Abstract
Objectives:
To assess the efficacy and feasibility of topical manuka honey application in chronic nonhealing discharging extraoral wounds.
Materials and Methods:
The study includes 15 patients (9 males and 6 females, mean age: 38.06, range: 20–50 years), presenting with the complaint of chronic nonhealing discharging extraoral wounds from January 2018 to January 2020. After wound irrigation with normal saline, manuka honey in conjunction with the antibiotic treatment was directly applied onto the surface of the wound and was then covered by an absorbent layer to contain the honey. Dressings were changed every alternate day for a week till there was complete cessation of pus discharge. Henceforth, the interval between dressings was increased to 1 week subsequently and was continued for 4 weeks. Assessment was done on the basis of discharge and depth of the wound before the procedure and weekly for 4 weeks.
Results:
The average depth of wound as seen at 15 sites after a week was 5.72 mm, and decrease in the average depth of wound seen at the end of the 4th week was 0.88 mm with complete wound epithelization. This was found to be statistically significant (P = 0.0001). No cases were reported with allergy, pain, infection, inflammation, and swelling on 1st, 2nd, 3rd, and 4thweek.
Conclusion:
Hence, the use of manuka honey as a wound dressing material in our study has proved to promote the growth of tissues for wound repair, suppress inflammation, and bring about rapid autolytic debridement.
Keywords: Debriding wounds, extraoral wounds, manuka honey
INTRODUCTION
Honey, one of the substances, has been used to treat infections and heal wounds with reports dating back as far as 4000 years ago.
Manuka (Leptospermum scoparium) is a tree, indigenous to New Zealand and South East Australia, and from the myrtle family, Myrtaceae. The honey produced from its flowers is a uni-floral honey largely produced in New Zealand.[1]
In a review of the literature, Moore showed that Manuka honey has “very special healing properties” and described it as “the best natural antibiotic in the world.”[2]
As per literature,[1,2,3,4,5,6,7,8] it was found that the topical application of honey clears existing wound infection rapidly; facilitates healing of deeply infected surgical wounds; and halts spreading necrotizing fasciitis. It has also promoted healing of infected wounds that were not responding to conventional therapy such as antibiotics and antiseptics, including wounds that were infected with antibiotic-resistant bacteria such as methicillin-resistant Staphyloccocus aureus (MRSA).[4]
The broad-spectrum antibacterial activity of manuka honey has been validated through numerous clinical trials and in vivo bacterial challenges ranging from oral infections, dermatitis and skin irritations, intestinal inflammation to nosocomial pathogens. The capacity of manuka honey with Unique Manuka factor 15 was investigated in reducing dental plaque and clinical levels of gingivitis.
Laboratory studies have also shown that manuka honey affects the molecular structure of various bacteria, namely, S. aureus (MRSA-15),[9,10,11] Pseudomonas aeruginosa,[12,13] Escherichia coli,[10] and Streptococcus pyogenes (Group A streptococci).[14]
MATERIALS AND METHODS
The study was conducted on 15 patients (9 males and 6 females, mean age: 38.06, range; 20–50 years), presenting with the complaint of chronic nonhealing discharging extraoral wounds due to posttraumatic, foreign body, wound infections caused by antibiotic-resistant strains such as S. aureus (MRSA), iatrogenic (postsurgical wounds) from January 2018 to January 2020 after approval from the institutional ethical committee.
Patients suffering from systemic illness, uncontrolled diabetes, allergic to honey or bees, known hypersensitivities, allergies, or idiosyncratic reactions to medications, pregnant, or lactating females were excluded from the study.
The study protocol was explained to the patients in detail and their consent was obtained.
The manuka honey dressings were used in conjunction with the antibiotic treatment as numerous studies have shown that, when manuka honey is used along with antibiotics, it brings about rapid healing.
Hence, following the standard Centers for Disease Control and Prevention[15] protocols for sterilization and asepsis, irrigation of the wound was done with normal saline then the manuka honey; presterilized by gamma radiations procured directly from New Zealand was directly applied onto the surface of the wound and was then covered by an absorbent layer to contain the honey. As the amount of honey needed to treat a wound depends on the amount of exudate, because the beneficial effects are reduced or lost if small amounts of honey are diluted by large amounts of exudate. The deeper the infection, the more honey will be needed to achieve an effective level of antibacterial activity diffusing deep into the wound tissues.
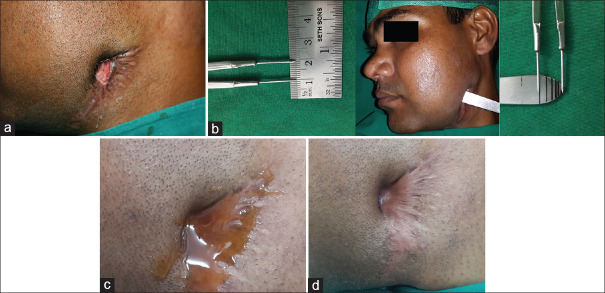
Patients were followed up and dressings were changed every alternate day for a week. At the end of the 1st week, the pus discharge ceased in all the cases and the depth of the wound reduced. The interval between dressings was increased to 1 week subsequently and was continued for 4 more weeks. The depth of the wound was measured every week up to 4 weeks. On the 4th week, there was complete epithelization of the wound [Figure 1a-d].
Figure 1.
(a) Chronic nonhealing infected wound at the left mandibular angle region (b) shows procedure of wound depth measurement (c) topical manuka honey application on the wound surface (d) complete epithilization of the wound
Assessment was done on the basis of discharge and depth of the wound before the procedure and weekly for 4 weeks.
Scar revision/fat grafting were done to elevate the depressed scar only for facial esthetic purpose.
RESULTS
Fifteen patients (9 males and 6 females, mean age: 38.06, range: 20–50 years) [Graphs 1 and 2].
Graph 1.
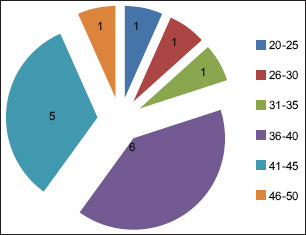
Age of 15 patients selected for the study
Graph 2.
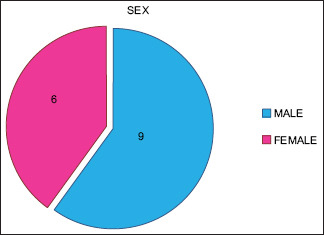
Nine male and 6 female patients included in the study
Two patients with chronic nonhealing wound on zygoma, 1 on maxilla, 1 on mandible symphysis region, 2 on mandible parasymphysis region, 3 on mandible body region, and 6 on mandible angle region [Graph 3] were treated with manuka honey dressings which showed complete cessation of pus discharge on the 1st week itself [Graph 4].
Graph 3.
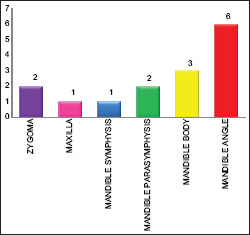
The 15 sites with intra oral - extra oral communication
Graph 4.
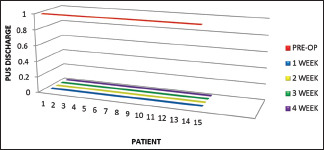
Complete cessation of pus discharge on 1st, 2nd, 3rd, and 4th week
The average depth of wound as seen at 15 sites after a week was 5.72 mm, and decrease in the average depth of wound seen at the end of the 4th week was 0.88 mm with complete wound epithelization. Moreover, it helped create an acidic wound environment, which favors wound healing. This was found to be statistically significant (P = 0.0001) [Graph 5].
Graph 5.
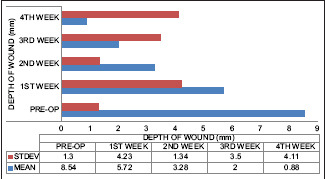
Marked reduction in the depth of the wound on subsequent follow-up
No cases were reported with allergy, pain, infection, inflammation, and swelling on 1st, 2nd, 3rd, and 4th week.
DISCUSSION
In recent times, it has been “rediscovered,” with numerous reports of animal model and clinical studies, case reports, and randomized controlled trials that show favorable rates alongside modern dressing materials in its effectiveness in managing wounds.[6]
In 2009, a study by Merckoll et al. showed the effects of honey on planktonic and biofilm-embedded bacteria which suggests that honey has a bactericidal effect against the wound pathogens that are grown in the laboratory as biofilms.[16]
Similarly, Alandejani et al., in 2009, illustrated biofilms of S. aureus and P. aeruginosa exposed to honey were inhibited in vitro.[17]
Methylglyoxal has been implicated in the inhibition of biofilms by Jervis-Bardy et al.; in 2011.[18] Methylglyoxal is the unique compound in the honey responsible for some of its potent antimicrobial properties. It is rich in glucose oxidase that catalyzes glucose to produce hydrogen peroxide which exerts antibacterial properties topically. D-glucono-δ-lactone is also produced which reduces the pH of the honey and in addition to the high sugar osmolarity exerts natural antibacterial properties and renders the honey shelf-stable. The low water activity of honey in general (0.6–0.75) also renders it uninhabitable for most microorganisms. Further, propolis another component of honey contains chiefly flavonoids (i.e., galangin and pinocembrin), phenolic acids, and their esters that contribute to its immunostimulant properties.[2] Biofilms of methicillin-sensitive S. aureus, MRSA, and vancomycin-resistant Enterococci can be prevented from forming and established biofilms can be inhibited – in vitro with varying concentrations of manuka honey as illustrated by Cooper et al., in 2011.[19]
Honey has also shown to be effective in inhibiting six isolates of P. aeruginosa forming biofilms in vitro by Cooper et al. in 2009[19] and one reference strain of S. pyogenes by Maddocks et al. in 2012.[7]
Proteases work optimally at an alkaline pH and manuka honey has been shown to reduce pH as illustrated by Gethin et al. in 2008; therefore this is likely to modulate protease activity in chronic wounds.[8]
According to Molan in 2009, the osmotic effect of honey has been thought to encourage lymphatic flow to devitalize tissue while reducing bacterial load.[20]
As stated by Gethin and Cowman in 2009, honey promotes autolytic debridement by bringing plasminogen into the wound environment, which is normally activated into active plasmin by plasminogen activator. In chronic wounds, the production of plasminogen activator inhibitor (PAI) by macrophages inactivates plasminogen activator and results in low levels of active plasmin. By inactivating PAI, honey allows plasminogen to become plasmin and in turn, digest fibrin and so lower the quantity of nonviable tissue.[21]
However, the use of honey in modern wound care is still met with some skepticism. Since the advent of evidence-based medicine, changing clinical practice depends on providing clinicians with appropriate levels of evidence of clinical efficacy. Although honey has become a first-line intervention in some wound care clinics, larger and better designed randomized controlled trials are needed to cement the role of honey in modern wound care.
CONCLUSION
With the increase in incidence of difficult to treat infection in our day-to-day practice and inability of the pharmaceutical companies to keep up with the pace of development of antibiotic resistance will only lead to increased costs, increased days of hospital stay, increased spread of multidrug-resistant infection, thereby leading to loss of productivity and economy as a whole. This should lead us, as health-care professionals to the increased use of adjuvant modalities to treat tenacious infections such as the use of manuka honey in combination with antibiotics.
Manuka honey as a wound dressing is useful in maintaining a moist wound environment and acts as an autolytic debriding agent in debriding wounds.
The rapid healing that was observed after topical honey application can be explained through a dual effect on the inflammatory response. First, honey prevents a prolonged inflammatory response by suppressing the production and propagation of inflammatory cells at the wound site; second, it stimulates the production of proinflammatory cytokine, allowing normal healing to occur and stimulating the proliferation of fibroblasts and epithelial cells.
Hence, the use of manuka honey as a wound dressing material in our study has proved to promote the growth of tissues for wound repair, suppress inflammation, and bring about rapid autolytic debridement.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Patel S, Cichello S. Manuka honey: An emerging natural food with medicinal use. Nat Prod Bioprospect. 2013;3:121–8. [Google Scholar]
- 2.Moore OA, Smith LA, Campbell F, Seers K, McQuay HJ, Moore RA. Systematic review of the use of honey as a wound dressing. BMC Complement Altern Med. 2001;1:2. doi: 10.1186/1472-6882-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogdanov S. Bee product Science In: Book of Honey as nutrient and functional food. 2009 [Google Scholar]
- 4.Haynes JS, Callaghan R. Properties of honey: Its mode of action and clinical outcomes. Wounds UK. 2011;7:50–7. [Google Scholar]
- 5.Molan P, Betts J. Using honey dressings: The practical considerations. Nurs Times. 2000;96:36–7. [PubMed] [Google Scholar]
- 6.Patricia VI, Fazlul HU. Systematic reviews on interventions with honey in cancer. J Clin Nurs. 2008;17:2604–23. [Google Scholar]
- 7.Maddocks SE, Lopez MS, Rowlands RS, Cooper RA. Manuka honey inhibits the development of Streptococcus pyogenes biofilms and causes reduced expression of two fibronectin binding proteins. Microbiology (reading) 2012;158:781–90. doi: 10.1099/mic.0.053959-0. [DOI] [PubMed] [Google Scholar]
- 8.Gethin GT, Cowman S, Conroy RM. The impact of Manuka honey dressings on the surface pH of chronic wounds. Int Wound J. 2008;5:185–94. doi: 10.1111/j.1742-481X.2007.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Cooper RA, Halas E, Molan PC. The efficacy of honey in inhibiting strains of Pseudomonas aeruginosa from infected burns. J Burn Care Rehabil. 2002;23:366–70. doi: 10.1097/00004630-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Blair SE, Cokcetin NN, Harry EJ, Carter DA. The unusual antibacterial activity of medical-grade Leptospermum honey: Antibacterial spectrum, resistance and transcriptome analysis. Eur J Clin Microbiol Infect Dis. 2009;28:1199–208. doi: 10.1007/s10096-009-0763-z. [DOI] [PubMed] [Google Scholar]
- 11.Henriques AF, Jenkins RE, Burton NF, Cooper RA. The intracellular effects of manuka honey on Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 2010;29:45–50. doi: 10.1007/s10096-009-0817-2. [DOI] [PubMed] [Google Scholar]
- 12.Henriques AF, Jenkins RE, Burton NF, Cooper RA. The effect of manuka honey on the structure of Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2011;30:167–71. doi: 10.1007/s10096-010-1065-1. [DOI] [PubMed] [Google Scholar]
- 13.Roberts AE, Maddocks SE, Cooper RA. Manuka honey is bactericidal against Pseudomonas aeruginosa and results in differential expression of oprF and algD. Microbiology (Reading) 2012;158:3005–13. doi: 10.1099/mic.0.062794-0. [DOI] [PubMed] [Google Scholar]
- 14.Cooper RA, Lindsay E, Molan PC. Testing the susceptibility to manuka honey of streptococci isolated from wound swab. J ApiProduct ApiMed Sci. 2011;;3:117–22. [Google Scholar]
- 15.Guideline for Disinfection and Sterilization in Healthcare Facilities; 2014, CDC [Google Scholar]
- 16.Merckoll P, Jonassen TØ, Vad ME, Jeansson SL, Melby KK. Bacteria, biofilm and honey: A study of the effects of honey on ‘planktonic’ and biofilm-embedded chronic wound bacteria. Scand J Infect Dis. 2009;41:341–7. doi: 10.1080/00365540902849383. [DOI] [PubMed] [Google Scholar]
- 17.Alandejani T, Marsan J, Ferris W, Slinger R, Chan F. Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol Head Neck Surg. 2009;141:114–8. doi: 10.1016/j.otohns.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Jervis-Bardy J, Foreman A, Bray S, Tan L, Wormald PJ. Methylglyoxal-infused honey mimics the anti-Staphylococcus aureus biofilm activity of manuka honey: Potential implication in chronic rhinosinusitis. Laryngoscope. 2011;121:1104–7. doi: 10.1002/lary.21717. [DOI] [PubMed] [Google Scholar]
- 19.Cooper R, Jenkins L, Rowlands R. Inhibition of biofilms through the use of manuka honey. Primary Care Diabetes Society. Wounds UK. 2011;;7:24–32. [Google Scholar]
- 20.Molan PC. Debridement of wounds with honey. J Wound Technol. 2009;5:12–6. [Google Scholar]
- 21.Gethin G, Cowman S. Manuka honey vs. hydrogel – A prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous ulcers. J Clin Nurs. 2009;18:466–74. doi: 10.1111/j.1365-2702.2008.02558.x. [DOI] [PubMed] [Google Scholar]