Abstract
INTRODUCTION:
Neoadjuvant imatinib (Neo-IM) therapy may facilitate R0 resection in primary gastrointestinal stromal tumors (GISTs) that are large or in difficult anatomic locations. While response to preoperative tyrosine kinase inhibitors is associated with better outcome in metastatic GIST, little is known about prognostic factors after Neo-IM in primary GIST.
STUDY DESIGN:
Patients with primary GIST with or without synchronous metastases who underwent Neo-IM were retrospectively analyzed from a prospective maintained institutional database for Response Evaluation Criteria in Solid Tumors (RECIST), tumor viability, and mitotic rate. Overall survival (OS) was estimated by Kaplan-Meier and compared by log-rank test. Cox proportionate hazard models were used for univariate and multivariate analysis.
RESULTS:
One hundred and fifty patients were treated for a median of 7.1 months (range 0.2–160). By RECIST, partial response, stable disease, and progressive disease were seen in 40%, 51%, and 9%, respectively. By pathologic analysis, ≤50% of the tumor was viable in 72%, and the mitotic rate was ≤5/50HPF in 74%. On multivariate analysis, RECIST response and tumor viability were not associated with OS, while post-treatment high mitotic rate (hazard ratio [HR] for death 5.3, CI 2.3–12.4), R2 margins (HR 6.0, CI 2.3–15.5), and adjuvant imatinib (HR 0.4, CI 0.2–0.9) were (p<0.05). 5 year OS was 81 vs. 38% for low vs. high mitotic rate; 81, 59, and 39% for R0, R1, and R2 margins; and 75 vs 61% for adjuvant vs. no adjuvant imatinib therapy (p<0.05).
CONCLUSIONS:
In primary GIST undergoing Neo-IM therapy, progression was uncommon, but substantial down-sizing occurred in the minority. High tumor mitotic rate and incomplete resection following Neo-IM were associated with poor outcome, while adjuvant imatinib was associated with prolonged survival.
Keywords: Gastrointestinal stromal tumor, GIST, imatinib, neoadjuvant, surgery
INTRODUCTION
It has been shown that up to 70% of GISTs may be cured by surgery alone[1], however, surgery for GIST varies widely in complexity. In some GISTs, small bowel resection or wedge partial gastrectomy is adequate, while others require esophagogastrectomy, pancreaticoduodenectomy, abdominoperineal resection, or extensive multivisceral resection. Given the morbidity associated with the latter procedures, there has been significant interest in downsizing tumors to facilitate less radical surgery.
Neoadjuvant imatinib (Neo-IM) has been reported in GIST, although mostly as a mix of primary and recurrent/metastatic GIST in the form of case reports, retrospective series, and several phase II clinical trials[2–14]. The largest retrospective series of locally advanced primary GIST pooled data from 10 European sarcoma centers on 161 patients treated with Neo-IM, of whom 83% underwent R0 resection [9]. Additionally, several phase II clinical trials of Neo-IM in primary GIST showed safety, feasibility, and R0 resection in most patients[5–8, 12], with two suggesting that Neo-IM allowed less morbid surgery[8, 12], as we had seen in our retrospective report on rectal GIST[14].
While the majority of these studies focus on the safety of Neo-IM and its association with R0 margins, less is known about whether traditional prognostic factors after resection of treatment naïve primary GIST (size, site, mitotic rate[15–19]) are relevant after Neo-IM, or whether adjuvant imatinib is associated with improved outcome after Neo-IM as it is after up front surgery[1, 20–24]. In the European retrospective study of Neo-IM, Rutkowski et al[9] reported that lack of adjuvant imatinib therapy and small bowel primary tumor site (bivariate anaylsis) were associated with worse outcome. However, it is unknown whether this holds true for overall survival, or how this relates to mitotic rate or tumor response to treatment. Existing data on this topic are limited to a study of Neo-IM in patients with recurrent/metastatic disease -- in which we reported the combined results of our institution and the Brigham and Women’s Hospital, detailing 400 metastasectomy operations performed in 323 patients after preoperative treatment with tyrosine kinase inhibitors (TKIs)[13]. Outcome was predicted by radiographic response, with median OS not reached for responsive disease, and 110, 59, and 24mo for stable, unifocal progressive, and multifocal progressive disease. Post-treatment mitotic rate ≥5/50 high-powered fields (HPF), R2 resection, and multifocal disease progression all predicted worse progression-free survival and OS on multivariate analyses.
In primary GIST, it is not known whether mitotic rate, size change, or pathologic response after Neo-IM are associated with outcome. Here, we characterize response parameters that are widely available: tumor size change as well as pathologic viability and mitotic rate, with the hypothesis that lack of response by these parameters would be associated with worse overall survival.
METHODS
We recently reported 1,000 patients who underwent surgery for GIST at our institution from July 1982 to April 2016 from a prospective institutional sarcoma database[25]. Diagnosis was confirmed by immunohistochemistry for CD117 (KIT) and sometimes DOG-1. The database was queried for age, sex, standard primary tumor clinicopathologic variables (site of origin, size, histologic subtype, presence of synchronous metastases), significant dates (date of surgery, recurrence, death or last follow-up), surgical margins (R2, grossly positive; R1, microscopically positive; R0, negative), and chemotherapy information (drug and treatment dates). Among patients whose initial surgery was for a primary tumor (n=788), those who underwent treatment with Neo-IM were selected for further analysis (n=150). Baseline characteristics of patients undergoing Neo-IM were compared to those who did not receive the drug preoperatively. Among patients undergoing Neo-IM, subgroup analysis was planned based on the presence or absence of synchronous metastases.
Treatment response was assessed using a modification of Response Criteria for Solid Tumors (RECIST[26]), mitotic rate, and tumor viability from the resected primary tumor post-treatment. For the modified RECIST (mRECIST) assessment, computed tomography (CT) measurements from the scan just prior to Neo-IM were compared to the final pathologic measurements. This slight modification using pathologic size was necessary for consistency since the timing of the most recent scan prior to surgery was highly variable. RECIST categories of progressive disease (PD), stable disease (SD), partial response (PR), and complete response (CR) were defined as ≥20% increase in tumor size, <20% increase to <30% decrease, ≥30% decrease, and complete disappearance of the lesion, respectively. The number of mitoses was counted by a pathologist from 50 HPFs[27]. Percent viability was visually estimated by the pathologist. All research was done under an Institutional Review Board approved protocol, in accordance with the Health Insurance Portability and Accountability Act.
OS was estimated among patients undergoing Neo-IM from the time of the initial surgery using the Kaplan-Meier method, with comparisons done by log-rank test. To compare factors between groups, Fisher’s exact test was used for categorical variables, and the Wilcoxon rank sum test for continuous variables. Univariable and multivariable Cox proportional hazard models were built for OS. P-values <0.05 were considered significant (two-sided). SAS 9.4 (SAS Institute, Inc, Cary, NC) and R version 3.4.0 were used for statistical analysis.
RESULTS
Among 1,000 patients with GIST who underwent surgery at our institution from 1982–2016[25], 788 had a primary tumor at the initial surgery, of which 128 had synchronous metastases. There were 150 of these patients who were treated with Neo-IM between 2001 and 2015 (Figure 1a, Table 1). Median follow-up was 3.8 years. Patients undergoing Neo-IM had more rectal and small bowel tumors, larger tumors, higher mitotic rate, and more commonly had positive margins and synchronous metastases. Neo-IM was administered for a median of 7.1 months (range 0.2–160) before resection. Radiologic response was evaluated by mRECIST, and primary tumors were evaluated by pathology for mitotic rate and tumor viability (Figure 1b). Among 138 patients in whom there were adequate data for mRECIST assessment, 13 (9%), 70 (51%), and 55 (40%) had PD, SD, and PR, respectively. There were no CR. By pathologic analysis, ≤50% of the tumor was viable in 72% (99 of 137 evaluable), and the mitotic rate was ≤5/50HPF in 74% (93 of 126 evaluable). mRECIST, mitotic rate, and tumor viability were not always concordant. For example, in patients who had PR, 40% had either residual high mitotic rate or tumor viability, possibly reflecting resistant subclones or less responsive tumor tissue.
Figure 1: Response to Neo-IM in 150 primary GISTs.
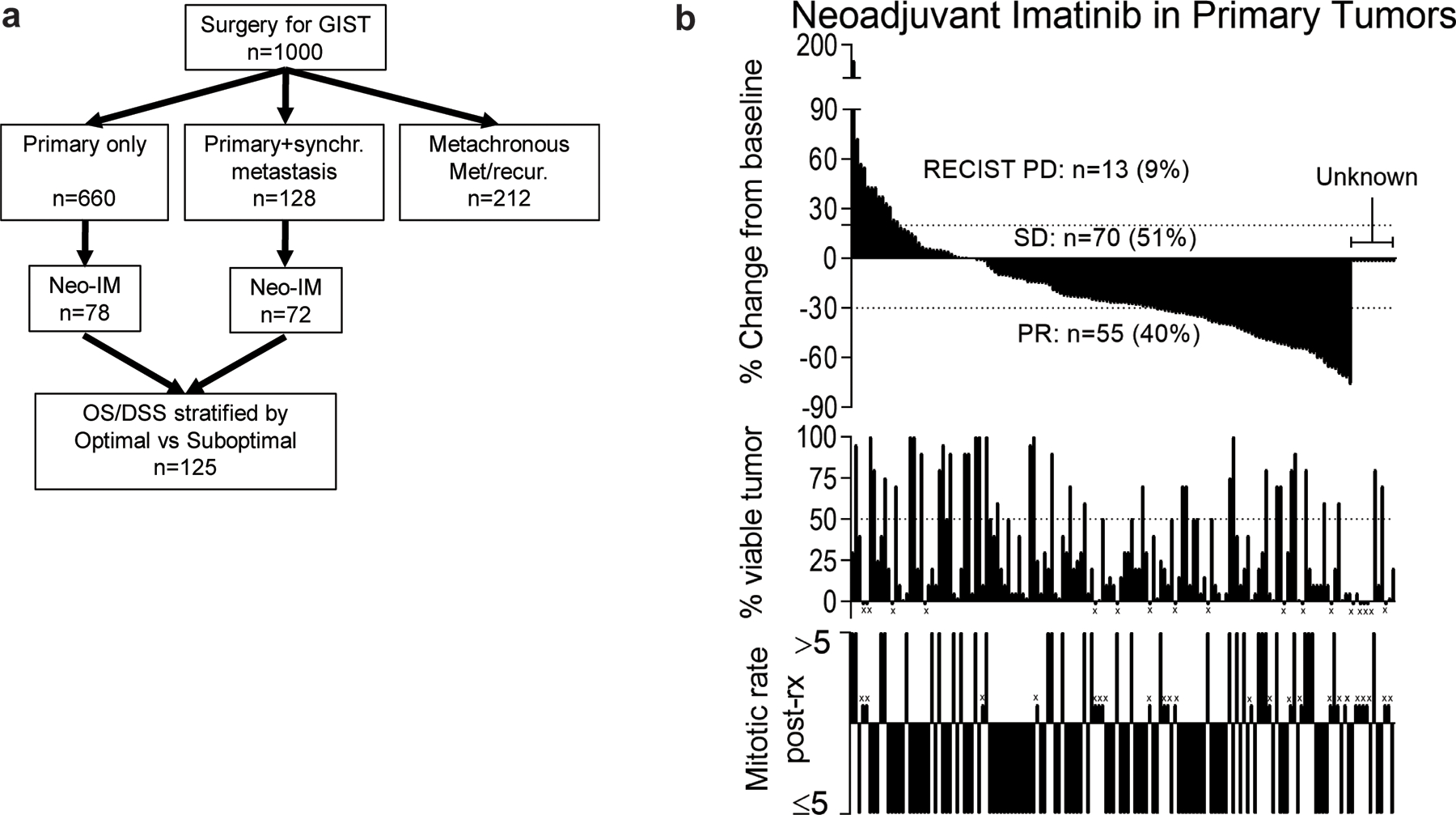
(a) Flowchart showing 1,000 GISTs treated surgically at a single institution[25], of which 150 had primary tumors and underwent treatment with neoadjuvant imatinib (Neo-IM). Note that only patients with complete data for response evaluation (n=125) were analyzed for survival in subsequent figures. (b) Response to Neo-IM in 150 patients by mRECIST (top), tumor viability (middle), and mitotic rate after resection (bottom). ‘x’ indicates no evaluable data for that factor. For mRECIST, viability, and mitotic rate there were 12, 17, and 26 unknown, respectively. Matched vertical lines in each graph represent the same patient.
Table 1:
Characteristics of 788 Primary GISTs.
| Total (n=788) |
Neo-IM (n=150) |
No Neo-IM (n=638) | p-value | ||
|---|---|---|---|---|---|
| Survivor follow-up (yrs) | Median (range) | 4.6 (0.0–27.7) | 3.8 (0.01–14.3) | 4.8 (0.0–27.7) | |
| Age at first surgery (yrs) | Median (range) | 62.5 (8.07–94.7) | 58.2 (23.0–86.2) | 64.1 (8.07–94.7) | <0.001 |
| Sex | F | 373 (47.3) | 61 (40.7) | 312 (48.9) | 0.070 |
| M | 415 (52.7) | 89 (59.3) | 326 (51.1) | ||
| Primary tumor site | Stomach | 526 (66.8) | 74 (49.3) | 452 (70.8) | <0.001 |
| Small Bowel | 171 (21.7) | 44 (29.3) | 127 (19.9) | ||
| Rectum | 44 (5.6) | 20 (13.3) | 24 (3.8) | ||
| Other | 47 (6) | 12 (8) | 35 (5.5) | ||
| Primary tumor size (cm) | ≤5 | 344 (43.8) | 28 (18.8) | 316 (49.6) | <0.001 |
| 5–10 | 235 (29.9) | 45 (30.2) | 190 (29.8) | ||
| >10 | 207 (26.3) | 76 (51) | 131 (20.6) | ||
| Unknown | 2 (N/A) | 1 (N/A) | 1 (N/A) | ||
| Primary tumor mitotic rate per 50 HPF (pre-treatment or untreated)a | ≤5 | 377 (63.2) | 19 (42.2) | 358 (65) | 0.003 |
| >5 | 219 (36.7) | 26 (57.7) | 193 (35) | ||
| Unknown | 192 (N/A) | 105 (N/A) | 87 (N/A) | ||
| Histologic variant | Spindle | 414 (75.3) | 95 (74.2) | 319 (75.6) | 0.93 |
| Epithelioid | 67 (12.2) | 16 (12.5) | 51 (12.1) | ||
| Mixed | 69 (12.5) | 17 (13.3) | 52 (12.3) | ||
| Unknown | 238 (N/A) | 22 (N/A) | 216 (N/A) | ||
| Marginb | R0 | 646 (82.1) | 95 (63.3) | 551 (86.5) | <0.001 |
| R1 | 69 (8.8) | 27 (18) | 42 (6.6) | ||
| R2 | 72 (9.1) | 28 (18.7) | 44 (6.9) | ||
| Unknown | 1 (N/A) | 0 (N/A) | 1 (N/A) | ||
| Synchronous metastases | No | 660 (83.8) | 78 (52) | 582 (91.2) | <0.001 |
| Yes | 128 (16.2) | 72 (48) | 56 (8.8) | ||
| Adjuvant imatinib Duration | No | 591 (75.1) | 58 (38.9) | 533 (83.5) | <0.001 |
| Yes | 196 (24.9) | 91 (61.1) | 105 (16.5) | ||
| Unknown | 1 (N/A) | 1 (N/A) | 0 (N/A) | ||
| Median (range) | 2.06 (0.01–11.6) | 2.32 (0.01–9.45) | 1.85 (0.17–11.6) | 0.34 | |
| Median (IQR) | 2.06 (0.84–3.88) | 2.32 (0.90–4.15) | 1.85 (0.77–3.47) | ||
| Mutationc | KIT exon 9 | 24 (5.7) | 13 (10.7) | 11 (3.7) | N/Ad |
| KIT exon 11 deletion | 171 (40.6) | 53 (43.8) | 118 (39.3) | ||
| KIT exon 11 other | 87 (20.7) | 18 (14.9) | 69 (23) | ||
| KIT exon 13 | 7 (1.7) | 3 (2.5) | 4 (1.3) | ||
| KIT exon 17 only | 1 (0.2) | 0 (0) | 1 (0.3) | ||
| KIT mult exons | 23 (5.5) | 15 (12.4) | 8 (2.7) | ||
| PDGFRA D842V / D842I | 20 (4.8) | 5 (4.1) | 15 (5) | ||
| PDGFRA other | 23 (5.5) | 3 (2.5) | 20 (6.7) | ||
| NF1 only | 4 (1) | 0 (0) | 4 (1.3) | ||
| SDH | 2 (0.5) | 0 (0) | 2 (0.7) | ||
| Wild type (WT) | 59 (14) | 11 (9.1) | 48 (16) | ||
| Unknown | 367 (N/A) | 29 (N/A) | 338 (N/A) | ||
For patients who underwent Neo-IM, only those who had pre-treatment mitotic rate available are listed, while the “No Neo-IM” values all reflect untreated tumors. Most patients in the Neo-IM group did not have a pre-treatment mitotic rate available, since this requires a core needle biopsy.
R2 margins include incomplete resection and tumor rupture
Mutational analysis in the earliest patients included only KIT and PDGFRA, thus some “wild type” may reflect alternate mutations/epimutations
Descriptive only, too many variables for statistical comparison. Numbers listed reflect percentage of all patients in whom mutation was known.
After excluding 25 patients lacking complete data for the 3 response parameters, univariate and multivariate analysis of OS were performed for standard clinicopathologic variables and response parameters(Table 2). After Neo-IM, multivariate analysis showed high post-treatment mitotic rate was independently associated with worse OS (HR 5.3 [CI 2.3–12.4], p<0.001). Surprisingly, neither mRECIST response nor post-treatment tumor viability correlated with OS by univariate or multivariate analysis (p>0.05). Other site (i.e., not stomach, small bowel, or rectum) was associated with worse OS on univariate analysis, but this association did not persist when other factors were controlled for in the multivariate analysis. Notably, the presence of synchronous metastases was not associated with OS (p=0.22) on univariate analysis. R2 margins were associated with worse OS (HR 6.0 [CI 2.3–15.5], p<0.001) on multivariate analysis (Table 2), while there was no association with survival for R1 margins. Adjuvant imatinib treatment was administered for a median of 2.3 years (IQR 0.9–4.2), and was associated with longer OS (HR 0.4 [CI 0.2–0.9], p=0.025).
Table 2:
Univariate and multivariate analysis of OS in 125 patients with primary GIST undergoing Neo-IM.
| Univariate | Multivariatea | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Class Value | Reference | HR | CI | p-value | HR | CI | p-value |
| Age at first surgery (yrs) | N/A | N/A | 1.04 | 1.01–1.06 | 0.009 | 1.05 | 1.01–1.09 | 0.013 |
| Sex | M | F | 1.62 | 0.81–3.25 | 0.18 | |||
| Primary tumor site | Small Bowel | Stomach | 1.67 | 0.80–3.52 | 0.18 | |||
| Rectum | Stomach | 0.42 | 0.06–3.23 | 0.41 | ||||
| Other | Stomach | 3.01 | 1.20–7.53 | 0.019 | ||||
| Primary tumor size (cm) | >10 | <=5 | 1.59 | 0.54–4.64 | 0.40 | |||
| 5–10 | <=5 | 1.29 | 0.40–4.23 | 0.67 | ||||
| Histologic variant | Epithelioid | Spindle | 1.18 | 0.44–3.16 | 0.74 | |||
| Mixed | Spindle | 1.55 | 0.65–3.67 | 0.32 | ||||
| Margin | R1 | R0 | 1.69 | 0.70–4.10 | 0.24 | 2.33 | 0.78–6.93 | 0.13 |
| R2 | R0 | 3.98 | 1.89–8.37 | <.001 | 6.00 | 2.32–15.5 | <0.001 | |
| Primary tumor post-treatment mitotic rate per 50 HPF | >5 | ≤5 | 4.14 | 2.10–8.16 | <.001 | 5.34 | 3.30–12.4 | <0.001 |
| Primary tumor viability | >50% | ≤50% | 1.61 | 0.80–3.25 | 0.18 | 1.20 | 0.48–3.00 | 0.69 |
| Primary tumor mRECIST | PD | SD | 1.14 | 0.38–3.40 | 0.82 | 0.34 | 0.04–2.73 | 0.31 |
| PR | SD | 1.00 | 0.49–2.08 | >0.95 | 0.72 | 0.32–1.61 | 0.43 | |
| Synchronous metastases | Synch met | No synch | 1.52 | 0.78–2.97 | 0.22 | |||
| Adjuvant imatinib | Yes | No | 0.46 | 0.24–0.91 | 0.026 | 0.41 | 0.19–0.89 | 0.025 |
Only the final multivariate model is shown.
Sub-analysis was performed for patients with primary tumors only (n=78; Supplemental tables 1-2) and those with primary tumors and synchronous metastases (n=72; Supplemental tables 3-4). Compared to those not undergoing Neo-IM, primary only (i.e., without metastasis) patients undergoing Neo-IM had more rectal and less stomach tumors, larger tumors, and more commonly had positive margins, while synchronous patients (i.e., those with metastasis) undergoing Neo-IM had similar characteristics (Supplemental tables 1 and 3). Both primary only and synchronous patients undergoing Neo-IM were more likely to receive adjuvant imatinib. Among those patients with complete data for response evaluation, multivariate analysis of the subgroups was not possible due to inadequate sample size and too few events, however, on univariate analysis, interesting differences from the overall group emerged. While mitotic rate remained statistically significant (or nearly so) for both groups, tumor viability was nearly significant for the primary only group (p=0.055, Supplemental Table 2), but not for the other group. mRECIST PD was associated with worse survival in synchronous but not primary only patients (p=0.028, Supplemental Table 4). Adjuvant imatinib was associated with longer OS in the synchronous group (HR 0.2 [CI 0.08–0.52], p<0.001), but there was only a trend in the primary only group (HR 0.5 [CI 0.16–1.67], p=0.27).
Kaplan-Meier curves are shown depicting OS stratified by mitotic rate, margin, tumor viability, RECIST response, synchronous metastasis, and adjuvant imatinib (Figure 2, Supplemental Figures 1 and 2 for subgroups). The 5 year OS was 81% for patients with post-treatment mitotic rate ≤5/50HPF compared to 38% for those >5/50HPF (p<0.001). The 5 year OS was 81, 59, and 39% (p<0.001) for R0, R1, and R2 margins (p<0.001). Likewise, 5 year OS was 75 vs 61% for adjuvant vs no adjuvant imatinib (p=0.022).
Figure 2: OS after Neo-IM for primary GIST.
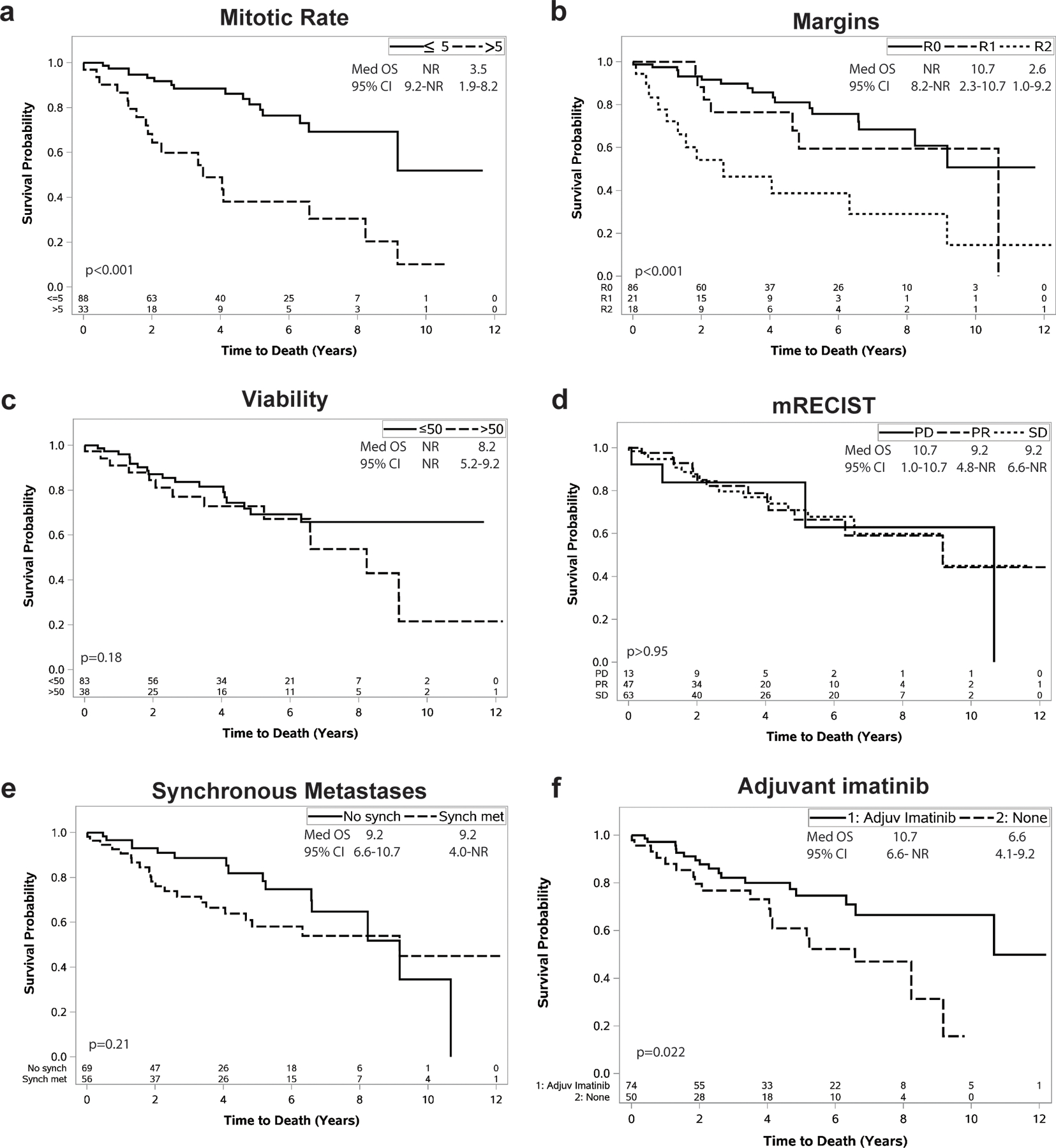
After completion of Neo-IM, overall survival (OS) following surgery is shown stratified by (a) mitotic rate, (b) margins, (c) tumor viability, (d) mRECIST, (e) presence of synchronous metastases, and (f) whether adjuvant imatinib was given. The number of patients at risk at each time point is shown on the x-axis. The inset in each graph denotes the median survival, 95% confidence interval of the median, and p-value value for a log-rank test comparing groups.
Mutational analysis was performed on the resected specimen in 81% of Neo-IM patients (Table 1, Supplemental table 5). Although formal analysis was not possible given the number of groups and sample size, high mitotic rate after Neo-IM was seen at similar proportions in most mutation subgroups (Supplemental table 5), ranging from 20–33% of each group. The exception was the group with multiple KIT exon mutations, of which 50% had high mitotic rate, possibly reflecting development of resistant subclones as these patients had undergone extended therapy compared to those with a single exon mutation (median 11.0 mo Neo-IM for multiple KIT mutations [IQR 7.4– 39.1; n=15] vs. 7.0 mo [IQR 4.8–12.9; n=87; p=0.007] for those with single mutations). Waterfall plots of mRECIST, tumor viability, and post-treatment mitotic rate stratified by mutation are shown in Supplemental Figure 3.
Among 72 patients with synchronous metastases, there were complete data to evaluate mRECIST, tumor viability, and mitotic rate in both the primary and metastasis in 52, 48, and 22 patients, respectively. For each parameter, the primary and metastatic tumor usually had concordant responses. From these, in the 49 patients with SD or PR in the primary tumor, 90% had SD or PR in the metastasis. Among the 33 primary tumors with tumor viability ≤50%, the majority (82%) had concordant responses in the metastasis.
Discussion
We present here the largest single-institution retrospective clinical series of neoadjuvant imatinib in primary GIST. The factors associated with outcome after resection of primary GIST in the pre-imatinib era have long been known, specifically, tumor site of origin, size, and mitotic rate[15–19, 28]. Neoadjuvant imatinib is increasingly being used for patients with locally advanced primary tumors requiring extensive multivisceral resection or tumors in difficult locations such as the gastroesophageal junction, duodenum, or rectum, as well as for patients with metastatic disease. However, it is currently unknown whether traditional clinicopathologic factors are associated with outcome after neoadjuvant imatinib treatment. Based on our experience with preoperative treatment in metastatic GIST[13], we hypothesized that tumor size change, post-treatment mitotic rate, and tumor viability would predict outcome in primary GIST. However, we found that among all patients with primary GIST undergoing Neo-IM, unlike in metastatic GIST, tumor size change was not associated with outcome, and neither was tumor viability. Post-treatment mitotic rate did correlate with outcome, with an estimated 5 year OS of 81 vs 38% for high vs low mitotic rate, respectively. As with metastatic GIST, incomplete (R2) resection in primary GIST was associated with poor outcome.
Some interesting differences emerged in the sub-analyses of primary only patients and those with a primary GIST and synchronous metastasis undergoing Neo-IM, although any conclusions must be tempered by the fact that sample size was too small for multivariate analysis. Mitotic rate remained the most important factor for both groups. In the 4 patients with RECIST progression of disease in the synchronous group, worse outcome was seen, although this was not seen in the primary only group. This is a small fraction of patients, and in the overall group RECIST response, particularly partial response, did not correlate with outcome as it did in metastatic patients[13]. That mRECIST status did not correlate with outcome was not surprising. It has been previously reported that RECIST criteria did not correlate with treatment response to imatinib[29]. Choi criteria (based on density and less stringent size change criteria) were more sensitive. Unexpectedly, though, tumor viability by pathologic analysis did not associate with outcome in the overall group, while it may be related to outcome after Neo-IM in primary only patients. That the effect was not stronger in the overall group underscores the fact that imatinib is essentially not curative, even in the micrometastatic setting as shown by the ACOSOG Z9001 placebo arm and the 1 year adjuvant imatinib arm converging in recurrence-free survival upon long-term follow up[21].
The decision to continue imatinib following surgery is multifactorial and not definitively answered by our data. In the overall group, adjuvant imatinib was associated with longer OS on multivariate analysis (HR 0.4 [CI 0.19–0.89], p=0.025), with 5 year OS 75 vs 61% for no adjuvant therapy, similar to what was seen for disease-free survival in one other study[9]. However, subgroup analysis showed that the synchronous metastasis group had the strongest effect (univariate HR 0.2 [CI 0.08–0.52], p<0.001), with only a trend in the primary only group (univariate HR 0.5 [CI 0.16–1.67], p=0.27). It is possible that the OS effect is blunted in the primary only group due to early salvage at recurrence with TKIs, which we postulate may be more easily accomplished in this group with less aggressive biology than in the synchronous group. For patients with primary GIST without metastases, adjuvant therapy is generally recommended for tumors that were originally large (gastric >10 cm or non-gastric >5cm) and those with a high mitotic rate. In most centers, including ours, mitotic rate is not routinely determined prior to imatinib initiation, which limits use of validated prognostic systems, such as our nomogram for tumor recurrence[17]. Mutation subtype also has prognostic value, although it is not performed as the standard of care before starting imatinib, which is why some patients in our series ultimately turned out to have a resistant mutation (e.g., PDGFRA D842V). It is now generally our practice to obtain mutational analysis prior to commencing neoadjuvant therapy. For patients with stable or progressing disease on Neo-IM and high residual tumor viability and high mitotic rate at resection, consideration should be given to other TKIs in the adjuvant setting. Our data support the practice of continuing imatinib postoperative for patients with primary GIST and synchronous metastasis, unless there is obvious evidence of resistance or the mutation itself is resistant. Overall, among response parameters we evaluated, our data suggest that any patient with high mitotic activity following Neo-IM is at high risk of tumor recurrence/progression and should be considered for further therapy.
Neo-IM is frequently prescribed with the goal of tumor size reduction to facilitate less radical and safer surgery, with some basis in the literature[8, 12, 14]. However, we found that after Neo-IM, the minority of patients (40%) had a size reduction of 30% of more, whereas most had stable disease. Despite the frequent lack of a reduction in tumor size, there are other potential benefits of Neo-IM for the surgeon, such as reducing vascularity and peritumoral inflammation.
In this study, we analyzed all 788 patients who underwent resection of primary tumors at our institution, of whom 150 underwent treatment with Neo-IM and half of these patients had synchronous primary tumors. Among all 788, without selection for Neo-IM, patients with synchronous metastases (n=128) had worse outcome than those with primary tumors only (n=660)[25]. However, of the patients who underwent Neo-IM, there was no association of synchronous metastases with outcome (p=0.22). We believe this may reflect the aggressive biology of primary tumors without synchronous metastases that were selected for Neo-IM, as these tumors were larger, located in high risk sites, and had a higher high mitotic rate than primary tumors who did not undergo Neo-IM.
Incomplete (R2) resection was associated with poor outcome on multivariate analysis in this study of patients undergoing Neo-IM, while microscopically positive margins (R1) were not different from negative (R0) margins. This is consistent with our larger cohort of 1000 patients, in which a similar pattern was seen in all groups (primary, primary with synchronous metastases, or metachronous recurrence/metastases)[25]. Inability to obtain a complete resection may reflect aggressive tumor biology. Based on this, we believe that surgical resection should only be attempted if R0/R1 resection is anticipated, as these data suggest little role for R2 debulking.
Our findings are limited by the retrospective nature of our study. Our survival outcomes were measured from the time of surgery. We had considered measuring outcome from the date of Neo-IM initiation, however, we abandoned this due to concern for immortal time bias[30]. While the actual margin achieved was clearly associated with outcome, our study does not delineate whether Neo-IM was associated with a greater likelihood of negative margins or less extensive surgery, as we had shown in a report of Neo-IM in rectal GIST[14]. Further analysis of the estimated extent of operation required before Neo-IM compared to what was actually done is beyond the scope of this current study, and ideally would come in the form of a randomized clinical trial.
Conclusions
In conclusion, tumor size change and viability after Neo-IM in primary GIST were not associated with outcome, whereas post-treatment mitotic rate and complete resection were the most important factors on multivariate analysis. Substantial tumor size reduction after Neo-IM occurred in the minority of patients. Following neoadjuvant therapy and resection, adjuvant imatinib therapy was associated with prolonged survival, although this effect was strongest in primary GIST with synchronous metastases.
Supplementary Material
Supplemental Figure 1: OS after Neo-IM for primary GIST without synchronous metastases.
After completion of Neo-IM, OS following surgery is shown stratified by (a) mitotic rate, (b) margins, (c) tumor viability, (d) mRECIST, and (e) whether adjuvant imatinib was given. The number of patients at risk at each time point is shown on the x-axis. The inset in each graph denotes the median survival, 95% confidence interval of the median, and p-value value for a log-rank test comparing groups. Note that for margins (c) the R2 group only had one patient and was thus combined with the R1 group for this figure.
Supplemental Figure 2: OS after Neo-IM for primary GIST with synchronous metastases.
After completion of Neo-IM, OS following surgery is shown stratified by (a) mitotic rate, (b) margins, (c) tumor viability, (d) mRECIST, and (e) whether adjuvant imatinib was given. The number of patients at risk at each time point is shown on the x-axis. The inset in each graph denotes the median survival, 95% confidence interval of the median, and p-value value for a log-rank test comparing groups.
Supplemental Figure 3: Treatment response stratified by mutation.
Response to Neo-IM in 150 patients is shown by (a) mRECIST, (b) tumor viability, and (c) mitotic rate after resection. ‘x’ indicates no evaluable data for that factor. For mRECIST, viability, and mitotic rate there were 12, 17, and 26 unknown, respectively. Mutation is depicted by color according to the key in the inset. Note that graphs a-c are sorted from least treatment response (left) to most response (right). Unknowns are shown at the far right of each graph.
Acknowledgments
Grant Support: The investigators were supported by NIH grant R01 CA102613, the David Foundation, Betsy Levine-Brown and Marc Brown, GIST Cancer Research Fund, and David and Monica Gorin (RPD), Kristen Ann Carr Fellowship (MJC), and P50 CA217694 (SS).
Footnotes
Twitter: @sloan_kettering @UKMarkey @UKSurgeryDept @pennsurgery
Meeting Presentation: Society of Surgical Oncology Annual Cancer Symposium 2017, Oral Presentation
Disclosures: Consulting fees from Blueprint Medicines, Novartis, Daiichi Sankyo, Loxo, GlaxoSmithKline Pharmaceuticals (William Tap). Dr. DeMatteo has a research grant from Blueprint Medicines. The other authors have no disclosures.
References
- 1.Corless CL, Ballman KV, Antonescu CR, Kolesnikova V, Maki RG, Pisters PW, Blackstein ME, Blanke CD, Demetri GD, Heinrich MC, von Mehren M, Patel S, McCarter MD, Owzar K, DeMatteo RP. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32(15):1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andtbacka RH, Ng CS, Scaife CL, Cormier JN, Hunt KK, Pisters PW, Pollock RE, Benjamin RS, Burgess MA, Chen LL, Trent J, Patel SR, Raymond K, Feig BW. Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Annals of surgical oncology 2007;14(1):14–24. [DOI] [PubMed] [Google Scholar]
- 3.Rubio J, Marcos-Gragera R, Ortiz MR, Miro J, Vilardell L, Girones J, Hernandez-Yague X, Codina-Cazador A, Bernado L, Izquierdo A, Colomer R. Population-based incidence and survival of gastrointestinal stromal tumours (GIST) in Girona, Spain. European journal of cancer 2007;43(1):144–8. [DOI] [PubMed] [Google Scholar]
- 4.DeMatteo RP, Maki RG, Singer S, Gonen M, Brennan MF, Antonescu CR. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Annals of surgery 2007;245(3):347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenberg BL, Harris J, Blanke CD, Demetri GD, Heinrich MC, Watson JC, Hoffman JP, Okuno S, Kane JM, von Mehren M. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. Journal of surgical oncology 2009;99(1):42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyon C, Sideris L, Leblanc G, Leclerc YE, Boudreau D, Dube P. Prolonged Therapy with Imatinib Mesylate before Surgery for Advanced Gastrointestinal Stromal Tumor Results of a Phase II Trial. Int J Surg Oncol 2012;2012:761576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Zhang Q, Blanke CD, Demetri GD, Heinrich MC, Watson JC, Hoffman JP, Okuno S, Kane JM, von Mehren M, Eisenberg BL. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Annals of surgical oncology 2012;19(4):1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hohenberger P LC, Wendtner CM, Hohenberger W, Pustowka A, Wardelmann E AE, Licht T. Neoadjuvant treatment of locally advanced GIST: results of APOLLON, a prospective, open label phase II study in KIT- or PDGFRA-positive tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012;30 (suppl). [Google Scholar]
- 9.Rutkowski P, Gronchi A, Hohenberger P, Bonvalot S, Schoffski P, Bauer S, Fumagalli E, Nyckowski P, Nguyen BP, Kerst JM, Fiore M, Bylina E, Hoiczyk M, Cats A, Casali PG, Le Cesne A, Treckmann J, Stoeckle E, de Wilt JH, Sleijfer S, Tielen R, van der Graaf W, Verhoef C, van Coevorden F. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Annals of surgical oncology 2013;20(9):2937–43. [DOI] [PubMed] [Google Scholar]
- 10.Bischof DA, Kim Y, Blazer DG 3rd, Behman R, Karanicolas PJ, Law CH, Quereshy FA, Maithel SK, Gamblin TC, Bauer TW, Pawlik TM. Surgical management of advanced gastrointestinal stromal tumors: an international multi-institutional analysis of 158 patients. Journal of the American College of Surgeons 2014;219(3):439–49. [DOI] [PubMed] [Google Scholar]
- 11.Bauer S, Rutkowski P, Hohenberger P, Miceli R, Fumagalli E, Siedlecki JA, Nguyen BP, Kerst M, Fiore M, Nyckowski P, Hoiczyk M, Cats A, Casali PG, Treckmann J, van Coevorden F, Gronchi A. Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib -- analysis of prognostic factors (EORTC-STBSG collaborative study). Eur J Surg Oncol 2014;40(4):412–9. [DOI] [PubMed] [Google Scholar]
- 12.Kurokawa Y, Yang HK, Cho H, Ryu MH, Masuzawa T, Park SR, Matsumoto S, Lee HJ, Honda H, Kwon OK, Ishikawa T, Lee KH, Nabeshima K, Kong SH, Shimokawa T, Yook JH, Doki Y, Im SA, Hirota S, Hahn S, Nishida T, Kang YK. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer 2017;117(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairweather M, Balachandran VP, Li GZ, Bertagnolli MM, Antonescu C, Tap W, Singer S, DeMatteo RP, Raut CP. Cytoreductive Surgery for Metastatic Gastrointestinal Stromal Tumors Treated With Tyrosine Kinase Inhibitors: A 2-institutional Analysis. Annals of surgery 2018;268(2):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavnar MJ, Wang L, Balachandran VP, Antonescu CR, Tap WD, Keohan M, Singer S, Temple L, Nash GM, Weiser MR, Guillem JG, Aguilar JG, DeMatteo RP, Paty PB. Rectal Gastrointestinal Stromal Tumor (GIST) in the Era of Imatinib: Organ Preservation and Improved Oncologic Outcome. Annals of surgical oncology 2017;24(13):3972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dematteo RP, Gold JS, Saran L, Gonen M, Liau KH, Maki RG, Singer S, Besmer P, Brennan MF, Antonescu CR. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008;112(3):608–15. [DOI] [PubMed] [Google Scholar]
- 16.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Annals of surgery 2000;231(1):51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold JS, Gonen M, Gutierrez A, Broto JM, Garcia-del-Muro X, Smyrk TC, Maki RG, Singer S, Brennan MF, Antonescu CR, Donohue JH, DeMatteo RP. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. The Lancet Oncology 2009;10(11):1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joensuu H, Vehtari A, Riihimaki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. The Lancet Oncology 2012;13(3):265–74. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Seminars in diagnostic pathology 2006;23(2):70–83. [DOI] [PubMed] [Google Scholar]
- 20.Casali PG, Le Cesne A, Poveda Velasco A, Kotasek D, Rutkowski P, Hohenberger P, Fumagalli E, Judson IR, Italiano A, Gelderblom H, Adenis A, Hartmann JT, Duffaud F, Goldstein D, Broto JM, Gronchi A, Dei Tos AP, Marreaud S, van der Graaf WT, Zalcberg JR, Litiere S, Blay JY. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33(36):4276–83. [DOI] [PubMed] [Google Scholar]
- 21.Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF, Patel S, McCarter MD, Polikoff JA, Tan BR, Owzar K, American College of Surgeons Oncology Group Intergroup Adjuvant GST. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373(9669):1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schutte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE, Schlemmer M, Bauer S, Wardelmann E, Sarlomo-Rikala M, Nilsson B, Sihto H, Monge OR, Bono P, Kallio R, Vehtari A, Leinonen M, Alvegard T, Reichardt P. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307(12):1265–72. [DOI] [PubMed] [Google Scholar]
- 23.Joensuu H, Eriksson M, Sundby Hall K, Reichardt A, Hartmann JT, Pink D, Ramadori G, Hohenberger P, Al-Batran SE, Schlemmer M, Bauer S, Wardelmann E, Nilsson B, Sihto H, Bono P, Kallio R, Junnila J, Alvegard T, Reichardt P. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34(3):244–50. [DOI] [PubMed] [Google Scholar]
- 24.Raut CP, Espat NJ, Maki RG, Araujo DM, Trent J, Williams TF, Purkayastha DD, DeMatteo RP. Efficacy and Tolerability of 5-Year Adjuvant Imatinib Treatment for Patients With Resected Intermediate- or High-Risk Primary Gastrointestinal Stromal Tumor: The PERSIST-5 Clinical Trial. JAMA Oncol 2018:e184060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavnar MJ, Seier K, Curtin C, Balachandran VP, Coit DG, Yoon SS, Crago AM, Strong VE, Tap WD, Gonen M, Antonescu CR, Brennan MF, Singer S, DeMatteo RP. Outcome of 1000 Patients With Gastrointestinal Stromal Tumor (GIST) Treated by Surgery in the Pre and Post-imatinib Eras. Annals of surgery 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92(3):205–16. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen M, El-Rifai W, L HLS, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Human pathology 2002;33(5):478–83. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Human pathology 2002;33(5):459–65. [DOI] [PubMed] [Google Scholar]
- 29.Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Charnsangavej C. We should desist using RECIST, at least in GIST. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2007;25(13):1760–4. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal P, Moshier E, Ru M, Ohri N, Ennis R, Rosenzweig K, Mazumdar M. Immortal Time Bias in Observational Studies of Time-to-Event Outcomes: Assessing Effects of Postmastectomy Radiation Therapy Using the National Cancer Database. Cancer Control 2018;25(1):1073274818789355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: OS after Neo-IM for primary GIST without synchronous metastases.
After completion of Neo-IM, OS following surgery is shown stratified by (a) mitotic rate, (b) margins, (c) tumor viability, (d) mRECIST, and (e) whether adjuvant imatinib was given. The number of patients at risk at each time point is shown on the x-axis. The inset in each graph denotes the median survival, 95% confidence interval of the median, and p-value value for a log-rank test comparing groups. Note that for margins (c) the R2 group only had one patient and was thus combined with the R1 group for this figure.
Supplemental Figure 2: OS after Neo-IM for primary GIST with synchronous metastases.
After completion of Neo-IM, OS following surgery is shown stratified by (a) mitotic rate, (b) margins, (c) tumor viability, (d) mRECIST, and (e) whether adjuvant imatinib was given. The number of patients at risk at each time point is shown on the x-axis. The inset in each graph denotes the median survival, 95% confidence interval of the median, and p-value value for a log-rank test comparing groups.
Supplemental Figure 3: Treatment response stratified by mutation.
Response to Neo-IM in 150 patients is shown by (a) mRECIST, (b) tumor viability, and (c) mitotic rate after resection. ‘x’ indicates no evaluable data for that factor. For mRECIST, viability, and mitotic rate there were 12, 17, and 26 unknown, respectively. Mutation is depicted by color according to the key in the inset. Note that graphs a-c are sorted from least treatment response (left) to most response (right). Unknowns are shown at the far right of each graph.


