Abstract
Primary pulmonary melanoma (PPM) is an entity recognized by the thoracic WHO classification. However, given the absence of native melanocytes in the lung and the known phenomenon of regression of cutaneous melanomas, the existence of PPM has remained controversial. Herein we investigate clinicopathologic and genomic features of lung-only melanomas with the goal to clarify their site of origin. We identified 10 melanomas involving exclusively lung with no current or previous cutaneous, uveal, or mucosal primaries. Four patients had solitary lesions with mean size of 5.1 cm (range 3.0–10.1 cm), meeting the criteria of PPM. Four patients had 2–3 lesions and 2 patients had >10 lesions. All cases underwent targeted next-generation sequencing interrogating up to 468 cancer genes, which revealed mean tumor mutation burden of 42.6 per megabase (range 1.8 to 126) and frequent mutations involving BRAF, NRAS, NF1, KIT, and KRAS – a genomic profile typical of UV-associated cutaneous melanoma. Mutational signature was assessable for eight cases harboring >20 mutations. This revealed that all evaluable cases harbored a dominant UV signature. In addition, one nonevaluable case harbored a GG > AA TERT promoter variant that is highly specific for UV-mutagenesis. As control groups, using the same methodology, a dominant UV signature was identified in 97% (470/486) of cutaneous melanomas, whereas no lung adenocarcinoma (n = 291) exhibited this signature. Notably, the clinical and pathologic features of solitary melanomas, especially those with large size and epithelioid morphology, closely mimicked primary lung carcinomas, highlighting a major potential for misdiagnosis. In conclusion, presence of a UV signature provides direct evidence that nearly all lung-only melanomas in this series, including solitary lesions meeting the strict criteria of PPM, represent metastases from occult cutaneous melanomas. This suggests that lung-only melanomas should be considered as likely metastatic even in the absence of a known primary melanoma elsewhere.
Introduction
Primary pulmonary melanoma (PPM) is an entity recognized by the thoracic World Health Organization classification [1], which has been described in multiple publications dating from 1916 [2–32]. PPMs are defined as malignant melanomas, as confirmed by immunohistochemistry (IHC) or electron microscopy, which exclusively involve the lung parenchyma with no evidence of current or previous primary cutaneous, uveal, or mucosal melanoma despite exhaustive clinical evaluation [1, 2]. Solitary lesion with a central location adjacent to the major airways is considered characteristic of PPM given the presumed origin from intrabronchial melanocytes [1, 2].
However, the existence of PPM as an entity has remained speculative and controversial. While the rare occurrence of bronchial blue nevi has been reported [33], there are no well-documented native melanocytes or benign melanocytic proliferations in either bronchial or alveolar epithelium [34–36], which contrasts with known existence of native melanocytes in the uvea and mucosal sites well established to give rise to primary melanomas, such as oral, sinonasal, anorectal, and vulvovaginal regions [37, 38]. While intrabronchial involvement by malignant melanocytes and nevi-like lesions has been suggested in cases of presumed PPM [2, 23, 39], whether this represents colonization versus true precursor lesions has not been firmly established. In addition, regression of cutaneous melanoma is a well-known phenomenon, with studies showing up to 8.7% of cases with regional metastasis to have complete spontaneous regression [40]. Last, melanomas of known cutaneous and occasionally mucosal origin may develop solitary metastatic lesions involving lung [41].
Overall, whether previously reported cases and analogous cases encountered in our practice of lung-only melanomas represent PPM or oligo-metastatic spread from regressed or undiagnosed primary melanoma elsewhere remains unclear. To clarify this question, we identified a set of such tumors and assessed their clinicopathologic and genomic features. In particular, we sought to determine whether an ultraviolet radiation (UV) mutational signature—a characteristic feature of the vast majority of melanomas originating from sun-exposed skin—was present in these cases. Mutational signature analysis is a recently-developed robust method that involves global annotation of the nature of synonymous and nonsynonymous mutations across the genome. UV-exposure mutagenesis is characterized by the predominance of C > T (or G > A) mutations at dipyrimidines (two adjacent pyrimidines) and an excess of CC > TT (or GG > AA) double substitutions [42–44]. This signature is strongly associated with tumors arising from UV-exposed skin [45, 46]. Signature analysis can be applied to clinical next-generation sequencing (NGS), and can serve as a helpful diagnostic tool to determine the etiology and site of origin of tumors [42, 47].
Herein, we describe clinicopathologic features and NGS profiles, including mutational signature analysis, for a set of lung-only melanomas encountered in our clinical practice. In this study, we included both the patients with solitary lesions that meet the strict definition of PPM, as well as the patients with oligo-nodular or multi-nodular lung-only melanomas. Although the latter scenarios are generally presumed to represent a metastatic process, the site of origin for such tumors has not been explored by molecular methods, and at least in theory, these could be speculated to represent intrapulmonary spread of PPM.
Materials and method
Case selection
This study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board. The surgical pathology archives were searched for cases of melanoma involving the lung with no known primary elsewhere at the time of diagnosis or during follow-up. Only cases that have undergone NGS (see below) were included. Clinical information was extracted from the electronic medical records. The following parameters were recorded: sex, age, smoking history, number and location of lung lesions on imaging, and size of the lung lesions. Special attention was paid to whether the patient had a history of cutaneous, ocular, or mucous membrane melanoma or reported nevi at presentation or during the available follow-up period. Cases with known invasive or in-situ cutaneous, ocular, or mucous membrane melanoma were excluded. In total, ten cases meeting the study criteria were identified.
Histology review
All hematoxylin-and-eosin (H&E) slides and previously performed IHC stains were retrieved from archives and centrally reviewed by two pathologists (CY and NR) to confirm the diagnosis, evaluate tumor morphology, assess presence of bronchial epithelial involvement or subepithelial nevus-like lesions, and review the expression of melanocytic markers (S100, SOX10, Melan-A, and HMB-45).
Next-generation sequencing (NGS) by Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) platform
All ten cases of lung-only melanomas included in this study were analyzed using MSK-IMPACT—an Illumina-based Hybrid Capture NGS platform, which interrogates somatic mutations in 341–468 cancer genes, as previously described [42]. The list of genes covered is provided in Supplementary Table 1. The profiles of sequenced tumors were reviewed to identify validated oncogenic drives relevant to melanoma, as defined by OncoKB [48].
Mutational signature analysis
Using MSK-IMPACT results, all synonymous and non-synonymous mutations were used to identify mutational signatures according to the distribution of the six substitution classes (C > A, C > G, C > T, T > A, T > C, T > G) and their trinucleotide context. For each sample, a weight corresponding to the fraction of mutations attributable to each of 30 mutational signatures was calculated using the method by Alexandrov et al. [46]. Computational pipeline used to compute these signatures is publicly available at (https://github.com/mskcc/mutation-signatures). Dominant mutational signature was defined as mutational signature accounting for greater than 40% of all identified mutations in a given sample, according to previously described method [42]. Only cases with a minimum of 20 total mutations were included in mutational signature analysis.
A control group of 605 cases of primary lung adenocarcinoma and 549 cases of cutaneous malignant melanoma with available MSK-IMPACT results were analyzed for mutational signatures using the same method.
Statistical analysis
Clinicopathologic parameters were compared using Fisher’s exact and Mann–Whitney U tests. Statistical analysis was conducted using R 3.3.2 (https://www.R-project.org/). Kaplan–Meier curves were plotted using GraphPad Prism version 8.42 (GraphPad Software, La Jolla, California, USA, www.graphpad.com).
Results
Clinical and radiologic characteristics
Clinicoradiologic characteristics of ten lung-only melanomas are summarized in Table 1. All patients were male, and their age ranged from 47 to 75 years (mean = 63.8 years). Radiologically, four patients had solitary lung lesions (Fig. 1a, b), four patients had 2–3 lesions, and 2 patients had >10 lesions (Fig. 1c). For solitary lesions, the mean tumor size was 5.1 cm (range = 3.0–10.1 cm). Of those, three tumors had a central, peribronchial location.
Table 1.
Clinical and radiologic characteristics of lung-only melanomas.
| Case | Sex | Age | Smoking history | Location | No. of lung lesions | Size (cm)a |
|---|---|---|---|---|---|---|
| 1 | Male | 73 | N/A | Central | 1 | 3.0 |
| 2 | Male | 68 | No | Central | 1 | 10.1 |
| 3 | Male | 52 | No | Central | 1 | 3.9* |
| 4 | Male | 61 | Yes | Peripheral | 1 | 3.2 |
| 5 | Male | 75 | No | Peripheral | 1 (lung), 2 (pleura) | 3.5* |
| 6 | Male | 70 | Yes | Central | 2 | 2.6 |
| 7 | Male | 47 | No | Central | 2 | 2.9 |
| 8 | Male | 71 | No | Peripheral | 3 | 7 |
| 9 | Male | 66 | No | Peripheral | >10 | 7.3* |
| 10 | Male | 55 | No | Central + Peripheral | >10 | 5.9* |
N/A information not available.
Size based on radiology if indicated by an asterisk; otherwise, the size is based on gross measurement of the resected tumor.
For cases with multiple lung lesions, the size is given for the largest mass.
Fig. 1. Radiologic and macroscopic features of lung-only melanomas.

Computed tomography (CT) images of three cases: (a) a 10.1 cm centrally located solitary lung mass; (b) a 3.2 cm solitary lung mass; and (c) multiple bilateral lung lesions. d Serial sections of a solitary lung mass exhibiting tan-yellow cut surfaces with central necrosis and hemorrhage. e, f Two separate lesions in one patient also demonstrate tan-yellow cut surfaces with central necrosis and hemorrhage.
There was no evidence of cutaneous, mucosal, or uveal melanoma in any patient on presentation or during the available follow-up, which had a mean length of 27.1 months (range 5–43 months).
On presentation, all 4 patients with solitary tumors and some patients with two lesions were suspected clinically to have primary lung carcinomas. This impression was compounded by incidental smoking history and hilar or intrapulmonary lymph node involvement in several patients (Tables 1 and 2).
Table 2.
Histological and immunohistochemical characteristics of lung-only melanomas.
| Case | Procedure | Histology | Microscopic pigmentation | Bronchial mucosa involvement | Thoracic lymph node metastasisa | Immunohistochemistry |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pan-cytokeratin | S100 | SOX10 | Melan-A | HMB45 | ||||||
| 1 | Lobectomy | Epithelioid > spindle | No | − | + | + (weak, focal) | + | N/A | + | N/A |
| 2 | Lobectomy | Epithelioid | No | − | + | − | + | + | + | + |
| 3 | Biopsy | Spindle | No | N/E | N/E | − | + | + | N/A | N/A |
| 4 | Wedge resection | Epithelioid | Yes | + | + | − | N/A | + | + | N/A |
| 5 | Biopsy | Spindle | No | N/E | N/E | − | + | + | + | + |
| 6 | Lobectomy | Epithelioid | Yes | + | − | N/A | + | N/A | N/A | + |
| 7 | Wedge resectionb | Epithelioid | Yes | + | − | N/A | + | + | N/A | + |
| 8 | Pneumonectomy | Epithelioid | No | − | − | N/A | + | N/A | N/A | + |
| 9 | Biopsy | Epithelioid > spindle | No | N/E | N/E | N/A | + | + | − | − |
| 10 | Biopsy | Epithelioid | Yes | N/E | N/E | N/A | N/A | + | + | N/A |
N/E not evaluable (no bronchial tissue or lymph nodes represented in biopsy specimens), N/A not available (IHC was not performed).
Pathologically confirmed.
Molecular testing performed on a pre-operative needle core biopsy.
Pathologic characteristics
Macroscopic examination revealed tumors with tan-yellow cut surfaces, central necrosis, and hemorrhage in some cases (Fig. 1d–f). Gross examination confirmed central location adjacent to bronchial structures in three cases.
Microscopically (Table 2), eight cases had entirely or predominant epithelioid cytomorphology (Fig. 2a, b) and two cases had only spindle cell morphology (Fig. 2c). Presence of melanin pigment was apparent microscopically in only 4 cases. The diagnosis of melanoma was confirmed by expression of 2–4 melanocytic markers by IHC (S100, SOX10, Melan-A, or HMB-45) in all cases. All tested cases labeled for SOX10 and S100. Notably, of five cases tested for pan-cytokeratins, four were negative, whereas one case had weak and focal but convincing labeling. Detailed IHC results are summarized in Table 2.
Fig. 2. Microscopic features of lung-only melanomas.
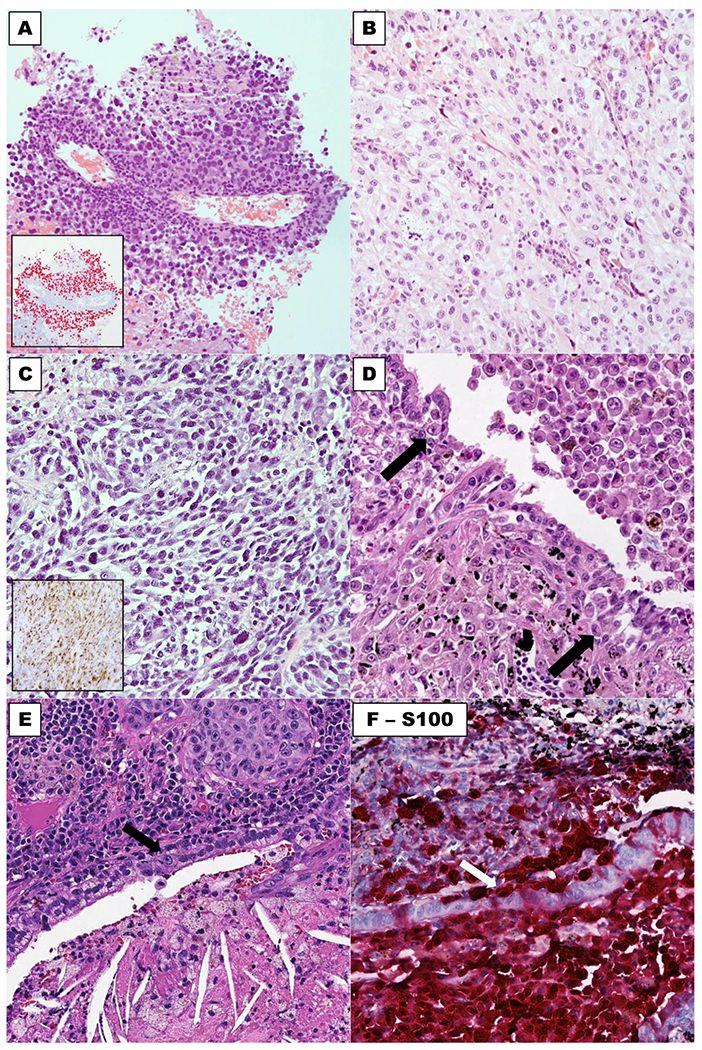
Majority of cases show predominant epithelioid cytomorphology, which may mimic non-small cell carcinoma (a, b), and some cases have spindle cell morphology (c). Expression of SOX10 and HMB45 is illustrated in insets in (a) and (c), respectively. An example (d) showing pigmented melanoma cells involving the bronchial epithelium in small nests (arrow: melanoma cells in bronchial epithelium). e and f illustrate H&E stain and S100, respectively, of involvement of bronchial epithelium adjacent to a lung-only melanoma. Arrows: melanoma cells in bronchial epithelium.
Bronchial epithelium adjacent to melanoma was evaluable for six resected cases, whereas no bronchial tissue was represented in four needle core biopsy cases. Bronchial epithelium was involved by melanoma in 3 of 6 evaluable cases (Fig. 2d–f), although their morphologic features were not diagnostic of melanoma in-situ. Subepithelial nevus-like lesions were not identified in any cases.
Next-generation sequencing: genomic alterations
Next-generation sequencing of ten lung-only melanomas revealed a mean of 43.6 nonsynonymous somatic mutations per case (range = 2–113). Tumors had mean tumor mutation burden (TMB) of 42.6 total mutations per megabase (range = 1.8–126). The following alterations in Mitogen-activated protein kinase (MAPK) drivers were identified: BRAF (n = 4), NRAS (n = 1), NF1 (n = 7), KIT (n = 1), and KRAS (n = 1) — a mutation profile that is characteristic of cutaneous melanoma. In addition, other oncogenic alterations commonly encountered in cutaneous melanoma included TP53 (n = 5) and CDKN2A (n = 1). No GNAQ or GNA11 mutations typical of uveal melanoma or primary pulmonary melanoma that may have arisen in association with a bronchial blue nevus were identified in any cases. Profiles of key mutations are summarized in Fig. 3, and detailed list of mutations is provided in Supplementary Table 2.
Fig. 3. OncoPrint of mutations in lung-only melanomas.
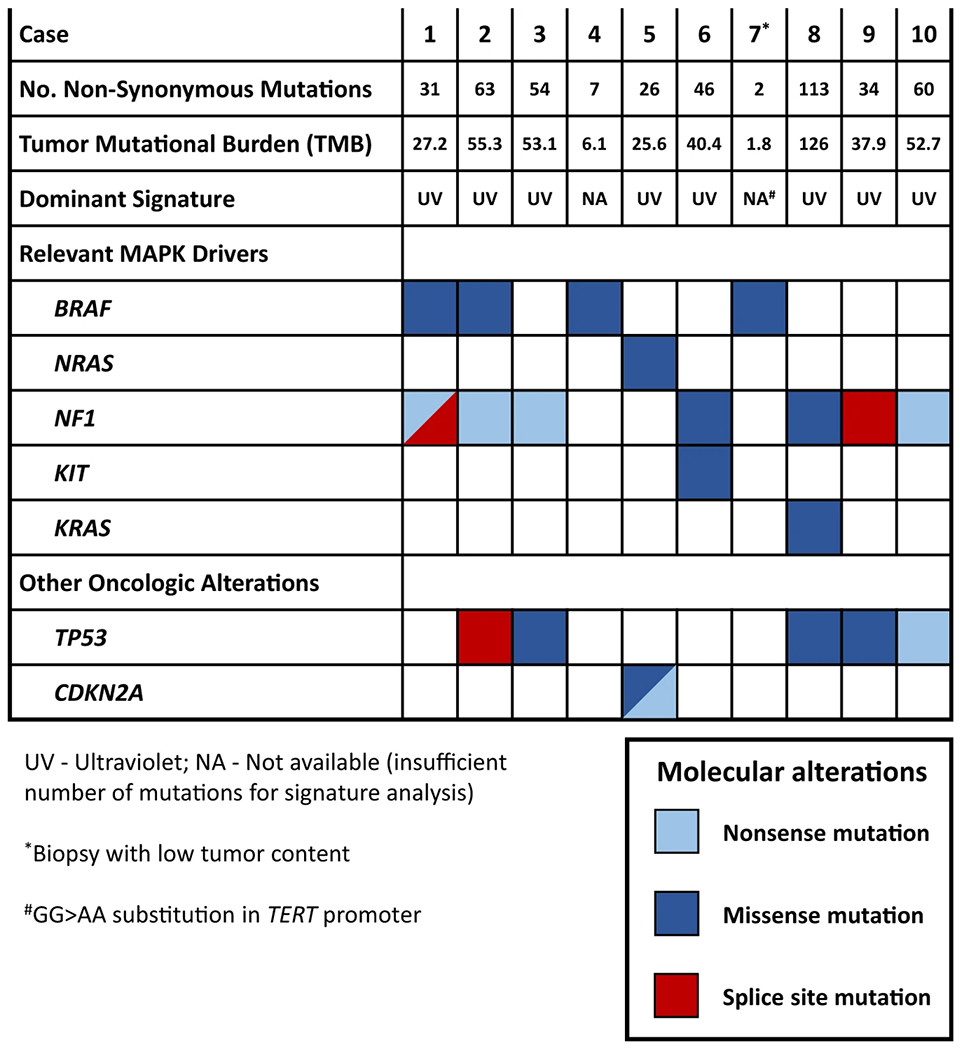
Shown are mutations relevant to melanoma. MAPK: Mitogen-activated protein kinase.
Mutational signature analysis
Mutational signature analysis was performed for eight cases harboring >20 total mutations, whereas two cases had insufficient number of mutations and were thus excluded. This analysis revealed that all 8 evaluable lung-only melanomas harbored a dominant UV signature (Fig. 4a, b). Notably, dominant UV signature was present in all 3 evaluable solitary tumors. As control groups, using the same methodology, we analyzed mutational signatures for 549 cutaneous melanomas and 605 lung primary lung adenocarcinomas. After excluding samples with less than 20 mutations, a total of 486 cutaneous melanomas and 291 lung adenocarcinomas were analyzed for dominant mutational signature. This revealed that cutaneous melanomas had a dominant UV signature in 97% (470/486) of cases, whereas none of lung adenocarcinomas (0/291) had a dominant UV signature (Fig. 4b; Supplementary Fig. 1).
Fig. 4. UV signature in lung-only melanoma with cutaneous melanoma and lung adenocarcinoma as control groups.
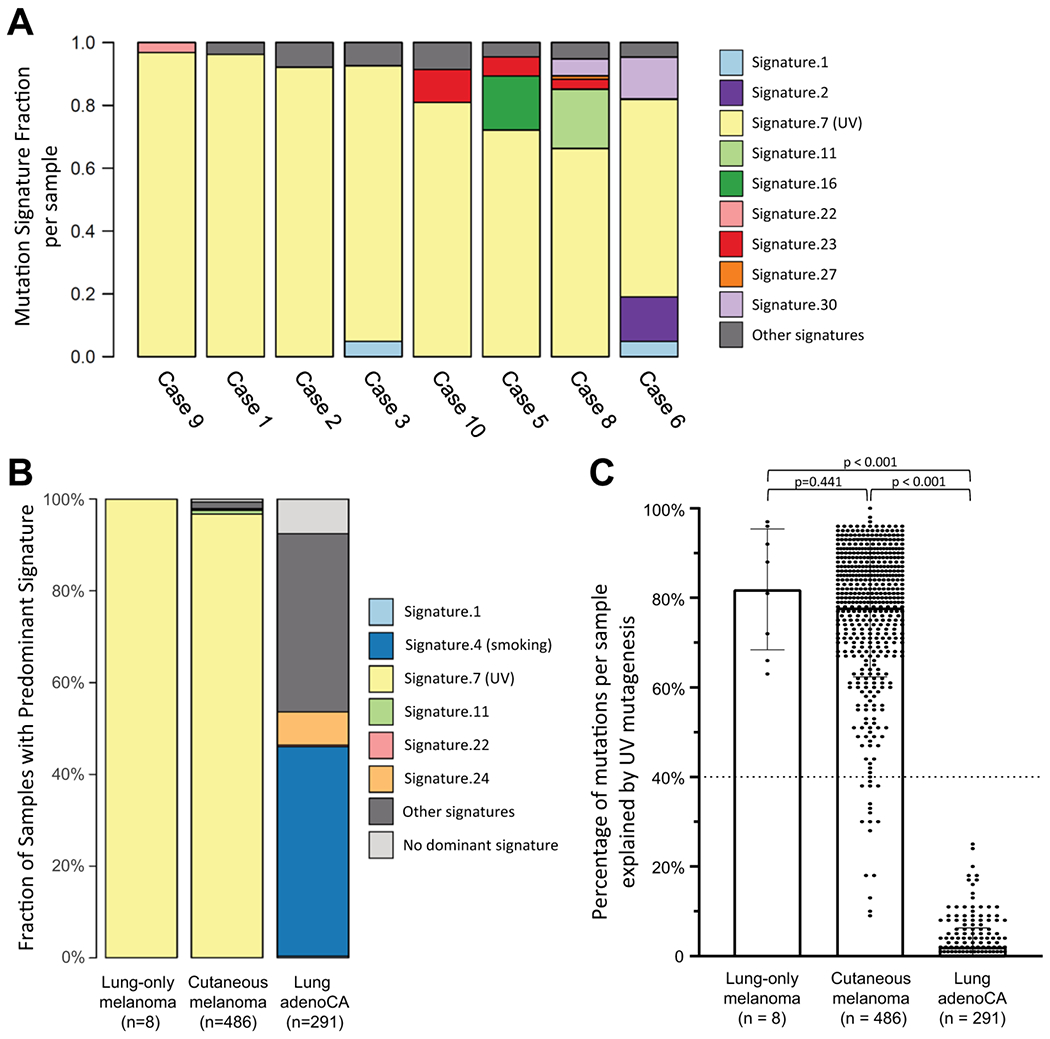
Two cases (case ID 4 and 7) had <20 total mutations and were excluded from this analysis (see Fig. 3). a Contribution of various signatures to each evaluable case of lung-only melanoma. Each bar represents a single case, and Y-axis represents fraction contributed by each mutational signature in individual case. b Fraction of evaluable cases characterized by various dominant signatures for lung-only melanoma vs. cutaneous melanoma vs. lung adenocarcinoma. c Dot plots showing percentage of UV-related mutations in each sample as a continuous variable. Dotted line at 40% indicate a threshold for “dominant signature”.
We also analyzed fraction of mutations in each sample explained by UV mutagenesis as a continuous variable (Fig. 4c). This showed that mutations attributed to UV-mutagenesis accounted for the majority of all mutations in all lung-only melanoma samples, which was similar to the distribution of UV-related mutations in cutaneous melanomas but was sharply distinct from lung adenocarcinomas (p < 0.00001).
Of the two cases excluded from signature analysis due to low number of mutations (case ID 4 and 7), one of the cases (case ID 7) was a core biopsy with low tumor content which likely contributed to low number of detected mutations. Nevertheless, this case harbored a BRAF exon 15 p.V600E mutation, commonly found in cutaneous melanomas, as well as a GG > AA substitution in TERT promoter (g.129588_1295299delinsTT). GG > AA dinucleotide substitution is highly suggestive of UV-exposure mutagenesis, and review of more than 45,000 solid tumors from the MSK-IMPACT clinical sequencing cohort [42] revealed that this specific variant in TERT promoter is found almost exclusively in cutaneous melanomas and cutaneous squamous cell carcinomas. Case 4 also harbored a BRAF p.V600E mutation, but no additional genomic findings were identified to establish the site of origin.
Clinical follow-up to assess progression
Clinical follow-up to assess progression after presentation of lung-only melanoma is summarized in Table 3. All but one patient received anti-PD-1 and/or anti-CTLA-4 inhibitor therapy, and most patients with 1–3 lesions had tumors surgically resected. For the entire set, extra-pulmonary metastatic lesions developed in 7 or 10 patients (involving brain, bone, pancreas, or liver), with 3 patients dying of disease during the available follow-up. Median overall survival for the full set of patients was 40 months (Supplementary Fig. 2). For the subgroup with solitary lesions, metastatic progression at extra-pulmonary site developed in 1 of 4 patients during available follow-up.
Table 3.
Clinical outcomes of lung-only melanomas.
| Case | Complete (R0) surgical resection | Systemic therapy | Recurrent or residual disease (time to recurrence, months) | Outcome | Follow-up (months) |
|---|---|---|---|---|---|
| 1 | Yes | Nivolumab | – | NED | 20 |
| 2 | Yes | Nivolumab | – | NED | 28 |
| 3 | Noa | Pembrolizumab | – | NED | 42 |
| 4 | Yes | Pembrolizumab Ipilimumab + Nivolumab | Bone metastasis (12) | AWD | 27 |
| 5 | No | Pembrolizumab Ipilimumab Binimetinib + Ribociclib | Brain metastasis (8), Liver metastasis (4) | DOD | 16 |
| 6 | Yes | Nivolumab | – | NED | 5 |
| 7 | Yes | Pembrolizumab Ipilimumab + Nivolumab | Pancreas (42) | AWD | 43 |
| 8 | Yes | Ipilimumab Nivolumab | Brain metastasis (32) | DOD | 40 |
| 9 | No | Ipilimumab Pembrolizumab | Lung (residual), Brain metastasis (5) | DOD | 29 |
| 10 | No | No systemic therapy | Lung (residual), Soft tissue (20) | AWD | 21 |
NED no evidence of disease, AWD alive with disease, DOD dead of disease.
Patient was treated with immunotherapy leading to complete radiologic response.
Discussion
In this study, we identified ten cases of lung-only melanomas—including four cases with solitary lung lesions—with no evidence of cutaneous, uveal, or mucosal primary lesions despite exhaustive clinical evaluation. Here we provide detailed clinicopathologic and genomic description of these tumors and utilize the NGS-based mutational signature analysis as a robust tool to clarify their site of origin.
The key observation in our study is that all evaluable cases of lung-only melanomas (n = 8) exhibited a dominant UV signature. In prior studies [49] and our large control groups, using identical methodology, a dominant UV signature was present in 97% of evaluable cutaneous melanomas, whereas none of 291 evaluable pulmonary adenocarcinomas exhibited a dominant UV signature, confirming the specificity of this method. Furthermore, most of these tumors had extremely high TMB and exhibited the profile of mutations—BRAF, NRAS, NF1, KIT, KRAS — characteristic of UV-associated cutaneous melanomas [50].
Although mutational signature could not be assessed for two cases with low total number of mutations, cutaneous origin for one of those case was strongly suggested by the presence of a dinucleotide GG > AA substitution in TERT promoter—an alteration that is highly specific for tumors of cutaneous origin. The low number of detected mutations in that case was likely a result of low tumor content in the core biopsy. The site of origin of the second case with low TMB (solitary peripheral melanoma) could not be established with certainty by our genomic analysis. However, genomic findings are not incompatible with cutaneous origin given that a minor subset (11%) of cutaneous melanomas in our control group analyzed by identical method similarly harbored <20 detectable mutations. Furthermore, this case harbored a BRAF p.V600E mutation, which is most commonly present in cutaneous melanomas, although it may occasionally occur in mucosal melanomas as well. Thus, although the possibility of metastasis from mucosal melanoma could be considered, detailed clinical evaluation did not identify evidence of mucosal primaries (such as oral or urogenital), and—unlike UV-associated cutaneous mela-nomas—spontaneous regression is uncharacteristic for non-UV associated melanomas. Another consideration would be the possibility of a true PPM, as could arise from a bronchial blue nevus. However, this case lacked GNAQ or GNA11 mutations characteristic of melanomas arising from such lesions.
Overall, these data provide direct evidence that nearly all cases of lung-only melanomas in our series, represent metastases of cutaneous melanoma in the setting of regressed primary tumors. Most remarkably, this conclusion extends to solitary, some extremely large (>10 cm), tumors. We note, however, that although none of the patients had known history of any melanocytic lesions, the possibility of a remote removal of such lesions cannot be entirely excluded. Solitary metastatic lesions or oligometastatic spread of melanoma is a well-documented phenomenon, which is particularly common in the lung. Overall, lung metastases are extremely common in patients with stage IV melanoma. In a study from our institution, of 1319 patients with stage IV melanoma, 89% had pulmonary metastases at some time point during the course of disease [51]. In addition, at the time of initial presentation, up to 36–42% of patients with stage IV melanoma have lung-only metastases [52, 53], and of those, 24–32% have solitary lung lesions [51, 54]. Thus, lung-only metastases, including solitary or oligo-metastatic lung lesions, are an exceedingly common initial presentation for patients with stage IV melanoma of known cutaneous origin. In combination with the relatively common phenomenon of regression of primary cutaneous melanomas, this can present an extremely perplexing clinical and pathological picture leading to an impression of PPM.
Our findings bring into question the concept of PPM given that several cases in our series met the strict criteria for this entity (solitary, central tumors), yet they harbored a UV signature. Since the original description in 1916, over 70 cases of PPM have been reported in the English literature [2–26]. Even though there is no reported evidence of native melanocytes in pulmonary airways or alveolar parenchyma [34], it was speculated that these tumors could arise from melanocytes that migrated to bronchial epithelium from lower respiratory anlage during embryogenesis [55–57]. In fact, several prior studies on PPM have documented intrabronchial melanocytic proliferations areas adjacent to PPM, which were interpreted as evidence of precursor lesions [2, 23, 39]. However, it has been noted that this phenomenon can be seen in metastatic melanomas as well [58]. Indeed, in our series we documented intrabronchial involvement in lung-only melanomas harboring a UV mutational signature. This further suggests that intrabronchial lesions may also be explained by pagetoid-like colonization by metastatic melanoma rather than melanoma insitu. In exploratory analysis, we evaluated bronchial and alveolar epithelium of five nonmelanoma lung resection specimens using H&E, SOX10, Melan-A, and tyrosinase stains, and did not identify any native melanocytes (data not shown).
Although the above considerations suggest that at least some previously reported cases of PPM may have also represented metastases from regressed cutaneous primaries, we cannot exclude the possibility of rare examples of true primary lung melanomas, such as melanomas that may have developed from a bronchial blue nevus. Genomic analysis that encompasses GNAQ and GNA11 mutations would thus be of interest in future studies of presumed PPM.
Although our study was focused on lung-only melanomas, we note that solitary metastasis of melanoma can less commonly occur in other visceral organs, such as liver. Thus, our findings could have implications for presumed primary melanomas in other visceral organs lacking native melanocytes.
This is the first study to report on comprehensive NGS for a set of lung-only melanomas, including four solitary lesions meeting the criteria of PPM. In prior literature, ten cases of presumed PPM were tested for mutations in 1–4 melanoma-related genes by single-gene or small panel approaches, revealing isolated mutations in NRAS and TP53 [10, 15, 28–32]. However, this is the first study to report on large-panel NGS covering up to 468 cancer genes and mutational signature analysis in these tumors.
We note that despite the large panel of genes covered by our platform, the number of detected mutations to infer signature is more limited compared with more comprehensive methods like whole-exome sequencing. Nevertheless, this approach yielded information on a dominant signature for the majority (8/10) of lung-only melanoma cases, with one case being limited by tumor content in the biopsy. Conversely, the high specificity of UV signature detection based on our panel is supported by highly distinct distribution of this signature in large control groups of skin melanoma versus lung carcinoma. In addition, using a set of control cases with whole-exome sequencing and MSK-IMPACT performed on the same sample, UV signature was highly-concordant in both methods (P < 0.001; Supplementary Fig. 3).
The clinical outcomes for patients with lung-only melanomas in our series were overall comparable to what is expected for patients with stage IV cutaneous melanoma treated with current approaches [59]. Despite small numbers, patients that presented with solitary lung lesions appeared to have lower rate of progression. This is in line with previous studies showing the number of metastatic lesions correlated with outcome in patients with stage IV melanoma both prior to and during the modern era of checkpoint inhibition [60, 61].
Importantly, our findings highlight a major potential for mis-diagnosis for lung-only melanoma as non-small cell lung carcinoma (NSCLC). Eight of our cases had epithelioid morphology, that can closely mimic NSCLC. Clinically, on presentation all 4 cases with solitary lung lesions were suspected to represent primary lung carcinomas. This was based on (1) the lack of evidence of primary tumors, (2) massive size of some tumors, with a mean size of 5.1 cm, with one solitary lesion measuring over 10 cm, (3) incidental smoking history in some patients, and (4) hilar and intrapulmonary lymph node involvement in several cases. In this clinical setting, a biopsy or even resection of a lung-only melanoma with epithelioid morphology and lack of apparent melanin pigment—as observed in the majority of cases—can present as a major pitfall for misdiagnosis as NSCLC. This issue is compounded by the possibility of aberrant keratin expression in melanomas, which is a phenomenon that is well documented in the literature [62–64]. In our series, one case of solitary lung-only melanoma with predominant epithelioid morphology exhibited labeling for pan-cytokeratin AE1/AE3, leading to initial diagnostic consideration of NSCLC. It is well recognized that a subset of NSCLC lacks glandular and squamous differentiation by morphology and IHC [65]. Thus, the possibility of large solitary metastasis from occult melanoma must be critically kept in this differential diagnosis and IHC work-up should include not only cytokeratins but also melanocytic markers.
Similar to our findings, application of mutational signature analysis is becoming recognized as instrumental for helping establish the site of origin of tumors of unknown or unexpected site of origin. For example, Sholl et al. [47] reported a case of cutaneous squamous cell carcinoma metastatic to the lung, initially diagnosed as a lung primary, but whose diagnosis was later clarified as metastasis from prior skin primary by presence of a strong UV signature. Interpretation of mutational signatures thus provides a new powerful diagnostic tool to clarify tumor origin.
In conclusion, the presence of UV signature provides strong support that most and possibly all lung-only melanomas represent metastases from regressed or undiagnosed cutaneous primaries and argues against the concept of PPM. We suggest that for practical purposes, all melanomas involving lung may be considered as likely metastatic even in the absence of a known primary melanoma elsewhere. Clinical presentation as solitary large (reaching >10 cm) masses frequently with epithelioid morphology can closely mimic primary lung carcinomas both clinicoradiologically and pathologically, and can represent a major diagnostic pitfall in practice.
Supplementary Material
Acknowledgements
This work was made possible in part by the infrastructural support from Marie-José and Henry R. Kravis Center for Molecular Oncology and the National Cancer Institute Cancer Center Core Grant P30-CA008748. NR and ML receive support from NIH P01 CA129243.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Part of this work was presented at the United States and Canadian Academy of Pathology meeting, Feb 29-March 05, 2020, Los Angeles, CA, USA.
Supplementary information The online version of this article (https://doi.org/10.1038/s41379-020-0594-0) contains supplementary material, which is available to authorized users.
References
- 1.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart, 4th ed. Lyon: International Agency for Research on Cancer, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Wilson RW, Moran CA. Primary melanoma of the lung: a clinicopathologic and immunohistochemical study of eight cases. Am J Surg Pathol. 1997;21:1196–202. [DOI] [PubMed] [Google Scholar]
- 3.Ost D, Joseph C, Sogoloff H, Menezes G. Primary pulmonary melanoma: case report and literature review. Mayo Clin Proc. 1999;74:62–6. [DOI] [PubMed] [Google Scholar]
- 4.Dountsis A, Zisis C, Karagianni E, Dahabreh J. Primary malignant melanoma of the lung: a case report. World J Surg Oncol. 2003;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kundranda MN, Clark CT, Chaudhry AA, Chan V, Daw HA. Primary malignant melanoma of the lung: a case report and review of the literature. Clin Lung Cancer. 2006;7:279–81. [DOI] [PubMed] [Google Scholar]
- 6.Maeda R, Isowa N, Onuma H, Miura H, Tokuyasu H, Kawasaki Y. Primary malignant melanoma of the lung with rapid progression. Gen Thorac Cardiovasc Surg. 2009;57:671–4. [DOI] [PubMed] [Google Scholar]
- 7.Pan X-D, Zhang B, Guo L-C, Gu D-M, Mao Y-Q, Li J, et al. Primary malignant melanoma of the lung in the elderly: case report and literature review. Chin Med J (Engl). 2010;123:1815–7. [PubMed] [Google Scholar]
- 8.Mahowald MK, Aswad BI, Okereke IC, Ng T. Long-term survival after pneumonectomy for primary pulmonary malignant melanoma. Ann Thorac Surg. 2015;99:1428–30. [DOI] [PubMed] [Google Scholar]
- 9.Postrzech-Adamczyk K, Chabowski M, Gluszczyk-Ferenc B, Wodzińska A, Muszczyńska-Bernhard B, Szuba A, et al. Malignant melanoma of the lung: case series. Kardiochirurgia Torakochirurgia Pol. 2015;12:72–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirai I, Tanese K, Obata S, Funakoshi T. A case of primary malignant melanoma of the lung responded to anti-PD-1 antibody therapy. Indian J Thorac Cardiovasc Surg. 2017;33:173–5. [Google Scholar]
- 11.Peng J, Han F, Yang T, Sun J, Guan W, Guo X. Primary malignant melanoma of the lung: A case report and literature review. Med (Baltim). 2017;96:e8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yunce M, Selinger S, Krimsky W, Harley DP. Primary malignant melanoma of the lung: a case report of a rare tumor and review of the literature. J Community Hosp Intern Med Perspect. 2018;8: 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azuma Y, Ono H, Kawabe K, Yanagimoto R, Suruda T, Minakata Y. Primary pulmonary melanoma diagnosed by semi-rigid thoracoscopy: Pulmonary melanoma diagnosis. Thorac Cancer. 2018; 9:1528–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figueroa Rodriguez F, Uddin A, Nasr J. Primary pulmonary malignant melanoma found while evaluating new onset cough: a case presentation and literature review. Case Rep Pulmonol. 2019;2019:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyriakopoulos C, Zarkavelis G, Andrianopoulou A, Papoudou-Bai A, Stefanou D, Boussios S, et al. Primary pulmonary malignant melanoma: report of an important entity and literature review. Case Rep. Oncol Med 2017;2017:8654326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, Zhao J, Yang Q, Xiong W, Zhen G, Xu Y, et al. Pulmonary melanoma and “crazy paving” patterns in chest images: a case report and literature review. BMC Cancer. 2016;16:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SR, Yoon H-Y, Jin GY, Choe YH, Park SY, Lee YC. Pulmonary malignant melanoma with distant metastasis assessed by positron emission tomography-computed tomography. Thorac Cancer. 2016;7:503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filippini A, Zorzi F, Bna’ C, Arnaboldi A, Sabatini T. Dark sputum: an atypical presentation of primary pulmonary malignant melanoma. Respir Med Case Rep. 2015;15:118–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang K-B, Hwang K-E, Jung J-W, Oh S-J, Park M-J, Jeong Y-H, et al. Primary pulmonary malignant melanoma: an unexpected tumor. Tuberc Respir Dis. 2015;78:272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Wang Y, Du J. Primary malignant melanoma of left lower lobe of lung: a case report and review of the literature. Oncol Lett. 2015;10:528–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byanju S, Chen J, Zhu D, Liao M. Primary pulmonary malignant melanoma: a case report and literature review. Med Rep Case Stud. 2018;03. 10.4172/2572-5130.1000155. [DOI] [Google Scholar]
- 22.Ouarssani A, Atoini F, Reda R, Lhou FA, Rguibi MI. Malignant melanoma of the lung: a case report. Pan Afr Med J. 2012;11:68. [PMC free article] [PubMed] [Google Scholar]
- 23.Gong L, Liu X-Y, Zhang W-D, Zhu S-J, Yao L, Han X-J, et al. Primary pulmonary malignant melanoma: a clinicopathologic study of two cases. Diagn Pathol. 2012;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neri S, Komatsu T, Kitamura J, Otsuka K, Katakami N, Takahashi Y. Malignant melanoma of the lung: report of two cases. Ann Thorac Cardiovasc Surg. 2011;17:170–3. [DOI] [PubMed] [Google Scholar]
- 25.Kunkel OF, Torrey Edward. Report of a case of primary melanotic sarcoma of lung presenting difficulties in differentiating from tuberculosis. N Y State J Med. 1916;16:198–201. [Google Scholar]
- 26.de Wilt JHW, Farmer SEJ, Scolyer RA, McCaughan BC, Thompson JF. Isolated melanoma in the lung where there is no known primary site: metastatic disease or primary lung tumour? Melanoma Res. 2005;15:531–7. [DOI] [PubMed] [Google Scholar]
- 27.Hibiya T, Tanaka M, Matsumura M, Aoki A, Ikegami T, Okudela K, et al. An NRAS mutation in primary malignant melanoma of the lung: a case report. Diagn Pathol. 2020;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe M, Yamamoto H, Hashida S, Soh J, Sugimoto S, Toyooka S, et al. Primary pulmonary melanoma: a report of two cases. World J Surg Oncol. 2015;13:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.dos Santos CL, Fernandes LR, Meruje M, Barata F. Primary pulmonary melanoma: the unexpected tumour. BMJ Case Rep 2013;2013. 10.1136/bcr-2013-200706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto Y, Kodama K, Maniwa T, Takeda M, Tanaka Y, Ozawa K, et al. Primary malignant melanoma of the lung: a case report. Mol Clin Oncol. 2017;7:39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Y, Bing Z, Xu X, Cui Y. Primary pulmonary malignant melanoma: case report and literature review. Thorac Cancer. 2018;9:1185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yabuki H, Kuwana K, Minowa M. Resection of primary malignant lung melanoma: a case report. Asian Cardiovasc Thorac Ann. 2018;26:710–2. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara G, Boscaino A, De Rosa G. Bronchial blue naevus. A previously unreported entity. Histopathology. 1995;26:581–3. [DOI] [PubMed] [Google Scholar]
- 34.Cagle P, Mace ML, Judge DM, Teague RB, Wilson RK, Greenberg SD. Pulmonary melanoma. Prim vs metastatic Chest. 1984; 85:125–6. [DOI] [PubMed] [Google Scholar]
- 35.Carstens PH, Kuhns JG, Ghazi C. Primary malignant melanomas of the lung and adrenal. Hum Pathol. 1984;15:910–4. [DOI] [PubMed] [Google Scholar]
- 36.Tomashefski JF Jr, Cagle PT, Farver CF, Fraire AE Dail and Hammar’s Pulmonary Pathology Volume II Neoplastic Lung Disease, 3rd ed. New York, NY 10013, USA: Springer Science +Business Media, LLC, 2008. [Google Scholar]
- 37.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–78. [DOI] [PubMed] [Google Scholar]
- 38.Mihajlovic M, Vlajkovic S, Jovanovic P, Stefanovic V. Primary mucosal melanomas: a comprehensive review. Int J Clin Exp Pathol. 2012;5:739–53. [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy VS, Mykytenko J, Giltman LI, Mansour KA. Primary malignant melanoma of the lung: review of literature and report of a case. Am Surg. 2007;73:287–9. [PubMed] [Google Scholar]
- 40.Smith JL, Stehlin JS. Spontaneous regression of primary malignant melanomas with regional metastases. Cancer. 1965;18: 1399–415. [DOI] [PubMed] [Google Scholar]
- 41.Harpole DH, Johnson CM, Wolfe WG, George SL, Seigler HF. Analysis of 945 cases of pulmonary metastatic melanoma. J Thorac Cardiovasc Surg. 1992;103:743–8. [PubMed] [Google Scholar]
- 42.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017; 23:703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfeifer GP, You Y-H, Besaratinia A. Mutations induced by ultraviolet light. Mutat Res. 2005;571:19–31. [DOI] [PubMed] [Google Scholar]
- 44.Cadet J, Sage E, Douki T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res. 2005;571:3–17. [DOI] [PubMed] [Google Scholar]
- 45.Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y, et al. The repertoire of mutational signatures in human cancer. Nature. 2020;578:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sholl LM, Do K, Shivdasani P, Cerami E, Dubuc AM, Kuo FC, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight. 2016;1:e87062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017;2017. 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brash DE. UV signature mutations. Photochem Photobio. 2015; 91:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuman HB, Patel A, Hanlon C, Wolchok JD, Houghton AN, Coit DG. Stage-IV melanoma and pulmonary metastases: factors predictive of survival. Ann Surg Oncol. 2007;14:2847–53. [DOI] [PubMed] [Google Scholar]
- 52.Balch CM, Soong SJ, Murad TM, Smith JW, Maddox WA, Durant JR. A multifactorial analysis of melanoma. IV. Prognostic factors in 200 melanoma patients with distant metastases (stage III). J Clin Oncol J Am Soc Clin Oncol. 1983;1:126–34. [DOI] [PubMed] [Google Scholar]
- 53.Essner R, Lee JH, Wanek LA, Itakura H, Morton DL. Contemporary surgical treatment of advanced-stage melanoma. Arch Surg Chic Ill 1960. 2004;139:961–6. [DOI] [PubMed] [Google Scholar]
- 54.Petersen RP, Hanish SI, Haney JC, Miller CC, Burfeind WR, Tyler DS, et al. Improved survival with pulmonary metasta-sectomy: an analysis of 1720 patients with pulmonary metastatic melanoma. J Thorac Cardiovasc Surg. 2007;133:104–10. [DOI] [PubMed] [Google Scholar]
- 55.Busuttil A Dendritic pigmented cells within human laryngeal mucosa. Arch Otolaryngol Chic Ill 1960. 1976;102:43–4. [DOI] [PubMed] [Google Scholar]
- 56.Goldman JL, Lawson W, Zak FG, Roffman JD. The presence of melanocytes in the human larynx. Laryngoscope. 1972;82:824–35. [DOI] [PubMed] [Google Scholar]
- 57.De La Pava S, Nigogosyan G, Pickren JW, Cabrera A. Melanosis of the esophagus. Cancer. 1963;16:48–50. [DOI] [PubMed] [Google Scholar]
- 58.Littman CD. Metastatic melanoma mimicking primary bronchial melanoma. Histopathology. 1991;18:561–3. [DOI] [PubMed] [Google Scholar]
- 59.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl J Med 2019;381:1535–46. [DOI] [PubMed] [Google Scholar]
- 60.Neuman HB, Patel A, Ishill N, Hanlon C, Brady MS, Halpern AC, et al. A single-institution validation of the AJCC staging system for stage IV melanoma. Ann Surg Oncol. 2008;15:2034–41. [DOI] [PubMed] [Google Scholar]
- 61.Davis EJ, Perez MC, Ayoubi N, Zhao S, Ye F, Wang DY, et al. Clinical correlates of response to anti-PD-1-based therapy in patients with metastatic melanoma. J Immunother 1997. 2019; 42:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarbo RJ, Gown AM, Nagle RB, Visscher DW, Crissman JD. Anomalous cytokeratin expression in malignant melanoma: one- and two-dimensional western blot analysis and immunohistochemical survey of 100 melanomas. Mod Pathol. 1990;3:494–501. [PubMed] [Google Scholar]
- 63.Miettinen M, Franssila K. Immunohistochemical spectrum of malignant melanoma. The common presence of keratins. Lab Invest. 1989;61:623–8. [PubMed] [Google Scholar]
- 64.Chen N, Gong J, Chen X, Xu M, Huang Y, Wang L, et al. Cytokeratin expression in malignant melanoma: potential application of in-situ hybridization analysis of mRNA. Melanoma Res. 2009;19:87–93. [DOI] [PubMed] [Google Scholar]
- 65.Rekhtman N, Travis WD. Large no more: the journey of pulmonary large cell carcinoma from common to rare entity. J Thorac Oncol. 2019;14:1125–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


