar]
- McClelland J, Kekic M, Bozhilova N, Nestler S, Dew T, David AS, Rubia K, Campbell IC, Schmidt U, & Van den Eynde F (2016b). A randomised controlled trial of neuronavigated repetitive transcranial magnetic stimulation (rTMS) in anorexia nervosa. PloS One, 11(3), e0148606. 10.1371/journal.pone.0148606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland J, Kekic M, Campbell IC, & Schmidt U (2016a). Repetitive transcranial magnetic stimulation (rTMS) treatment in enduring anorexia nervosa: A case series. European Eating Disorders Review, 24(2), 157–163. 10.1002/erv.2414 [DOI] [PubMed] [Google Scholar]
- McLaughlin NCR, Didie ER, Machado AG, Haber SN, Eskandar EN, & Greenberg BD (2013). Improvements in anorexia symptoms after deep brain stimulation for intractable obsessive-compulsive disorder. Biological Psychiatry, 73(9), e29–e31. 10.1016/j.biopsych.2012.09.015 [DOI] [PubMed] [Google Scholar]
- Moher D (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- Murray SB, Loeb KL, & Le Grange D (2018). Treatment outcome reporting in anorexia nervosa: Time for a paradigm shift? Journal of Eating Disorders, 6(1), 10. 10.1186/s40337-018-0195-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SB, Quintana DS, Loeb KL, Griffiths S, & Le Grange D (2019). Treatment outcomes for anorexia nervosa: A systematic review and meta-analysis of randomized controlled trials. Psychological Medicine, 49(4), 535–544. 10.1017/S0033291718002088 [DOI] [PubMed] [Google Scholar]
- Pugh J, Tan J, Aziz T, & Park RJ (2018). The moral obligation to prioritize research into Deep brain stimulation over brain lesioning procedures for severe enduring anorexia nervosa. Frontiers in Psychiatry, 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safadi Z, Grisot G, Jbabdi S, Behrens TE, Heilbronner RS, McLaughlin NCR, Mandeville J, Versace A, Phillips ML, Lehman JF, Yendiki A, & Haber SN (2018). Functional segmentation of the anterior limb of the internal capsule” linking white matter abnormalities to specific connections. Journal of Neuroscience, 38(8), 2106–2117. 10.1523/JNEUROSCI.2335-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, & Rauch S (2000). Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. The Psychiatric Clinics of North America, 23(3), 563–586. 10.1016/S0193-953X0570181-7 [DOI] [PubMed] [Google Scholar]
- Schebendach JE, Mayer LE, Devlin MJ, Attia E, Contento IR, Wolf RL, & Walsh BT (2008). Dietary energy density and diet variety as predictors of outcome in anorexia nervosa. The American Journal of Clinical Nutrition, 87(4), 810–816. 10.1093/ajcn/87.4.810 [DOI] [PubMed] [Google Scholar]
- Schmidt U, & Campbell IC (2013). Treatment of eating disorders cannot remain “brainless”: The case for brain-directed treatments. European Eating Disorders Review, 21(6), 425–427. 10.1002/erv.2257 [DOI] [PubMed] [Google Scholar]
- Steinglass JE, Walsh BT, & Walsh BT (2015). Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nature Neuroscience, 18(11), 1571–1573. 10.1038/nn.4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober M (2004). Pathologic fear conditioning and anorexia nervosa: On the search for novel paradigms. International Journal of Eating Disorders, 35(4), 504–508. 10.1002/eat.20029 [DOI] [PubMed] [Google Scholar]
- Strober M, Freeman R, Lampert C, & Diamond J (2007). The association of anxiety disorders and obsessive compulsive personality disorder with anorexia nervosa: Evidence from a family study with discussion of nosological and neurodevelopmental implications. International Journal of Eating Disorders, 40(S3), S46–S51. 10.1002/eat.20429 [DOI] [PubMed] [Google Scholar]
- Titova OE, Hjorth OC, Schiöth HB, & Brooks SJ (2013). Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: A meta-analysis of VBM studies. BMC Psychiatry, 13(1), 1. 10.1186/1471-244X-13-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eynde F, Guillaume S, Broadbent H, Campbell IC, & Schmidtm U (2013). Repetitive transcranial magnetic stimulation in anorexia nervosa: A pilot study. European Psychiatry, 28(2), 98–101. 10.1016/j.eurpsy.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Wang J, Chang C, Geng N, Wang X, & Gao G (2013). Treatment of intractable anorexia nervosa with inactivation of the nucleus accumbens using stereotactic surgery. Stereotactic and Functional Neurosurgery, 91(6), 364–372. 10.1159/000348278 [DOI] [PubMed] [Google Scholar]
- Watson HJ, & Bulik CM (2012). Update on the treatment of anorexia nervosa: Review of clinical trials, practice guidelines and emerging interventions. Psychological Medicine, 43 (12), 2477–2500. 10.1017/S0033291712002620 [DOI] [PubMed] [Google Scholar]
- Watson HJ, Yilmaz Z, Thornton LM, Hübel C, Coleman JRI, Gaspar HA, Bryois J, Hinney A, Leppä VM, Mattheisen M, Medland SE, Ripke S, Yao S, Giusti-Rodríguez P, Hanscombe KB, Purves KL, Adan RAH, Alfredsson L, Ando T, Baker JH, … Bulik CM (2019). Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nature Genetics, 51(8), 1207–1214. 10.1038/s41588-019-0439-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Bischoff-Grethe A, Melrose AJ, Irvine Z, Torres L, Bailer UF, Simmons A, Fudge JL, McClure SM, Ely A, & Kaye WH (2015). Hunger does not motivate reward in women remitted from anorexia nervosa. Biological Psychiatry, 77(7), 642–652. 10.1016/j.biopsych.2014.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Van Dyck-Lippens PJ, Santegoeds R, Van Kuyck K, Gabriëls L, Lin G, Pan G, Li Y, Li D, Zhan S, Sun B, & Nuttin B (2013). Deep-brain stimulation for anorexia nervosa. World Neurosurgery, 80(3–4), S29.e1–S29.e10. 10.1016/j.wneu.2012.06.039 [DOI] [PubMed] [Google Scholar]
- Zhang HW, Li DY, Zhao J, Guan YH, Sun BM, & Zuo CT (2013). Metabolic imaging of deep brain stimulation in anorexia nervosa. Clinical Nuclear Medicine, 1. 10.1097/RLU.0000000000000261 [DOI] [PubMed] [Google Scholar]
- Zis P, Shafique F, Hadjivassiliou M, Blackburn D, Venneri A, Iliodromiti S, Mitsikostas D-D, & Sarrigiannis PG (2020). Safety, tolerability, and nocebo phenomena during transcranial magnetic stimulation: A systemic review and meta-analysis of placebo-controlled clinical trials. Neuromodulation: Technology at the Neural Interface, 23 (3), 291–300. 10.1111/ner.12946 [DOI] [PubMed] [Google Scholar]


