Abstract
Early life trauma dramatically increases the risk of developing major depressive disorder (MDD), and is associated with a markedly decreased adult treatment response to antidepressants. Novel treatment approaches are required to treat childhood trauma-associated MDD. Recent studies suggest that the (R,S)-ketamine (ketamine) metabolite, (2R,6R)-hydroxynorketamine (HNK), exerts fast- and long-lasting antidepressant-like effects without ketamine’s NMDAR-inhibition-associated adverse side-effect profile. We investigated the therapeutic potential of (2R,6R)-HNK against behavioral despair produced by a novel live-predator stress exposure during adolescence. Male and female C57BL/6J mice were exposed to a live snake or control conditions at post-natal (PND) days 31, 45 and 61. In order to assess the enduring consequences of trauma-exposure, at a minimum of 14 days following the last exposure, mice received inescapable shocks followed by a session with available escape options twenty-four hours later. Mice that manifested enduring escape deficits (helplessness) were treated with vehicle or (2R,6R)-HNK (20 mg/kg, i.p.), 24 hr prior to retesting for reversal of escape deficits. We found that a significantly greater number of mice developed the helpless phenotype when they were exposed to the live predator and that the helpless phenotype was reversed in mice treated with (2R,6R)-HNK. There were no sex differences in the response to predator-stress exposure or (2R,6R)-HNK treatment. The live-predator model developed in this study provides an opportunity to further refine our understanding of the neurobiological substrates impacted by adolescent trauma and improve treatment strategies. The demonstrated efficacy of (2R,6R)-HNK in this model suggests a novel therapeutic intervention for a treatment-resistant population.
Keywords: depression; adolescent trauma; antidepressant; ketamine; metabolite; (2R,6R)-hydroxynorketamine; (2R,6R)-HNK
Introduction
Early life trauma significantly increases the risk for psychiatric illness across a broad range of diagnostic categories (Anda et al., 2006; Chapman et al., 2004; Felitti et al., 1998; Kessler et al., 2010; Park et al., 2014; Powers et al., 2016; Widom et al., 2007), and can significantly alter symptom complexity (Cloitre et al., 2009), as well as treatment trajectories within these diagnostic categories (Agnew-Blais and Danese, 2016; Gould et al., 2012; Heim et al., 2010; Nanni et al., 2012; Nemeroff et al., 2003; Shin et al., 2013). For example, 87% of major depressive disorder (MDD) patients without a history of childhood trauma successfully respond to classical antidepressant treatment, while only 17% of the patients with childhood trauma respond (Williams et al., 2016). Thus, novel antidepressants utilizing alternative mechanisms of action are required to better treat childhood trauma-induced MDD.
(R,S)-Ketamine (ketamine) has demonstrated rapid and robust efficacy as an antidepressant in treatment-refractory patients following a single administration (e.g. Murrough et al., 2013; Singh et al., 2016; Zarate et al., 2006). Ketamine is rapidly metabolized to a number of metabolites including the hydroxynorketamines (HNKs) (e.g., Can et al., 2016; Portmann et al., 2010; Zanos et al., 2018a). In mice, metabolism of peripherally-administered ketamine to its (2S,6S;2R,6R)-HNK metabolite was found necessary for its full antidepressant-like actions (Zanos et al., 2016). The (2R,6R)-HNK metabolite has been identified to rapidly cross the blood-brain barrier and exert rapid antidepressant-like effects in many assays (Cavalleri et al., 2018; Chou et al., 2018; Collo et al., 2018; Fred et al., 2019; Fukumoto et al., 2019; Fukumoto et al., 2017; Highland et al., 2019; Lumsden et al., 2020; Pham et al., 2018; Wray et al., 2018; Yao et al., 2018; Ye et al., 2019; Zanos, P. et al., 2019; Zanos et al., 2016). In contrast with ketamine, (2R,6R)-HNK has a low potency to bind to N-methyl-D-aspartate receptors (NMDAR), and to inhibit NMDAR function (Lumsden et al., 2020; Moaddel et al., 2013; Morris et al., 2017; Suzuki et al., 2017; Zanos et al., 2016). Presumably as a consequence of minimal NMDAR inhibition, (2R,6R)-HNK lacks locomotor stimulant effects at doses up to 125 mg/kg (i.p.) (Zanos et al., 2016) and 450 mg/kg following oral administration (Highland et al., 2019), is devoid of self-administration properties at doses and conditions where ketamine is self-administered, and does not disrupt pre-pulse inhibition at doses up to 375 mg/kg in mice (Zanos et al., 2016). Although (2R,6R)-HNK’s antidepressant-relevant actions are independent of direct NMDAR inhibition, they involve early and sustained activation of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) by enhancing presynaptically-released glutamate (Riggs et al., 2019; Zanos et al., 2016) via a mechanism convergent with mGlu2/3 receptor signaling (Zanos, Panos et al., 2019).
Here, we hypothesized that (2R,6R)-HNK would have efficacy to reverse adult behavioral despair produced by a live-predator stress exposure during adolescence. We utilized a novel animal stress model (snake exposure) that directly exposes mice to close contact with a live predator (life-threatening event) without confounding physical harm. Mice were exposed to live predator stress twice during adolescence and then once again during early adulthood. In mice with an adult helpless phenotype (assessed two weeks following last stressor), (2R,6R)-HNK’s efficacy to reverse adult behavioral despair was determined.
Methods
Experimental design
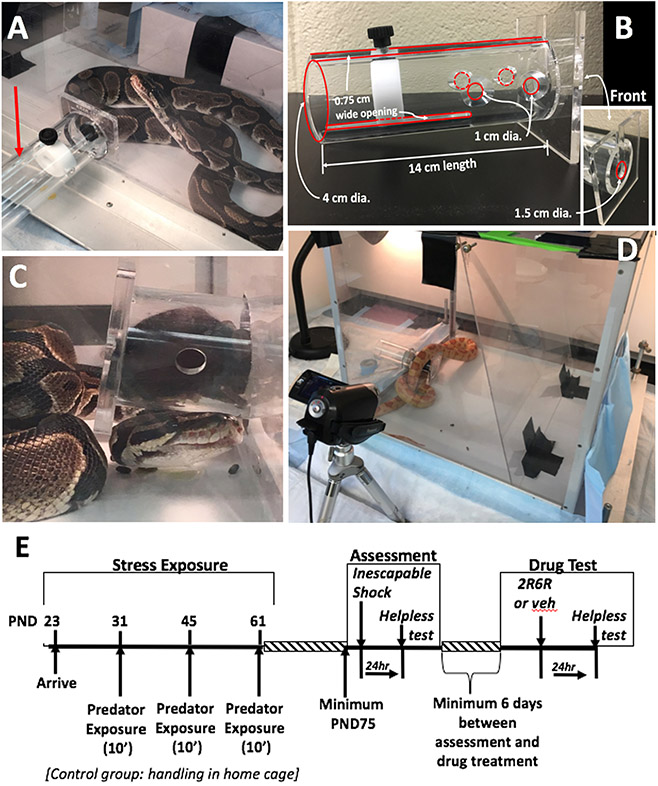
Mice were exposed to live predator stress (snake) twice during adolescence (post natal day (PND) 31 and 45) then again at early adulthood, PND61 (Figure 1E). Behavioral assessment of helplessness (see description below) was initiated when mice were at least PND75. All experiments were performed in a randomized manner. Experimenters performing the injections and the behavioral assessments were blind to the treatment group assignments. All procedures were approved by the Institutional Care and Use Committee of the University of Maryland School of Medicine and conducted in accordance with the guidelines described in the Guide for the Care and Use of Laboratory Animals, Eighth Edition (2011).
Figure 1. Mouse exposure to live predator.
(A) A restraint tube (red arrow) holding a mouse was inserted into the larger chamber holding the live snake. (B) The restraint tube has several holes and narrow openings that allow the snake to come in close contact with the mouse yet not physically harm it. The black knob connects to a disk that moves forward to constrain the mouse to the front of the tube. (C & D) A ball python and albino corn snake are shown interacting with a mouse. (E) Experiment timeline. Mice arrived at post-natal day (PND) 23, acclimated for one week prior to a 10 min. predator exposure (or handling in control group) at three separate times separated by ~ 2 week intervals. At a minimum of 2 weeks following the last exposure, mice were assessed for their susceptibility to develop helpless behavior following exposure to inescapable foot shock stress. A minimum of 6 days the effects of 24 hr pretreatment with vehicle or (2R,6R)-HNK reversed the helpless phenotype.
Subjects and snakes
Male (n = 27) and female (n = 26) C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were used, and provided with water and food ad libidum. Snakes were loaned to the laboratory from a local store (House of Tropicals, Glen Burnie, MD) on a 12 hr basis. A variety of snake species were used (Gulf-Hammock, Rusty, Corn or Albino Rat Snake, Ball Python) based on availability and the longest feed interval. All snakes were 60-100 cm in length.
Exposure to a live predator
Mice were randomly divided into a control group (n = 12, male; 10, female adolescent mice) or a stress group (n = 15, male; 16, female adolescent mice). Control mice were housed two per cage throughout the duration of the experiment as were the predator-exposed mice. Figure 1E illustrates the experimental timeline. Mice arrived at post-natal day (PND) 23, housed two per cage and allowed to acclimate for one week prior to the initial predator exposure. Mice were exposed to the live predator at PND 31, 45 and 61. Control mice were handled (picked up, handled, weighed and tail marked) in their home-room at the same time. Here, we define ‘predator + restraint’ as the traumatic event. Tonic peri-traumatic immobility is associated with PTSD severity (Fiszman et al., 2008; Lima et al., 2010); in this manner, the tube increases the uncontrollable nature of the event, augmenting the trauma and increasing the likelihood of a relevant outcome.
Mice were placed in a restraint tube (PLAS Labs Inc, Lansing, MI) that contained several small openings that allowed the snake to come in close proximity (touching the mouse with its nose and tongue through the openings) but did not allow the snake to get an open mouth inside the container holding the mouse (see Figure 1 A-C). A movable disk was positioned to constrain the mouse to the front portion of the restraint tube. The tube containing the mouse was then inserted into a large arena (see Figure 1) that held the live snake. A Plexiglass sheet was used to confine the snake to a 35 L x 40 W x 40 H cm portion of the chamber containing the mouse. The duration of each exposure was 10 minutes.
Behavioral testing
To assess the effects of adolescent predator exposure on behavioral despair, we employed a modified helpless paradigm in which mice were exposed to inescapable footshocks and then assessed as to whether they failed to avoid or escape the shock when subsequently given the opportunity to do so. This is a well-characterized preclinical model to assess behaviors relevant to depression with established predictive validity in medications development (Ramaker and Dulawa, 2017; Zanos et al., 2016) and proven utility in negative construct circuitry investigation (Maier and Seligman, 2016).
At a minimum of 16 days following the last snake exposure (range = 16-35 days; PND75+) mice were assessed for development of footshock stress-induced helpless behavior. Experiments were conducted in a two-chamber shuttle box (Med Associates, interior compartments each measuring 20.5 L x 16.5 W x 21.3 H cm) equipped with a centered computer-controlled guillotine door. The shuttle box was contained within a sound-attenuating cabinet.
Inescapable stress (Day 1 of the protocol):
Mice were placed on one side of the shuttle box with the guillotine door closed (house lights off) and subjected to 120 inescapable foot shocks (0.56 mA for 15 sec). The interval between consecutive shocks varied between trials on a variable interval (VI30-60 sec) schedule. Shock was scrambled and verified at the grid floor across all rods. Based upon the variable inter-trial interval and shock duration, the absolute minimum and maximum session length was 60-150 min, respectively. During trials mice were observed to confirm they received shock, and that they did not identify an escape mechanism within the apparatus. At the end of the session, mice were returned to their home cage.
Test for escape deficits (Day 2 of the protocol):
Twenty to 24 hours following the inescapable shock procedure, the animals were evaluated for escape deficits over the course of 30 trials total. During the first 5 trials the door separating the two compartments was opened concurrently with presentation of a footshock (0.56 mA). Crossing into the alternate chamber immediately terminated the shock. Following these first 5 trials, trials 6 through 30 had a 3 sec delay before the door was opened for the mouse to be able to escape to the opposite, non-shocked, side of the chamber. The trial was terminated when the mouse moved to the alternate chamber. If a trial ended in failure (mouse did not cross chambers), the shock was terminated after 15 sec and the door was closed.
Test for effectiveness of (2R,6R)-HNK to reverse escape deficits:
Individual mice that met the criteria for helplessness (≥ 30% failure rate during last 10 trials) were then used to assess the effectiveness of (2R,6R)-HNK to reverse this escape deficit. At a minimum of 6 days following their screening test (range 6-35 days; average (±S.E.M) 18.4±3.7 and 19.7±4.0, vehicle- and drug-treated, respectively), helpless mice were treated with either vehicle (saline) or (2R,6R)-HNK (20 mg/kg, i.p.) 24 hrs prior to an additional test for escape deficits identical to the procedure described above. The (2R,6R)-HNK dose chosen for this study was based on the higher dose we have previously used to elicit antidepressant effects of both ketamine and HNK in C57BL/6J mice (Zanos et al, 2016). We have previously shown that (2R,6R)-HNK is rapidly metabolized and is not longer detectable in the mouse brain by 4 hours hours after administration (Zanos et al., Nature 2016). Thus, the pretreatment time reflects the hypothesized role of long-lasting neuroadaptations and synaptic plasticity (Abdallah et al., 2015; Duman, 2018; Zanos et al., 2018b). Thus, the behavioral actions observed 24 hours after treatment are attributed to these long-lasting neurobiological changes, rather than lasting exposure to the active metabolite (2R,6R)-HNK.
Drugs
(2R,6R)-HNK hydrochloride was synthesized and characterized internally at the National Institutes of Health National Center for Advancing Translational Sciences (Rockville, Maryland). Absolute and relative stereochemistry for (2R,6R)-HNK was confirmed by small molecule x-ray crystallography (Zanos et al., 2016). (2R,6R)-HNK was dissolved in 0.9% saline and administered at a volume of 0.1 ml/kg.
Statistical analyses
The primary outcome was the percent of escape failures recorded during the test for escape deficits. Data were tested for equal group variance. To determine the consequences of adolescent-stress exposure, for the first assessment test that the mice received (Day 2 of protocol), a two-way analysis of variance (ANOVA) (condition x sex) was used to compare the percent of overall trial failures as a function of trauma exposure and sex. Only those mice failing to escape ≥30% of their final 10 trials were considered helpless and included in the next separately conducted test that treated mice with vehicle or drug. To confirm that mice were still helpless after the waiting period prior to treatment, a one-way repeated measures ANOVA (percent of overall trials failed during first exposure and second test exposure) was used to compare the number of failures as a function of delayed repeated exposure in the vehicle-treated group. We expect that if vehicle-treated subjects were still exhibiting the helpless phenotype there would be no difference between the number of failures during their first test that determined their helpless state and the next test in which they were used as the control comparison against the (2R,6R)-HNK)-treated group. The effect of (2R,6R)-HNK on escape deficits was assessed using a two-way ANOVA (drug x sex), which compared the percent of overall trial failures as a function of drug treatment (vehicle or (2R,6R)-HNK) and sex. A two-way repeated measures ANOVA was also used to confirm the analysis by comparing the number of escape failures from the initial test assessment to the number of escape failures in the second, drug- or vehicle-treatment test (second test). All statisticall analyses were performed using JMP Statistical Analysis Software (SAS Institute Inc, Carey, NC). Significance was assigned at p < 0.05.
Results
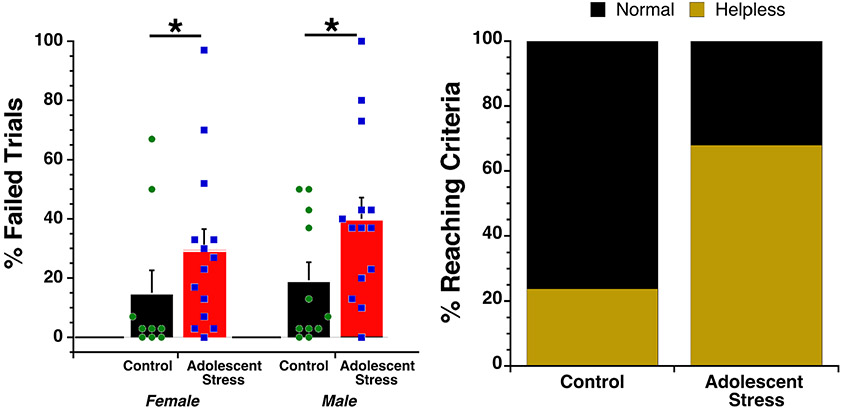
Mice exposed to adolescent stress manifested escape deficits at a significantly higher rate than non-stressed controls (F(Adolescent stress) = 5.16, df = 1, 45, p = 0.028; Figure 2A). There was no main effect of sex and (p = 0.367) and no interaction between stress history and sex (p = 0.614). The likelihood that mice experiencing adolescent stress would fail ≥30% of their final 10 trials, the criteria for moving into the treatment phase, differed significantly between control and adolescent stress conditions. Male and female mice from within each condition were equally likely to reach criteria: 3 out of 11 male controls, 3 out of 10 female controls, 10 out of 14 male adolescent stress, 10 out of 14 female adolescent stress (sex collapsed within condition chi2 likelihood ratio = 9.69, p = 0.002; Figure 2B). Four mice were removed from the experimental analysis due to equipment failure (1 control male; 2 female and 1 male adolescent stress). We conclude that exposure to a live predator stress during adolesence significantly increased susceptibility to the development of escape deficits following inescapable shock in adulthood. Given the constraints of our throughput, mice were tested at variable intervals (range = 16-35 days; PND75+) following the last predator exposure (or control handling). The effects were evident weeks after their last predator exposure (average 25 days) suggesting sustained effects induced by the predator exposure protocol.
Figure 2. Helpless assessment in adolescent-stress exposed mice.
(A) Mean percentage of trials (out of 30) mice failed to escape shock when escape options were available was significantly higher in adolescent-stress exposed mice. The sex of the mouse did not influence the outcome. Error bars represent S.E.M. Dots represent individual animals. (B) 72.4% of the adolescent-stress exposed mice (20/28, male and female combined) met helpless criteria compared to only 28.6% of the control mice (6/21 male and female combined).
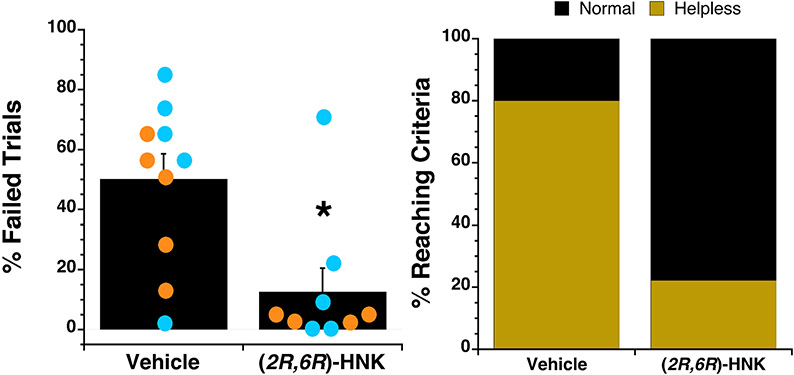
At least six days following the first helpless assessment (average 19 days, range 6-35 days), mice failing to escape ≥30% of their final 10 trials received vehicle or (2R,6R)-HNK treatment. We note that few control mice met this criteria (n = 3 each male, female) so this portion of the analysis focused only on adolescent stress-exposed animals. Adolescent-stressed mice that met failure criteria received either vehicle or 20 mg/kg (2R,6R)-HNK (i.p.) 24hr prior to being re-assessed in the helpless test (n = 10 each for vehicle and (2R,6R)-HNK). The inescapable shock protocol was not performed again prior to the drug or vehicle treatment. The helpless phenotype remained for the duration of the inter-test interval as evidenced by the finding that the incidence of escape failures in subjects between the first assessment test (Day 2 of the drug-naive asessment protocol) and second test (six-plus days later following vehicle treatment) did not statistically differ (p = 0.750). In contrast, subjects treated with (2R,6R)-HNK manifested significantly fewer escape failures than their vehicle treated counterparts during their second helpless test (F(Drug) = 10.45, df = 1, 15, p = 0.006) (Figure 3). Repeated measures analysis contrasting the first test and the second test confirms the direct comparison between the two gropus, only the (2R,6R)-HNK treated mice decreased escape failures in their second test (F(Drug) = 6.75, df = 1, 15, p = 0.020; F(Drug x Test) = 4.64, df = 1, 15, p = 0.048). There was no effect of sex on this outcome (p = 0.212). One (2R,6R)-HNK-treated female mouse was not included in the analysis due to equipment malfunction.
Figure 3. (2R,6R)-HNK treatment (20 mg/kg, i.p.) of the helpless phenotype in adolescent-stress exposed mice.
(A) The percentage of trials (out of 30) mice failed to escape shock when escape options were available was significantly lower in (2R,6R)-HNK compared to vehicle pretreated mice. Error bars represent S.E.M. Orange and blue dots represent individual female and male mice, respectively. (B) 80.0% of the vehicle-treated mice (8/10, male and female combined) met helpless criteria compared to only 22.2% of the vehicle-treated mice (2/9; male and female combined).
The likelihood that mice treated with (2R,6R)-HNK would fail ≥30% or more of their final 10 trials was signficantly less than that of vehicle-treated animals (sex collapsed within condition chi2 likelihood ratio = 6.74, p = 0.009). There was no correlation between the number of days following initial assessment for helplessness (Day 2 of the protocol) and failure rates in either the vehicle- or (2R,6R)-HNK-treated groups (r2 = 0.06 and 0.12, respectively).
Discussion
The purpose of the current studies was to investigate the potential of (2R,6R)-HNK to diminish susceptibility to adult stress-associated behavioral despair (helplessness) induced by adolescent stress. Developmentally-sensitive modifications have been made in DSM-5 for childhood trauma and PTSD, however there is no set criteria for what constitutes severe stress (trauma) in adolescents. In adults, a risk factor for the development of PTSD is described as exposure to actual or threatened death, serious injury or sexual violation. In a translationally-relevant animal model, the distinct neural response engaged by the stress event is important. Stress can differentially impact CNS function depending upon the type of stressor, its relevance to the rodent and controllability. For example, water submersion and live cat exposure both result in an anxious phenotype but the neuronal activation pattern consequent to each stressor is quite different (Adamec et al., 2012a, b). Synthetic fox odor (trimethylthiazoline (TMT)) and cat fur both elicit avoidance but TMT does not elicit the same emotional response as evidenced by differential response to anxiolytic drugs and failure to condition to the stimulus (Blanchard et al., 2003). Thus, a stimulus may be aversive but not transduced by similar mechanisms or carry the overtones of threat or danger. To this end, we developed an animal stress model (snake exposure) that directly exposes a rodent to close contact with a live predator (life-threatening event) without confounding physical harm. Unpublished studies using this model in Wistar rats suggests an array of long-lasting deficits that are present at adulthood (manuscript in prep). In the present study, male and female C57BL/6J mice were exposed to a live snake or control conditions during adolsence at PND days 31, 45 and then once again during early adulthood at PND 61. The consequences of this form of adolescent stress on an adult uncontrollable stress-induced helpless phenotype was assessed, and the effects of (2R,6R)-HNK on the adolescent stress-induced helpless phenotype was determined.
We report three primary findings. First, a greater proportion of mice developed a stress-induced helpless phenotype as an adult when they were exposed to a live snake during adolescence compared to the control non-stressed mice. Second, the helpless phenotype was reversed with a single administration of (2R,6R)-HNK. Third, there were no sex differences in either the response to live predator exposure nor in the effectiveness of the (2R,6R)-HNK treatment to reverse helpless behaviors. Live-predator exposure can give rise to the perception of a life-threatening event that has a different profile than other stressors (Adamec et al., 2012a; Adamec et al., 2005; Paschoalin-Maurin et al., 2018), and when introduced during adolescence has the potential to result in adult maladaptive behavior that is exhibited under non-stressful conditions and/or revealed following stressful conditions. The behavioral assessments conducted in these studies were designed to capitalize on the latter condition, an increased susceptibility to adult stress-induced helplessness. When mice were exposed to an uncontrollable stressor during adulthood, animals that experienced adolescent live predator stress were much more likely to exhibit behavioral despair. While clinical studies have found a sex difference in response to adverse chidhood experiences (Evans et al., 2020; Evans et al., 2017) we found no sex-differences in response to live-predator exposure during adolescence. This may be due to the pronounced universal effect of predator-stress, the timing of the stressor events or the particular phenotype under investigation (helplessness vs DSM-labeled depression). There are numerous approaches to induce a helpless phenotype similar to what we observed, all require the core element of sustained stress. It is the detection of control that activates circuitry that in turn prevents passivity and anxiety (see Maier and Seligman, 2016). In vehicle-treated adolescent-stressed mice the adult stress-induced helpless phenotype was expressed in the majority of mice (but not all) and sustained during the time between test and retest after treatment. Clinically, the individual response to childhood trauma is variable and often times leads to complex poly-diagnosis in adulthood (Dvir et al., 2014; Ford et al., 2013; McLaughlin et al., 2020). Although speculative, it is likely that in our model, the sustained duration of helplessness was similarly influenced by the previously experienced uncontrollable adolescent stress during sensitive times of neurodevelopment. Further studies will be required to determine behavioral and neurobiological differences that underlie adolescent versus adult stress-induced susceptibility to helplessness.
In adolescent-stressed mice, administration of (2R,6R)-HNK resulted in a robust decrease in escape failures compared to their initial test values and compared to the adolescent-stressed mice given vehicle. The lack of sex differences following (2R,6R)-HNK administration in the present study contrasts with sex differences in the potency of ketamine in behavioral models, where ketamine is more potent in females (Carrier and Kabbaj, 2013; Franceschelli et al., 2015; Zanos et al., 2016). However, we have previously provided evidence that sex differences in response to ketamine may be due to the metabolic production of (2R,6R)-HNK, which is greater in females than in males (Zanos et al., 2016). Given the same exposure to the metabolite there was no outcome difference in the current experiment, however a limitation of the current study is that only a single dose of (2R,6R)-HNK was used, thus not allowing for intepretations related to differences in potency of (2R,6R)-HNK between male and female mice.
We have previously reported antidepressant-relevant behavioral effects of (2R,6R)-HNK in mice, with a minimally effective dose ranging between 3 and 10 mg/kg (i.p.) in a number of behavioral tests including the forced swim test, novelty-supressed feeding test, and reversal of chronic corticosterone-induced anhedonia, all in CD-1 mice (Highland et al., 2019; Lumsden et al., 2019; Zanos et al., 2019; Zanos et al., 2016). (2R,6R)-HNK has also been shown to reverse deficits in social defeat-induced social interaction 24 h after administration (Zanos et al., 2016) and learned helplessness 24 h following both i.p. and oral administration to mice (Highland et al., In press; Zanos et al., 2016). In addition, Chou et al. reported reversal of despair and anhedonia behaviors induced by inescapable shock to previously stress naïve rats up to 21 days after a single i.p. administration of (2R,6R)-HNK (10 mg/kg) (Chou et al., 2018). The present study adds to these previous studies by demonstrating efficacy in reversing helpless behavior that was specifically enhanced by prior exposure to adolescent stress. In addition, the present study is the first, to our knowledge, to compare in the learned helplessness paradigm the behavioral effects of (2R,6R)-HNK in both male and female mice. Added importance to these findings is the observation that classic antidepressants are ineffective in the majority of patients with MDD and childhood trauma history (Williams et al., 2016). While we have not directly contrasted the efficacy of a classic antidepressant with that of (2R,6R)-HNK in this model, the demonstration of (2R,6R)-HNK efficacy may be a step towards realizing effective treatment options in this treatment-resistant population. Overall, the current findings add important knowledge to previous findings demonstrating robust antidepressant-relevant activity of (2R,6R)-HNK by a number of research groups (Cavalleri et al., 2018; Chou et al., 2018; Collo et al., 2018; Fred et al., 2019; Fukumoto et al., 2019; Fukumoto et al., 2017; Highland et al., 2019; Lumsden et al., 2019; Pham et al., 2018; Pham et al., 2017; Wray et al., 2018; Yao et al., 2018; Ye et al., 2019; Zanos et al., 2019; Zanos et al., 2016), however, see (Shirayama and Hashimoto, 2018; Yamaguchi et al., 2018; Yang et al., 2017).
Rapid-acting antidepressant drugs (e.g. ketamine) and putative rapid-acting antidepressant compounds (e.g. (2R,6R)-HNK, mGlu2 receptor antagonists etc.) can exert acute neurobiological actions that induce long-lasting neuroadaptations and synaptic plasticity changes hypothesized to be responsible for the sustained effects of these drugs (Abdallah et al., 2015; Duman, 2018; Zanos et al., 2018b). Thus, the behavioral actions observed 24 hours after treatment are likely attributed to these long-lasting neurobiological changes, rather than lasting exposure to the active metabolite (2R,6R)-HNK. Important consideration for the treatment of adolescent trauma is to identify pharmacotherapies that will lack serious side effects. Ketamine, despite its breakthrough potential for treating depression, has limited use due to its dissociative properties (Krystal et al., 1994), as well as its recreational abuse (Sassano-Higgins et al., 2016).
In contrast with ketamine, (2R,6R)-HNK was shown to lack adverse effects in preclinical models. In particular, it has been shown to lack locomotor stimulant effects at doses up to 125 mg/kg i.p. and 450 mg/kg orally (Highland et al., 2019). In addition, it does not support i.v. self-administration at doses and conditions that support (R,S)-ketamine self-administration and does not disrupt pre-pulse inhibition at doses up to 375 mg/kg in mice (Zanos et al., 2016), The majority of ketamine’s adverse side-effects appear to be mediated by NMDAR inhibition (Zanos et al., 2018a). In contrast, (2R,6R)-HNK has limited to no affinity/potency to inhibit the NMDAR at antidepressant-relevant concentrations (Lumsden et al., 2019; Moaddel et al., 2013; Morris et al., 2017; Suzuki et al., 2017; Zanos et al., 2016). Although the exact mechanism of action of (2R,6R)-HNK as an antidepressant remains to be determined, there is evidence suggesting that its actions to enhance presynaptic glutamate release probability (Riggs et al., 2019) converge with the metabotropic glutamate receptor subtype 2 (mGlu2) signaling (Zanos et al., 2019) and its behavioral effects, consistent with theories of rapid antidepressant action (see Alt et al., 2006) require AMPAR activity (Zanos et al., 2016), activity-dependent release of BDNF and downstream activation of mTORC1 (Fukumoto et al., 2019). (2R,6R)-HNK has not yet been tested in humans as a therapeutic for depression treatment. However, two studies have unexpectably identified a negative correlation between (2R,6R;2S,6S)-HNK plasma levels antidepressant responses in depressed subjects who received ketamine treatment (Farmer et al., 2020;Grunebaum et al., 2019), while one study identified no such relationship (Zarate et al., 2012).
A region of interest for ketamine’s effects is suggested by ongoing studies in rats indicating that the lateral habenula – rostromedial tegmentum function is altered as a consequences of adolescent predator exposure (in preparation) and that LHb hyperexcitability occurs after a much earlier life stress in the form of maternal deprivation (Tchenio et al., 2017, Shepard et al., 2018). Importantly, ketamine has been shown to reverse late adolescent (PND42-50) LHb hyperexcitability and immobility in the forced swim test consequent to early life stress (Shepard et al., 2018), and additional demonstrated habenula-involved conditions such as congenitally-susceptible helplessness and acute adult stress-induced helplessness (Dolzani et al., 2018; Shepard et al., 2018; Yang et al., 2018).
Adolescent exposure to a live predator produced enduring alterations in a behavioral construct relevant to psychiatric illness, especially MDD. The model developed in the present study provides an opportunity to improve our understanding of the neurobiological substrates impacted by adolescent trauma and improve treatment strategies. The demonstrated effectiveness of (2R,6R)-HNK in this animal model of adolescent stress exposure suggests a novel therapeutic intervention opportunity in this treatment-resistant MDD population.
FUNDING SOURCES
This work was supported by NIH MH107615 and VA Merit awards 1I01BX004062 and 101BX003631-01A1 to TDG.
Role of funding sources:
Funding sources have not participiated in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Declaration of interest
P.Z., and T.D.G. are listed as co-authors in patent applications related to the pharmacology and use of (2R,6R)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation and post-traumatic stress disorder. G.I.E., J.D.T and C.L.M report no conflict of interest.
References
- Abdallah CG, Sanacora G, Duman RS, Krystal JH, 2015. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 66, 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamec R, Toth M, Haller J, Halasz J, Blundell J, 2012a. Activation patterns of cells in selected brain stem nuclei of more and less stress responsive rats in two animal models of PTSD - predator exposure and submersion stress. Neuropharmacology 62(2), 725–736. [DOI] [PubMed] [Google Scholar]
- Adamec R, Toth M, Haller J, Halasz J, Blundell J, 2012b. A comparison of activation patterns of cells in selected prefrontal cortical and amygdala areas of rats which are more or less anxious in response to predator exposure or submersion stress. Physiol Behav 105(3), 628–638. [DOI] [PubMed] [Google Scholar]
- Adamec RE, Blundell J, Burton P, 2005. Neural circuit changes mediating lasting brain and behavioral response to predator stress. Neurosci Biobehav Rev 29(8), 1225–1241. [DOI] [PubMed] [Google Scholar]
- Agnew-Blais J, Danese A, 2016. Childhood maltreatment and unfavourable clinical outcomes in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry 3(4), 342–349. [DOI] [PubMed] [Google Scholar]
- Alt A, Nisenbaum ES, Bleakman D, Witkin JM, 2006. A role for AMPA receptors in mood disorders. Biochem Pharmacol 71(9), 1273–1288. [DOI] [PubMed] [Google Scholar]
- Anda RF, Brown DW, Felitti VJ, Dube SR, Giles WH, 2008. Adverse childhood experiences and prescription drug use in a cohort study of adult HMO patients. BMC Public Health 8, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH, 2006. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci 256(3), 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ, 2003. Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion. Prog Neuropsychopharmacol Biol Psychiatry 27(8), 1177–1185. [DOI] [PubMed] [Google Scholar]
- Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, Cheer JF, Frost DO, Huang XP, Gould TD, 2016. Effects of Ketamine and Ketamine Metabolites on Evoked Striatal Dopamine Release, Dopamine Receptors, and Monoamine Transporters. J Pharmacol Exp Ther 359(1), 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M, 2013. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70, 27–34. [DOI] [PubMed] [Google Scholar]
- Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, Spano PF, Collo G, 2018. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry 23(4), 812–823. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Wheaton AG, Anda RF, Croft JB, Edwards VJ, Liu Y, Sturgis SL, Perry GS, 2011. Adverse childhood experiences and sleep disturbances in adults. Sleep Med 12(8), 773–779. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF, 2004. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 82(2), 217–225. [DOI] [PubMed] [Google Scholar]
- Chou D, Peng HY, Lin TB, Lai CY, Hsieh MC, Wen YC, Lee AS, Wang HH, Yang PS, Chen GD, Ho YC, 2018. (2R,6R)-hydroxynorketamine rescues chronic stress-induced depression-like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology 139, 1–12. [DOI] [PubMed] [Google Scholar]
- Cloitre M, Stolbach BC, Herman JL, van der Kolk B, Pynoos R, Wang J, Petkova E, 2009. A developmental approach to complex PTSD: childhood and adult cumulative trauma as predictors of symptom complexity. J Trauma Stress 22(5), 399–408. [DOI] [PubMed] [Google Scholar]
- Collo G, Cavalleri L, Chiamulera C, Merlo Pich E, 2018. (2R,6R)-Hydroxynorketamine promotes dendrite outgrowth in human inducible pluripotent stem cell-derived neurons through AMPA receptor with timing and exposure compatible with ketamine infusion pharmacokinetics in humans. Neuroreport 29(16), 1425–1430. [DOI] [PubMed] [Google Scholar]
- Danese A, Baldwin JR, 2017. Hidden Wounds? Inflammatory Links Between Childhood Trauma and Psychopathology. Annu Rev Psychol 68, 517–544. [DOI] [PubMed] [Google Scholar]
- Dolzani SD, Baratta MV, Moss JM, Leslie NL, Tilden SG, Sorensen AT, Watkins LR, Lin Y, Maier SF, 2018. Inhibition of a Descending Prefrontal Circuit Prevents Ketamine-Induced Stress Resilience in Females. eNeuro 5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, 2018. Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide. F1000Research 7, F1000 Faculty Rev-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir Y, Ford JD, Hill M, Frazier JA, 2014. Childhood maltreatment, emotional dysregulation, and psychiatric comorbidities. Harv Rev Psychiatry 22(3), 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EA, Goff SL, Upchurch DM, Grella CE, 2020. Childhood adversity and mental health comorbidity in men and women with opioid use disorders. Addict Behav 102, 106149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EA, Grella CE, Upchurch DM, 2017. Gender differences in the effects of childhood adversity on alcohol, drug, and polysubstance-related disorders. Soc Psychiatry Psychiatr Epidemiol 52(7), 901–912. [DOI] [PubMed] [Google Scholar]
- Farmer CA, Gilbert JR, Moaddel R, George J, Adeojo L, Lovett J, Nugent AC, Kadriu B, Yuan P, Gould TD, Park LT, Zarate CA Jr., 2020. Ketamine metabolites, clinical response, and gamma power in a randomized, placebo-controlled, crossover trial for treatment-resistant major depression. Neuropsychopharmacology. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS, 1998. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study., AMEPRE. pp. 245–258. [DOI] [PubMed] [Google Scholar]
- Fiszman A, Mendlowicz MV, Marques-Portella C, Volchan E, Coutinho ES, Souza WF, Rocha V, Lima AA, Salomao FP, Mari JJ, Figueira I, 2008. Peritraumatic tonic immobility predicts a poor response to pharmacological treatment in victims of urban violence with PTSD. J Affect Disord 107(1-3), 193–197. [DOI] [PubMed] [Google Scholar]
- Ford JD, Grasso D, Greene C, Levine J, Spinazzola J, van der Kolk B, 2013. Clinical significance of a proposed developmental trauma disorder diagnosis: results of an international survey of clinicians. J Clin Psychiatry 74(8), 841–849. [DOI] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM, 2015. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and "depressed" mice exposed to chronic mild stress. Neuroscience 290, 49–60. [DOI] [PubMed] [Google Scholar]
- Fred SM, Laukkanen L, Brunello CA, Vesa L, Goos H, Cardon I, Moliner R, Maritzen T, Varjosalo M, Casarotto PC, Castren E, 2019. Pharmacologically diverse antidepressants facilitate TRKB receptor activation by disrupting its interaction with the endocytic adaptor complex AP-2. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Fogaca MV, Liu RJ, Duman C, Kato T, Li XY, Duman RS, 2019. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc Natl Acad Sci U S A 116(1), 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi J. i., Hashimoto K, Chaki S, 2017. Antidepressant Potential of (R)-Ketamine in Rodent Models: Comparison with (S)-Ketamine. Journal of Pharmacology and Experimental Therapeutics 361(1), 9–16. [DOI] [PubMed] [Google Scholar]
- Gearon JS, Kaltman SI, Brown C, 2003. Traumatic life events and PTSD among women with substance use disorders and schizophrenia, Psychiatric Services. pp. 523–528. [DOI] [PubMed] [Google Scholar]
- Gould F, Clarke J, Heim C, Harvey PD, Majer M, Nemeroff CB, 2012. The effects of child abuse and neglect on cognitive functioning in adulthood. J Psychiatr Res 46(4), 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum MF, Galfalvy HC, Choo TH, Parris MS, Burke AK, Suckow RF, Cooper TB, Mann JJ, 2019. Ketamine metabolite pilot study in a suicidal depression trial. J Psychiatr Res 117, 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy A, Emsley R, Freeman D, Bebbington P, Garety PA, Kuipers EE, Dunn G, Fowler D, 2016. Psychological Mechanisms Mediating Effects Between Trauma and Psychotic Symptoms: The Role of Affect Regulation, Intrusive Trauma Memory, Beliefs, and Depression., schizophrenia Bulletin. pp. S34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff CB, 2010. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol 52(7), 671–690. [DOI] [PubMed] [Google Scholar]
- Highland JN, Morris PJ, Zanos P, Lovett J, Ghosh S, Wang AQ, Zarate CA Jr., Thomas CJ, Moaddel R, Gould TD, 2019. Mouse, rat, and dog bioavailability and mouse oral antidepressant efficacy of (2R,6R)-hydroxynorketamine. J Psychopharmacol 33(1), 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Aguilar-Gaxiola S, Alhamzawi AO, Alonso J, Angermeyer M, Benjet C, Bromet E, Chatterji S, de Girolamo G, Demyttenaere K, Fayyad J, Florescu S, Gal G, Gureje O, Haro JM, Hu CY, Karam EG, Kawakami N, Lee S, Lepine JP, Ormel J, Posada-Villa J, Sagar R, Tsang A, Ustun TB, Vassilev S, Viana MC, Williams DR, 2010. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry 197(5), 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr., Charney DS, 1994. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of general psychiatry 51(3), 199–214. [DOI] [PubMed] [Google Scholar]
- Lima AA, Fiszman A, Marques-Portella C, Mendlowicz MV, Coutinho ES, Maia DC, Berger W, Rocha-Rego V, Volchan E, Mari JJ, Figueira I, 2010. The impact of tonic immobility reaction on the prognosis of posttraumatic stress disorder. J Psychiatr Res 44(4), 224–228. [DOI] [PubMed] [Google Scholar]
- Lumsden EW, Troppoli TA, Myers SJ, Zanos P, Aracava Y, Kehr J, Lovett J, Kim S, Wang FH, Schmidt S, Jenne CE, Yuan P, Morris PJ, Thomas CJ, Zarate CA Jr., Moaddel R, Traynelis SF, Pereira EFR, Thompson SM, Albuquerque EX, Gould TD, 2019. Antidepressant-relevant concentrations of the ketamine metabolite (2R,6R)-hydroxynorketamine do not block NMDA receptor function. Proc Natl Acad Sci U S A 116(11), 5160–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Seligman ME, 2016. Learned helplessness at fifty: Insights from neuroscience. Psychol Rev 123(4), 349–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Martin KR, Etkin A, 2015. Childhood trauma exposure disrupts the automatic regulation of emotional processing, …. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Colich NL, Rodman AM, Weissman DG, 2020. Mechanisms linking childhood trauma exposure and psychopathology: a transdiagnostic model of risk and resilience. BMC Med 18(1), 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, Rosenberg A, Tran T, Xiao Y, Zarate CA, Wainer IW, 2013. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in alpha7 nicotinic acetylcholine receptors. Eur J Pharmacol 698(1-3), 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris PJ, Moaddel R, Zanos P, Moore CE, Gould TD, Zarate CA Jr., Thomas CJ, 2017. Synthesis and N-Methyl-d-aspartate (NMDA) Receptor Activity of Ketamine Metabolites. Org Lett 19(17), 4572–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Vandeleur C, Rodgers S, Rössler W, Castelao E, Preisig M, Ajdacic-Gross V, 2015. Childhood adversities as specific contributors to the co-occurrence of posttraumatic stress and alcohol use disorders., Psychiatry Research. pp. 251–256. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ, 2013. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170(10), 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni V, Uher R, Danese A, 2012. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry 169(2), 141–151. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP Jr., Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB, 2003. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A 100(24), 14293–14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Hong JP, Bae JN, Cho S-J, Lee D-W, Lee J-Y, Chang SM, Jeon HJ, Hahm B-J, Lee YM, Seong S, Cho MJ, 2014. Impact of childhood exposure to psychological trauma on the risk of psychiatric disorders and somatic discomfort: single vs. multiple types of psychological trauma., Psychiatry Research. pp. 443–449. [DOI] [PubMed] [Google Scholar]
- Paschoalin-Maurin T, Dos Anjos-Garcia T, Falconi-Sobrinho LL, de Freitas RL, Coimbra JPC, Laure CJ, Coimbra NC, 2018. The Rodent-versus-wild Snake Paradigm as a Model for Studying Anxiety- and Panic-like Behaviors: Face, Construct and Predictive Validities. Neuroscience 369, 336–349. [DOI] [PubMed] [Google Scholar]
- Pham TH, Defaix C, Xu X, Deng S-X, Fabresse N, Alvarez J-C, Landry DW, Brachman RA, Denny CA, Gardier AM, 2018. Common neurotransmission recruited in (R,S)-ketamine and (2R,6R)-hydroxynorketamine-induced sustained antidepressant-like effects. Biol Psychiatry 84(1), e3–e6. [DOI] [PubMed] [Google Scholar]
- Pham TH, Defaix C, Xu X, Deng SX, Fabresse N, Alvarez JC, Landry DW, Brachman RA, Denny CA, Gardier AM, 2017. Common Neurotransmission Recruited in (R,S)-Ketamine and (2R,6R)-Hydroxynorketamine-Induced Sustained Antidepressant-like Effects. Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- Portmann S, Kwan HY, Theurillat R, Schmitz A, Mevissen M, Thormann W, 2010. Enantioselective capillary electrophoresis for identification and characterization of human cytochrome P450 enzymes which metabolize ketamine and norketamine in vitro. J Chromatogr A 1217(51), 7942–7948. [DOI] [PubMed] [Google Scholar]
- Powers A, Etkin A, Gyurak A, Bradley B, Jovanovic T, 2015. Associations Between Childhood Abuse, Posttraumatic Stress Disorder, and Implicit Emotion Regulation Deficits: Evidence From a Low-Income, Inner-City Population. Psychiatry 78(3), 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers A, Fani N, Cross D, Ressler KJ, Bradley B, 2016. Childhood trauma, PTSD, and psychosis: Findings from a highly traumatized, minority sample., Child Abuse Negl. pp. 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Dulawa SC, 2017. Identifying fast-onset antidepressants using rodent models. Mol Psychiatry 22(5), 656–665. [DOI] [PubMed] [Google Scholar]
- Riggs LM, Aracava Y, Zanos P, Fischell J, Albuquerque EX, Pereira EFR, Thompson SM, Gould TD, 2019. (2R,6R)-hydroxynorketamine rapidly potentiates hippocampal glutamatergic transmission through a synapse-specific presynaptic mechanism. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs LM, Aracava Y, Zanos P, Fischell J, Albuquerque EX, Pereira EFR, Thompson SM, Gould TD, 2019. (2R,6R)-hydroxynorketamine rapidly potentiates hippocampal glutamatergic transmission through a synapse-specific presynaptic mechanism. Neuropsychopharmacology June19 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassano-Higgins S, Baron D, Juarez G, Esmaili N, Gold M, 2016. A Review of Ketamine Abuse and Diversion. Depress Anxiety 33(8), 718–727. [DOI] [PubMed] [Google Scholar]
- Shepard RD, Langlois LD, Browne CA, Berenji A, Lucki I, Nugent FS, 2018. Ketamine Reverses Lateral Habenula Neuronal Dysfunction and Behavioral Immobility in the Forced Swim Test Following Maternal Deprivation in Late Adolescent Rats. Front Synaptic Neurosci 10, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SH, Miller DP, Teicher MH, 2013. Exposure to childhood neglect and physical abuse and developmental trajectories of heavy episodic drinking from early adolescence into young adulthood. Drug Alcohol Depend 127(1-3), 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K, 2018. Lack of antidepressant effects of (2R,6R)-hydroxynorketamine in a rat learned helplessness model: comparison with (R)-ketamine. International Journal of Neuropsychopharmacology 21(1), 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L, 2016. A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. Am J Psychiatry 173(8), 816–826. [DOI] [PubMed] [Google Scholar]
- Sitko K, Bentall RP, Shevlin M, O'Sullivan N, Sellwood W, 2014. Associations between specific psychotic symptoms and specific childhood adversities are mediated by attachment styles: an analysis of the National Comorbidity Survey., Psychiatry Research. pp. 202–209. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM, 2017. Effects of a ketamine metabolite on synaptic NMDAR function. Nature 546(7659), E1–E3. [DOI] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ, 2007. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up., Arch Gen Psychiatry. pp. 49–56. [DOI] [PubMed] [Google Scholar]
- Williams LM, Debattista C, Duchemin A-M, Schatzberg AF, Nemeroff CB, 2016. Childhood trauma predicts antidepressant response in adults with major depression: data from the randomized international study to predict optimized treatment for depression, Translational Psychiatry. Nature Publishing Group, pp. e799–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NH, Schappi JM, Singh H, Senese NB, Rasenick MM, 2018. NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J. i., Toki H, Qu Y, Yang C, Koike H, Hashimoto K, Mizuno-Yasuhira A, Chaki S, 2018. (2R,6R)-Hydroxynorketamine is not essential for the antidepressant actions of (R)-ketamine in mice. Neuropsychopharmacology 43, 1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K, 2017. (R)-Ketamine Shows Greater Potency and Longer Lasting Antidepressant Effects Than Its Metabolite (2R,6R)-Hydroxynorketamine. Biol Psychiatry 82(5), e43–e44. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, Hu H, 2018. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554(7692), 317–322. [DOI] [PubMed] [Google Scholar]
- Yao N, Skiteva O, Zhang X, Svenningsson P, Chergui K, 2018. Ketamine and its metabolite (2R,6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Mol Psychiatry 23(10), 2066–2077. [DOI] [PubMed] [Google Scholar]
- Ye L, Ko CY, Huang Y, Zheng C, Zheng Y, Chou D, 2019. Ketamine metabolite (2R,6R)-hydroxynorketamine enhances aggression via periaqueductal gray glutamatergic transmission. Neuropharmacology 157, 107667. [DOI] [PubMed] [Google Scholar]
- Zanos P, Highland JN, Liu X, Troppoli TA, Georgiou P, Lovett J, Morris PJ, Stewart BW, Thomas CJ, Thompson SM, Moaddel R, Gould TD, 2019. (R)-Ketamine exerts antidepressant actions partly via conversion to (2R,6R)-hydroxynorketamine, while causing adverse effects at sub-anaesthetic doses. Br J Pharmacol 176(14), 2573–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Highland JN, Stewart BW, Georgiou P, Jenne CE, Lovett J, Morris PJ, Thomas CJ, Moaddel R, Zarate CA, Gould TD, 2019. (2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions. Proc Natl Acad Sci U S A 116(13), 6441–6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr., Gould TD, 2016. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533(7604), 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr., Gould TD, 2018a. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev 70(3), 621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Thompson SM, Duman RS, Zarate CA Jr., Gould TD, 2018b. Convergent Mechanisms Underlying Rapid Antidepressant Action. CNS Drugs 32(3), 197–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, Moaddel R, Wainer IW, 2012. Relationship of ketamine's plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry 72(4), 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, 2006. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of General Psychiatry 63(8), 856–864. [DOI] [PubMed] [Google Scholar]