Abstract
Purpose of Review:
In this review, we discuss targets of interest in Triple-negative breast cancer (TNBC), approved targeted agents and the results of the clinical trials that led to their approval. Additionally, we review ongoing clinical trials evaluating the use of novel targeted agents in the treatment of TNBC.
Recent Findings:
TNBC accounts for 15–20% of all breast cancer cases and is associated with worse clinical outcomes. Patients have a higher risk of metastatic recurrence and inferior overall survival compared to other breast cancer subtypes. Cytotoxic chemotherapy has historically been the mainstay of treatment for TNBC. In recent years, we have seen a surge in clinical trials investigating the use of targeted agents in TNBC and now have approval for targeted therapies in select patients. Inhibitors of PARP (olaparib and talazoparib), PD-L1 (atezolizumab) and an antibody drug conjugate targeting Trop-2 (sacituzumab govitecan-hziy) are now approved for the use in select groups of patients with TNBC.
Summary:
Various novel targeted agents as monotherapy, dual targeted combinations, and chemotherapy combinations are currently under investigation. The results are promising and may significantly improve patient outcomes in TNBC.
Keywords: triple-negative breast cancer, targeted therapies, immunotherapy, novel agents
Introduction
Triple-negative breast cancer (TNBC) is defined by ASCO/CAP guidelines as having negative expression (<1%) for the estrogen receptor (ER) and progesterone receptor (PR) by immunohistochemistry (IHC), and no overexpression of human epidermal growth factor receptor 2 (HER2) oncogene [1]. TNBC accounts for approximately 15–20% of all breast cancer cases and is associated with worse overall survival (OS) compared to hormone receptor-positive and HER2-positive breast cancer [2–4]. Patients with localized TNBC have a risk of metastatic recurrence of about 30–50% with the highest risk of recurrence in the first three years from date of diagnosis [4–6]. Patients with metastatic TNBC have a median survival of 13–18 months [7, 8]. FDA-approved therapies for TNBC have been limited to cytotoxic chemotherapy, an antibody drug conjugate targeting trophoblast cell-surface antigen 2 (Trop-2), PARP inhibitors in patients with deleterious BRCA 1/2 gene mutations, and atezolizumab, a PD-L1 inhibitor in patients with PD-L1-positive tumors [9–12] (Table I).
Table I.
FDA-approved Targeted Drugs in Triple-Negative Breast Cancer
| Drug | Indication | Pivotal Trial |
|---|---|---|
| Atezolizumab | In combination with nab-paclitaxel for unresectable locally advanced or metastatic TNBC whose tumors are PD-L1-positive (PD-L1 stained tumor infiltrating immune cells ≥1%) | Impassion130 (NCT02425891) |
| Olaparib | Deleterious or suspected deleterious gBRCAm, HER-2 negative metastatic breast cancer previously treated with chemotherapy in the neoadjuvant, adjuvant, or metastatic setting | OlympiAD (NCT02000622) |
| Talazoparib | Deleterious or suspected deleterious gBRCAm, HER-2 negative locally advanced or metastatic breast cancer | EMBRCA (NCT01945775) |
| Sacituzumab govitecan-hziy | Metastatic TNBC who received at least two prior lines of therapy for metastatic disease | IMMU-132-01 (NCT01631552) |
Abbreviations: gBRCAm: germline BRCA-mutated
TNBC is a molecularly heterogenous disease which can be further characterized into four subtypes including two basal-like subtypes, basal-like 1 (BL1) and basal-like 2 (BL2), a mesenchymal subtype (M) and a luminal androgen receptor (LAR) subtype [13]. Each subtype varies in histology, natural history, response to therapy and prognosis suggesting unique tumor biology [13–15]. About 60% of TNBC is basal-like, 25% M subtype, and 15% LAR subtype [13–16]. However, single-cell genomic analysis demonstrates that multiple subtypes may exist within a single tumor [13–16].
The BL1 subtype is characterized by an increase in cell cycle and DNA damage response gene expression [13]. BL1 tumors appear to be higher grade and have the highest response to chemotherapy with a pathologic complete response (pCR) rate of 41% [13]. BL2 tumors are characterized by growth factor signaling and myoepithelial markers and have the poorest response to chemotherapy with a significantly lower pCR rate of 18% [13]. TNBC of the M subtype is characterized by infiltrating lymphocytes and tumor-associated mesenchymal cells which may suggest an immunosuppressive microenvironment [13]. The LAR subtype is characterized by luminal gene expression and androgen receptor signalling and also has a poor response to chemotherapy with a pCR rate of 29% likely due to a decreased rate of cell proliferation [13].
Understanding the extent of heterogeneity in TNBC is critical for the development of new targeted therapeutics that may significantly improve outcomes in this area of unmet need. In this article, we discuss therapeutic targets of interest in TNBC, agents that have been recently FDA approved, and review ongoing clinical trials evaluating these novel therapies.
PARP Inhibitors
Poly (ADP-ribose) polymerase (PARP) is a family of enzymes responsible for repair of single-stranded DNA breaks [17]. PARP inhibitors block and trap PARP1 and 2 isoforms on DNA which interferes with DNA replication resulting in double-stranded DNA breaks, DNA damage, cell-cycle arrest, and apoptosis [17, 18]. BRCA1 and 2 tumor suppressor proteins are responsible for homologous recombination (HR) repair of double-stranded DNA breaks and can result in cell survival despite use of PARP inhibitors [18]. BRCA 1/2-mutated cancers lose the ability to repair double-stranded DNA breaks and are therefore more sensitive to PARP inhibition and other DNA damaging drugs (Figure 1) [18]. The inability of a cell to undergo proper HR repair is not limited to BRCA 1/2-mutated cancers and has also been found in non-BRCA-mutated tumors [19]. This characteristic has been described as homologous-repair deficient (HRD) [19]. It is important to note that there is lack of consensus in the definition of HRD; however, in general, HRD has been characterized by loss of heterozygosity, telomeric allelic imbalance and large-scale transitions [19].
Figure 1.
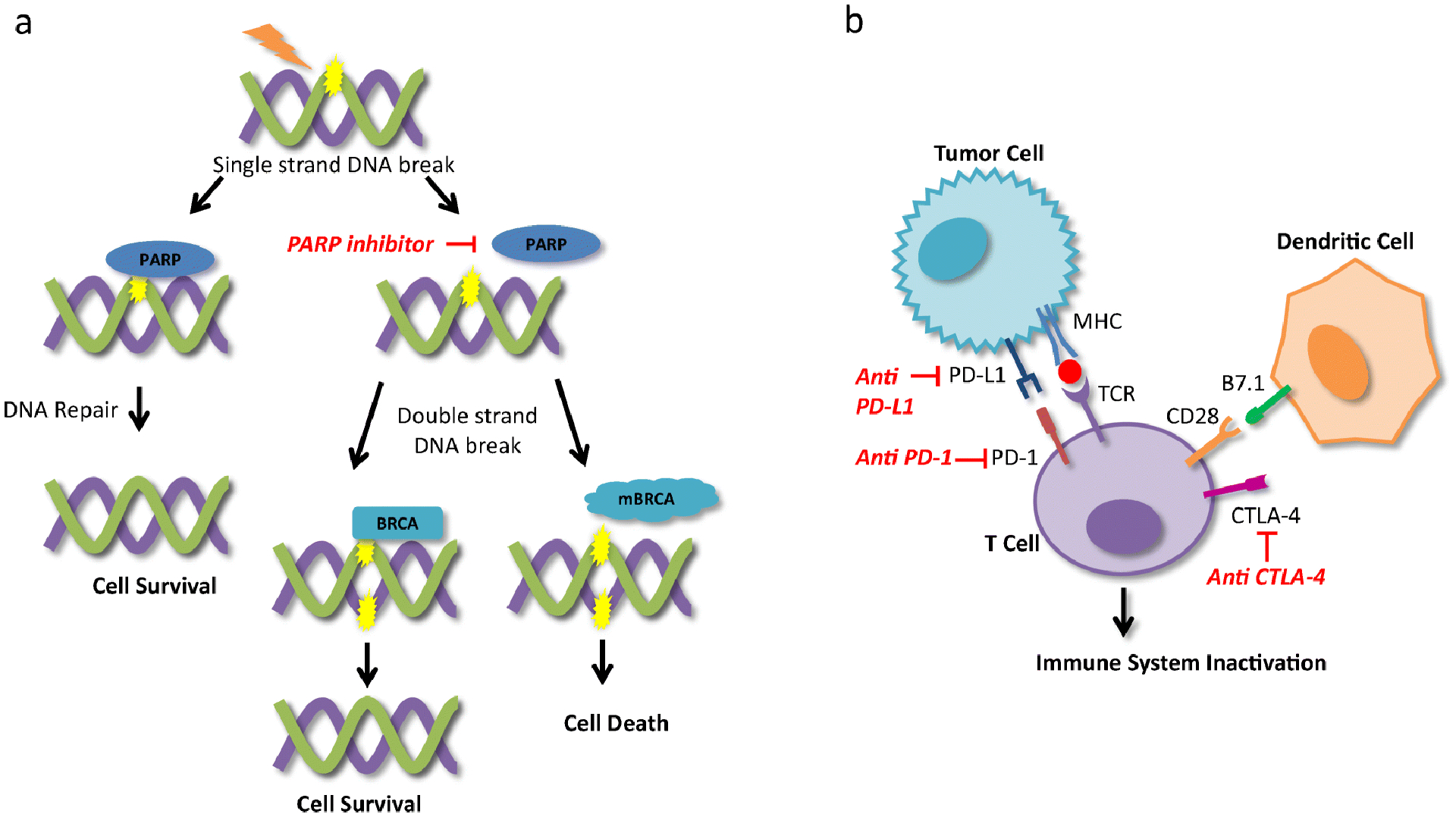
Mechanism of action of PARP inhibitors and immune checkpoint inhibitors. (A) The PARP enzymes repair single-stranded DNA breaks which allow for cell survival. PARP inhibitors prevent PARP from repairing single-stranded DNA breaks which result in double-stranded DNA breaks. BRCA proteins repair double-stranded DNA breaks via homologous recombination and allow for cell survival however mutated BRCA proteins lose the ability repair double-stranded DNA breaks resulting in cell death. (B) Interaction between PD-L1 on tumor cells and PD-1 on T cells results in immune system inactivation and tumor cell survival. CTLA-4 on T cells competes with CD28 for B7 ligands on antigen presenting cells and when bound to B7 results in immune system inactivation. PD-L1, PD-1 and CTLA-4 blockade results in immune system activation and anti-tumor response. PARP: Poly ADP-ribose polymerase, BRCA: breast cancer gene, mBRCA: mutated BRCA gene, PD-L1: programmed-death ligand 1, PD-1: programmed cell death protein 1, CTLA-4: cytotoxic T-lymphocyte-associated antigen 4; MHC: major histocompatibility complex, TCR: T cell receptor.
Germline BRCA 1/2-mutations occur in 10–20% of TNBC whereas somatic BRCA 1/2-mutations have been reported in 3–5% of TNBC [19]. At this time, olaparib and talazoparib are the only PARP inhibitors approved for use in patients with deleterious or suspected deleterious germline BRCA 1/2-mutated metastatic HER2-negative breast cancer [20–23]. In the OlympiAD trial, patients with a BRCA 1/2-mutation and HER2-negative breast cancer who received no more than two prior lines of chemotherapy for metastatic disease had a superior objective response rate (ORR) of 60% vs 29% with olaparib compared to treatment of physician’s choice (TPC) which included capecitabine, eribulin, or vinorelbine [22]. In addition, median PFS was improved with olaparib compared to TPC at 7.0 months vs 4.2 months (HR 0.58; 95% CI, 0.43–0.80; p<0.001) [22]. The EMBRACA trial also showed benefit in ORR and PFS with talazoparib over TPC (capecitabine, eribulin, gemcitabine, or vinorelbine) in patients with a germline BRCA 1/2-mutation and advanced HER2-negative breast cancer who received up to three prior lines of chemotherapy [23]. In the intention-to-treat population, ORR was 62.6% with talazoparib vs 27.2% with TPC (odds ratio 5.0; 95% CI, 2.9–8.8; p<0.001) [23]. The median PFS improved from 5.6 months with TPC to 8.6 months with talazoparib (HR 0.54; 95% CI, 0.55–1.06; p=0.11) [23]. Notably, DNA-damaging platinum agents, such as carboplatin, were not available in the TPC arm and as such we do not know how PARP inhibitors compare to this group of cytotoxic therapy.
The use of PARP inhibitors in somatic BRCA-mutated and non-BRCA-mutated HRD TNBC is under investigation. The TBB trial was a phase II study exploring the activity of talazoparib in BRCA 1/2-wild-type advanced HER2-negative breast cancer with underlying HRD due to pathogenic germline mutation in another gene in the HR pathway [24]. Patients had an ORR of 25% [24]. The RUBY trial assessed the use of rucaparib in 37 patients with germline BRCA 1/2-wild-type metastatic HER2-negative breast cancer with HRD defined as having a high loss of heterozygosity score [25]. This trial showed a clinical benefit rate (CBR) of 13.5% [25]. The TBCRC 048 phase II study showed that patients with metastatic breast cancer with germline or somatic mutations in the DNA damage response pathway genes have a ORR of 29.6 – 38.5% to olaparib monotherapy [26]. In particular, patients with germline PALB2 and somatic BRCA 1/2 mutations predicted response with an observed response as long as 16.4 months [26]. The NOBROLA study is an ongoing phase II clinical trial evaluating the use of olaparib in patients with BRCA 1/2-wild-type metastatic breast cancer that also exhibit HRD as determined by a tissue based test [27].
In a similar population, PARP inhibition was evaluated in the neoadjuvant setting and in combination with other agents. The GeparOLA phase II study evaluated the efficacy and toxicity of olaparib in combination with paclitaxel compared to paclitaxel plus carboplatin followed by epirubicin and cyclophosphamide in the neoadjuvant setting [28]. The trial included patients with HER2-negative breast cancer with either a germline or somatic BRCA 1/2-mutation or high HRD score [28]. Patients treated with olaparib in combination with paclitaxel had a similar pCR of 55.1% (90% CI, 44.5%–65.3%) compared to 48.6% with carboplatin and paclitaxel (90% CI, 34.3%–63.2%) [29]. The VIOLETTE study is an ongoing phase II trial investigating the use of olaparib in combination with DNA damage response (DDR) inhibitors [30]. The DDR agents under investigation include a WEE1 inhibitor, AZD1775, and an ataxia telangiectasia and Rad3-related protein inhibitor, AZD6738 [30]. Patients with metastatic TNBC are eligible and stratified by alterations in HRD-related genes into BRCA 1/2-mutated, non-BRCA HRD mutated, and non-HRD mutated [30]. Although further research is necessary, these studies indicate that PARP inhibitors may be effective in somatic BRCA 1/2-mutated and BRCA 1/2-wild-type HRD TNBC, in addition to germline BRCA 1/2-mutated TNBC which is its only indication at this time.
The efficacy of PARP inhibitors in combination with immunotherapy as maintenance therapy is also being investigated. The KEYLYNK-009 phase III clinical trial is currently recruiting patients with advanced TNBC on first-line gemcitabine, carboplatin and pembrolizumab, an anti-PD-1 antibody, who will be randomized to maintenance therapy with the same drugs versus olaparib plus pembrolizumab (NCT04191135). The DORA phase II trial is an ongoing study exploring the efficacy of olaparib alone or in combination with durvalumab, an anti-PD-L1 antibody, as maintenance therapy [31]. Patients with advanced TNBC who derive clinical benefit from platinum-based chemotherapy in the first or second-line setting are randomized to olaparib alone or in combination with durvalumab [31]. These studies will help us to understand the effects of combining PARP inhibitors with immune checkpoint inhibitors.
Immunotherapy Agents
Immune Checkpoint Inhibitors in Metastatic TNBC
Immunotherapy agents use the host immune system to control disease and recently, there has been extensive research into the use of different immune modalities in TNBC. Tumor-infiltrating lymphocytes (TILs) are representative of tumoral immune response and can be found within the tumor itself in surrounding stroma [32]. In TNBC, a greater number of TILs predicts improved response to chemotherapy [32].
Immune checkpoint inhibitors are antibodies that block immune checkpoint proteins, programmed cell death protein 1 (PD-1), programmed-death ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) resulting in the activation of anti-tumor T-cell activity (figure 1) [33]. TNBC is associated with higher levels of TILs and PD-L1 expression compared to other breast cancer subtypes [34]. Approximately 40% of patients with metastatic TNBC are PD-L1-positive [35].
The IMpassion130 trial led to the approval of atezolizumab in combination with nab-paclitaxel for the treatment of PD-L1-positive, metastatic TNBC [11, 36]. Atezolizumab is the only immunotherapeutic agent currently approved for the treatment of TNBC and is an immune checkpoint inhibitor that binds to PD-L1 [35–37]. In the IMpassion130 trial, patients with locally advanced or metastatic TNBC with no prior treatment in the metastatic setting were randomized to atezolizumab plus nab-paclitaxel or nab-paclitaxel plus placebo [36]. In the PD-L1-positive subgroup, ORR was 58.9% and PFS was significantly prolonged with atezolizumab plus nab-paclitaxel compared to nab-paclitaxel plus placebo (7.5 months vs 5.0 months, stratified HR for progression or death 0.62; 95% CI, 0.49–0.78; p<0.001) [36]. While the improvement in PFS was modest, there was a subset of patients who received long-term benefit. This regimen is now a standard first line therapy for patients with PD-L1-positive metastatic TNBC for whom nab-paclitaxel is an appropriate chemotherapy agent.
The KEYNOTE-355 trial evaluated the efficacy of adding pembrolizumab to chemotherapy compared to chemotherapy plus placebo in locally recurrent, inoperable, and metastatic TNBC in the first-line setting [38]. This trial included more chemotherapy options compared to Impassion130. Patients could receive nab-paclitaxel, paclitaxel, or gemcitabine with carboplatin [38]. Interim analysis showed improvement in PFS with the addition of pembrolizumab to chemotherapy compared to chemotherapy plus placebo in patients with PD-L1 positive tumors (CPS≥10) (9.7 months vs 5.6 months, HR 00.65; 95% CI, 0.49–0.86; p=0.0012) [38]. The study is ongoing to collect overall survival data however these findings suggest that there is a role for pembrolizumab to be added to chemotherapy in the first-line treatment of PD-L1-postive, metastatic TNBC. If approved, this regimen could provide more flexibility in chemotherapy partners for immunotherapy, but this trial used a different assay to determine PD-L1-positivity with the CPS score which will need to be incorporated into clinical practice.
Checkpoint inhibition as maintenance following chemotherapy in patients with metastatic TNBC was evaluated in the phase II SAFIR02-IMMUNO trial. The results were mixed with the use of maintenance durvalumab after response to induction chemotherapy [39]. In the overall population including patients with locally advanced or metastatic HER2-negative breast cancer, who received first or second line chemotherapy, there was no improvement in PFS with durvalumab. Moreover, outcomes were better in the chemotherapy alone arm (2.7 months vs 4.6 months with chemotherapy alone, HR 1.40; p = 0.047) [39]. However, in the TNBC subgroup, there was a significant improvement in overall survival (OS) with maintenance durvalumab (21 months vs 14 months with chemotherapy alone, HR 0.54; p = 0.0377) [39]. As a result, further evaluation of the potential benefit of durvalumab as maintenance therapy is warranted in the TNBC population in a larger clinical trial before this strategy can be considered.
Immune Checkpoint Inhibitors in the Neoadjuvant Setting
In the neoadjuvant setting, the GeparNuevo phase II clinical trial evaluated the addition of durvalumab to neoadjuvant chemotherapy in patients with TNBC [40]. Patients were randomized to durvalumab or placebo given every 4 weeks with nab-paclitaxel followed by epirubicin and cyclophosphamide. Initially patients were enrolled to start durvalumab or placebo two weeks prior to chemotherapy (window-phase), however, due to concern for delay in starting chemotherapy, the start date of durvalumab and placebo was changed to day 1 of chemotherapy after 117 patients were already enrolled [40]. Patients treated with durvalumab had an increase in pCR rate at 53.4% compared to 44.2% with placebo (95% CI, 33.5% – 55.3%; unadjusted continuity corrected X2 p=0.287) [40]. Interestingly, the improvement in pCR rate appeared to be limited to the subgroup of patients treated in the window-phase as the pCR rate in this group was 61.0% compared to 41.4% in patients in the non-window cohort (95%CI, 1.06–4.64, p=0.035) [40]. These findings raise question about whether there may be a benefit to receiving immunotherapy prior to chemotherapy and what changes in the tumor microenvironment may have led to this improved response to chemotherapy.
The phase III trial KEYNOTE-522 found that the addition of pembrolizumab to neoadjuvant chemotherapy in patients with stage II or III TNBC resulted in improved pCR rate. Patients received 4 cycles of paclitaxel and carboplatin every three weeks and were randomized to placebo or pembrolizumab [41]. This was followed by an additional four cycles of pembrolizumab or placebo with doxorubicin or epirubicin plus cyclophosphamide. After definitive surgery, patients received adjuvant pembrolizumab or placebo every 3 weeks for up to 9 cycles [41]. Patients treated with pembrolizumab in combination with chemotherapy had a pCR rate of 64.8% compared to 51.2% with placebo plus chemotherapy (95% CI, 5.4–21.8; p <0.001) [41]. The use of pembrolizumab in this setting is currently under FDA review and we await longer term follow-up for disease-free survival and overall survival in these patients.
In contrast to KEYNOTE-522, there was no significant difference in pCR rate with the addition of atezolizumab to neoadjuvant chemotherapy with carboplatin and nab-paclitaxel for patients with TNBC as discovered in the NeoTRIPaPDL1 trial [42]. The difference in results may be explained by the use of different immunotherapy agents and chemotherapy regimens. Atezolizumab binds to PD-L1 and pembrolizumab binds to PD-1 which may result in more complete pathway inhibition through PD-L1 and PD-L2. In addition, patients in KEYNOTE-522 were treated with an anthracycline and cyclophosphamide prior to surgery rather than in the adjuvant setting as in NeoTRIPaPDL1 [41, 42]. The I-SPY2 trial is an ongoing phase II trial for patients with high risk, stage II and III breast cancer evaluating multiple investigational arms in parallel including an arm in which patients with HER2-negative breast cancer are treated with pembrolizumab in addition to standard taxane and anthracycline based neoadjuvant chemotherapy [43]. Preliminary results estimate an increase in pCR rate with the addition of pembrolizumab to neoadjuvant chemotherapy in TNBC (60% vs 22%) [43]. Final results of this study may help clarify the benefit of adding immunotherapy to neoadjuvant chemotherapy.
Novel Immunotherapy Approaches
The efficacy of checkpoint inhibition in combination with other novel agents that may improve response is also being explored. The IMPRIME 1 phase II clinical trial evaluated the effects of an immune activator, Imprime PGG, and pembrolizumab [44]. Imprime PGG is a beta glucan agonist that binds to anti-beta glucan antibodies (ABA) and forms an immune complex which activates the innate immune system to activate antigen-presenting cells and increase tumor specific T-cell activation [44]. Patients with metastatic TNBC and an ABA level of greater than or equal to 20 mcg/mL were treated with weekly PGG and pembrolizumab every 3 weeks [44]. The ORR rate was 15.9% (95% CI, 7.9% – 29.4%) and disease control rate at 24 weeks was 25.0% (95% CI, 14.6% – 39.4%) [45]. Median PFS was 2.7 (95% CI, 1.35 – 4.04) months and OS 16.4 months (95% CI, 11.1 –19.2) [45].
KEYNOTE-890 is an ongoing trial evaluating the use of tavokinogene telseplasmid in patients with inoperable TNBC previously treated with chemotherapy [46]. Patients receive pembrolizumab and the tumor is injected with tavokinogene telseplasmid followed by electroporation in the same region [46]. Intratumoral tavokinogene telseplasmid is a plasmid encoding the proinflammatory cytokine IL-12 and electroporation is suspected to enhance tumor immunogenicity and boost response to immunotherapy [46]. Preliminary data showed an ORR of 28.6% in patients with a median of 3 prior lines of therapy which is promising compared to historical controls with pembrolizumab alone in patients with previously-treated TNBC [46, 47].
In early TNBC, adagloxad simolenin is an immune stimulant being investigated in a phase III clinical trial for patients with early-stage TNBC at high risk of recurrence defined as patients with residual invasive disease after neoadjuvant treatment or patients with 4 or more axillary lymph nodes with invasive carcinoma treated with adjuvant chemotherapy [48]. The compound is Globo H hexasaccharide epitope linked to carrier protein keyhole limpet hemocyanin (KLH) and is administered with saponin-based adjuvant OBI-821 [48]. Globo H is a tumor associated antigen which is sometimes expressed on TNBC cells and adagloxad simolenin may help to stimulate T cell response to Globol H-expressing tumor cells. Patients included in this trial must have tumors that express Globo H using a validated IHC assay [48]. This is a first-in-class immune activator that may help lower the 20–30% risk of recurrence in this population of patients who did not achieve pCR with neoadjuvant chemotherapy [5]. Together, these novel immune approaches may lead to expansion of the patient population with TNBC benefiting from immunotherapy and overcoming resistance to single agent immune check-point inhibition.
Phosphoinositide 3-kinse (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin (mTOR) pathway
The PI3K/AKT/mTOR (PAM) pathway is a major pathway involved in cell proliferation and survival, motility regulation, metabolism, and migration of breast cancer cells [49, 50]. The pathway is activated when a growth factor or ligand binds to membrane associated tyrosine kinase. AKT is then phosphorylated which further activates its downstream effector, mTOR, leading to protein synthesis and cell growth [50]. Phosphatase and tensin homolog (PTEN) and proline-rich inositol polyphosphates are proteins that both downregulate PI3K (figure 2) [51].
Figure 2.
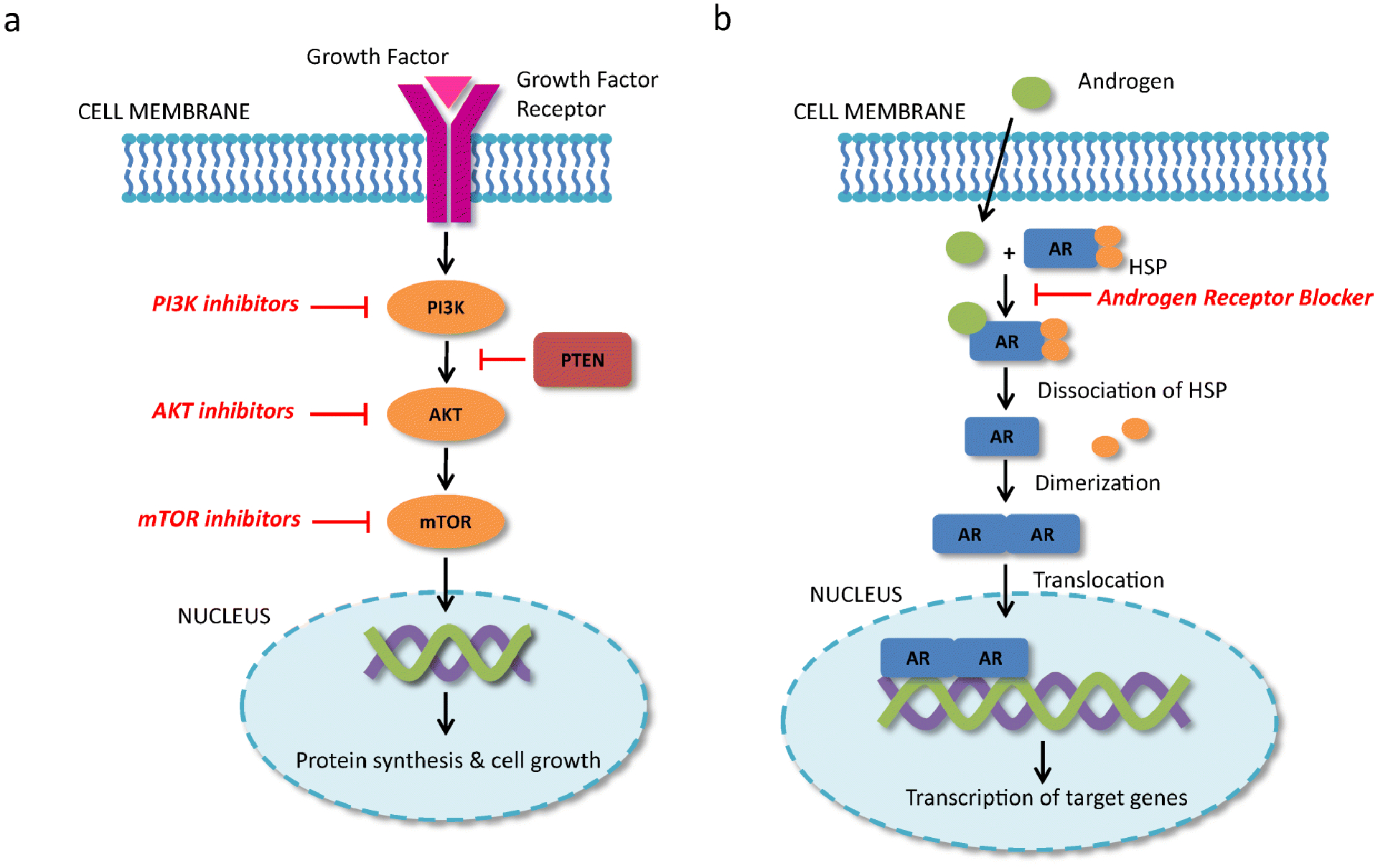
Mechanism of action of PI3K/AKT/mTOR (PAM) pathway inhibitors and androgen receptor blockers. (A) Growth factor binds to growth factor receptor in the tumor cell membrane resulting in activation of the PI3K, AKT, and mTOR. Activation of mTOR results in protein synthesis and cell growth. PTEN downregulates PI3K and results in decreased activation of the PAM pathway. PI3K, AKT, and mTOR inhibitors block the activation of this pathway and results in cell death. (B) Androgens bind to cytoplasmic androgen receptors resulting in activation. Heat shock proteins bound to the androgen receptor dissociate and the androgen receptor dimerizes. The dimer is translocated to the nucleus where it binds to the promoter region and results in gene transcription. Androgen receptor blockers block androgen binding to androgen receptor. PI3K: phosphoinositide 3-kinse, AKT: protein kinase B, mTOR: mechanistic target of rapamycin, PTEN: phosphatase and tensin homolog. AR: Androgen receptor, HSP: heat shock protein
In TNBC, activation of the PAM pathway is mainly mediated by PIK3CA mutations resulting in AKT-independent or AKT-dependent mechanisms, or PTEN mutations inactivating PTEN [50, 52]. Although PIK3CA mutations occurs less frequently in TNBC compared to other breast cancer subtypes, about 13% of patients have PIK3CA mutations [53]. PTEN deficiency appears to be more prevalent in TNBC and has been reported in up to 66% of TNBC [50, 52, 53]. The PAM pathway contains multiple targetable sites for novel agents in TNBC.
Alpelisib is a small molecule, α-specific, PI3K inhibitor approved for the use in combination with fulvestrant in hormone receptor positive, HER2 negative, PIK3CA-mutated, advanced or metastatic breast cancer, however, there are no FDA approved agents targeting the PAM pathway for TNBC [54]. In a phase I/II trial, patients with metastatic HER2-negative breast cancer were treated with alpelisib and nab-paclitaxel [55]. In patients evaluable for response, ORR was 57% with a median PFS of 9 months (95% CI, 6–12) [55]. Forty percent of the patients had a PIK3CA mutation and had a significantly better PFS at 13 months compared to 7 months in patients without a PIK3CA mutation (HR =0.39; p = 0.03) [55]. There is a phase III trial, not yet recruiting, designed to study the efficacy of alpelisib in combination with nab-paclitaxel in patients with advanced TNBC with PIK3CA mutation or PTEN loss without PIK3CA mutation (NCT04251533).
The use of AKT inhibitors in TNBC is promising. The LOTUS trial was a phase II study evaluating the use of ipatasertib, and oral AKT inhibitor, in patients with previously untreated locally advanced or metastatic TNBC [56]. Patients were randomized to receive paclitaxel plus ipatasertib or placebo [56]. In the intention-to-treat population, the median PFS was 6.2 months with ipatasertib compared to 4.9 months with placebo (95% CI, 0.37–0.98, p = 0.037) and CBR was 48% with ipatasertib compared to 37% with placebo [56]. In the subgroup of patients with PIK3CA/AKT1/PTEN-altered tumors, median PFS was increased to 9.0 months compared to 4.9 months with placebo (95% CI 0.20–0.99, p=0.041) [56]. Updated OS data showed that in the intention-to-treat population, median OS was 25.8 months with ipatasertib compared to 16.9 months with placebo (HR 0.81; 95% CI 0.53–1.23) [57]. This data supports further evaluation of ipatasertib in the IPATunity 130 trial which is an ongoing phase III study evaluating the efficacy of ipatasertib plus paclitaxel in patients with PIK3CA/AKT1/PTEN-altered TNBC [52]. In addition, the IPATunity170 trial is a phase III trial evaluating the efficacy of ipatasertib in combination with atezolizumab and paclitaxel in previously untreated locally advanced or metastatic TNBC (NCT04177108). In the neoadjuvant setting, the FAIRLANE trial did not show a statistically significant increase in pCR rate or ORR with the addition of ipatasertib to paclitaxel in early TNBC [58]. However, all patients treated with ipatasertib who had a pCR had a PIK3CA/AKT1/PTEN mutation so further study of the effects of AKT inhibition in early PIK3CA/AKT1/PTEN-altered TNBC is also of interest [58].
The PAKT trial was a phase II study which also evaluated the use of an AKT inhibitor, capivasertib, in patients with untreated TNBC [59]. Patients were randomized to paclitaxel plus cavpivasertib or placebo, and patients who received cavpivasertib were found to have a significant increase in PFS and OS [59]. Median PFS was 5.9 months with cavpivasertib vs 4.2 months with placebo (95% CI, 0.50–1.08; p = 0.06) and median OS was 19.1 months with capivasertib compared to 12.6 months with placebo (95% CI, 0.37–0.99; p = 0.04) [59]. In patients with PIK3CA/AKT1/PTEN-altered tumors, PFS increased to 9.3 months with capivasertib compared to 3.7 months with placebo (95% CI, 0.11–0.79; p = 0.01) [59]. It is now being investigated in a phase III clinical trial (NCT03997123).
In addition to combining PAM pathway inhibitors with chemotherapy or immunotherapy, ongoing studies are evaluating the effects of dual inhibition of the PAM pathway, which may overcome resistance to one drug. Gedatolisib is a dual PI3K/mTOR inhibitor that is being evaluated in phase I/II clinical trials in the metastatic setting [52]. The PAM pathway also interacts with the RAS/RAS/MEK/ERK (MAPK) pathways which also promote cell survival [60]. ONC201 is a dual AKT and ERK inhibitor under investigation in metastatic TNBC [52]. In summary, multiple inhibitors of this pathway are in phase III trials and may lead to PIK3CA or AKT becoming targetable mutations in TNBC.
Androgen Receptor
The androgen receptor (AR) is a steroid hormonal receptor that when activated may lead to cell proliferation, differentiation, apoptosis or angiogenesis depending on concurrent signalling pathways and tissue type [61, 62]. Androgens bind to the intracellular AR in the cytoplasm resulting in phosphorylation and dimerization of the AR [61, 62]. The dimer is translocated to the nucleus where it binds to the promoter region, interacts with transcription factors, and results in transcription of target genes (figure 2) [61, 62].
In breast cancer, androgens have different effects on ER-positive cells compared to ER-negative cells [61]. Preclinical data shows that in ER-negative, AR-positive breast cancer cell lines, androgens stimulate cell proliferation and AR antagonist decrease cell proliferation [61, 63]. The opposite effect was seen in patients with ER-positive, AR-positive breast cancer cell lines [61, 64]. AR expression has been reported in 70–90% of all breast cancer and in 10%–50% in TNBC, more commonly in the LAR subtype [61].
Enzalutamide, a potent AR inhibitor, was evaluated in a single-arm, phase II trial in patients who had AR-positive, locally advanced or metastatic TNBC [65]. AR-positivity was defined as AR expression greater than 0% on IHC [65]. In the evaluable subgroup, the CBR was 33% at 16 weeks (95% CI, 23% – 45%) and median PFS was 3.3 months (95% CI, 1.9 – 4.1 months) [65]. This supports further studies evaluating the use of enzalutamide in advanced TNBC. Ongoing clinical trials are also evaluating the use of AR inhibitors, enzalutamide and bicalutamide, in the neoadjuvant and adjuvant settings as well as monotherapy or in combination with PARP inhibitors, checkpoint inhibitors and PI3K targeted agents in the metastatic setting [52].
Antibody-Drug Conjugates
Antibody-drug conjugates (ADC) are humanized monoclonal antibodies bound to a cytotoxic drug via a linker [66]. The ADC binds to a specific cell surface marker, becomes internalized via antigen-mediated endocytosis then undergoes degradation and linker cleavage which releases the cytotoxic agent causing cell death (figure 3) [66]. This approach allows for targeted delivery of potent cytotoxic drug to cancer cells in attempts to limit off-target toxicity [66].
Figure 3.
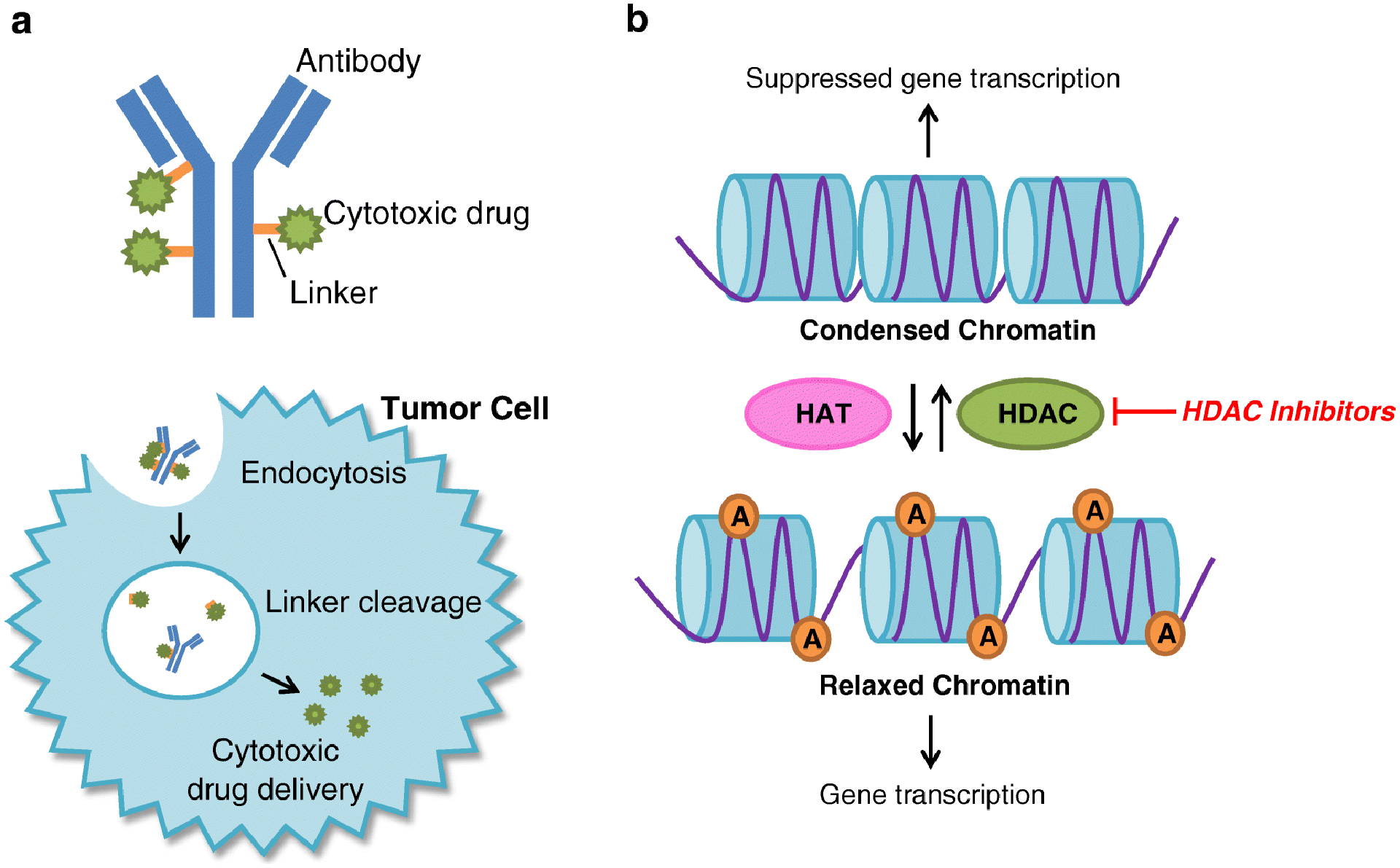
Mechanism of action of antibody-drug conjugates and HDAC inhibitors. (A) Antibody drug conjugates are humanized monoclonal antibodies bound to a cytotoxic drug by a linker. It binds to a tumor cell specific marker and is engulfed via endocytosis. The linker is degraded and the cytotoxic agent is cleaved from the antibody delivering the cytotoxic agent into the tumor cell which results in cell death. (B) Histone acetylation by HATs relaxes chromatin allowing for gene transcription. HDACs remove acetyl groups which results in condensed chromatin and suppression of gene transcription. In tumor cells, suppression of gene transcription of tumor suppressor and DNA repair genes can allow for tumor growth. HDAC inhibitors prevent removal of acetyl groups by HDACs leaving chromatin in its relaxed state allowing for gene transcription. A: acetyl group, HAT: Histone acetyltransferase, HDAC: histone deacetylase.
Sacituzumab govitecan-hziy was recently approved for patients with metastatic TNBC who have received two prior lines of therapy. This drug is an ADC linking SN-38, a topoisomerase I inhibitor, to humanized Trop-2 monoclonal antibody, hRS7 IgG1κ, via cleavable CL2A linker [67]. Accelerated approval was based on a phase I/II clinical trial during which 108 patients with metastatic TNBC were treated with sacituzumab govitecan-hziy days 1 and 8 of 21 day cycles. The ORR was 33.3% (95% CI, 24.6– 43.1) with a CBR of 45.4%. Median PFS was 5.5 months (95%C CI, 4.1–6.3) and OS was 13.0 months (95% CI, 11.2 – 13.7) [67]. These results were especially promising as patients included in the study were heavily pretreated with a median of 3 prior lines of therapy [67]. The phase III trial, ASCENT, randomizing patients with metastatic TNBC with at least 2 prior lines of therapy to sacituzumab govitecan-hziy or treatment of physician’s choice is ongoing.
Ladiratuzumab vedotin binds LIV1, a transmembrane cell adhesion molecule, and delivers monomethyl auristatin E, a microtubule disrupting agent [68]. It was evaluated in a phase I study for patients with unresectable, locally advanced, metastatic TNBC who have received at least two prior lines of chemotherapy [69]. In the TNBC cohort, ORR was 25.0% and median PFS was 13 weeks. Evaluation of this drug is in progress and it is also being tested in combination with pembrolizumab in patients with locally advanced or metastatic TNBC [68]. Preliminary results of the phase Ib/II study combining ladiratuzumab vendotin with pembrolizumab in the first-line treatment of patients with unresectable locally-advanced or metastatic TNBC denote an ORR of 54% [70]. Patients included in this study were not preselected for PD-L1 expression demonstrating that there may be a role for immunotherapy regardless of PD-L1 status [68]. Pending final analysis, further study of ladiratuzumab vendotin may be worthwhile.
Histone deacetylase (HDAC) inhibitors
Histone acetylation by histone acetyltransferases allows for chromatin relaxation and active gene transcription, while histone deacetylases remove the acetyl group and lead to compressed chromatin structure which suppresses gene transcription [71]. HDAC levels are increased in certain cancer types, which may contribute to tumorigenesis by inhibiting expression of tumor suppressor and DNA repair genes [71, 72]. HDAC inhibitors, therefore, create a HRD like state, inhibit tumor growth, and result in apoptosis of cancer cells (figure 3) [71–73]. The use of HDAC inhibitors in combination with platinum chemotherapy and PARP inhibition may be especially effective in HRD tumors. This approach is currently being investigated in phase I and II clinical trials.
Conclusions
Patients with TNBC have varying responses to standard chemotherapy and characterization of molecular subtypes has aided in clarifying tumor behavior. Despite classification into subgroups, similar molecular and epigenetic targets can be found across these TNBC subtypes. This heterogeneity has resulted in difficulty identifying targeted therapies that are effective for many patients. However, the recent surge in clinical trials evaluating targets in TNBC will likely result in continued growth of the armamentarium for the treatment of TNBC.
Recent data suggest that the population of patients with TNBC who derive benefit from PARP inhibitors may be expanded to include somatic BRCA 1/2-mutated and BRCA 1/2-wild type, HRD TNBC with results from ongoing trials. HDAC inhibitors may be efficacious in a similar population. There is strong data to suggest that pembrolizumab may improve pCR when added to neoadjuvant chemotherapy and excitingly, there are multiple novel immune activators that are being used in combination with immune checkpoint inhibitors that may improve response and efficacy in TNBC. In addition, response to immunotherapy may not be limited to PD-L1-positive tumors in the neoadjuvant setting and multiple approaches are under investigation to improve response to immunotherapy in so called “cold” tumors. In AR-positive TNBC, AR blockade has shown some effect and there are multiple targets within the PI3K/AKT/mTOR pathway that may result in successful anti-neoplastic effects with a few drugs being evaluated in phase III clinical trials. Attention to uncovering biomarkers and developing novel targeted therapies for TNBC may significantly improve patient outcomes and treatment tolerability for patients with early stage and advanced TNBC.
Acknowledgements
This investigation was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32CA236734-01. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Jodi A. Kagihara, Elena Shagisultanova, Anosheh Afghahi, and Jennifer R. Diamond declare that they have no conflict of interest
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–95. doi: 10.1200/jco.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Yang J, Peng L, Sahin AA, Huo L, Ward KC, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2017;161(2):279–87. doi: 10.1007/s10549-016-4059-6. [DOI] [PubMed] [Google Scholar]
- 3.Ismail-Khan R, Bui MM. A review of triple-negative breast cancer. Cancer Control. 2010;17(3):173–6. doi: 10.1177/107327481001700305. [DOI] [PubMed] [Google Scholar]
- 4.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.Ccr-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Kuroi K, Toi M, Ohno S, Nakamura S, Iwata H, Masuda N, et al. Prognostic significance of subtype and pathologic response in operable breast cancer; a pooled analysis of prospective neoadjuvant studies of JBCRG. Breast Cancer. 2015;22(5):486–95. doi: 10.1007/s12282-013-0511-1. [DOI] [PubMed] [Google Scholar]
- 6.Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147–59. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 7.Gobbini E, Ezzalfani M, Dieras V, Bachelot T, Brain E, Debled M, et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer. 2018;96:17–24. doi: 10.1016/j.ejca.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9(1):29–33. doi: 10.3816/CBC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- 9.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, Andre F, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)dagger. Ann Oncol. 2018;29(8):1634–57. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Network NCC: Breast Cancer (Version 3.2019). https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf (2019). Accessed.
- 11.Tecentriq (atezolizumab) [package insert]. San Francisco, CA: Genentech, Inc; 2019. [Google Scholar]
- 12.Immunomedics, Inc. Trodelvy (sacituzumab govitecan-hziy) [package insert]. U.S. Food and Drug Administration website. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761115s000lbl.pdf. April 2020. Accessed May 11, 2020. . [Google Scholar]
- 13.Lehmann BD, Jovanović B, Chen X, Estrada MV, Johnson KN, Shyr Y, et al. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS One. 2016;11(6):e0157368. doi: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: the immunotherapy era. BMC Med. 2019;17(1):90. doi: 10.1186/s12916-019-1326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azim HA, Ghosn M, Oualla K, Kassem L. Personalized treatment in metastatic triple-negative breast cancer: The outlook in 2020. Breast J. 2020;26(1):69–80. doi: 10.1111/tbj.13713. [DOI] [PubMed] [Google Scholar]
- 16.Gao R, Davis A, McDonald TO, Sei E, Shi X, Wang Y, et al. Punctuated copy number evolution and clonal stasis in triple-negative breast cancer. Nat Genet. 2016;48(10):1119–30. doi: 10.1038/ng.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmer AS, Gillard M, Lipkowitz S, Lee JM. Update on PARP Inhibitors in Breast Cancer. Curr Treat Options Oncol. 2018;19(5):21. doi: 10.1007/s11864-018-0540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turk AA, Wisinski KB. PARP inhibitors in breast cancer: Bringing synthetic lethality to the bedside. Cancer. 2018;124(12):2498–506. doi: 10.1002/cncr.31307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belli C, Duso BA, Ferraro E, Curigliano G. Homologous recombination deficiency in triple negative breast cancer. Breast. 2019;45:15–21. doi: 10.1016/j.breast.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Pifzer Inc. Talzenna (talazoparib) [package insert]. U.S. Food and Drug Administration website. www.accessdata.fda.gov/drugsatfda_docs/label/2018/211651s000lbl.pdf. Revised October 2018. Accessed April 21, 2020. . [Google Scholar]
- 21.AstraZeneca Pharamceuticals LP. Lynparza (olaparib). [package insert]. U.S. Food and Drug Administration website. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208558s001lbl.pdf. RevisedJanuary2018. AccessedApril 21, 2020. [Google Scholar]
- 22.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377(6):523–33. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]; *The OlympiAD trial which led to the FDA approval of olaparib
- 23.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med. 2018;379(8):753–63. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]; *The EMBRACA trial which led to the FDA approval of talazoparib
- 24.Gruber JJ, Afghahi A, Hatton A, Scott D, McMillan A, Ford JM, et al. Talazoparib beyond BRCA: A phase II trial of talazoparib monotherapy in BRCA1 and BRCA2 wild-type patients with advanced HER2-negative breast cancer or other solid tumors with a mutation in homologous recombination (HR) pathway genes. Journal of Clinical Oncology. 2019;37(15_suppl):3006-. doi: 10.1200/JCO.2019.37.15_suppl.3006. [DOI] [Google Scholar]
- 25.Patsouris A, Tredan O, Nenciu D, Tran-Dien A, Campion L, Goncalves A, et al. RUBY: A phase II study testing rucaparib in germline (g) BRCA wild-type patients presenting metastatic breast cancer (mBC) with homologous recombination deficiency (HRD). Journal of Clinical Oncology. 2019;37(15_suppl):1092-. doi: 10.1200/JCO.2019.37.15_suppl.1092. [DOI] [Google Scholar]
- 26.Tung NM. TBCRC 048: A phase II study of olaparib monotherapy in metastatic breast cancer patients with germline or somatic mutations in DNA damage response (DDR) pathway genes (Olaparib Expanded). In: Nadine M Tung MERSVCAS-MPKMRNPDSTJBES-HYME, Beth Israel Deaconess Medical C, Dana-Farber Cancer Institute BMA, Memorial Sloan Kettering Cancer Center NYNY, Dana-Farber Cancer Institute BMA, Northwestern University Feinberg School of Medicine CIL, et al. , editors. ASCO Virtual Scientific Program: American Society of Clinical Oncology; 2020. [Google Scholar]
- 27.Aguirre E, Amillano K, Cortés A, Juan MJ, Márquez A, Ruiz M, et al. Abstract CT165: A two-stage Simon Design phase II study for NOn-BRCA metastatic BReast cancer (MBC)patients with homologous recombination deficiency treated with OLAparib single agent.(NOBROLA study). Cancer Research. 2018;78(13 Supplement):CT165-CT. doi: 10.1158/1538-7445.Am2018-ct165. [DOI] [Google Scholar]
- 28.Fasching PA, Jackisch C, Rhiem K, Schneeweiss A, Klare P, Hanusch C, et al. GeparOLA: A randomized phase II trial to assess the efficacy of paclitaxel and olaparib in comparison to paclitaxel/carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients (pts) with HER2-negative early breast cancer (BC) and homologous recombination deficiency (HRD). Journal of Clinical Oncology. 2019;37(15_suppl):506-. doi: 10.1200/JCO.2019.37.15_suppl.506. [DOI] [Google Scholar]
- 29.Elias AD. Triple-negative breast cancer: a short review. Am J Clin Oncol. 2010;33(6):637–45. doi: 10.1097/COC.0b013e3181b8afcf. [DOI] [PubMed] [Google Scholar]
- 30.Tutt A, Stephens C, Frewer P, Pierce A, Rhee J, So K, et al. VIOLETTE: A randomized phase II study to assess DNA damage response inhibitors in combination with olaparib (Ola) vs Ola monotherapy in patients (pts) with metastatic, triple-negative breast cancer (TNBC) stratified by alterations in homologous recombination repair (HRR)-related genes. Journal of Clinical Oncology. 2018;36(15_suppl):TPS1116-TPS. doi: 10.1200/JCO.2018.36.15_suppl.TPS1116. [DOI] [Google Scholar]
- 31.Sammons S, Tan TJY, Traina TA, Kim S-B, Im Y-H, Bachelder C, et al. Dora: A randomized phase II multicenter maintenance study of olaparib alone or olaparib in combination with durvalumab in platinum responsive advanced triple-negative breast cancer (aTNBC). Journal of Clinical Oncology. 2019;37(15_suppl):TPS1113-TPS. doi: 10.1200/JCO.2019.37.15_suppl.TPS1113. [DOI] [Google Scholar]
- 32.Garcia-Teijido P, Cabal ML, Fernandez IP, Perez YF. Tumor-Infiltrating Lymphocytes in Triple Negative Breast Cancer: The Future of Immune Targeting. Clin Med Insights Oncol. 2016;10(Suppl 1):31–9. doi: 10.4137/cmo.S34540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z, Li M, Jiang Z, Wang X. A Comprehensive Immunologic Portrait of Triple-Negative Breast Cancer. Transl Oncol. 2018;11(2):311–29. doi: 10.1016/j.tranon.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams S, Diamond JR, Hamilton E, Pohlmann PR, Tolaney SM, Chang CW, et al. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer With 2-Year Survival Follow-up: A Phase 1b Clinical Trial. JAMA Oncol. 2019;5(3):334–42. doi: 10.1001/jamaoncol.2018.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379(22):2108–21. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]; *The IMpassion130 trial which led to the FDA approval of atezolizumab plus nab-paclitaxel
- 37.Lee HT, Lee SH, Heo YS. Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology. Molecules. 2019;24(6). doi: 10.3390/molecules24061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortes J, Cescon DW, Rugo HS. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol. 2020;38(suppl 15; abstr 1000). doi: 10.1200/JCO.2020.38.15_suppl.1000. [DOI] [PubMed] [Google Scholar]
- 39.Dalenc F, Garberis I, Filleron T, et al. : Durvalumab compared to maintenance chemotherapy in patients with metastatic breast cancer: Results from phase II randomized trial SAFIR02-IMMUNO. 2019 San Antonio Breast Cancer Symposium. Abstract GS3–02. PresentedDecember12, 2019. [Google Scholar]
- 40.Loibl S, Untch M, Burchardi N, Huober J, Sinn BV, Blohmer JU, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Annals of Oncology. 2019;30(8):1279–88. doi: 10.1093/annonc/mdz158. [DOI] [PubMed] [Google Scholar]
- 41.Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382(9):810–21. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 42.Gianni L, Huang CS, Egle D, et al. : Pathologic complete response to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer: NeoTRIPaPDL1 Michelangelo randomized study. 2019 San Antonio Breast Cancer Symposium. Abstract GS3–04. PresentedDecember12, 2019. [DOI] [PubMed] [Google Scholar]
- 43.Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncology. 2020. doi: 10.1001/jamaoncol.2019.6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Day S, Borges VF, Chmielowski B, Rao RD, Abu-Khalaf MM, Stopeck A, et al. An open label, multicenter phase II study combining imprime PGG (PGG) with pembrolizumab (P) in previously treated metastatic triple-negative breast cancer (mTNBC). Journal of Clinical Oncology. 2019;37(15_suppl):2550-. doi: 10.1200/JCO.2019.37.15_suppl.2550. [DOI] [Google Scholar]
- 45.O’Day SJ, Borges VF, Chmielowski B, et al. IMPRIME 1 (NCT02981303): A novel phase 2 study in second-line +, metastatic triple negative breast cancer patients shows promising clinical benefit for the combination of the immune checkpoint inhibitor, pembrolizumab (pembro), with the novel innate immune activator, Imprime PGG. Presented at: 2020 AACR Virtual Annual Meeting; April27-28, 2020. Abstract CT073. [Google Scholar]
- 46.Telli ML, Wapnir I, Devitt B, et al. : Phase II, open-label study of intratumoral tavokinogene telseplasmid (tavo) plus electroporation in combination with intravenous pembrolizumab therapy in patients with inoperable locally advanced or metastatic triple-negative breast cancer (KEYNOTE-890/OMS-I141). 2019 San Antonio Breast Cancer Symposium. Abstract P3–09-04. PresentedDecember12, 2019. [Google Scholar]
- 47.Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397–404. doi: 10.1093/annonc/mdy517. [DOI] [PubMed] [Google Scholar]
- 48.Rugo HS, Barry WT, Moreno-Aspitia A, Lyss AP, Cirrincione C, Leung E, et al. Randomized Phase III Trial of Paclitaxel Once Per Week Compared With Nanoparticle Albumin-Bound Nab-Paclitaxel Once Per Week or Ixabepilone With Bevacizumab As First-Line Chemotherapy for Locally Recurrent or Metastatic Breast Cancer: CALGB 40502/NCCTG N063H (Alliance). J Clin Oncol. 2015;33(21):2361–9. doi: 10.1200/jco.2014.59.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Massihnia D, Galvano A, Fanale D, Perez A, Castiglia M, Incorvaia L, et al. Triple negative breast cancer: shedding light onto the role of pi3k/akt/mtor pathway. Oncotarget. 2016;7(37):60712–22. doi: 10.18632/oncotarget.10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan MA, Jain VK, Rizwanullah M, Ahmad J, Jain K. PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: a review on drug discovery and future challenges. Drug Discov Today. 2019;24(11):2181–91. doi: 10.1016/j.drudis.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Costa RLB, Han HS, Gradishar WJ. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Res Treat. 2018;169(3):397–406. doi: 10.1007/s10549-018-4697-y. [DOI] [PubMed] [Google Scholar]
- 52.Chan JJ, Tan TJY, Dent RA. Novel therapeutic avenues in triple-negative breast cancer: PI3K/AKT inhibition, androgen receptor blockade, and beyond. Therapeutic Advances in Medical Oncology. 2019;11:1758835919880429. doi: 10.1177/1758835919880429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Millis SZ, Gatalica Z, Winkler J, Vranic S, Kimbrough J, Reddy S, et al. Predictive Biomarker Profiling of > 6000 Breast Cancer Patients Shows Heterogeneity in TNBC, With Treatment Implications. Clin Breast Cancer. 2015;15(6):473–81.e3. doi: 10.1016/j.clbc.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 54.Novartis Pharmaceuticals Corporation. Piqray (alpelisib) [package insert]. U.S. Food and Drug Administration website. www.accessdata.fda.gov/drugsatfda_docs/label/2019/212526s000lbl.pdfRevised. May 2019. Accessed April 29, 2020. . [Google Scholar]
- 55.Sharma P, Abramson VG, O’Dea A, Pathak HB, Pessetto ZY, Wang YY, et al. Clinical and biomarker results from phase I/II study of PI3K inhibitor BYL 719 (alpelisib) plus nab-paclitaxel in HER2-negative metastatic breast cancer. Journal of Clinical Oncology. 2018;36(15_suppl):1018-. doi: 10.1200/JCO.2018.36.15_suppl.1018. [DOI] [Google Scholar]
- 56.Kim SB, Dent R, Im SA, Espié M, Blau S, Tan AR, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360–72. doi: 10.1016/s1470-2045(17)30450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Abstract 1.
- 58.Oliveira M, Saura C, Nuciforo P, Calvo I, Andersen J, Passos-Coelho JL, et al. FAIRLANE, a double-blind placebo-controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple-negative breast cancer. Annals of Oncology. 2019;30(8):1289–97. doi: 10.1093/annonc/mdz177. [DOI] [PubMed] [Google Scholar]
- 59.Schmid P, Abraham J, Chan S, Wheatley D, Brunt AM, Nemsadze G, et al. Capivasertib Plus Paclitaxel Versus Placebo Plus Paclitaxel As First-Line Therapy for Metastatic Triple-Negative Breast Cancer: The PAKT Trial. Journal of Clinical Oncology. 2020;38(5):423–33. doi: 10.1200/jco.19.00368. [DOI] [PubMed] [Google Scholar]
- 60.Dey N, De P, Leyland-Jones B. PI3K-AKT-mTOR inhibitors in breast cancers: From tumor cell signaling to clinical trials. Pharmacol Ther. 2017;175:91–106. doi: 10.1016/j.pharmthera.2017.02.037. [DOI] [PubMed] [Google Scholar]
- 61.Gerratana L, Basile D, Buono G, De Placido S, Giuliano M, Minichillo S, et al. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treat Rev. 2018;68:102–10. doi: 10.1016/j.ctrv.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 62.Venema CM, Bense RD, Steenbruggen TG, Nienhuis HH, Qiu S-Q, van Kruchten M, et al. Consideration of breast cancer subtype in targeting the androgen receptor. Pharmacology & Therapeutics. 2019;200:135–47. doi: 10.1016/j.pharmthera.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 63.Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25(28):3994–4008. doi: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
- 64.Ni M, Chen Y, Lim E, Wimberly H, Bailey Shannon T, Imai Y, et al. Targeting Androgen Receptor in Estrogen Receptor-Negative Breast Cancer. Cancer Cell. 2011;20(1):119–31. doi: 10.1016/j.ccr.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O’Shaughnessy J, et al. Enzalutamide for the Treatment of Androgen Receptor–Expressing Triple-Negative Breast Cancer. Journal of Clinical Oncology. 2018;36(9):884–90. doi: 10.1200/jco.2016.71.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J Natl Cancer Inst. 2019;111(6):538–49. doi: 10.1093/jnci/djz035. [DOI] [PubMed] [Google Scholar]
- 67.Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. New England Journal of Medicine. 2019;380(8):741–51. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]; *The study which led to the FDA approval of sacituzumab govitecan-hziy
- 68.Han HS, Alemany CA, Brown-Glaberman UA, Pluard TJ, Sinha R, Sterrenberg D, et al. SGNLVA-002: Single-arm, open label phase Ib/II study of ladiratuzumab vedotin (LV) in combination with pembrolizumab for first-line treatment of patients with unresectable locally advanced or metastatic triple-negative breast cancer. Journal of Clinical Oncology. 2019;37(15_suppl):TPS1110-TPS. doi: 10.1200/JCO.2019.37.15_suppl.TPS1110. [DOI] [Google Scholar]
- 69.Modi S, Pusztal L, Forero A, et al. Phase 1 study of the antibody-drug conjugate ladiratuzumab vedotin (SGN-LIV1A) in patients with heavily pretreated triple-negative metastatic breast cancer. Poster presented at: 2017 San Antonio Breast Cancer Symposium; December5-9, 2017; San Antonio, Texas. Poster PD3–14. [Google Scholar]
- 70.Han H, Diab S, Alemany C, et al. Open label phase 1b/2 study of ladiratuzumab vedotin in combination with pembrolizumab for first-line treatment of patients with unresectable locally-advanced or metastatic triple-negative breast cancer. December10-14, 2019; San Antonio, Texas. Poster PD1–06. [Google Scholar]
- 71.Garmpis N, Damaskos C, Garmpi A, Kalampokas E, Kalampokas T, Spartalis E, et al. Histone Deacetylases as New Therapeutic Targets in Triple-negative Breast Cancer: Progress and Promises. Cancer Genomics Proteomics. 2017;14(5):299–313. doi: 10.21873/cgp.20041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee A, Djamgoz MBA. Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treat Rev. 2018;62:110–22. doi: 10.1016/j.ctrv.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Marijon H, Lee DH, Ding L, Sun H, Gery S, de Gramont A, et al. Co-targeting poly(ADP-ribose) polymerase (PARP) and histone deacetylase (HDAC) in triple-negative breast cancer: Higher synergism in BRCA mutated cells. Biomedicine & Pharmacotherapy. 2018;99:543–51. doi: 10.1016/j.biopha.2018.01.045. [DOI] [PubMed] [Google Scholar]


