Abstract
Despite widespread use of conventional diagnostic methods in orthopaedic applications, limitations still exist in detection and diagnosing many pathologies especially at early stages when intervention is most critical. The use of biomaterials to develop diagnostics and theranostics, including nanoparticles and scaffolds for systemic or local applications, has significant promise to address these shortcomings and enable successful clinical translation. These developments in both modular and holistic design of diagnostic and theranostic biomaterials may improve patient treatments for myriad orthopaedic applications ranging from cancer to fractures to infection.
Clinical Rationale for Orthopaedic Diagnostics and Theranostics
Orthopaedic diseases and disorders are the second leading cause of disability worldwide and result in $880 billion in direct healthcare costs annually in the US alone [1,2]. As life expectancy increases, bone-related diseases and disorders have become increasingly prevalent. This trend will have significant impact on the rates of conditions such as osteoporosis, which affects >50% of Americans over the age of 50 [3].
Early and efficient detection and treatment of bone-related diseases and disorders is important to limit morbidity and mortality. For example, metastasis of aggressive cancers, such as osteosarcoma, severely impacts survival rates. Indeed, osteosarcoma survival rates fall from 70–80% to only 20–30% after metastatic progression [4]. Early diagnosis is critical for improving overall prognosis, as time to osteosarcoma diagnosis is correlated with greater rates of metastases and poorer prognosis [5,6]. Furthermore, early initiation of treatment can delay onset, number, and size of pulmonary metastases, increase the success of tumor resections, and improve overall survival [4,7,8]. Additionally, bone metastases from lung, prostate, and breast cancer require early detection to circumvent severe complications such as pathologic fractures or spinal cord compression [9].
Orthopaedic imaging plays a key role in current clinical diagnoses and disease progression and/or treatment monitoring. Modalities such as radiographs (x-rays), computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and nuclear imaging studies, including scintigraphy, single-photon emission CT (SPECT), and positron emission tomography (PET), are the mainstays of bone-related diagnostics. These techniques have distinct advantages and limitations, as detailed in Table 1. Specific to orthopaedics, features of bone-related disorders can be nonspecific and overlap with other etiologies, making accurate diagnosis a challenge [10]. Surgical management of bone-related pathologies can lead to anatomic changes and often requires hardware, which can also significantly interfere with image quality, limiting treatment monitoring [10]. Moreover, traditional diagnostic techniques may have a limited ability to detect bone disorders at early stages when intervention is most efficacious at preventing or mitigating progression [10,11].
Table 1:
Advantages and Disadvantages of Clinical Diagnostic Modalities*
| Modality | Advantages | Disadvantages |
|---|---|---|
| Radiography | High spatial resolution | Uses ionizing radiation (minor exposure) |
| (x-ray) | Portability | Superimposition of structures |
| Less expensive | Lower sensitivity | |
| CT | Volumetric data with multiplanar/3D reconstructions | Uses ionizing radiation (moderate exposure) |
| High spatial resolution | Lower soft tissue contrast compared to MRI | |
| Gives detailed information about complex fractures | Less useful for soft tissue evaluation | |
| Few contraindications when compared to MRI | ||
| MRI | High soft tissue contrast/spatial resolution | Expensive |
| Does not use ionizing radiation | Longer scanning time | |
| Better for evaluation of occult fractures, infections, articular cartilage, ligaments, and soft tissues | Image artifact from metal surgical implants | |
| Contraindications (pacemakers, claustrophobia, etc.) | ||
| Ultrasound | Does not use ionizing radiation | Operator dependent |
| Less expensive | Limited evaluation of deep structures | |
| Portability | Lower resolution | |
| Ability for dynamic examinations and procedural guidance | ||
| Nuclear Medicine (Scintigraphy, SPECT, PET) | Accessibility to functional information | Uses ionizing radiation/radiotracers |
| High sensitivity | Low specificity | |
| Low spatial resolution/anatomic localization | ||
| Expensive |
Adapted from Kamar and Hayashi (2016) [12]
CT=Computed tomography; MRI=Magnetic resonance imaging; SPECT=Single-photon emission computed tomography; PET=Positron emission tomography
Given the limitations of traditional bone diagnostic techniques, there is clear clinical rationale for developing advanced diagnostics and theranostics. In particular, biomaterials designed to target or localize to bone or disease sites are promising to increase the precision and prognostic value for cancer, osteoporosis, non-unions, fracture, and/or infection (Figure 1A). Furthermore, orthopaedic theranostics may have even greater clinical impact. Multimodal imaging can be employed to overcome the limitations of individual modalities, mediated through the use of multifunctional biomaterials. Herein, we discuss current advances and the promise in future biomaterials designs that can be used systemically or locally for diagnosing and treating for bone-related disorders (Figure 1B, C).
Figure 1: Overview of clinical needs for diagnostic/theranostic biomaterials and example materials.
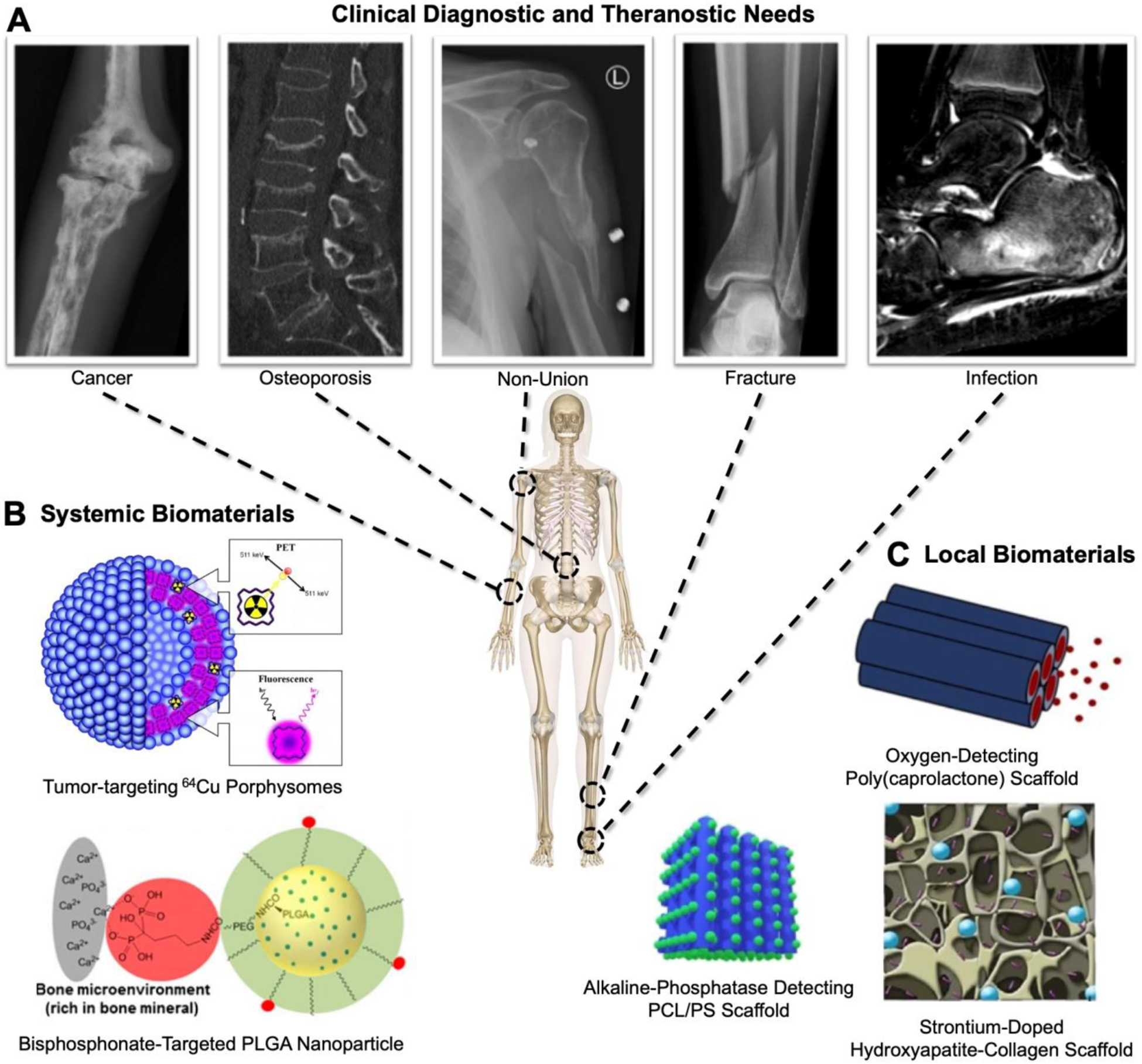
A) Representative clinical images of orthopaedic applications. B, C) Systemic and local biomaterials highlighted in this review. PLGA: Poly(lactic acid-co-glycolic acid), PCL/PS: Poly(caprolactone)/calcium silicate. Porphysome is reproduced from [13] (https://pubs.acs.org/doi/10.1021/nn400669r) with permission. Further permission related to the material excerpted should be directed to the ACS. BP-targeted PLGA Nanoparticles reprinted with permission from [14]: Low SA, Galliford CV, Yang J, Low PS, Kopeček J. Biodistribution of Fracture-Targeted GSK3β Inhibitor-Loaded Micelles for Improved Fracture Healing. Biomacromolecules. 2015;16(10):3145–53. doi: 10.1021/acs.biomac.5b00777. Copyright 2015 American Chemical Society. Alkaline phosphatase detecting PCL/PS Scaffold Originally published in [15] reprinted from Chemical Engineering Journal, 408, Yang C, Gao X, Younis MR, Blum NT, Lei S, Zhang D, Luo Y, Huang P, J Lin J, Non-invasive monitoring of in vivo bone regeneration based on alkaline phosphatase-responsive scaffolds, 127959, Copyright 2021, with permission from Elsevier under CC BY-NC 4.0 license #5036610232181. Oxygen-detecting poly(caprolactone) scaffolds reprinted with permission from [16]: Schilling K, El Khatib M, Plunkett S, Xue J, Xia Y, Vinogradov SA, Brown E, Zhang X. Electrospun Fiber Mesh for High-Resolution Measurements of Oxygen Tension in Cranial Bone Defect Repair. ACS Appl Mater Interfaces. 2019 Sep 18;11(37):33548–33558. Copyright 2019 American Chemical Society. Strontium-doped hydroxyapatite-collagen scaffold image [17] is reproduced under CC BY-NC 4.0 license #5036610940442. Center skeleton is from [18].
Biomaterials design considerations for bone diagnostics and theranostics
Biomaterials, including polymers [19,20], liposomes [13,21], gold nanoparticles (NPs) [22–24], mesoporous silica (MeSi) NPs [25–27], quantum dots (QDs) [28,29], upconversion (UC) NPs [30–32], hydroxyapatite [33–39], and superparamagnetic or ferromagnetic elements: nickel, iron (SPIONs), cobalt, and their combinations [40–42], are commonly used for diagnostics and theranostics (Figure 2, Table 2). Indeed, a recent review discusses diagnostic and theranostic biomaterials development for bone tumors [43]. However, far fewer approaches exist for other orthopaedic applications, which have unique diagnostic and theranostic requirements including deep and dense tissue penetration, robust, tissue-specific accumulation, and confounding factors from implants or other treatments. Diagnosing osteoporosis, for example, requires methods that can accurately measure skeletal biomechanics and physiochemical properties (i.e., size, shape, structural properties), while diagnosing osteomyelitis may be challenged by orthopaedic hardware and anatomical location [44]. These requirements are met through multifunctional biomaterials designed for both systemic administration and local applications that primarily enhance and extend the capabilities of existing orthopaedic diagnostic techniques.
Figure 2: Schematic representation of systemically delivered NP-based biomaterials used in diagnostics and theranostic for orthopaedic applications detailing the features that can be modulated.
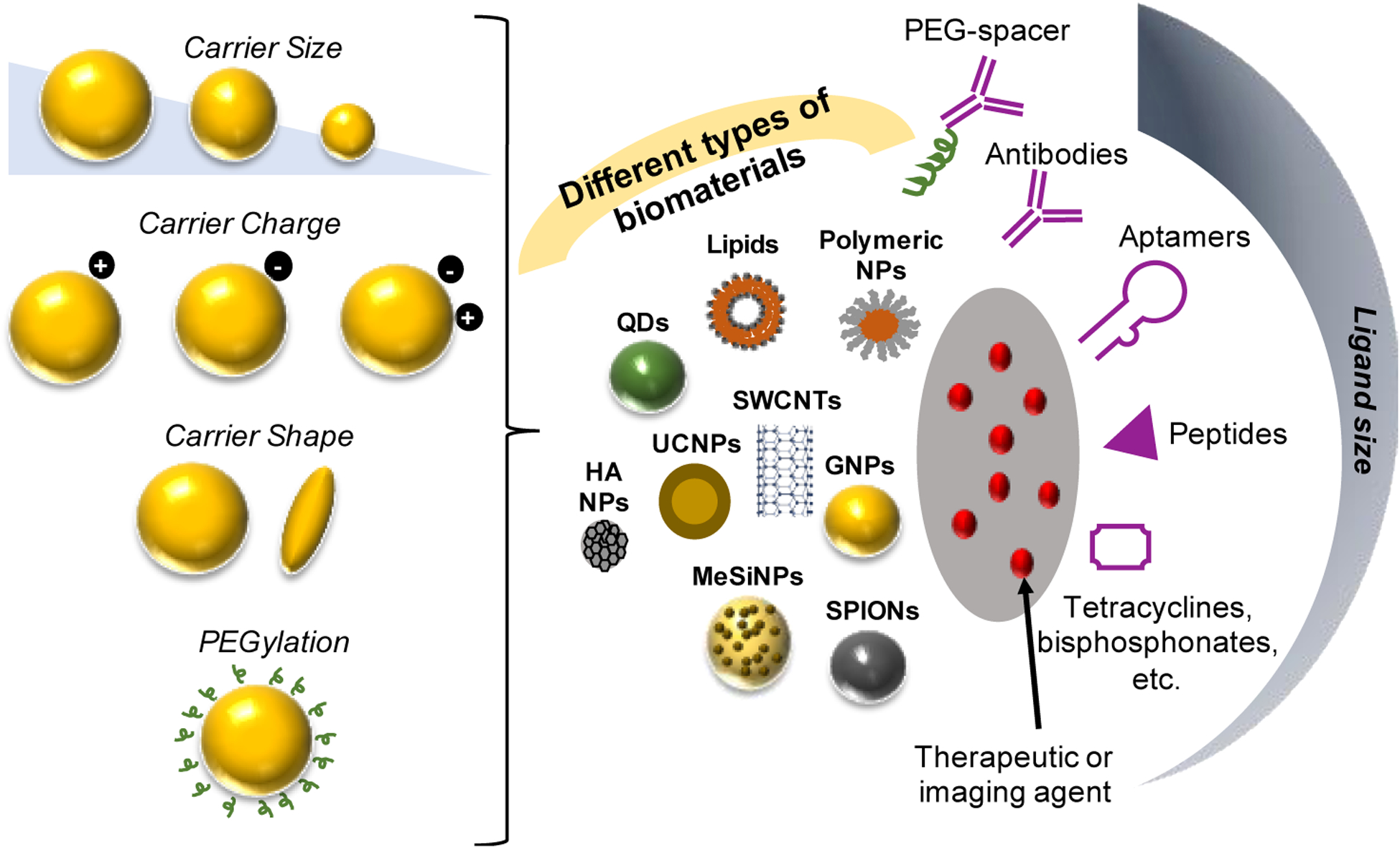
SWCNT, single-walled carbon nanotubes; QDs, quantum dots; UCNPs, upconversion nanoparticles; GNPs, gold nanoparticles, SPIONs, superparamagnetic nanoparticles, MeSiNPs, mesoporous silica nanoparticles; HA NPs, hydroxyapatite nanoparticles. Figure adapted from [49].
Table 2:
Advantages and Limitations of Existing and Emerging Biomaterials Diagnostics and Theranostics [50–57]
| Biomaterial | Advantages | Limitations |
|---|---|---|
| Polymers | Flexible compositions, functionalities, and morphologies to enable drug/tracer incorporation and targeting | Translation can be challenging due to difficult scale-up of manufacturing processes |
| Tunable size, shape, responsive behavior, and degradation | No inherent diagnostic capabilities | |
| Lipid-based NPs | Flexible compositions, functionalities, and morphologies to enable drug/tracer incorporation and targeting | No inherent diagnostic capabilities |
| Gold NPs | Favorable photostability | Limited surface area for drug/tracer, targeting group, and stabilizing molecule incorporation |
| Tunable absorptive/scattering properties based on size and shape | Rapid clearance | |
| Excellent biocompatibility | Toxicity | |
| Simple conjugation of targeting groups and/or therapeutic molecules via thiol chemistry | ||
| Superparamagnetic iron oxide (SPIONs) | Biocompatible | Dose accumulation over time leading to toxicity |
| Exhibit in vivo biodistribution tunability based on size and surface modifications | ||
| Detectable via MRI, which enables robust resolution | ||
| Induction of localized heating in magnetic fields | ||
| Upconversion nanoparticles (UCNPs) | Absorb multiple photons to produce high energy anti-Stokes luminescence | Non-negligible heating of exposed tissues |
| Minimal tissue autofluorescence | Low brightness relative to other NIR fluorescent probes | |
| Enable multiplexed imaging | ||
| Highly resistant to photobleaching | ||
| Quantum dots | High quantum yield | Sometimes formed from cytotoxic elements |
| Photostable | Rapid clearance | |
| High signal-to-noise ratio | ||
| Simultaneous excitation of multiple wavelengths | ||
| Hydroxyapatite | Biocompatible | Rapid clearance |
| Bioactive | ||
| Osteoconductive | ||
| Excellent drug loading capabilities | ||
| Mesoporous silica | High cargo loading capacity, controllable release | Cargo leakage |
| NPs | Excellent chemical, thermal, and mechanical stability | Biocompatibility and toxicity |
| Tunable size and porosity | ||
| Flexible morphology |
Systemically administered biomaterials for bone diagnostics and theranostics
Systemically administered biomaterials, often in the form of NPs, are commonly employed for bone diagnostics/theranostics (Figure 2 and Table 2). NPs have many advantages as diagnostic/theranostic biomaterials platforms. NPs can be systemically administered noninvasively and, if properly designed, can achieve tissue specificity through targeting moieties [43]. NPs are inherently multifunctional and/or modular to enable tissue targeting/selectivity, enable sustained and/or responsive drug release, and protect sensitive drug payloads from degradation [45]. NP chemistry can also be altered to modulate circulation half-life, for example, through poly(ethylene glycol) functionalization [46,47]. NPs can be developed to deliver synergistic, multidrug combinations of myriad drug cargos (hydrophobic or hydrophilic drugs/contrast agents, nucleic acids, etc.), and/or to localize heating or radiation in conjunction with multimodal imaging [45]. Additionally, some NP compositions, namely metallic NPs, are inherently magnetic or fluorescent for imaging [48].
Facile incorporation of bone targeting, either through passive or active mechanisms, is another advantage of NPs that can lead to the specificity and contrast necessitated by bone diagnostics/theranostics (Figure 2). Indeed, systemically delivered small molecule biodistribution to bone is poor with <1% of doses successfully reaching bone [14]. Tissue specificity arises from the incorporation of targeting moieties and/or enhanced permeability and retention (EPR) due to tumor growth, infection, or injury [58–61]. For example, first generation small molecule radiotracer-based bone diagnostics were developed based on molecules with bone affinity including 18F-NaF, 99mTc-methylenediphosphonate (MDP), and 99mTc-hydroxymethylenediphosphonate [62–66]. Similarly, facile incorporation of bone targeting moieties is a key asset of NPs to provide specificity and contrast necessitated by bone diagnostics/theranostics (Figure 2). For bone targeting of NPs, bisphosphonates (BP) are commonly used. BP bind generally to bone tissue due to high affinity for hydroxyapatite [67,68] and also to regions of high metabolic activity, such as primary bone tumors (e.g., osteosarcoma) or metastases [69,70]. Other targeting groups have been explored, including phytic acid, aspartic acid and glutamic acid and (co)polypeptides thereof, tetracycline, as well as aptamers and other peptides with affinity to the bone matrix or relevant cells, as recently reviewed [43].
Macroscale biomaterials for diagnostics and theranostics
Macroscale or bulk biomaterials placed locally within or juxtaposed to bone during surgical procedures also have significant value for diagnostics and theranostics. These include tissue engineering scaffolds, bone fixation devices or implants, and drug reservoirs using a variety of biomaterials including poly(caprolactone), poly(lactic acid), poly(glycolic acid), poly(methyl methacrylate), poly(butyl terephthalate), poly(carbonate), hydroxyapatite, calcium phosphate, and calcium silicate [71,72]. The general design principals and biomaterials used for implantable orthopaedic devices can be found in recent reviews [71–73]. Common orthopaedic biomaterials are adapted for diagnostic/theranostic applications through the incorporation of contrast agents and responsive moieties, which enables sensing of the local microenvironment, therapeutic efficacy, or the rate and/or extent of degradation of scaffolds. Due to localized placement, macroscale biomaterials are subject to different delivery constraints (e.g., surgical placement or injectable localization) compared to systemically administered biomaterials, which can lessen the risk for off-target effects and background and may also enable use of more varied materials with greater quantities/varieties of detection moieties. Diagnostic capabilities are often employed in biomaterials development in the pre-clinical stage to better understand in vivo interactions and provide examples of potential future clinical applications.
Biomaterial Diagnostics and Theranostics in Development for Orthopaedic Applications
The goal of biomaterials-based diagnostics is to improve existing detection modalities, either through enhanced contrast or increased pathological specificity. Examples of diagnostic and theranostic biomaterials developed for both systemic and local orthopaedic applications since the year 2010 are highlighted in Table 3, with selected examples discussed in subsequent sections.
Table 3.
Examples of biomaterials explored for bone diagnostics and theranostics from 2010–2021. Additional examples pertaining to bone tumor diagnostics and diagnostics can be found in the following review [43].
| Biomaterial Type | Diagnostic/Therapeutic Entity | Surface modification | Rationale for Use in Bone Applications | Clinical application | Diagnostic Modality* | Ref |
|---|---|---|---|---|---|---|
| SPIONs | Iron-oxide NPs | 1,5-dihydroxy-1,5,5-tris-phosphono-pentyl-phosphonic acid (di-HMBPs) | High affinity for calcium ions/hydroxyapatite | Osteoporosis | MRI | [74] |
| Iron-oxide NPs | Alendronate | High affinity for hydroxyapatite | Bone metabolic activity | MRI | [75] | |
| 99mTc labeled iron-oxide NPs | Alendronate | High affinity for hydroxyapatite | Bone | SPECT/PET-MRI | [76,77] | |
| 99mTc labeled PEGylated iron-oxide NPs | Bisphosphonate | High affinity for hydroxyapatite | Bone | SPECT/PET-MRI | [77] | |
| Iron-doped hydroxyapatite NPs | N/A | Bone substitute | Bone | SPECT/PET MRI | [34] | |
| Carbon nanotubes | 99mTc labeled carbon nanotubes | Alendronate | High affinity for hydroxyapatite | Active bone metabolism | Photoacoustic imaging | [78] |
| Quantum dots | Quantum dots | Various antibodies | Binding to unique cell populations in bone marrow | Targeted cell imaging | NIR | [79] |
| Ag2S QD/ Doxorubicin | Alendronate | High affinity for hydroxyapatite | Bone tumors | NIR | [80] | |
| Gold NPs | Gold NPs | Glutamic acid | Targets microcracks by chelating with calcium ions | Damaged bone | CT | [81] |
| Gelatin methacrylate -gold NP scaffold | N/A | N/A | Bone defects | CT | [20] | |
| Gold nanorods | Tumor specific oligopeptides | Binding to osteosarcoma cells | Osteosarcoma | Photoacoustic imaging | [82] | |
| Mesoporous silica NPs | Mesoporous silica NPs loaded with ammonia borate | N/A | Nanocomposites respond to acidic environment of the tumor to release H2 improving contrast between osteosarcoma and surrounding healthy bone | Osteosarcoma | CT | [33] |
| Mesoporous silica NPs/ gold nanorods | Zoledronic acid | High affinity for hydroxyapatite | Bone metastasis | Photoacoustic imaging | [83] | |
| Mesoporous silica NPs containing an iron oxide core-immobilized with BMP-2, coated with calcium phosphate/ Bone morphogenic protein 2 (BMP-2) | N/A | Resemblance to native bone | Critical sized defects | MRI | [84] | |
| Mesoporous silica-coated bismuth sulfide NPs/ Doxorubicin | RGD-peptide | Target tumor vasculature and tumor cells | Osteosarcoma | NIR/CT | [85] | |
| Hydroxyapatite | Silicate-substituted HAp doped with Eu(III) and Bi(III)A | N/A | Bone substitute | Bone | Luminescence | [39] |
| Calcium deficient hydroxyapatite scaffold labeled with NIR probe | N/A | Bone substitute | Monitor bone healing | NIR | [86] | |
| Hydroxyapatite NPs/ Ho-166 | N/A | High affinity to bone | Bone cancer | Unclear | [38] | |
| Folic acid modified hydroxyapatite NPs/ 64Cu | Medronic acid (MDP) | High affinity to areas of active bone metabolism | Osteosarcomas and other bone disorders | PET | [36] | |
| Nano-hydroxyapatite rods/ Molybdenum oxide | N/A | Bone substitute | Bone infection | Fluorescence | [37] | |
| Eu(III)/Gd(III) doped hydroxyapatite nanorods/ Ibuprofen (as model drug) | N/A | Bone substitute | Bone | Luminescence/MRI/CT | [35] | |
| Iron-doped hydroxyapatite alginate-gelatin scaffold/ Bone morphogenic protein 2 (BMP- 2) | N/A | Osteoconductive properties of scaffold | Localized monitoring of cell infiltration in bone | MRI | [87] | |
| Upconversion nanoparticles (UCNPs) | 18F labeled NaGdF4:Yb, Er UCNPs | Etidronic acid, alendronic acid, and nitrile (trimethylphosphonic acid) | High affinity for hydroxyapatite | Bone | MRI/PET | [88] |
| Yb(III)/Ho(III) doped UCNPs | N/A | Apatite component of UCNP mimics bone | Bone regeneration | Luminescence | [30] | |
| NaYF4:Yb3+,Er3+ UCNPs | Positive and negative charged polymers | Use for tracking without affecting intrinsic cell properties | Cell localization | Fluorescence | [31] | |
| NaYbF4:Gd3+/Er3+ UCNPs | Iminodiacetate | Chelates exposed calcium ions | Damaged bone | Gemstone spectral CT | [89] | |
| Mesoporous silica-coated upconversion NPs doped with gadolinium (III)/ Plumbagin and poly (acrylic acid) | Zoledronic acid | High affinity for hydroxyapatite | Bone metastasis | MRI/luminescence | [32] | |
| Polymer-based systems | Electrospun polycaprolactone fibers encapsulating PtP-C343 (phosphorescent probe) | N/A | Electrospun fibers mimic ECM | Bone healing | Two-photon phosphorescence lifetime microscopy | [16] |
| PLGA-PEG loaded with iron-oxide NPs and NIR dye | Alendronate | High affinity for hydroxyapatite | Bone | MRI/NIR | [90] | |
| Fluorescein isothiocyanate labeled PEGylated Poly(y-benzyl-l-glutamate)(PBLG) | Alendronate | High affinity for hydroxyapatite | Bone | Fluorescence | [91] | |
| Poly(trimethylene carbonate)-b-poly(glutamic acid) loaded with SPIONS | HER2 antibody | Tumor targeting | Bone metastasis | MRI | [19] | |
| 99mTc labeled polymers | N/A | Binds to bone surface | Bone metastasis | PET | [92] | |
| SPION coated with chitosan-PEG copolymer | HER2 antibody | Binds to metastatic tumor cells | Bone metastasis | MRI | [19] | |
| Polycaprolactone/calcium silicate, (PCL/CS) scaffold labeled with hemicyanine dye | N/A | Alkaline phosphatase (ALP) responsive dye | Monitor bone healing | NIR/photoacoustic imaging | [15] | |
| Poly(lactide-co-glycolide) (PLGA) loaded with SPIONS/ Paclitaxel | Alendronate | High affinity for hydroxyapatite | Bone tumors | MRI | [93] | |
| HAp composite PLGA scaffold/ SPIONs | N/A | Bone substitute | Bone defect | X-ray | [94] | |
| Iron-doped polydopamine NPs/ 7-ethyl-10- hydroxycamptothecin (SN38) | Alendronate | High affinity for hydroxyapatite | Bone tumor/osteolysis | MRI | [95] | |
| Chitosan-grafted PEG copolymer with SPION core | HER2/neu | Tumor targeting | Bone metastasis | MRI | [96] | |
| Lipid-based systems | 64Cu-porphysomes | N/A | Binds to metastatic tumor cells | Bone metastasis | PET/fluorescence | [13] |
| PEGylated liposomes loaded with CdSe QDs and iron oxide NPs | cRGDyk peptide | Binds to metastatic tumor cells | Bone metastasis | MRI/fluorescence | [21] |
Abbreviations: MRI = magnetic resonance imaging; SPIONs = superparamagnetic iron oxide nanoparticles; HAp = hydroxyapatite; NIR = near infrared imaging; PET = positron emission tomography; ECM = extracellular matrix; HER2 = human epidermal growth factor receptor 2; PLGA = poly(lactic acid-co-glycolic acid); PEG = poly(ethylene glycol); Yb = Ytterbium; Ho = Holmium; Gd = Gadolinium; Er = Erbium; Eu = Europium; Bi = Bismuth; CT = computed tomography; RGD = arginine-glycine-aspartic acid.
denotes imaging modality used in the paper but is not an exhaustive list of the potential diagnostic applications.
Systemic Diagnostics and Theranostics
Several examples of orthopaedic diagnostics and theranostics in development involve systemic delivery of biomaterials (Table 3). Systemic diagnostic and theranostics are defined here as technologies injected intravenously, subcutaneously, intradermally, or otherwise. One critical hurdle for systemically delivered diagnostics/theranostics is tissue specificity to ensure high levels of contrast between dysfunctional and healthy tissues for reliable disease detection. Despite this hurdle, a variety of designs developed in the absence of targeting ligands have resulted in dramatic improvements in traditional diagnostics. For example, porphyrin-lipid conjugates combined with small fractions of radioactive 64Cu were used to detect metastatic bone lesions via porphyrin fluorescence and 64Cu PET/CT imaging in a prostate cancer animal model [13]. While traditional PET scans are limited to detection of metastatic lesions larger than 1 cm, the porphysome technology improved sensitivity to 2 mm. Porphysomes also accumulated at naïve bone, suggesting inherent bone diagnostic capabilities, which may be useful for adapting the technology to other orthopaedic applications (e.g., bone mineral density, stress fracture detection). In another example, PEGylated MeSiNPs were used to deliver ammonia borate (AB), which releases H2 in acidic environments, resulting in negative CT contrast [33]. While a clinical contrast agent was unable to differentiate between osteosarcoma and healthy bone in a rat model, intravenous injection of MeSiNPs enabled 20x greater contrast on CT [33], therefore highlighting the utility of MeSiNPs-AB diagnostics.
To achieve the goal of high tissue specificity and diagnostic or theranostic sensitivity, bone-targeted biomaterials have been developed based on existing clinical diagnostic modalities. For example, gold NPs functionalized with bone-targeting glutamic acid were developed to non-invasively detect bone microdamage, which is indicative of impending bone fracture but is undetectable using x-ray [81]. Ex vivo x-ray revealed preferential binding to damaged bone owing to glutamic acid binding to exposed calcium versus healthy bone tissue, suggesting the utility of gold NPs for bone diagnostics and theranostics. For cell targeting, HER2 antibody conjugated PEGylated polymersomes loaded with SPIONs were used to detect tumor boundaries in bone metastasis compared to untargeted controls in a breast cancer metastasis animal model [19]. Following the idea of tissue targeting, theranostic alendronate-functionalized iron-doped poly(dopamine) NPs were used to image tumor shrinkage via MRI [95]. Compared to untargeted controls, the NPs suppressed tumor growth and reduced osteolytic bone damage in an orthotopic bone tumor model due to combined chemo-photothermal therapy of iron and the therapeutic efficacy of the loaded drug, 7-ethyl-10-hydroxycamptothecin [95]. BP-functionalized, PEGylated 99mTc-SPIONs exhibited enhanced accumulation at bone compared to untargeted controls [77] and leveraged multimodality to overcome SPION sensitivity issues, enabling successful longitudinal monitoring in animal studies. In the absence of the radiolabel, improved BP-PEG-SPION sensitivity was attributed to the small NP size (~20 nm), hydrophilicity of BP and PEG that allow for proton relaxation at the iron oxide NP surface, and the presence of the PEG coating, which prevented aggregation. This approach may be useful to enhance diagnostic capabilities of MRI for detecting osteoporotic bone and bone metabolic activity.
Emerging technologies such as photoacoustic (PA) and NIR imaging combined with biomaterial diagnostics and theranostics present unique opportunities for orthopaedic applications. PA imaging can reach tissue depths up to 5–6 cm with outstanding spatial resolution of ~5 μm. Improved capabilities of PA was exploited using PEGylated gold nanorods targeted with osteosarcoma-specific peptides to visualize neovascularization with high contrast via PA imaging, enabling differentiation between tumor and healthy tissue [97]. To diagnose and treat bone metastasis in an animal model of breast cancer, gold nanorods were incorporated within MeSiNPs functionalized with zoledronic acid and imaged photoacoustically [83]. MicroCT revealed tumor size, and osteolysis was reduced compared to untreated controls. NIR imaging allows for deep tissue penetration with minimal tissue autofluorescence. To diagnose and treat bone metastases, zoledronic acid targeted MeSi-coated UCNPs doped with gadolinium were injected in a bone metastatic breast cancer model [32]. Upconversion luminescence and NIR imaging revealed that loaded UCNPs reduced tumor size compared to empty UCNP controls. The theranostic effects of UCNPs were further verified using microCT, which revealed reduced osteolysis [32]. Similarly, QDs have been incorporated within cell-targeted NPs to characterize the heterogeneous bone marrow cellular repertoire [79]. To achieve dual-modality, RGD-functionalized liposomes co-loaded with iron-oxide and CdSe QDs were developed [21]. In a prostate cancer bone metastasis model, QD MRI signal was 1.5-fold higher than iron-oxide NPs alone and tumor fluorescence was higher than untargeted liposomes. QDs have also been used to detect prostate cancer-related delta/notch-like epidermal growth factor-related receptor expression, which has prognostic value for bone metastases [98]. Core-shell silica NPs (C dots) are emerging as alternative materials to quantum dots [99]. This class of biomaterials covalently integrate organic fluorophores into the core of core-shell silica NPs resulting in enhanced brightness (~2–3x vs. QDs) and photostability. For example, C dots were recently used to label cancer cells to study early-stage bone metastasis [100].
Local Diagnostics and Theranostics
Implantable biomaterials are adapted to local diagnostics or theranostics through the addition of therapeutics or contrast agents (Figure 1). There are several recent examples of bone diagnostics incorporated within traditional tissue engineering scaffolds. Electrospun poly(caprolactone) scaffolds encapsulating porphyrin-based sensors for oxygen tension, which is correlated with healing, have been used to monitor bone regeneration using two-photon microscopy [16]. A limitation of this approach is that sensitivity may decrease in humans in which scaffold placement will be deeper versus murine models. In an alternative system, MRI was used to image gadolinium-doped HA NPs incorporated into electrospun poly(caprolactone) scaffolds to track in vitro bone regeneration [101] as well as nano-hydroxyapatite-Alginate-Gelatin scaffolds incorporating SPIONs for detection of cellular infiltration and scaffold mineralization [87]. Bone morphogenic protein 2 was immobilized within iron oxide core mesoporous silica beads and incorporated within a calcium phosphate cement, enhancing contrast and improving bone regeneration for up to 8 weeks following theranostic implantation [84]. Finally, gold NPs entrapped within gelatin methacrylate (GelMA) hydrogels were developed to enable imaging during the process of bone regeneration within a condyle defect [20], enabling greater resolution of bone microarchitecture compared to GelMA hydrogels alone. The ability to assess bone regeneration and/or integration using engineered scaffolds can improve monitoring and enable earlier detection of treatment failures.
Future Directions
Biomaterials-based diagnostics and theranostics have great promise for orthopaedic applications. However, significant opportunities and challenges remain. Refinement of biomaterials designed for diagnostics and theranostics is necessary to meet the demands of orthopaedic applications. In general, conventional theranostic and diagnostic biomaterials are combinations of existing technologies. This modular approach that integrates therapeutic, diagnostic, and targeting moieties enables a high degree of tunability in the resulting diagnostic/theranostic at the cost of complexity. This principle also applies to diagnostic agents embedded within biomaterials for local applications, which allows independent tuning of the scaffold properties and detection modalities. Complementary to this trend of isolating functionalities is that of more holistic design tailored to orthopaedic diagnostics/theranostics. As opposed to adapting existing formulations for additional diagnostic/theranostic functionality, inherently multifunctional biomaterials allow highly integrated designs with fewer components that may ultimately allow for greater reproducibility in design, manufacturing, and testing. An example of holistic design is 64Cu-porphysome technology, where the biomaterial complexes with Cu and is also fluorescent. Not only will holistic design likely provide greater efficacy but may also streamline FDA approval due to its simplicity.
Several difficult to detect orthopaedic conditions are well suited for diagnosis and treatment with high-contrast and high-specificity NPs, including tumors, osteomyelitis, and osteoporosis. Nevertheless, myriad challenges remain for systemically administered biomaterials including NPs. For example, bone possesses unique barriers to NP delivery including limited vascularization, large volume, and high density. Bone can also have disease/disorder-specific challenges to delivery, including sub-micrometer canaliculi, which can serve as a reservoir for bacteria [102,103]. More generally, NP protein adsorption reduces targeting efficacy and shifts biodistribution to the reticuloendothelial system, which contributes to poor bone accumulation [70]. Improvements in surface chemistry to modulate protein adsorption and maintain targeting specificity, which have recently been reviewed elsewhere [104–106], are critical to improve diagnostic/theranostic resolution as well as safety and efficacy.
For local applications, the addition of diagnostic or theranostic capabilities to conventional orthopaedic implants/treatments also has great potential benefit. In particular, augmenting local biomaterials or implants would enable rapid intervention and/or minimize the need for additional invasive procedures. Incorporating sensing functionalities within surgical implants may enable early detection of complications such as poor graft integration, infection, nonunion, or implant loosening. Vascularization and host cell infiltration are important metrics for successful tissue integration/regeneration [107,108] and valuable to inform clinical decision-making regarding need for revision surgery. However, current clinical diagnostics lack resolution to enable imaging of microvessels and cells in vivo [109,110]. Similarly, implant-associated bacterial infections are difficult to detect due to limited specificity of existing diagnostic modalities versus normal post-surgical inflammation [111]. Incorporating bacteria-responsive materials within implants or antibiotic PMMA beads could allow for enhanced infection detection and treatment post-operatively [112]. While these technologies have not yet been developed, locally delivered theranostics represent an important area of exploration in the future.
Diagnostic requirements differ between research and clinical applications. Many of the biomaterials described here have fluorescent detection modalities, which provide unique diagnostic information including bacterial detection [37] and metabolite or protein sensing [16,86]. While efficacious for research purposes, fluorescence is not a standard clinical detection modality and is limited by tissue penetration. Rather, x-rays/CT, magnetic imaging, and ultrasound are clinical mainstays that can be augmented to improve contrast and specificity for orthopaedic applications through development of diagnostic and theranostic biomaterials.
Clinical translation of the orthopaedic diagnostic and theranostic biomaterials discussed here will be challenging. Nanoparticle diagnostic agents have been used clinically for other applications and provide a road map for orthopaedic applications [113]. However, the newness (>95% of publications to date with “theranostics” as a keyword in Web of Science are from 2012 or later) and complexity of theranostics makes their path to regulatory approval unclear. Early theranostics relied on relatively simple radioactive payloads and chelating agents and do not provide good models for the translation of more complex biomaterials approaches necessitated by orthopaedic applications. Nevertheless, for successful translation of orthopaedic diagnostics or theranostics, demonstration of safety and efficacy in large animal models is critical due to the difficulties associated with imaging in bone due to depth, complexity, and size. As the biomaterials field continues to grow, the goal is to integrate the advantages offered through the systems described herein to advance orthopaedic diagnostics and theranostics and ultimately improve patient quality of life.
Conclusions
Early diagnosis and treatment of orthopaedic pathologies is important to treat diseases and injuries effectively and limit undue morbidity. However, traditional diagnostic modalities are subject to limitations, prompting the need for advances in orthopaedic diagnostic and theranostic biomaterials. Bone presents unique challenges for diagnostics and theranostics compared to other tissues due to its unique composition and limited accessibility. Several promising biomaterials strategies have emerged for bone diagnostic and theranostic applications, though work in this area is still relatively new. While current clinically approved applications are limited, the rapid development and expansion of diagnostic and therapeutic modalities within biomaterials offers tremendous promise for clinical use.
Acknowledgements
This work was supported by the National Science Foundation (CAREER Award CBET1450987 and DMR2103553 (to D.S.W.B.)), National Institutes of Health (NIH) P30 AR069655, R01 AR064200, R01 AR056696 (to D.S.W.B.), R01 AR071363 (to R.C.), F31 CA228391 (to M.A.F.), F31 AR076874 (to C.O.), Orthopaedic Research and Education Foundation Grant 20-072 (to B.E.H. and D.S.W.B.), Orthopaedic Trauma Association Grant 6272 (to B.E.H. and D.S.W.B.), University of Rochester CTSA award number UL1 TR002001 (to D.S.W.B.), University Research Award (to D.S.W.B.), and a UR Drug Discovery Grant (to D.S.W.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
There are no competing interests to declare.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Weinstein SL: The Burden of Musculoskeletal Conditions. J Bone Joint Surg Am 2016, 98:1331. [DOI] [PubMed] [Google Scholar]
- 2.Rasker JJ: Rheumatology in general practice. Br J Rheumatol 1995, 34:494–497. [DOI] [PubMed] [Google Scholar]
- 3.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B: The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 2014, 29:2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson JL, Turner SP: Bone Cancer: Diagnosis and Treatment Principles. Am Fam Physician 2018, 98:205–213. [PubMed] [Google Scholar]
- 5.Kim MS, Lee SY, Cho WH, Song WS, Koh JS, Lee JA, Yoo JY, Shin DS, Jeon DG: Prognostic effects of doctor-associated diagnostic delays in osteosarcoma. Arch Orthop Trauma Surg 2009, 129:1421–1425. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida S, Celaire J, Pace C, Taylor C, Kaneuchi Y, Evans S, Abudu A: Delay in diagnosis of primary osteosarcoma of bone in children: Have we improved in the last 15 years and what is the impact of delay on diagnosis? J Bone Oncol 2021, 28:100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu J, Zhang C, Zhu K, Zhang L, Cai T, Zhan T, Luo X: Treatment-Related Prognostic Factors in Managing Osteosarcoma around the Knee with Limb Salvage Surgery: A Lesson from a Long-Term Follow-Up Study. Biomed Res Int 2019, 2019:3215824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poudel RR, Tiwari V, Kumar VS, Bakhshi S, Gamanagatti S, Khan SA, Rastogi S: Factors associated with local recurrence in operated osteosarcomas: A retrospective evaluation of 95 cases from a tertiary care center in a resource challenged environment. J Surg Oncol 2017, 115:631–636. [DOI] [PubMed] [Google Scholar]
- 9.Kitagawa Y, Ito T, Mizuno Y, Sudo Y, Kim Y, Tsunoda R, Miyamoto M, Takai S: Challenges in the Diagnosis of bone Metastasis in Patients without a History of Malignancy at Their First Clinic Visit. J Nippon Med Sch 2018, 85:271–278. [DOI] [PubMed] [Google Scholar]
- 10.Math KR, Berkowitz JL, Paget SA, Endo Y: Imaging of Musculoskeletal Infection. Rheum Dis Clin North Am 2016, 42:769–784. [DOI] [PubMed] [Google Scholar]
- 11.Lambers FM, Kuhn G, Muller R: Advances in multimodality molecular imaging of bone structure and function. Bonekey Rep 2012, 1:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar Y, Hayashi D: Role of Imaging in Musculoskeletal Care. Curr Phys Med Rehabil Rep 2016:28–36. [Google Scholar]
- 13.Liu TW, MacDonald TD, Jin CS, Gold JM, Bristow RG, Wilson BC, Zheng G: Inherently Multimodal Nanoparticle-Driven Tracking and Real-Time Delineation of Orthotopic Prostate Tumors and Micrometastases. ACS Nano 2013, 7:4221–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low SA, Galliford CV, Yang J, Low PS, Kopeček Ji: Biodistribution of fracture-targeted GSK3β inhibitor-loaded micelles for improved fracture healing. Biomacromolecules 2015, 16:3145–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang C, Gao X, Younis MR, Blum NT, Lei S, Zhang D, Luo Y, Huang P, Lin J: Non-invasive monitoring of in vivo bone regeneration based on alkaline phosphatase-responsive scaffolds. Chemical Engineering Journal 2021, 408. [Google Scholar]; 3D printed poly(caprolactone)/calcium silicate scaffolds loaded with a near-infrared (NIR) dye were developed to detect alkaline phosphatase activity via NIR fluorescence /photoacoustic dual imaging.
- 16.Schilling K, El Khatib M, Plunkett S, Xue J, Xia Y, Vinogradov SA, Brown E, Zhang X: Electrospun Fiber Mesh for High-Resolution Measurements of Oxygen Tension in Cranial Bone Defect Repair . ACS Applied Materials & Interfaces 2019, 11:33548–33558. [DOI] [PMC free article] [PubMed] [Google Scholar]; A multifunctional oxygen tension-reporting poly(caprolactone) electrospun matrix was developed to enable real-time high-resolution 3D mapping of oxygen tension during bone regeneration, which may prove useful to e monitor and augment regenerative strategies.
- 17.Oryan A, Baghaban Eslaminejad M, Kamali A, Hosseini S, Sayahpour FA, Baharvand H: Synergistic effect of strontium, bioactive glass and nano- hydroxyapatite promotes bone regeneration of critical- sized radial bone defects. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2019, 107:50–64. [DOI] [PubMed] [Google Scholar]
- 18.Taylor T: Skeletal System. Edited by: Innerbody Research; 2020. vol 2021.] [Google Scholar]
- 19.Pourtau L, Oliveira H, Thevenot J, Wan Y, Brisson AR, Sandre O, Miraux S, Thiaudiere E, Lecommandoux S: Antibody-Functionalized Magnetic Polymersomes: In vivo Targeting and Imaging of Bone Metastases using High Resolution MRI. Advanced Healthcare Materials 2013, 2:1420–1424. [DOI] [PubMed] [Google Scholar]
- 20.Celikkin N, Mastrogiacomo S, Walboomers X, Swieszkowski W: Enhancing X-ray Attenuation of 3D Printed Gelatin Methacrylate (GelMA) Hydrogels Utilizing Gold Nanoparticles for Bone Tissue Engineering Applications. Polymers 2019, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, Chen Z, Zhu L: cRGD-conjugated magnetic-fluorescent liposomes for targeted dual-modality imaging of bone metastasis from prostate cancer. Journal of Liposome Research 2014, 25:89–100. [DOI] [PubMed] [Google Scholar]
- 22.Boisselier E, Astruc D: Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev 2009, 38:1759–1782. [DOI] [PubMed] [Google Scholar]
- 23.Manohar S, Ungureanu C, Van Leeuwen TG: Gold nanorods as molecular contrast agents in photoacoustic imaging: the promises and the caveats. Contrast Media Mol Imaging 2011, 6:389–400. [DOI] [PubMed] [Google Scholar]
- 24.Seekell K, Crow MJ, Marinakos S, Ostrander J, Chilkoti A, Wax A: Hyperspectral molecular imaging of multiple receptors using immunolabeled plasmonic nanoparticles. Journal of biomedical optics 2011, 16:116003–116003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Zhang Y, Feng N: Mesoporous silica nanoparticles: synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin Drug Deliv 2019, 16:219–237. [DOI] [PubMed] [Google Scholar]
- 26.Mai WX, Meng H: Mesoporous silica nanoparticles: A multifunctional nano therapeutic system. Integr Biol (Camb) 2013, 5:19–28. [DOI] [PubMed] [Google Scholar]
- 27.Tang F, Li L, Chen D: Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater 2012, 24:1504–1534. [DOI] [PubMed] [Google Scholar]
- 28.Larson DR, Zipfel WR, Williams RM, Clark SW, Bruchez MP, Wise FW, Webb WW: Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 2003, 300:1434–1436. [DOI] [PubMed] [Google Scholar]
- 29.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S: Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307:538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Chen H: Yb3+/Ho3+Co-Doped Apatite Upconversion Nanoparticles to Distinguish Implanted Material from Bone Tissue. ACS Applied Materials & Interfaces 2016, 8:27458–27464. [DOI] [PubMed] [Google Scholar]
- 31.DiMasi JA, Grabowski HG, Hansen RW: Innovation in the pharmaceutical industry: New estimates of R&D costs. Journal of Health Economics 2016, 47:20–33. [DOI] [PubMed] [Google Scholar]
- 32.Qiao H, Cui Z, Yang S, Ji D, Wang Y, Yang Y, Han X, Fan Q, Qin A, Wang T, et al. : Targeting Osteocytes to Attenuate Early Breast Cancer Bone Metastasis by Theranostic Upconversion Nanoparticles with Responsive Plumbagin Release. ACS Nano 2017, 11:7259–7273. [DOI] [PubMed] [Google Scholar]
- 33.Meng X, Zhang H, Zhang M, Wang B, Liu Y, Wang Y, Fang X, Zhang J, Yao Z, Bu W: Negative CT Contrast Agents for the Diagnosis of Malignant Osteosarcoma. Advanced Science 2019, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]; To improve osteosarcoma imaging, mesoporous silica nanoparticles were developed to deliver H2 to act as a negative contrast agents and different tumor from healthy tissue
- 34.Adamiano A, Iafisco M, Sandri M, Basini M, Arosio P, Canu T, Sitia G, Esposito A, Iannotti V, Ausanio G, et al. : On the use of superparamagnetic hydroxyapatite nanoparticles as an agent for magnetic and nuclear in vivo imaging. Acta Biomaterialia 2018, 73:458–469. [DOI] [PubMed] [Google Scholar]
- 35.Chen F, Huang P, Zhu Y-J, Wu J, Zhang C-L, Cui D-X: The photoluminescence, drug delivery and imaging properties of multifunctional Eu3+/Gd3+ dual-doped hydroxyapatite nanorods. Biomaterials 2011, 32:9031–9039. [DOI] [PubMed] [Google Scholar]
- 36.Cipreste MF, Mussel WdN, Batista da Silva J, Freitas Marques MBd, Batista RJC, Gastelois PL, Macedo WAdA, Sousa EMBd: A new theranostic system for bone disorders: Functionalized folate-MDP hydroxyapatite nanoparticles with radiolabeled copper-64. Materials Chemistry and Physics 2020, 254. [Google Scholar]
- 37.Placente D, Ruso JM, Baldini M, Laiuppa JA, Sieben JM, Santillán GE, Messina PV: Self-fluorescent antibiotic MoOx–hydroxyapatite: a nano-theranostic platform for bone infection therapies. Nanoscale 2019, 11:17277–17292. [DOI] [PubMed] [Google Scholar]
- 38.Silva F, Almeida J, Oliveira E, Albernaz M, Rossi A, Santos-Oliveira R: Nano-Hydroxyapatite Doped with Ho-166 as Drug Delivery System for Bone Cancer Therapy and Diagnosis: Developing a Theragnostic Radiopharmaceuticals. Anti-Cancer Agents in Medicinal Chemistry 2017, 17:355–358. [DOI] [PubMed] [Google Scholar]
- 39.Targonska S, Sikora M, Marycz K, Smieszek A, Wiglusz RJ: Theranostic Applications of Nanostructured Silicate-Substituted Hydroxyapatite Codoped with Eu3+ and Bi3+ Ions—A Novel Strategy for Bone Regeneration. ACS Biomaterials Science & Engineering 2020, 6:6148–6160. [DOI] [PubMed] [Google Scholar]
- 40.Rosen JE, Chan L, Shieh DB, Gu FX: Iron oxide nanoparticles for targeted cancer imaging and diagnostics. Nanomedicine 2012, 8:275–290. [DOI] [PubMed] [Google Scholar]
- 41.Thorek DL, Chen AK, Czupryna J, Tsourkas A: Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Eng 2006, 34:23–38. [DOI] [PubMed] [Google Scholar]
- 42.Wang YX, Hussain SM, Krestin GP: Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol 2001, 11:2319–2331. [DOI] [PubMed] [Google Scholar]
- 43.Gao X, Li L, Cai X, Huang Q, Xiao J, Cheng Y: Targeting nanoparticles for diagnosis and therapy of bone tumors: Opportunities and challenges. Biomaterials 2021, 265:120404. [DOI] [PubMed] [Google Scholar]; A comprehensive review of biomaterial diagnostics and theranostics designed for bone cancer applications.
- 44.Hake ME, Oh JK, Kim JW, Ziran B, Smith W, Hak D, Mauffrey C: Difficulties and challenges to diagnose and treat post-traumatic long bone osteomyelitis. Eur J Orthop Surg Traumatol 2015, 25:1–3. [DOI] [PubMed] [Google Scholar]
- 45.Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, et al. : Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology 2018, 16:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veronese FM, Pasut G: PEGylation, successful approach to drug delivery. Drug Discov Today 2005, 10:1451–1458. [DOI] [PubMed] [Google Scholar]
- 47.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM: PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Advanced Drug Delivery Reviews 2016, 99:28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dadfar SM, Roemhild K, Drude NI, von Stillfried S, Knuchel R, Kiessling F, Lammers T: Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv Drug Deliv Rev 2019, 138:302–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benoit DSW, Overby CT, Sims KR, Ackun-Farmmer M: Drug Delivery Systems. In Biomaterials Science, edn 4th. Edited by Wagner WR, Sakiyama-Elbert SE, Zhang G, Yaszemski MJ: Academic Press; 2020:1237–1266. [Google Scholar]
- 50.Liechty WB, Kryscio DR, Slaughter BV, Peppas NA: Polymers for Drug Delivery Systems. Annual Review of Chemical and Biomolecular Engineering 2010, 1:149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohammadi-Samani S, Ghasemiyeh P: Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Research in Pharmaceutical Sciences 2018, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arvizo R, Bhattacharya R, Mukherjee P: Gold nanoparticles: opportunities and challenges in nanomedicine. Expert Opinion on Drug Delivery 2010, 7:753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernández-Hernández AA, Aguirre-Álvarez G, Cariño-Cortés R, Mendoza-Huizar LH, Jiménez-Alvarado R: Iron oxide nanoparticles: synthesis, functionalization, and applications in diagnosis and treatment of cancer. Chemical Papers 2020, 74:3809–3824. [Google Scholar]
- 54.Liang G, Wang H, Shi H, Wang H, Zhu M, Jing A, Li J, Li G: Recent progress in the development of upconversion nanomaterials in bioimaging and disease treatment. Journal of Nanobiotechnology 2020, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matea C, Mocan T, Tabaran F, Pop T, Mosteanu O, Puia C, Iancu C, Mocan L: Quantum dots in imaging, drug delivery and sensor applications. International Journal of Nanomedicine 2017, Volume12:5421–5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jafari S: Application of Hydroxyapatite Nanoparticle in the Drug Delivery Systems. Journal of Molecular Pharmaceutics & Organic Process Research 2015, 03. [Google Scholar]
- 57.Bharti C, Gulati N, Nagaich U, Pal A: Mesoporous silica nanoparticles in target drug delivery system: A review. International Journal of Pharmaceutical Investigation 2015, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alakhov V, Klinski E, Lemieux P, Pietrzynski G, Kabanov A: Block copolymeric biotransport carriers as versatile vehicles for drug delivery. Expert Opin Biol Ther 2001, 1:583–602. [DOI] [PubMed] [Google Scholar]
- 59.Torchilin VP: Structure and design of polymeric surfactant-based drug delivery systems. J Control Release 2001, 73:137–172. [DOI] [PubMed] [Google Scholar]
- 60.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC: Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev 2014, 66:2–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Newman MR, Ackun-Farmmer M, Baranello MP, Sheu TJ, Puzas JE, Benoit DSW: Fracture-Targeted Delivery of beta-Catenin Agonists via Peptide-Functionalized Nanoparticles Augments Fracture Healing. ACS Nano 2017, 11:9445–9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jadvar H, Desai B, Conti PS: Sodium 18F-Fluoride PET/CT of Bone, Joint, and Other Disorders. Seminars in Nuclear Medicine 2015, 45:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iagaru AH, Mittra E, Colletti PM, Jadvar H: Bone-Targeted Imaging and Radionuclide Therapy in Prostate Cancer. J Nucl Med 2016, 57:19S–24S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blau M, Nagler W, Bender MA: Fluorine-18: a new isotope for bone scanning. J Nucl Med 1962, 3:332–334. [PubMed] [Google Scholar]
- 65.Messa C, Goodman WG, Hoh CK, Choi Y, Nissenson AR, Salusky IB, Phelps ME, Hawkins RA: Bone metabolic activity measured with positron emission tomography and [18F]fluoride ion in renal osteodystrophy: correlation with bone histomorphometry. J Clin Endocrinol Metab 1993, 77:949–955. [DOI] [PubMed] [Google Scholar]
- 66.Wong KK, Piert M: Dynamic bone imaging with 99mTc-labeled diphosphonates and 18F-NaF: mechanisms and applications. J Nucl Med 2013, 54:590–599. [DOI] [PubMed] [Google Scholar]
- 67.Pan H, Sima M, Kopeckova P, Wu K, Gao S, Liu J, Wang D, Miller SC, Kopecek J: Biodistribution and pharmacokinetic studies of bone-targeting N-(2-hydroxypropyl)methacrylamide copolymer-alendronate conjugates. Mol Pharm 2008, 5:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RGG, Ebetino FH: Novel insights into actions of bisphosphonates on bone: Differences in interactions with hydroxyapatite. Bone 2006, 38:617–627. [DOI] [PubMed] [Google Scholar]
- 69.Swami A, Reagan MR, Basto P, Mishima Y, Kamaly N, Glavey S, Zhang S, Moschetta M, Seevaratnam D, Zhang Y, et al. : Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc Natl Acad Sci U S A 2014, 111:10287–10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thamake SI, Raut SL, Gryczynski Z, Ranjan AP, Vishwanatha JK: Alendronate coated poly-lactic-co-glycolic acid (PLGA) nanoparticles for active targeting of metastatic breast cancer. Biomaterials 2012, 33:7164–7173. [DOI] [PubMed] [Google Scholar]
- 71.Navarro M, Michiardi A, Castano O, Planell J: Biomaterials in orthopaedics. Journal of the royal society interface 2008, 5:1137–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I, Grigolo B: Scaffolds for bone tissue engineering: state of the art and new perspectives. Materials Science and Engineering: C 2017, 78:1246–1262. [DOI] [PubMed] [Google Scholar]
- 73.Brydone A, Meek D, Maclaine S: Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine 2010, 224:1329–1343. [DOI] [PubMed] [Google Scholar]
- 74.Lalatonne Y, Monteil M, Jouni H, Serfaty JM, Sainte-Catherine O, Lièvre N, Kusmia S, Weinmann P, Lecouvey M, Motte L: Superparamagnetic Bifunctional Bisphosphonates Nanoparticles: A Potential MRI Contrast Agent for Osteoporosis Therapy and Diagnostic. Journal of Osteoporosis 2010, 2010:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Panahifar A, Mahmoudi M, Doschak MR: Synthesis and in Vitro Evaluation of Bone-Seeking Superparamagnetic Iron Oxide Nanoparticles as Contrast Agents for Imaging Bone Metabolic Activity. ACS Applied Materials & Interfaces 2013, 5:5219–5226. [DOI] [PubMed] [Google Scholar]
- 76.Torres Martin de Rosales R, Tavaré R, Glaria A, Varma G, protti A, Blower Pj: 99mTc-Bisphosphonate-Iron Oxide Nanoparticle Conjugates for Dual-Modality Biomedical Imaging. Bioconjugate Chemistry 2011, 22:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sandiford L, Phinikaridou A, Protti A, Meszaros LK, Cui X, Yan Y, Frodsham G, Williamson PA, Gaddum N, Botnar RM, et al. : Bisphosphonate-Anchored PEGylation and Radiolabeling of Superparamagnetic Iron Oxide: Long-Circulating Nanoparticles forin VivoMultimodal (T1 MRI-SPECT) Imaging. ACS Nano 2012, 7:500–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Genady AR, Fong D, Slikboer SR, El-Zaria ME, Swann R, Janzen N, Faraday A, McNelles SA, Rezvani M, Sadeghi S, et al. : 99mTc-Functionalized Single-Walled Carbon Nanotubes for Bone Targeting. ACS Applied Nano Materials 2020, 3:11819–11824. [Google Scholar]; This paper introduces a promising bone-targeted diagnostic biomaterial based on a radiotracer and carbon nanotubes, and uses photoacoustic imaging to confirm biodistribution to bone.
- 79.Han HS, Niemeyer E, Huang Y, Kamoun WS, Martin JD, Bhaumik J, Chen Y, Roberge S, Cui J, Martin MR, et al. : Quantum dot/antibody conjugates for in vivo cytometric imaging in mice. Proc Natl Acad Sci U S A 2015, 112:1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li C, Zhang Y, Chen G, Hu F, Zhao K, Wang Q: Engineered Multifunctional Nanomedicine for Simultaneous Stereotactic Chemotherapy and Inhibited Osteolysis in an Orthotopic Model of Bone Metastasis. Advanced Materials 2017, 29. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z, Ross RD, Roeder RK: Preparation of functionalized gold nanoparticles as a targeted X-ray contrast agent for damaged bone tissue. Nanoscale 2010, 2. [DOI] [PubMed] [Google Scholar]
- 82.Ma Z, Qin H, Chen H, Yang H, Xu J, Yang S, Hu J, Xing D: Phage display-derived oligopeptide-functionalized probes for in vivo specific photoacoustic imaging of osteosarcoma. Nanomedicine: Nanotechnology, Biology and Medicine 2017, 13:111–121. [DOI] [PubMed] [Google Scholar]
- 83.Sun W, Ge K, Jin Y, Han Y, Zhang H, Zhou G, Yang X, Liu D, Liu H, Liang X-J, et al. : Bone-Targeted Nanoplatform Combining Zoledronate and Photothermal Therapy To Treat Breast Cancer Bone Metastasis. ACS Nano 2019, 13:7556–7567. [DOI] [PubMed] [Google Scholar]; MeSi NPs entrapping gold nanorods and functionalized with zoledronic acid showed excellent bone targeting in vivo and reduced osteoclastogenesis and increased osteoblastogenesis in vitro. Photothermal ablation via near-infrared irradiation initiated tumor cell apoptosis and reduced pain and bone resorption, providing a promising theranostic strategy for tumor metastases.
- 84.Ventura M, Sun Y, Cremers S, Borm P, Birgani ZT, Habibovic P, Heerschap A, van der Kraan PM, Jansen JA, Walboomers XF: A theranostic agent to enhance osteogenic and magnetic resonance imaging properties of calcium phosphate cements. Biomaterials 2014, 35:2227–2233. [DOI] [PubMed] [Google Scholar]
- 85.Lu Y, Li L, Lin Z, Li M, Hu X, Zhang Y, Peng M, Xia H, Han G: Enhancing Osteosarcoma Killing and CT Imaging Using Ultrahigh Drug Loading and NIR- Responsive Bismuth Sulfide@Mesoporous Silica Nanoparticles. Advanced Healthcare Materials 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]; RGD-targeted MeSi-coated bismuth sulfide NPs (Bi2S3@MSN NPs) exhibited excellent accumulation in osteosarcoma cells. High accumulation enabled efficient computed tomography (CT) imaging and tumor ablation through activation of apoptosis. Bi2S3@MSN NPs are a promising theranostic platform for bone metastases.
- 86.Park CS, Ha TH, Kim M, Raja N, Yun H-s, Sung MJ, Kwon OS, Yoon H, Lee C-S: Fast and sensitive near-infrared fluorescent probes for ALP detection and 3d printed calcium phosphate scaffold imaging in vivo. Biosensors and Bioelectronics 2018, 105:151–158. [DOI] [PubMed] [Google Scholar]
- 87.Sajesh KM, Ashokan A, Gowd GS, Sivanarayanan TB, Unni AKK, Nair SV, Koyakutty M: Magnetic 3D scaffold: A theranostic tool for tissue regeneration and non-invasive imaging in vivo. Nanomedicine: Nanotechnology, Biology and Medicine 2019, 18:179–188. [DOI] [PubMed] [Google Scholar]
- 88.Alonso-de Castro S, Ruggiero E, Lekuona Fernández A, Cossío U, Baz Z, Otaegui D, Gómez-Vallejo V, Padro D, Llop J, Salassa L: Functionalizing NaGdF4:Yb,Er Upconverting Nanoparticles with Bone-Targeting Phosphonate Ligands: Imaging and In Vivo Biodistribution. Inorganics 2019, 7. [Google Scholar]
- 89.Wang Y, Jiang C, He W, Ai K, Ren X, Liu L, Zhang M, Lu L: Targeted Imaging of Damaged Bone in Vivo with Gemstone Spectral Computed Tomography. ACS Nano 2016, 10:4164–4172. [DOI] [PubMed] [Google Scholar]
- 90.Wyss PP, Herrera LC, Bouteghmes NS, Sarem M, Reichardt W, Leupold J, Hennig J, Shastri VP: Nanoprobes for Multimodal Visualization of Bone Mineral Phase in Magnetic Resonance and Near-Infrared Optical Imaging. ACS Omega 2016, 1:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Özcan İ Bouchemal K, Segura- Sánchez F, Özer Ö, Güneri T, Ponchel G: Synthesis and characterization of surface- modified PBLG nanoparticles for bone targeting: In vitro and in vivo evaluations. Journal of Pharmaceutical Sciences 2011, 100:4877–4887. [DOI] [PubMed] [Google Scholar]
- 92.Patricio BF, Albernaz Mde S, Sarcinelli MA, de Carvalho SM, Santos-Oliveira R, Weissmuller G: Development of novel nanoparticle for bone cancer. J Biomed Nanotechnol 2014, 10:1242–1248. [DOI] [PubMed] [Google Scholar]
- 93.Hasani-Sadrabadi MM, Dashtimoghadam E, Bahlakeh G, Majedi FS, Keshvari H, Van Dersarl JJ, Bertsch A, Panahifar A, Renaud P, Tayebi L, et al. : On-chip synthesis of fine-tuned bone-seeking hybrid nanoparticles. Nanomedicine 2015, 10:3431–3449. [DOI] [PubMed] [Google Scholar]
- 94.Li M, Liu J, Cui X, Sun G, Hu J, Xu S, Yang F, Zhang L, Wang X, Tang P: Osteogenesis effects of magnetic nanoparticles modified-porous scaffolds for the reconstruction of bone defect after bone tumor resection. Regenerative Biomaterials 2019, 6:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y, Huang Q, He X, Chen H, Zou Y, Li Y, Lin K, Cai X, Xiao J, Zhang Q, et al. : Multifunctional melanin-like nanoparticles for bone-targeted chemo-photothermal therapy of malignant bone tumors and osteolysis. Biomaterials 2018, 183:10–19. [DOI] [PubMed] [Google Scholar]; Alendronate-conjugated polydopamine NPs (PDA-ALN) were augmented with Fe to improve magnetic resonance contrast of bone tumors. Data suggest greater accumulation at osteolytic bone lesions via ALN-targeting and near-infrared irradiation-triggered chemotherapeutic drug release through localized heating and acidic conditions. The theranostic hindered tumor growth and reduced osteolysis suggesting potential for advancement of the theranostic for orthopaedic cancers.
- 96.Kievit FM, Stephen ZR, Veiseh O, Arami H, Wang T, Lai VP, Park JO, Ellenbogen RG, Disis ML, Zhang M: Targeting of Primary Breast Cancers and Metastases in a Transgenic Mouse Model Using Rationally Designed Multifunctional SPIONs. ACS Nano 2012, 6:2591–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manohar S, Ungureanu C, Van Leeuwen TG: Gold nanorods as molecular contrast agents in photoacoustic imaging: the promises and the caveats. Contrast Media & Molecular Imaging 2011, 6:389–400. [DOI] [PubMed] [Google Scholar]
- 98.Wang L, Wu Q, Zhu S, Li Z, Yuan J, Liu L, Yu D, Xu Z, Li J, Sun S, et al. : Quantum dot-based immunofluorescent imaging and quantitative detection of DNER and prognostic value in prostate cancer. Cancer Biomarkers 2018, 22:683–691. [DOI] [PubMed] [Google Scholar]
- 99.Choi J, Burns AA, Williams RM, Zhou Z, Flesken-Nikitin A, Zipfel WR, Wiesner U, Nikitin AY: Core-shell silica nanoparticles as fluorescent labels for nanomedicine. Journal of Biomedical Optics 2007, 12. [DOI] [PubMed] [Google Scholar]
- 100.Chiou AE, Hinckley JA, Khaitan R, Varsano N, Wang J, Malarkey HF, Hernandez CJ, Williams RM, Estroff LA, Weiner S, et al. : Fluorescent Silica Nanoparticles to Label Metastatic Tumor Cells in Mineralized Bone Microenvironments. Small 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent paper detailing the development of fluorescent silica nanoparticles to simulataneously image cells, bone marrow, and minearalized matrix.
- 101.Ganesh N, Ashokan A, Rajeshkannan R, Chennazhi K, Koyakutty M, Nair SV: Magnetic Resonance Functional Nano-Hydroxyapatite Incorporated Poly(Caprolactone) Composite Scaffolds for In Situ Monitoring of Bone Tissue Regeneration by MRI. Tissue Engineering Part A 2014, 20:2783–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Mesy Bentley KL, Trombetta R, Nishitani K, Bello- Irizarry SN, Ninomiya M, Zhang L, Chung HL, McGrath JL, Daiss JL, Awad HA: Evidence of Staphylococcus aureus deformation, proliferation, and migration in canaliculi of live cortical bone in murine models of osteomyelitis. Journal of Bone and Mineral Research 2017, 32:985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lavrador P, Gaspar VM, Mano JF: Stimuli-responsive nanocarriers for delivery of bone therapeutics–Barriers and progresses. Journal of Controlled Release 2018, 273:51–67. [DOI] [PubMed] [Google Scholar]
- 104.Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Aberg C, Mahon E, Dawson KA: Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nature Nanotechnology 2013, 8:137–143. [DOI] [PubMed] [Google Scholar]
- 105.Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, Schlenk F, Fischer D, Kiouptsi K, Reinhardt C, et al. : Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol 2013, 8:772–781. [DOI] [PubMed] [Google Scholar]
- 106.Corbo C, Molinaro R, Parodi A, Toledano Furman NE, Salvatore F, Tasciotti E: The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine 2016, 11:81–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hoffman MD, Xie C, Zhang X, Benoit DS: The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing. Biomaterials 2013, 34:8887–8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Y, Hoffman MD, Benoit DS: Matrix metalloproteinase (MMP)-degradable tissue engineered periosteum coordinates allograft healing via early stage recruitment and support of host neurovasculature. Biomaterials 2021, 268:120535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ladd ME, Bachert P, Meyerspeer M, Moser E, Nagel AM, Norris DG, Schmitter S, Speck O, Straub S, Zaiss M: Pros and cons of ultra-high-field MRI/MRS for human application. Progress in nuclear magnetic resonance spectroscopy 2018, 109:1–50. [DOI] [PubMed] [Google Scholar]
- 110.Upputuri PK, Sivasubramanian K, Mark CSK, Pramanik M: Recent developments in vascular imaging techniques in tissue engineering and regenerative medicine. BioMed research international 2015, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nodzo SR, Bauer T, Pottinger PS, Garrigues GE, Bedair H, Deirmengian CA, Segreti J, Blount KJ, Omar IM, Parvizi J: Conventional diagnostic challenges in periprosthetic joint infection. JAAOS-Journal of the American Academy of Orthopaedic Surgeons 2015, 23:S18–S25. [DOI] [PubMed] [Google Scholar]
- 112.Mei J, Hong Y, Lam JW, Qin A, Tang Y, Tang BZ: Aggregation- induced emission: the whole is more brilliant than the parts. Advanced materials 2014, 26:5429–5479. [DOI] [PubMed] [Google Scholar]
- 113.Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M, Hamblin MR: Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine 2019, 14:93–126. [DOI] [PMC free article] [PubMed] [Google Scholar]


