Abstract
Background and Objectives
Acute upper gastrointestinal bleeding (UGIB) is a common problem that can cause significant morbidity and mortality. We aimed to compare the performance of the ABC score (ABC), the AIMS65 score (AIMS65), the Glasgow-Blatchford score (GBS), and the pre-endoscopic Rockall score (pRS) in predicting 90-day mortality or rebleeding among patients with acute UGIB.
Methods
This was a prospective multicenter study conducted at 20 tertiary hospitals in China. Data were collected between June 30, 2020 and February 10, 2021. An area under the receiver operating characteristic curve (AUC) analysis was used to compare the performance of the four scores in predicting 90-day mortality or rebleeding.
Results
Among the 1072 patients included during the study period, the overall 90-day mortality rate was 10.91% (117/1072) and the rebleeding rate was 12.03% (129/1072). In predicting 90-day mortality, the ABC and pRS scores performed better with an AUC of 0.722 (95% CI 0.675–0.768; P<0.001) and 0.711 (95% CI 0.663–0.757; P<0.001), respectively, compared to the AIMS-65 (AUC, 0.672; 95% CI, 0.624–0.721; P<0.001) and GBS (AUC, 0.624; 95% CI, 0.569–0.679; P<0.001) scores. In predicting rebleeding in 90 days, the AUC of all scores did not exceed 0.70.
Conclusion
In patients with acute UGIB, ABC and pRS performed better than AIMS-65 and GBS in predicting 90-day mortality. The performance of each score is not satisfactory in predicting rebleeding, however. Newer predictive models are needed to predict rebleeding after UGIB.
Keywords: acute upper gastrointestinal bleeding, mortality, rebleeding, preendoscopic scoring systems, risk stratification
INTRODUCTION
Acute upper gastrointestinal bleeding (UGIB) is a common reason for seeking care in emergency departments (ED) and being admitted to acute care hospitals worldwide, with an incidence of 80–150 per 100,000 people each year.[1,2,3,4,5] Despite improvements in management over the past decades, acute UGIB remains a common, life-threatening condition with mortality ranging from 5% to 11%.[1,2,3,4,5] In China, there are approximately one million UGIB patients admitted to hospitals each year, with an UGIB-specific death rate estimated to be between 4%–14%.[6]
Around the world, timely treatment of patients with acute UGIB is highly dependent on emergency interventions and intensive care. If we can classify UGIB patients early while also accurately estimating their prognosis, the hope is that emergency resuscitation, endoscopy, and interventions can be performed in a timely manner on the correct patients, reducing patient mortality.[5–6] The European Society of Gastrointestinal Endoscopy, the Asia Pacific Working Group, and the International Consensus Group also recommend risk scoring for UGIB patients.[7,8,9]
Risk scores are usually used to predict UGIB patients’ outcomes. Outcomes of interest include death, the need for interventions to stop the bleeding (such as endoscopic haemostasis, interventional radiology or surgery), rebleeding, red blood cell (RBC) transfusion requirements, and readmission to hospital.[10–11] Mortality and rebleeding are mostly selected outcome indicators due to their relative objectiveness. There are a variety of risk scores that can be used for acute UGIB patients, each with multiple studies analyzing their outcomes. Studies of risk scores have been developed in both geographically and pathologically diverse populations, including all-comers with UGIB and some restricted to subgroups such as nonvariceal UGIB (NVUGIB) or variceal UGIB (VUGIB). Each is powered to detect different outcomes, and the robustness of external validation varies considerably. In addition, many of these predictive tools relies on endoscopic results and are, therefore, not ideal for early evaluation of patients. Nevertheless several risk scores can be applied prior to endoscopy results, and are particularly useful in the ED risk stratification of acute UGIB patients. Among them, the most notable scales are the pre-endoscopic Rockall score (pRS), the Glasgow-Blatchford score (GBS), the AIMS65 score, and the ABC score. There have been many studies on the outcomes of UGIB and continued studies on which scoring systems can better predict mortality.
Although some scoring systems have been validated in an ethnically Chinese population, there are relatively few large-scale, prospective studies.[12–13] It is important to identify UGIB patients who are at high risk of serious adverse consequences. Therefore, there is an urgent need for an effective and easy-to-use scoring method to identify those UGIB patients who are at a high risk of death in a Chinese population given the prevalence and mortality of cases in China. This was a prospective, multicenter study to evaluate the performance of the ABC, AIMS-65, GBS, and pRS scores in predicting the risk of death and rebleeding in Chinese acute UGIB patients.
MATERIALS AND METHODS
Study Design and Participants
This study was performed as a multicentre, prospective, non-interventional real-word prospective study. The study sample included all nontrauma adult (age ≥18 years) patients diagnosed with UGIB who were admitted to the hospital via the emergency department (ED) between June 30, 2020, and February 10, 2021. The diagnosis of UGIB was based on the presence of hematemesis, vomiting of coffee ground material, melena, or bloody content on nasogastric aspiration. Patients who refused to sign the participation consent form were excluded. This study was conducted at 20 tertiary hospitals from 20 (out of an invited 31) provinces, autonomous regions, or independent municipalities and was approved by the Institutional Review Boards of all 20 hospitals. Informed consent was obtained from all enrolled patients. The clinical trial registration number is ChiCTR1900028676.
Study outcome
The outcomes evaluated were the frequency of death or recurrent bleeding. Such outcomes were monitored from admission to the hospital or the onset of bleeding at admission to the hospital (for in-hospital patients) for up to 90 days. 90-day mortality was the primary investigated outcome; “bleeding-related” death was defined as any death occurring within 90 days of the bleeding episode. Rebleeding was defined by recurrent vomiting of fresh blood, melena, or both, with either shock or a decrease in hemoglobin concentration of at least 20 g/L 24 hours following initial treatment and stabilization. All data were recorded for the full duration of the initial medical encounter. To ensure the completeness of follow-up information, the study doctor called all patients or their families at 90 days.
Covariates
Variables included age, gender, vital signs at triage, comorbidities, relevant past medical history, any concomitant intake of medications in the six months preceding the bleeding episode, time elapsed from the onset of bleeding, physical examination findings and laboratory data (hemodynamic data, nasogastric tube use, complete blood count, and coagulation profile results), and any resuscitative measures employed. Endoscopic reports included identification of the bleeding lesion or stigmata of recent hemorrhage in the lesion. The presence of fresh blood in the stomach in such amounts to hamper the endoscopic diagnosis of the source of bleeding was annotated and underscored as “bleeding source not identified with endoscopic diagnosis.” Being unable to successfully conclude an already started endoscopic procedure with the intention to treat a source of hemorrhage was defined as a “failed intention to endoscopic treatment.” Both surgical and angiographic therapies were recorded as well as any pharmacologic therapy administered for hemorrhage.
Data Collection and Quality Control
All patients were enrolled consecutively without any personal tendency. At each site, a “lead” consultant represented the project locally. At each hospital, the coordinator identified subjects in the ED, inpatient wards, endoscopy unit, operating theatre, blood transfusion records, and hospital admission data. Specially trained research assistants collected data directly from the patient's point of care into case report forms. The coordinator was then responsible for checking and returning a completed form for each patient correctly identified. All data were denominalized and downloaded into a central database on a monthly basis. They were reviewed at a single location for internal logic of patient flow and biological plausibility. All data queries were resolved within 30 days following the original data entry. To help assure the internal validity of the registry, there was an independent data validation of a random subset of all information collected; the quality of the data was validated on a quarterly basis by randomly comparing 5% of all records to the source data recorded in the hospital charts. Personnel from a clinical research organization trained all research staff at a common start-up meeting and at each initial on-site visit prior to the first patient entry. On that occasion, all endoscopists participating in the registry were invited to review a wide set of video images of different bleeding stigmata to estimate inter-rater variability and find as much agreement as possible on the diagnosis of stigmata.
Missing data
The prevalence and pattern of missing data was evaluated and found not to be missing completely at random (Little's test: P<0.001). This study did not impute missing values.
Explanatory variables
The scores of interest were preendoscopic Rockall score (pRS), Glasgow–Blatchford score (GBS), AIMS65 score, and ABC score. Each of these scores was calculated using the information available at the time of presentation to the ED and were used as continuous and categorical measures separately in our analysis. Table 1 presents all specific risk factors and scoring algorithms included in each scoring system.
Table 1.
Preendoscopic Rockall score, Glasgow–Blatchford score, AIMS65 score, and ABC score
| Risk system | Risk factor | Score | |
|---|---|---|---|
| pRS | Age (yr) | <60 | 0 |
| 60–79 | 1 | ||
| ≥ 80 | 2 | ||
| Shock | HR >100 bpm | 1 | |
| SBP <100 mmHg | 2 | ||
| Comorbidity | IHD, CHF, any major comorbidity renal failure, liver failure | 2 | |
| metastatic malignancy | 3 | ||
| GBS | BUN (mmol/L) | ≥ 6.5 < 8.0 | 2 |
| ≥ 8.0 < 10.0 | 3 | ||
| 10.0–24.9 | 4 | ||
| ≥ 25.0 | 6 | ||
| HGB (g/dL) for men | ≥ 12 < 13 | 1 | |
| ≥ 10 < 12 | 3 | ||
| <10 | 6 | ||
| HGB (g/dL) for women | ≥ 10 to <12 | 1 | |
| <10 | 6 | ||
| SBP (mmHg) | 100–109 | 1 | |
| 90–99 | 2 | ||
| <90 | 3 | ||
| Other factors | Pulse ≥ 100 bpm | 1 | |
| Melena | 1 | ||
| Syncope | 2 | ||
| Liver disease or Heart failure | 2 | ||
| AIMS65 | Albumin (g/L) | <30 | 1 |
| INR | >1.5 | 1 | |
| Mental status | Altered | 1 | |
| SBP (mmHg) | <90 | 1 | |
| Age (yr) | >65 | 1 | |
| ABC | Age (yr) | 60–74 | 1 |
| ≥ 75 | 2 | ||
| Blood test | Urea >10 mmol/L | 1 | |
| Albumin <30 g/L | 2 | ||
| Creatinine 100–150 μmol/L | 1 | ||
| Creatinine >150 μmol/L | 2 | ||
| Comorbidity | Altered mental status | 2 | |
| Liver cirrhosis | 2 | ||
| Disseminated malignancy | 4 | ||
| ASA score 3 | 1 | ||
| ASA score ≥ 4 | 3 | ||
pRS: preendoscopic Rockall risk score; GBS: Glasgow–Blatchford score; AIMS65: AIMS65 score; ABC: ABC score; BUN: blood urea nitrogen; SBP: systolic blood pressure; HR: heart rate; HGB: hemoglobin; IHD: ischemic heart disease; CHF: congestive heart failure; INR: international normalized ratio; ASA: American Society of Anaesthesiologists.
Statistical Analysis
Continuous and categorical variables were, respectively, described by percentages and mean ± standard deviation. Student's t-test and Chi-square test were used for comparing continuous and categorical data, respectively, between the two groups (90-day mortality versus no 90-day mortality; rebleeding versus no rebleeding). Receiver operating curve (ROC) analysis was employed to compare the performance between the four score systems in predicting 90-day mortality and 90-day rebleeding. All analyses were conducted using statistical software packages R (The R Foundation) and Empowerstats (Solutions, Inc., Boston, MA). Statistical significance was accepted at the two-sided level of 0.05.
RESULTS
Patient characteristics
Demographic and clinical characteristics, treatments, and outcomes of the participants are shown in Table 2. Of the initial sample, 60 patients were excluded due to lower gastrointestinal bleeding, 56 patients were excluded due to unavailable laboratory data, and 12 were excluded due to loss of follow-up. Finally, a total of 1072 UGIB patients from 20 hospitals in 20 (out of a surveyed 31) provinces, autonomous regions, or independent municipalities were enrolled in the cohort study. During the study period, all patients enrolled presented first to the ED (i.e. there were no enrollments of already admitted patients).
Table 2.
Patient demographics
| Factor | N=1072 | Factor | N=1072 |
|---|---|---|---|
|
|
|
||
| Mean ± SD / N (%) | Mean ± SD / N (%) | ||
| Age (yr) | 61.41 ± 15.77 | Accompanying symptoms | |
| Male gender | 779 (72.67) | Chest pain | 66 (6.16) |
| Body mass index (kg/m2) | 23.02 ± 4.88 | Confusion | 34 (3.17) |
| Comorbidity | Syncope | 93 (8.68) | |
| None | 77(7.18) | Laboratory (on admission) | |
| Coronary heart disease | 149 (13.90) | Hemoglobin | 89.51 ± 29.78 |
| Atrial fibrillation | 24 (2.24) | Albumin | 34.10 ± 8.09 |
| Hypertension | 346 (32.28) | Hemoglobin | 89.51 ± 29.78 |
| Cirrhosis | 271 (25.28) | Albumin | 34.10 ± 8.09 |
| Diabetes mellitus | 197 (18.38) | INR | 1.36 ± 1.35 |
| Chronic respiratory disease | 30 (2.80) | BUN | 11.89±8.16 |
| Chronic kidney disease | 46 (4.29) | Serum creatinine | 94.61±108.12 |
| Stroke | 93 (8.68) | No endoscopy | 261 (24.35) |
| Gastrointestinal tumors | 54 (5.04) | Endoscopy | |
| Any malignancies except GI | 59 (5.50) | Varices bleeding | 202 (18.84) |
| Medication | Peptic ulcer bleeding | 412 (38.43) | |
| PPI | 93 (8.68) | Esophagitis/gastritis/duodenitis | 189 (17.63) |
| Aspirin | 145 (13.53) | Mallory–Weiss syndrome | 38 (3.54) |
| NSAIDs | 83 (7.74) | Upper gastrointestinal tumors | 26 (2.43) |
| Anticoagulant drugs | 31 (2.89) | Other finding | 50 (4.66) |
| Vital signs and consciousness | No abnormality seen | 7 (0.65) | |
| SBP (mmHg) | 115.86 ± 23.79 | Treatment after admission | |
| DBP (mmHg) | 69.13±27.62 | RBC transfusion | 506 (47.20) |
| HR (bpm) | 92.20 ± 19.40 | Endoscope treatment | 319 (29.76) |
| GCS | 14.85 ± 0.78 | Interventional radiography | 43 (4.01) |
| Onset symptoms | Emergency surgery | 28 (2.61) | |
| Hematesis | 559 (52.15) | Outcome | |
| Coffee ground material | 75 (7.00) | 90-Day mortality | 117 (10.91) |
| Melaena | 827 (77.15) | Rebleeding | 129 (12.03) |
SD: standard deviation; GI: gastrointestinal; PPI: proton pump inhibitor; NSAIDs: nonsteroidal anti-inflammatory drugs; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; GCS: Glasgow coma scale; INR: international normalized ratio; BUN: blood urea nitrogen; RBC: red blood cell.
The mean age of patients was 61.41 ± 15.77 years, with 779 (72.67%) being men. No endoscopic investigations were done on 261 (24.3%) patients because either the patient declined the procedure or the treating physician decided on the procedure for clinical reasons. The etiologies of acute and non-acute UGIB included peptic ulcer (38.43%), varices (18.84%), esophagitis/gastritis/duodenitis, (9.70%), Mallory-–Weiss syndrome (3.54%), upper gastrointestinal tumors (2.43%), and all others (4.66%). Other causes of bleeding included Dieulafoy's lesion, postsurgical anastomotic, post-endoscopic sphincterotomy, angiodysplasia, and diverticulum. More than half of the enrolled patients presented with melena (77.15%) or hematesis (52.15%). Two hundred and seventy-one (25.28%) patients had a history of cirrhosis, while 54 (5.04%) showed a history of gastrointestinal tumors. 145 (13.53%) patients had taken aspirin daily for the past 6 months, and 93 (8.68%) patients had taken a proton pump inhibitor (PPI). Regardless of the cause of UGIB, a red blood cell transfusion was performed in 506 (47.20%) patients and an endoscopic intervention in 319 (29.76%) respectively. 43 (4.01%) patients had interventional angiography therapy performed, and 28 (2.61%) patients underwent emergency surgery. At the 90-day follow-up, 117 (10.91%) patients died and 129 (12.03%) patients experienced rebleeding.
Comparison of scoring systems in predicting mortality and rebleeding
According to the AUC analysis, the predicted 90-day mortality for ABC, AIMS65, GBS, and pRS scores were found to be statistically significant for the estimation of mortality (AUROC; ABC =0.722, AIMS65 =0.672, GBS =0.624, pRS=0.711, all P<0.001)) (Tables 3 and 4 and Figure 1). When comparing the AUROCs between the scoring systems, the pRS score and ABC score were superior to GBS and AIMS65 scores (P = 0.001). There was no significant difference in the area under the ROC curves between the pRS score and the ABC score (P = 0.656).
Table 3.
Univariate analysis pRS, GBS, AIMS65, and ABC with risk of 90-day mortality and rebleeding
| Risk scores | N=1072 | 90-Day mortality | Rebleeding | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean ± SD | OR | 95% CI | P value | OR | 95% CI | P value | |
| pRS | 2.03 ± 1.39 | 1.79 | 1.55–2.07 | <0.0001 | 1.51 | 1.32–1.72 | <0.0001 |
| GBS | 9.06 ± 3.57 | 1.130 | 1.07–1.20 | <0.0001 | 1.07 | 1.01–1.13 | 0.0132 |
| AIMS65 | 0.99 ± 0.92 | 1.97 | 1.62–2.39 | <0.0001 | 1.37 | 1.14–1.65 | 0.0009 |
| ABC | 2.72± 1.96 | 1.480 | 1.35–1.63 | <0.0001 | 1.25 | 1.15–1.37 | <0.0001 |
CI: confidence interval pRS: preendoscopic Rockall risk score; GBS: Glasgow–Blatchford score; AIMS65: AIMS65 score; ABC: ABC score.
Table 4.
Comparison of pRS, GBS, AIMS65, and ABC with estimated optimal cutoff values for 90-day death
| Test | AUROC | 95% CI | Cutoff value | Specificity(%) | Sensitivity(%) | PPV(%) | NPV(%) |
|---|---|---|---|---|---|---|---|
| pRS | 0.711 | 0.664–0.758 | 2.5 | 67.12 | 64.10 | 19.28 | 93.85 |
| GBS | 0.624 | 0.569–0.679 | 10.5 | 62.93 | 57.26 | 15.91 | 92.32 |
| AIMS65 | 0.672 | 0.624–0.721 | 0.5 | 36.44 | 87.18 | 14.39 | 95.87 |
| ABC | 0.722 | 0.675–0.768 | 2.5 | 55.18 | 76.07 | 17.21 | 94.95 |
CI: confidence interval; pRS: preendoscopic Rockall risk score; GBS: Glasgow–Blatchford score; AIMS65: AIMS65 score; ABC: ABC score.
Figure 1.
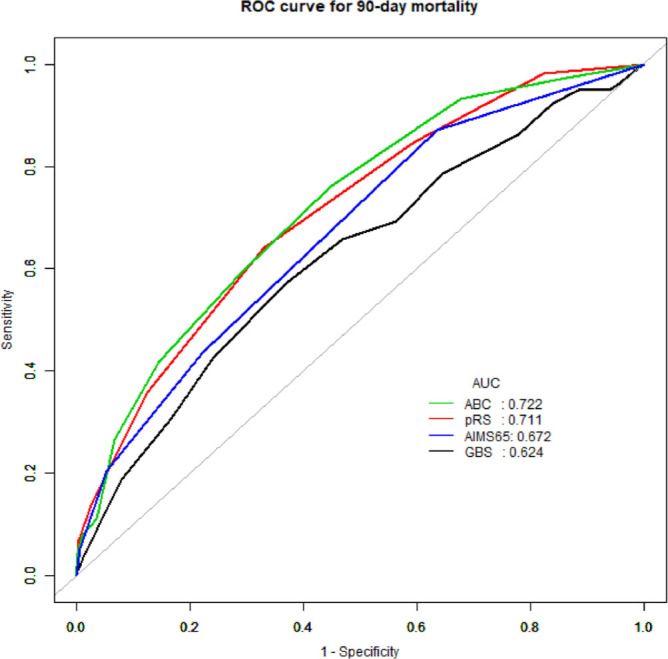
Area under receiver operating characteristic (AUROC) curve for pre-Rockall score, Glasgow–Blatchford score, AIMS65 score, and ABC score in predicting outcome of 90-day mortality.
All four scores had significant differences in predicting rebleeding (AUROC; ABC =0.645, AIMS65 =0.585, GBS =0.571, pRS=0.661, all P <0.05) (Tables 3 and 5 and Figure 2). Consistent with the 90-day mortality prediction results, the pRS score and ABC score were better than GBS and AIMS65 in predicting rebleeding (P = 0.013 and P = 0.008), but there were no significant differences between pRS score and ABC score (P = 0.922).
Table 5.
Comparison of pRS, GBS, AIMS65, and ABC with estimated optimal cutoff values for rebleeding within 90 days
| Test | AUROC | 95% CI | Cutoff value | Specificity(%) | Sensitivity(%) | PPV(%) | NPV(%) |
|---|---|---|---|---|---|---|---|
| pRS | 0.661 | 0.615–0.707 | 1.5 | 40.72 | 82.95 | 15.27 | 91.06 |
| GBS | 0.571 | 0.519–0.623 | 9.5 | 52.92 | 62.02 | 16.07 | 94.58 |
| AIMS65 | 0.585 | 0.537–0.634 | 0.5 | 35.52 | 78.29 | 14.25 | 92.29 |
| ABC | 0.645 | 0.596–0.694 | 2.5 | 54.72 | 69.77 | 17.41 | 92.97 |
CI: confidence interval; pRS: preendoscopic Rockall risk score; GBS: Glasgow–Blatchford score; AIMS65: AIMS65 score; ABC: ABC score.
Figure 2.
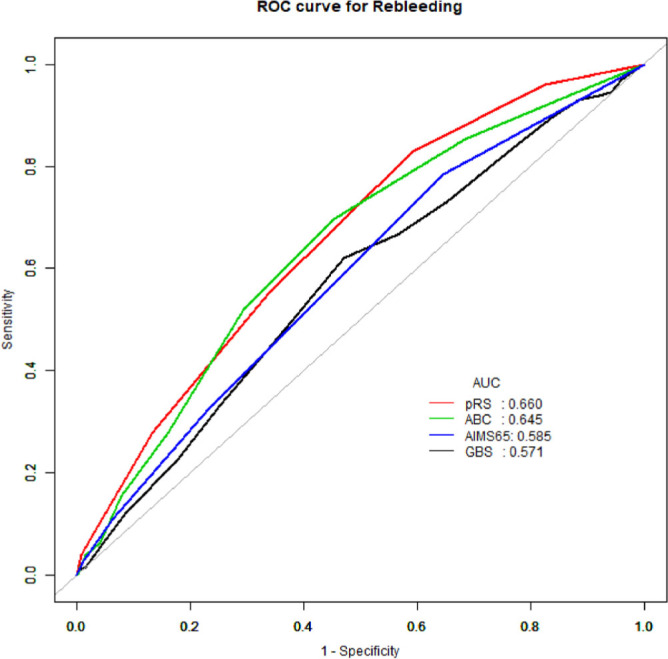
Area under receiver operating characteristic (AUROC) curve for pre-Rockall score, Glasgow–Blatchford score, AIMS65 score, and ABC score in predicting outcome of rebleeding within 90 days.
The cutoff values that maximized the sum of the sensitivity and specificity for predicting mortality in each score were generated from the ROC curves and were selected for further analysis. As shown in Tables 3 and 4, the cutoff for the pRS score were determined to be 2.5. At this value, the sensitivity of the pRS score was 67.12% and specificity was 64.10%. The cutoff for the ABC score was determined to be 2.5. The sensitivity was 76.06% and specificity was 55.18% at this value. Tables 3 and 4 show the AUROCs, 95% confidence intervals, cutoff scores, sensitivities, specificities, positive likelihood ratios, and negative likelihood ratios for all scores used in identifying patients with acute UGIB who died or experienced rebleeding.
DISCUSSION
Acute UGIB is a critical condition to diagnose and manage in the ED. Despite the improvement of intensive care technology and advancements in endoscopic treatment of UGIB, mortality remains significant. In our study, although we selected 90-day mortality as the primary outcome, the all-cause mortality (10.91%) we found in our sample is very similar to rates reported by previous studies (10%, 30-day mortality or in-hospital mortality).[14–15] Acute UGIB is an emergency that may need early treatment; consequently, accurate risk stratification is the key to appropriately managing patients with acute UGIB in the ED. The prognostic scoring system has the potential to ensure a more objective and repeatable risk assessment than individual clinical judgments; and the scores can be easily and repeatably communicated to different clinicians responsible for the management of UGIB patients.[16–17] Knowing which patients are at true elevated risk of morbidity or mortality can guide limited resources (such as emergent endoscopy and ICU beds) to their most useful ends.
Almost three decades after the creation of the Rockall score, many risk stratification schemes have been published.[18,19,20,21] Because medical treatment in the ED aims to rapidly intervene and stabilize UGIB patients prior to knowing endoscopic results, some popular UGIB scores cannot be used in ED.
In this study, we compared the accuracy of the four scoring systems that do not utilize apriori endoscopic results, we chose representatives of prognostic multifactorial scoring systems, including three widely used scores (pRS, GBS, AIMS65) and a new ABC scoring system, in a prospective collected cohort of patients with acute UGIB. The results show that all prognostic tools predict 90-day mortality and rebleeding in ED patients with acute UGIB. Nevertheless, the pRS and ABC performed better than GBS and AIMS65. To date, this study is the largest multicentre, real-world study of such scores for UGIB in Chinese EDs.
pRS is a risk score system that removes endoscopy from RS, which was derived in 1996 from 4185 cases of AUGIB in the United Kingdom.[18]. It has been externally verified in large-scale UGIB populations. Just like RS, the strength of pRS score lies in the prediction of death, with AUROCs of 0.65–0.93. In previous research reports of more than 500 samples, there were only two reports with pRS AUROC > 0.8, and one of the patients included was limited to peptic ulcer bleeding.
The GBS has also been extensively assessed, in even larger populations than those of pRS.[12,22–23] The GBS was derived based on 1748 patients with in order to predict the need for in-hospital interventions.[19] It has varying performance when predicting death with AUROCs between 0.63 and 0.80. Only three reports showed the AUROC of GBS for predicting death was more than 0.7.[12,23–24] The largest study comparing the performance of pRS and GBS was conducted by Oakland et al. in 10,639 UGIB patients, finding that pRS was no better than GBS in predicting mortality (AUROC 0.67 for GBS and 0.70 for pRS; P = 0.21). [23] In the report of Stanley et al., pRS and GBS were equivalent in predicting mortality.[24] However, the results of Yang et al. and Budimir et al. showed that pRS was more effective than GBS in predicting death,[25–26] which is consistent with what we found in our study. Current guidelines recommend that patients with a GBS ≤ 1 are suitable for early discharge, [7] suggesting that the role of GBS is to identify low-risk patients and help them be discharged from the emergency department.
The AIMS65 score is a relatively simple scoring method developed by using data from 29,222 patients admitted to 187 hospitals in the United States in 2011.[20] They found that AIMS65 reliably predicted mortality (AUROC > 0.8). In the initial external verification, AIMS65 also performed impressively on mortality prediction (AUROC = 0.77).[20] AIMS65 had a relative paucity of external validation studies and performed consistently well at predicting death with AUROCs >0.75 in three studies.[20,12,22] There are conflicting reports on the efficacy of AIMS65. Stanley et al. found that AIMS65 was superior at predicting mortality than GBS (AUROC 0.77 versus 0.64).[22] In a retrospective study of the Chinese population by Gu et al., they also found that AIMS65 is superior to GBS in predicting mortality.[12] However, some studies have reached the opposite conclusion. The ABC score is a newly discovered scoring system and currently lacks large-scale external verification, but it looks attractive because its calculation process is not complicated, and it is applicable to all patients with gastrointestinal bleeding.[21] In our study, we found that the AUROC for predicting the mortality of ABC score and pRS score was >0.7. Although the ABC score verified in our population is less than the equivalent value in the initial study, it is still better than GBS and AIMS65. Different from the previous research results, the performance of AIMS65 scores in our population is not satisfactory. It is speculated that this may be the result of different populations and etiological composition.
Compared with the prediction of mortality, most scores have relatively few studies on rebleeding, and some scores perform little better than chance alone at predicting rebleeding.[22–23,25,26,27,28,30] Rebleeding was less well predicted in pRS, with AUROCs consistently of <0.65 in the seven studies that reported this outcome.[23.25] Regarding the prediction of GBS for rebleeding, the results of two researches (Budimir et al. and Bryant et al.) showed the AUROCs to be >7.0.[26,28] In previous studies, the AIMS65 score did not predict rebleeding as well as it predicted mortality, and Stanley et al. reported AUROCs of 0.60 and 0.75 for rebleeding.[22] A study of 433 UGIB patients found that AIMS65 was inferior to GBS at predicting rebleeding.[29] In our study, although the four risk scores are effective in predicting rebleeding, it is consistent with the results of most previous studies, that is, the AUROCs of 4 scores are all less than 0.7. It may indicate that it is difficult to directly predict and evaluate whether rebleeding will occur in UGIB people through vital signs or biochemical indicators at admission. We may need to use multiple risk scores to predict different results of UGIB, or develop new models to improve methodology.
To date, this study is a large-scale, multicentre, real-world study of the upper gastrointestinal bleeding population in China. Unlike the 30-day mortality or in-hospital mortality selected by most previous studies, we chose the 90-day mortality and rebleeding rate as the outcome indicators in this study to avoid missing information on patients with adverse events after discharge.
When acute UGIB patients are admitted to the hospital, most of them will receive high-density treatments (such as fluid resuscitation, high-dose proton pump inhibitor therapy, and blood transfusion), therefore pateints’ biochemistry laboratory test results may change drastically over the course of their ED stay. With the progression of any UGIB bleeding and continuous clinical interventions, it may not be practical to directly predict the patient's prognosis based on the risk score calculated based on the results at the time of admission. Nevertheless, some critically ill patients with unstable hemodynamics cannot complete endoscopy during hospitalization, so if we absolutely rely on the results of endoscopy to judge the prognosis, there will be a bias. These also explain that in the 30 years since the RS was fist delineated, although there have been many risk scores for assessing prognosis or hospital intervention of UGIB patients, the effectiveness of these scores has been variable in different external validation populations without a clearly superior score.
In the future, we may need to develop a new predictive model that can better identify high-risk UGIB patients when they are admitted to the hospital, and it can monitor the progress of the disease in real time, which would be of more practical help to clinical practice.
The main limitation of this study is that this is a real-world study, and all clinical management decisions are still made by the responsible physician. Although the participating hospitals in the study adopted standard clinical management protocols, some treatment options may differ between different cases. In addition, we did not collect the specific number of days of treatment the patients had before dying (i.e. mortality was a binary variable) in our study. Perhaps if we had more variables, we could have provided even more granular data comparing the four scoring systems. We hope that future studies can further examine the performance of these scoring systems.
CONCLUSION
The pRS, GBS, AIMS65, and ABC scores were all acceptable for predicting 90-day death or rebleed among UGIB patients in a large Chinese population. The pRS and ABC scores performed better than GBS and AIMS65 scores in predicting the 90-day death and rebleeding. All four scoring systems had only moderate efficacy for predicting rebleeding.
REFERENCES
- 1.Kamboj AK, Hoversten P, Leggett CL. Upper Gastrointestinal Bleeding: Etiologies and Management. Mayo Clin Proc. 2019;94:697–703. doi: 10.1016/j.mayocp.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Fouad TR, Abdelsameea E, Abdel-Razek W, Attia A, Mohamed A, Metwally K. et al. Upper gastrointestinal bleeding in Egyptian patients with cirrhosis: Post-therapeutic outcome and prognostic indicators. J Gastroenterol Hepatol. 2019;34:1604–10. doi: 10.1111/jgh.14659. [DOI] [PubMed] [Google Scholar]
- 3.Christensen S, Riis A, Norgaard M, Sorensen HT, Thomsen RW. Short-term mortality after perforated or bleeding peptic ulcer among elderly patients: a population-based cohort study. BMC Geriatr. 2007;7:8. doi: 10.1186/1471-2318-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327–35. doi: 10.1136/gut.2010.228437. [DOI] [PubMed] [Google Scholar]
- 5.Laine L, Yang H, Chang SC, Datto C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol. 2012;107:1190–5. doi: 10.1038/ajg.2012.168. [DOI] [PubMed] [Google Scholar]
- 6.Zhong M, Chen WJ, Lu XY, Qian J, Zhu CQ. Comparison of three scoring systems in predicting clinical outcomes in patients with acute upper gastrointestinal bleeding: a prospective observational study. J Dig Dis. 2016;17:820–8. doi: 10.1111/1751-2980.12433. [DOI] [PubMed] [Google Scholar]
- 7.Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M. et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2015;47:a1–46. doi: 10.1055/s-0034-1393172. [DOI] [PubMed] [Google Scholar]
- 8.Sung JJ, Chiu PW, Chan FKL, Lau JY, Goh KL, Ho LH. et al. Asia-Pacific Working group consensus on non-variceal upper gastrointestinal bleeding: an update 2018. Gut. 2018;67:1757–68. doi: 10.1136/gutjnl-2018-316276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barkun AN, Almadi M, Kuipers EJ, Laine L, Sung J, Tse F. et al. Management of Nonvariceal upper gastrointestinal bleeding: guideline recommendations from the International consensus group. Ann Intern Med. 2019;171:805. doi: 10.7326/M19-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crooks C, Card T, West J. Reductions in 28-day mortality following hospital admission for upper gastrointestinal hemorrhage. Gastroenterology. 2011;141:62–70. doi: 10.1053/j.gastro.2011.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abougergi MS, Travis AC, Saltzman JR. The in-hospital mortality rate for upper GI hemorrhage has decreased over 2 decades in the United States: a nationwide analysis. Gastrointest Endosc. 2015;81:882–8.e1. doi: 10.1016/j.gie.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Gu L, Xu F, Yuan J. Comparison of AIMS65, Glasgow-Blatchford and Rockall scoring approaches in predicting the risk of in-hospital death among emergency hospitalized patients with upper gastrointestinal bleeding: a retrospective observational study in Nanjing, China. BMC Gastroenterol. 2018;18:98. doi: 10.1186/s12876-018-0828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Y, Shen J, Zhang F, Zhou X, Tang Z, You T. Scoring systems used to predict mortality in patients with acute upper gastrointestinal bleeding in the ED. Am J Emerg Med. 2018;36:27–32. doi: 10.1016/j.ajem.2017.06.053. [DOI] [PubMed] [Google Scholar]
- 14.Lassen A, Hallas J, Schaffalitzky de Muckadell OB. Complicated and uncomplicated peptic ulcers in a Danish county 1993–2002: a population-based cohort study. Am J Gastroenterol. 2006;101:945–53. doi: 10.1111/j.1572-0241.2006.00518.x. [DOI] [PubMed] [Google Scholar]
- 15.Chiu PW, Ng EK. Predicting poor outcome from acute upper gastrointestinal hemorrhage. Gastroenterol Clin North Am. 2009;38:215–30. doi: 10.1016/j.gtc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Shung DL, Au B, Taylor RA, Tay JK, Laursen SB, Stanley AJ. et al. Validation of a Machine Learning Model That Outperforms Clinical Risk Scoring Systems for Upper Gastrointestinal Bleeding. Gastroenterology. 2020;158:160–7. doi: 10.1053/j.gastro.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bardou M, Barkun AN. Editorial: how can we best promote the routine use of scores that are accurate at predicting outcomes in patients with upper gastrointestinal bleeding? Aliment Pharmacol Ther. 2020;51:305–6. doi: 10.1111/apt.15574. [DOI] [PubMed] [Google Scholar]
- 18.Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316–21. doi: 10.1136/gut.38.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318–21. doi: 10.1016/S0140-6736(00)02816-6. [DOI] [PubMed] [Google Scholar]
- 20.Saltzman JR, Tabak YP, Hyett BH, Sun X, Travis AC, Johannes RS. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc. 2011;74:1215–24. doi: 10.1016/j.gie.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Laursen SB, Oakland K, Laine L, Bieber V, Marmo R, Redondo-Cerezo E. et al. ABC score: a new risk score that accurately predicts mortality in acute upper and lower gastrointestinal bleeding: an international multicentre study. Gut. 2021;70:707–16. doi: 10.1136/gutjnl-2019-320002. [DOI] [PubMed] [Google Scholar]
- 22.Stanley AJ, Laine L, Dalton HR, Ngu JH, Schultz M, Abazi R. et al. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study. BMJ. 2017;356:i6432. doi: 10.1136/bmj.i6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oakland K, Kahan BC, Guizzetti L, Martel M, Bryant RV, Brahmania M. et al. Development, validation, and comparative assessment of an international scoring system to determine risk of upper gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2019;17:1121e9. doi: 10.1016/j.cgh.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 24.Stanley AJ, Dalton HR, Blatchford O, Ashley D, Mowat C, Cahill A. et al. Multicentre comparison of the Glasgow Blatchford and Rockall Scores in the prediction of clinical end-points after upper gastrointestinal haemorrhage. Aliment Pharmacol Ther. 2011;34:470e5. doi: 10.1111/j.1365-2036.2011.04747.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang HM, Jeon SW, Jung JT, Lee DW, Ha CY, Park KS. et al. Comparison of scoring systems for nonvariceal upper gastrointestinal bleeding: a multicenter prospective cohort study. J Gastroenterol Hepatol. 2016;31:119e25. doi: 10.1111/jgh.13057. [DOI] [PubMed] [Google Scholar]
- 26.Budimir I, Stojsavljevic S, Barsic N, Biscanin A, Mirosevic G, Bohnec S. et al. Scoring systems for peptic ulcer bleeding: which one to use? World J Gastroenterol. 2017;23:7450e8. doi: 10.3748/wjg.v23.i41.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Custodio Lima J, Garcia Montes C, Kibune Nagasako C, Soares Ruppert Reis GF, Meirelles Dos Santos JO, Guerrazzi F. et al. Performance of the Rockall scoring system in predicting the need for intervention and outcomes in patients with nonvariceal upper gastrointestinal bleeding in a Brazilian setting: a prospective study. Digestion. 2013;88:252e7. doi: 10.1159/000356313. [DOI] [PubMed] [Google Scholar]
- 28.Bryant RV, Kuo P, Williamson K, Yam C, Schoeman MN, Holloway RH. et al. Performance of the Glasgow-Blatchford score in predicting clinical outcomes and intervention in hospitalized patients with upper GI bleeding. Gastrointest Endosc. 2013;78:576e83. doi: 10.1016/j.gie.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Curdia Goncalves T, Barbosa M, Xavier S, Boal Carvalho P, Firmino Machado J, Magalhaes J. et al. Optimizing the risk assessment in upper gastrointestinal bleeding: comparison of 5 scores predicting 7 outcomes. GE Port J Gastroenterol. 2018;25:299e307. doi: 10.1159/000486802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oakland K. Risk stratification in upper and upper and lower GI bleeding: Which scores should we use? Best Pract Res Clin Gastroenterol. 2019;42–43:101613. doi: 10.1016/j.bpg.2019.04.006. [DOI] [PubMed] [Google Scholar]


