Abstract
The bypass graft is the mainstream of surgical intervention to treat vascular diseases. Ideal bypass materials, yet to be developed, require mechanical properties, availability, clinically feasible manufacturing logistics, and bioactivities with precise physicochemical cues defined to guide cell activities for arterial regeneration. Such needs instigated our fabrication of vascular grafts, which consist of coaxial, nanostructured fibers exhibiting a polycaprolactone (PCL) core and a photoclickable, 4-arm thiolated polyethylene glycol-norbornene (PEG-NB) sheath. The graft strength and bioactivity were modulated by the PCL concentration and the peptides (RGD, transforming growth factor β-1 or TGF-β1) conjugated to thiol–ene of PEG-NB, respectively. Structural, physical, and mechanical characterizations demonstrated that the fibrous grafts mimicked the key features of the native extracellular matrix, including a crosslinked fiber network for structural stability, viscoelasticity emulating arteries, hydration property, and high porosity for cell infiltration. Meanwhile, these grafts displayed strength and toughness exceeding or meeting surgical criteria. Furthermore, the grafts with higher PCL concentration (3 vs 1.8%) showed thicker fibers, lower porosity and pore size, and increased elastic and storage moduli. Graft bioactivity was determined by the mesenchymal stem cell (MSC) behaviors on the grafts and arterial regeneration in vivo using interposition grafting. Results showed that the cell adhesion and proliferation increased with the RGD density (25 vs 5 mM). After 1 week implantation, all peptide-functionalized PCL/PEG-NB grafts with or without MSC preseeding, as opposed to PCL grafts, showed expeditious endothelial lining, abundant vascular cell infiltration, and matrix production. Compared to RGD grafts, RGD/TGF-β1 grafts enhanced MSC differentiation into smooth muscle cells in vitro and developed thicker smooth muscle cell layers in vivo. Overall, the versatile porous vascular grafts offer superior properties and tunability for future translation.
Keywords: vascular graft, regeneration, multi-armed polymer, coaxial electrospun fiber, polyethylene glycol-norbornene, stiffness
Graphical Abstract

1. INTRODUCTION
Vascular diseases, such as coronary artery disease, peripheral vascular disease, and cerebrovascular disease, are leading causes of morbidity and mortality in developed countries.1 To treat vascular diseases, a mainstream of surgical intervention is the bypass graft, which is used in more than 1,000,000 cases every year in the U.S. with ~400 K coronary bypass procedures alone.2 Bypass vascular grafts primarily use autologous blood vessels or artificial material grafts made of polytetrafluoroethylene. Problems with these grafts include the poor availability of autologous grafts and the high failure rate of artificial materials, specifically for small-diameter grafts. Such graft shortage has driven a long-lasting exploration for alternative grafts, which ideally can be readily available in proper dimensions, compliant, strong, and biologically active to promote arterial regeneration for long-term patency. With the goal of offering an alternative approach to small-diameter bypass grafts, the fabrication of tissue-engineered grafts has been under intensive research in the last 2 decades.2,3 Though various bioengineered or tissue-engineered vascular grafts have emerged,4-6 there is still a long way to overcome a number of difficulties, before the success may be achieved in future clinical practices.7,8 Some difficulties lie in creating grafts that simultaneously satisfy all critical requirements, including mechanical properties, bioactivities, availability, biomanufacturing requirements related to problems from asepsis to logistics including time and cost, and most importantly superior grafting performances in vivo.
To achieve immediate grafting mechanics and bioactivity, a typical tissue-engineered scaffold for arterial grafting should withstand hemodynamic stresses derived from pulsatile arterial flow and enable the regeneration of arterial cells and extracellular matrices (ECMs).2 This often involves extended cell culture, which unfortunately increases the chance of contamination and raises issues of manufacturing logistics and clinical care. To reduce culture requirements, synthetic vascular grafts for in situ vascular remodeling are being investigated.7 The direct use of synthetic or natural materials is limited for such application because of their susceptibility of thrombosis or infection, resistance to cell infiltration, or lack of arterial strength or compliance.9,10 To overcome these issues, new hybrid graft scaffolds, which are mechanically compliant and strong to function as native arteries, highly porous to allow cell penetration, and bioactive to regenerate arterial cells and neo-vessel tissues, would present an attractive solution for vascular surgery.
To this end, we have combined a versatile hydrogel material, 4-arm, end-functionalized polyethylene glycol-norbornene (PEG-NB) with a stiff, biodegradable material, polycaprolactone (PCL), into a porous fibrous hybrid scaffold and utilized the photoclickable “thiol–ene” chemistry to achieve control over local physicochemical environments. Different from approaches that blend different fibers or layer multiple materials, which result in low adhesion between materials of distinct properties and thus undefined material defects in heterogeneous scaffolds,11 the formation of hybrid materials in this study employs coaxial electrospinning to enhance the material adhesions with nanoscale interactions. The improved interactions in the hybrid materials can benefit the mechanical stability. We previously developed porous scaffolds uniquely structured with coaxial fibers consisting of a PCL core and a PEG-thiol–ene sheath12 and demonstrated that their physicochemical properties vary with the polymerization time and/or molecular weight, affecting cell adhesion in vitro and cell infiltration in subcutaneous implants. Herein, we further developed these scaffolds into tubular vascular grafts with new manufacturing protocols and different peptides that interact with cells. We assessed the influences of PCL concentration on the graft mechanics and the effects of surface peptides on vascular differentiation, cell infiltration, and matrix production in vitro and in vivo.
2. EXPERIMENTAL SECTION: MATERIALS AND METHODS
2.1. Materials.
All polymers and chemicals were purchased from Sigma-Aldrich Inc. (St Louis, MO) unless specified otherwise.
2.2. Fabrication of Tubular Vascular Grafts Consisting of Coaxial Microfibers.
The apparatus used to obtain tubular grafts made of coaxial fibers was developed in-house. Our previous paper described the detailed procedure of vascular graft fabrication.12 A high-voltage ES30P 10 W power supply (Gamma High Voltage Research, Ormond Beach, FL) and syringe pumps (Pump 11 Plus, Harvard Apparatus, Boston, MA) were used. The polymer solutions consisted of 1.8 or 3 wt % PCL and 3 wt % 4-arm PEG-NB 5 kDa (synthesized in-house). The solutions were prepared by dissolving PCL (for core solution) and PEG-NB 5 kDa, poly(ethylene glycol) dithiol (PEG-SH, JenKem Technology, Dallas, TX), PEO, and Irgacure 2959 (Ciba Speciality Chemicals, Basel, Switzerland) (for sheath solution) in the solvent 1,1,1,3,3,3 hexafluoro-2-propanol (HFP, CovaChem, Loves Park, IL). Using our custom-made electrospinning apparatus, the fibers were deposited onto a grounded aluminum rotating mandrel placed at a distance of 10 cm perpendicular to the needle. The mandrel was rotated at 350 rpm. The obtained samples were kept dry at room temperature until further use.
2.3. Crosslinking of Fibrous Vascular Grafts.
Crosslinking of the coaxial fiber vascular grafts was performed as described in our previous paper.12 Briefly, the vascular grafts composed of coaxial fibers were placed in vacuum (Vacuum Atmospheres Company, OMNI-LAB, Hawthorne, CA) for 15 min and subsequently crosslinked by UV light for 90 min. During this process, the vascular grafts constantly rotated to assure uniform cross-linking throughout the graft. Right after the crosslinking process, the vascular grafts were rinsed with phosphate-buffered saline (PBS) and removed from the mandrel. Mechanical characterizations, including tensile test and rheometry measurement, were performed on the grafts. The grafts were also lyophilized for 48 h for imaging under scanning electron microscopy (SEM). For both cell culture and in vivo implantation, either CRGDS (GenScript Inc., Piscataway, NJ) at 5 or 25 mM or a combination of CRGDS at 25 mM and transforming growth factor β-1 (TGF-β1, PeproTech, Rocky Hill, NJ) at 50 nM was conjugated to graft samples. TGF-β1 was used to functionalize vascular grafts, with the purpose of differentiating MSCs into smooth muscle cells.
2.4. Electron Microscopy Imaging of Fiber Structures.
Transmission electron spectroscopy (TEM) and SEM were used to characterize electrospun grafts. The nanostructure of the coaxial fibers was imaged with TEM, as described in our previous paper.12 The TEM fiber sample preparation was done by directly depositing the as-spun fibers on carbon-coated TEM grids attached to the rotating mandrels. A FEI Tecnai T12 SpiritBT (FEI, Hillsboro, OR) was used with a CCD camera AMT XR41. Sheath-to-fiber diameter ratio analysis was performed similarly to our previous study,12 using NIH ImageJ.
To examine fiber morphology in the vascular graft materials, aluminum substrates coated with the electrospun fibers were mounted on brass stubs and sputter-coated with Pt/Pd (Cressington Sputter Coater 108auto with Rotary Vane Vacuum Pump VRL 100-3.5, Cressington Scientific Instruments, Watford, UK). Imaging was performed at 5 kV by using a field-emission SEM (Hitachi SU3500, Hitachi High Technologies America, Schaumburg, IL) with NPGS e-beam lithography capability. Uncrosslinked as-spun dry fibers, as well as dry and wet crosslinked electrospun fibers, were analyzed. The preparation of the water-swollen fibers in the wet state was done as previously described.12 Additionally, the analysis of the average pore size, porosity, and fiber diameter was performed from SEM images in a similar way as previously described.12
2.5. Mechanical and Biochemical Characterizations of Fibrous Vascular Grafts.
2.5.1. Tensile Testing.
Vascular grafts were cut into 1.5 cm × 2 mm rectangular pieces and tested for their tensile strength as described in our previous paper.12 Stress–strain measurement was performed at a crosshead speed of 5 mm/min under the condition of 37 °C and 65% for relative humidity. A 250 N load cell (MTS Systems Corp., Eden Prairie, MN) was used. The tensile measurements were used to estimate the burst pressure (BP) of vascular grafts, which was calculated using the following equation
where BP is the calculated average BP, T is the maximum tensile strength, x is half of the outside graft diameter, and y is half of the inner graft diameter or graft lumen.
2.5.2. Suture Strength Testing.
Vascular grafts were cut into 1.5 cm × 2 mm rectangular pieces and tested for their suture strength. The samples were prepared and tested as done for tensile testing, with the main difference being the insertion of a suture into a graft end. More specifically, a suture was inserted 2 mm from one end of the graft and through the graft wall forming a half loop. The other end of the graft was attached to the lower clamp of the tensile testing system. The suture was attached to a 250 N load cell pulling at a rate of 5 mm/min. Suture retention was documented as the force that was needed in order to tear the graft wall.
2.5.3. Rheometry Testing.
To prepare the vascular graft samples for rheometry, the hydrated vascular grafts were cut open, and then, a circle of 8 mm diameter was punched out for analysis. Rheometry testing was performed on an ARES/ARG2 rheometer (TA Instrument, New Castle, DE) as previously described.12 Samples were analyzed on ARES using the plate geometry (diameter = 8 mm; gap set between 0.3 and 0.8 mm). Strain sweep measurements were performed in the range of 0.1–10% strain with 10 points each decade of strain (e.g., 0.1–1%).
2.5.4. Hydrogel Surface Characterization with Atomic Force Microscopy and Modified ELISA.
To further determine the physicochemical properties of functionalized coaxial fiber hydrogel surfaces, the hydrogels were hydrated and measured with atomic force microscopy (AFM) (5420 Scanning Probe Microscope, Keysight Technologies Inc., Santa Rosa, CA, USA), as previously described.13 To confirm the successful thiolation and the tethering of TGF-β1 to the PEG hydrogel network, immunofluorescent staining and modified ELISA method were used.13
2.6. Cell Attachment and Culture on Coaxial Fiber Vascular Grafts.
Human mesenchymal stem cells (hMSCs) from Texas A&M Regenerative Institute were seeded on coaxial fiber graft scaffolds with a seeding density of 2.5 × 104 cells/cm2 at 37 °C and 5% CO2 for 24 h (1 day) to test cell attachment, 96 h (4 days) to test cell proliferation, and 144 h (6 days) to test cell differentiation. Cells at passage 2 were used. Prior to cell seeding, the grafts were cut open, and graft scaffolds were treated with 25 mM CRGDS or CRGDS + TGF-β1, which were covalently conjugated to the PEG-NB upon UV exposure for 10 min. In order to identify an optimal CRGDS concentration for cell attachment, the vascular graft samples were also treated with 5 mM CRGDS. After peptide binding, the samples were processed in the following sequence: rinse with PBS, sterilization with 70% ethanol for 15 min, and rinse three times with sterile PBS. The cell culture medium was composed of MEM α modified with 16 mL of l-glutamine (US Biological Life Sciences, Marblehead, MA), 16.5% fetal bovine serum (Corning, 35-0101-CV), 1% penicillin/streptomycin, and 0.05% gentamicin added only during cell attachment (first 24 h). To investigate cell adhesion, proliferation, and morphology, the scaffolds seeded with cells were fixed in 4% formaldehyde and stained with DAPI and F-actin after cultured for predetermined times. The stained cells were imaged with a Nikon fluorescent microscope at 10× magnification.
2.7. Cell Proliferation and Differentiation Assays.
A CCK assay was performed on day 4 to assess cell viability and proliferation. Additionally, to quantitatively evaluate cell proliferation, the cell number in each scaffold was calculated on day 4 using NIH ImageJ. To evaluate MSC differentiation into smooth muscle cell, the scaffolds seeded with cells were rinsed with PBS after 144 h of cell culture, fixed in 4% formaldehyde, and stained with immunofluorescence, using both anti-α smooth muscle actin (α-SMA) (ab7817, Abcam, Cambridge, MA) and antismooth muscle myosin heavy chain (SM-MHC) (sc-6956, Santa Cruz Biotechnology, Dallas, TX) antibodies, with DAPI counterstain. Confocal microscopy was used to image the stained cells, at 10× and 40× magnification. The intensity of each signal on the vascular graft scaffolds was determined using ImageJ by dividing the total mean gray value from all the cells in the image by the total number of cells. This way, we obtained an average mean gray value from each signal.
2.8. Gene Expression Assessment.
We extracted mRNA for cDNA synthesis and quantitative polymerase chain reaction (qPCR) to evaluate genetic expression of cultured cells. The mRNA extraction and purification were completed using Direct-zol RNA Miniprep Plus kit (ZYMO Research Corp, Irvine, CA) following the product instructions. The concentration of purified mRNA, with a final volume in 50 μL of dnase/rnase-free distilled water, was measured with NanoDrop 2000 (Thermo Fisher). The cDNA synthesis was performed using iScript cDNA synthesis Kit (Bio-Rad) according to the product instructions. The qPCR analysis was performed using iTaq universal SYBR Green Supermix (Bio Rad) following the manufacturer’s instructions. Expression of candidate genes, including α-SMA, MHC, elastin, and collagen I, was normalized to human GAPDH or HPRT. Thermal cycling protocol was performed as follows; 5 min denaturing step at 95 °C, 20 s annealing/extension and plate read at 60 °C for 45 cycles followed by Melt curve analysis from 65 to 95 °C (0.5 °C increments) at 3 s/step. Data calculations were done following the Livak method of ΔΔCq analysis. Three biological replicates were used for expression analysis of each gene.
2.9. Vascular Graft Preparation and Implantation.
Animal experiments were performed according to the IACUC requirements (approval number: 1407.02 at the University of Colorado) and complied with the NIH’s Guidelines for the Care and Use of Laboratory Animals. The PCL fiber-based vascular graft (n = 7, with 2 failures due to deceased rats and 5 successfully explanted) was used as a control. Four groups of coaxial fiber grafts (n = 3–5 for each group, all successfully explanted) were evaluated: PCL/PEG-NB + RGD, PCL/PEG-NB + RGD + seeded rat bone-marrow-derived stromal cells (rMSCs) before implantation, PCL/PEG-NB + RGD/TGF-β1, and PCL/PEG-NB + RGD/TGF-β1 + seeded rMSCs before implantation. To minimize the risk of thrombosis during graft implantation, heparin was added into the core of the coaxial fibers by mixing 1% heparin into the PCL solution. The prepared vascular grafts were cut into 1.5 cm in length. Graft functionalization with CRGDS and TGF-β1 was done by submerging the graft pieces into the solutions containing either CRGDS or CRGDS + TGF-β1 peptides for 30 min and then using 15 min of UV light to covalently bind the peptides to the grafts. During this process, the vascular grafts constantly rotated to assure uniform crosslinking throughout the graft.
Prior to implantation, grafts were sterilized the same way as done for the in vitro studies. Briefly, after peptide binding, the grafts were processed in the following sequence: rinse with PBS, sterilization with 70% ethanol for 15 min, and rinse again three times with sterile PBS. The grafts were then stored in 2× penicillin–streptomycin and 1% heparin in PBS until their implantation.
To seed allogenic cells into the graft lumen, rMSCs isolated from femur bone marrow were used at passage 2. The rMSCs were cultured in Dulbecco’s modified Eagle’s medium media supplemented with 10% stem cell qualified fetal bovine serum from Atlanta Biologicals (Flowery Branch, GA) and 1% penicillin–streptomycin from Thermo Fisher to grow until confluency. The centrifuge method was used to seed rMSCs onto the surface of the graft lumen. After peptide binding, each graft was inserted into a hollow aluminum jacket. Then, the cell suspension was introduced into the graft lumen, and the two ends were sealed tightly with plastic plugs. The plugged graft-jacket set was then inserted in the center of a 15 mL conical tube. The tube was centrifuged for 5 min at 1500 rpm. The stability of the mandrel in the tube was necessary to achieve a uniform cell seeding during centrifugation. The rMSCs seeded onto the surface of the graft lumen were cultured for 24 h before graft implantation. At that point, some MSC preseeded vascular grafts were fixed and stained with DAPI and F-actin to evaluate rMSC attachment onto the vascular graft inner surface. Successful cell adhesion was observed (Figure S1).
The vascular graft evaluation was performed on Sprague Dawley rats (ENVIGO, Indianapolis, IN) at 8–9 months old at the time of the implantation, weighing ~400 g. Anesthesia in rats was induced with 5% isoflurane gas per liter O2 (Vet One) until fully anesthetized and maintained with 2% isoflurane gas. The surgical site was cleaned and disinfected with povidone-iodine (Medline Industries Inc, Northfield, IL). Abdominal incision was made down the midline into the abdominal cavity to access abdominal aorta. The abdominal aorta was dissected away from the inferior vena cava and clamped off upstream and downstream of the implantation site. A small section of aorta was removed to where the graft was anastomosed in an end-to-end manner using twelve continuous surgical stitches at each anastomosis site of the graft. After completion of the anastomosis, the clamps were removed to restore arterial perfusion and the anastomosis examined for leakage. The incision was then closed in a standard manner using running stitches. The animals received 50/50 mix of lidocaine 1–2% with 0.5% bupivacaine on incision line as well as 4 mg/kg body weight dose of meloxicam (0.3 mg/kg) after the surgery. Their movements and signs of distress were closely monitored for the first 24 h and twice a day in the following two days. During these examinations, no rats that had been implanted with coaxial fiber vascular grafts were found to experience any obvious discomfort, edema, swelling, weakened thrill, or other neurologic disorders. No complications such as hemorrhage were revealed upon explantation. The grafts were retrieved after 7 days of the implantation. Only two rats that were implanted with grafts made of PCL fibers (control group) had complications and died during the first 48 h.
2.10. Graft Explantation and Assessment.
Prior to graft explantation, the vascular graft morphology and blood flow through the grafts were obtained with ultrasonographs and Doppler (Figure S2), which were taken with the ACUSON SEQUOIA 512 ultrasound system (Siemens Medical Solutions, Mountain View, CA). Rats were anesthetized and shaved around the area of implant with ultrasound gel applied. After imaging, rats were immediately euthanized via CO2 asphyxiation, and samples including the graft and neighboring arteries were taken.
The explanted grafts were embedded with OCT and cryosectioned into 8–20 μm thick sections. The sections were stained with Masson’s trichrome, which was performed by the Histology Core at University of Colorado. Both the middle of the graft and the anastomosis sections were cryosectioned and stained. Imaging was done using a light microscope (Nikon, Melville, NY). To determine the presence of endothelial cells and macrophages in the grafts, immunofluorescent staining was done, respectively, with anti-von Willebrand factor (vWF, ab8822, Abcam, Cambridge, MA) conjugated to FITC (Abcam, Cambridge, MA) and anti-CD68 (6A324, Santa Cruz Biotechnology, Dallas, TX) together with secondary antibody mIgG (Santa Cruz Biotechnology, Dallas, TX). All antibodies were used with 1:100 dilution in TBS. Finally, coverslips were applied with vectashield mounting medium containing DAPI for counterstain (Vector Laboratories, Burlingame, CA) and imaged with a fluorescent microscope (Nikon, Melville, NY). To determine the presence of smooth muscle cells in the grafts, immunohistochemistry was performed with anti-α-SMA stain (ab7817, Abcam, Cambridge, MA) and ImmunoCruz ABC kit (Santa Cruz Biotechnology, Dallas, TX) by following the manufacturer’s protocol. Slides were finally dehydrated with increasing concentrations of ethanol solutions and cleared with HistoClear prior to mounting with coverslips and imaging using a light microscope (Nikon, Melville, NY).
2.11. Second Harmonic Generation and Two-Photon Imaging.
To examine the ECM production in explanted vascular graft implants, second harmonic generation and two-photon excitation fluorescence (SHG-TPEF) imaging, a dual multiphoton imaging modality, was employed to respectively determine the fibrillar collagen and elastin contents as previously described.12 The dual-modality multiphoton laser scanning microscope system (Radiance 2000 MP, Bio-Rad Laboratories Inc, Hercules, CA) included a femtosecond pulsed laser system (Spectra-Physics, MaiTai wideband, mode-locked Ti:Sapphire laser system) and a dichroic mirror (AT455 DC, Chroma Technology Corp, Bellows Falls, VT).
2.12. Data Analysis.
One-way analysis of variance test (ANOVA) was used to analyze the statistical differences among several groups, with each group having a sample size of at least 3 (n ≥ 3). To compare the means between two groups, Student’s t-test was used. The significance levels were set at p < 0.05 (*, #, ^, or $), p < 0.01 (**, ##, ^^, or $$), and p < 0.001 (***, ###, ^^^, or $$$). Results were presented as mean ± standard deviation.
3. RESULTS
3.1. Structure of Hybrid Coaxial Microfibers.
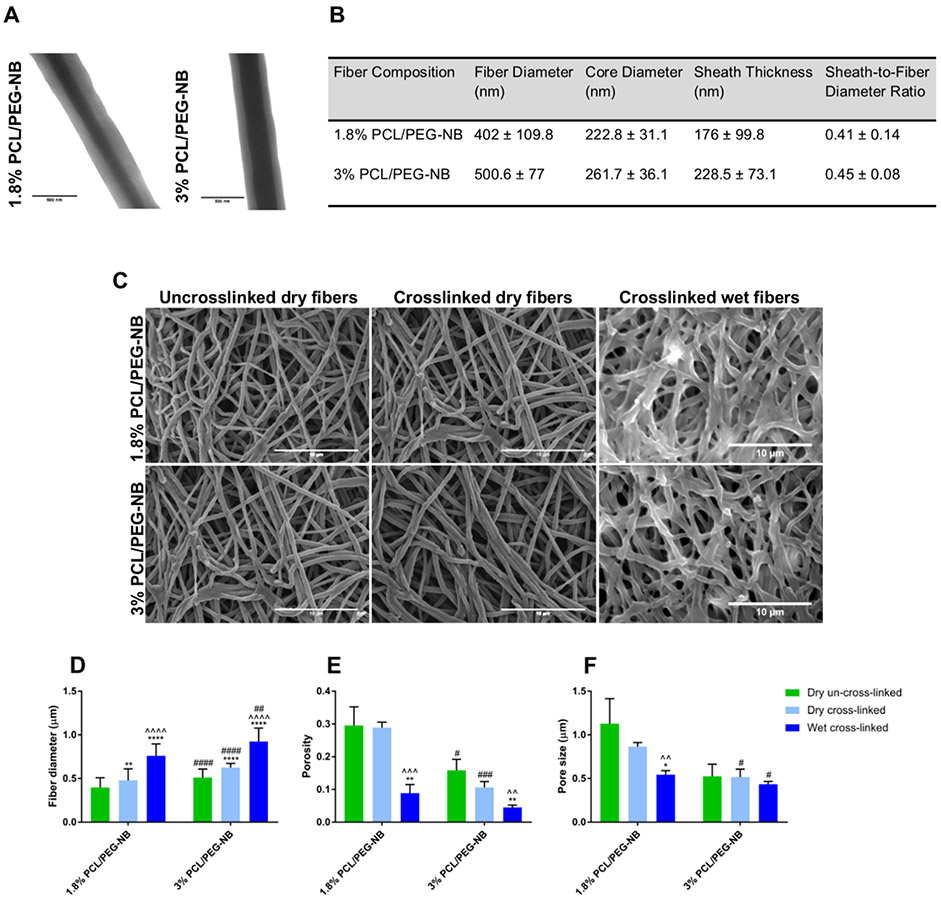
The morphology of coaxial fibers in the fabricated tubular grafts is shown in Figure 1. In order to form continuous fibers, appropriate flow rates of sheath and core fluids in accordance with optimal mandrel rotation were identified by performing parametric investigations of the coaxial electrospinning procedure. The development of optimal coaxial PCL/PEG-NB fibers was demonstrated in both cases. Figure 1A shows the TEM images of coaxial fibers displaying a sheath of PEG-NB and a core made of 1.8 or 3% PCL, with a thicker core in 3% PCL/PEG-NB compared with 1.8% PCL/PEG-NB. The thicker core is probably due to more viscous solution of higher PCL concentration and faster evaporation of the solvent.14-16 Figure 1B lists the diameters of fibers and their core, the sheath thickness, all of which were obtained from TEM images, as well as the sheath-to-fiber diameter ratios. It was found that the sheath-to-fiber diameter ratios were similar for 1.8 and 3% PCL/PEG-NB. Though 3% PCL/PEG-NB was characterized by a thicker core, both the sheath thickness and overall fiber diameter appeared larger as well, therefore leading to a similar ratio to 1.8% PCL/PEG-NB. Compared with 1.8% PCL/PEG-NB, stronger core-sheath interactions between PCL and PEG-NB could occur in 3% PCL/PEG-NB because of more concentrated PCL, leading to a thicker sheath. It was also observed that the sheath was not entirely uniform through the coaxial fibers, which could potentially be due to unequal interfacial energies between the sheath and the core constituents,17 having an impact on their interactions.
Figure 1.
Illustration of coaxial hybrid fibers imaged with electron microscopes and quantitatively evaluated based on the images. (A) TEM images of coaxially-electrospun fibers. Scale bar = 500 nm. (B) Quantitative analysis of the fiber micro/nanostructures. (C) SEM images of the uncrosslinked and crosslinked coaxial fiber scaffolds, which were measured in dry or wet states. Scale bar = 10 μm. (D) Fiber diameter of the uncrosslinked and crosslinked coaxial fibers, before and after hydration. (E,F) Porosity (E) and pore size (F) of the uncrosslinked and crosslinked coaxial fibers, before and after hydration. “*”: p < 0.05 compared to dry uncrosslinked, “^”: p < 0.05 compared to dry crosslinked, and “#”: p < 0.05 compared to 1.8% PCL/PEG-NB. Additional significance levels were set at p < 0.01 (“**”, “^^” or “##”), p < 0.001 (“***”, “^^^” or “###”), and p < 0.0001 (“****”, “^^^^” or “####”).
Figure 1C shows the SEM images of uncrosslinked coaxial fibers and crosslinked fibers in dry or hydrated states, with quantitative analyses in Figure 1D-F. Consistent with TEM results, SEM images showed that the coaxial fibers of 3% PCL/PEG-NB display larger diameters than 1.8% PCL/PEG-NB. Additionally, both fibers in the hydrated state exhibited significantly increased diameters, likely due to water absorption. Results also showed slightly smaller diameter for uncrosslinked fibers compared to crosslinked ones. Interestingly, the fiber diameter of PCL fibers (Figure S3) was ~4–7 times smaller than crosslinked coaxial fibers. Regarding their porosity and pore size (Figure 1E,F), results showed that 3% PCL/PEG-NB scaffold is less porous and has smaller pore size with respect to 1.8% PCL/PEG-NB scaffold, leading to a stronger and less permeable scaffold. This could be due to denser 3% PCL/PEG-NB mat with thicker fibers. Importantly, for vascular graft applications, reduced porosity might help to retain blood after implantation. Nevertheless, because of fully hydrated sheath, coaxial fiber grafts, different from traditional grafts made of hydrophobic polymer (e.g., PCL, polytetrafluoroethylene), withhold high water content to prevent fluid leakage while allowing molecular diffusion or cell migration. The lower porosity and pore size found in the hydrated fibers could be due to swollen fibers that have absorbed water. Compared to hybrid fiber scaffolds, the PCL fiber scaffold was found to have significantly lower porosity and pore size, with ~7–15 times reduction in the pore size compared to crosslinked coaxial fibers’ scaffolds.
3.2. Coaxial Fiber Vascular Grafts Are Mechanically Strong, Compliant, and Robust to Suture.
To characterize physical and mechanical behaviors of tubular vascular grafts, tensile tests performed in hydrated conditions, suture retention tests, rheometry tests, and AFM measurements were used. Results from tensile tests on the grafts consisting of 1.8 or 3% PCL/PEG-NB coaxial fibers and the 3% PCL fiber graft are shown in Table 1 with stress–strain curves illustrated in Figure 2A. Results demonstrated that both coaxial fiber grafts are strong, extremely elastic and tough. Compared with that of coaxial fiber grafts, the Young’s modulus of PCL fiber grafts was ~7–15 times higher, but their strain at fracture was ~3-4 times lower. Interestingly, Figure 2A shows that coaxial fiber grafts tend to fracture slowly, while PCL grafts break abruptly in a brittle manner. The absence of the hydrogel sheath results in stiff, brittle behaviors of PCL fiber grafts. Furthermore, when compared with 1.8% PCL/PEG-NB, 3% PCL/PEG-NB grafts showed increased stiffness, ultimate strength, and strain-at-break, which may be caused by the structural differences in the two PCL/PEG-NB grafts, that is, a thicker core of 3% PCL/PEG-NB. Therefore, 3% PCL/PEG-NB grafts were selected over 1.8% PCL/PEG-NB grafts for our in vitro and in vivo studies.
Table 1.
Tensile Test Results Showing Graft Mechanical Propertiesa
| fiber composition | maximum stress (kPa) | maximum strain (%) | Young’s modulus (kPa) |
|---|---|---|---|
| PCL | 10711.8 ± 2654.9 | 74.3 ± 10.2 | 15859.9 ± 3543.8 |
| 1.8% PCL/PEG-NB | 978.8 ± 415.5 (***) | 197.1 ± 17.2 (***) | 1081.8 ± 460.6 (**) |
| 3% PCL/PEG-NB | 2702.8 ± 369.8 (** ###) | 304 ± 15.1 (*** ###) | 2310.8 ± 284.3 (** ##) |
“*”: p < 0.05 compared to PCL fibers, “#”: p < 0.05 compared to 1.8% PCL/PEG-NB. The statistical significance levels were also set at p < 0.01 (** or ##) and p < 0.001 (*** or ###).
Figure 2.
Tensile and viscoelastic characteristics of PCL/PEG-NB coaxially spun fibrous grafts. (A) Illustration of stress–strain curves for coaxial 1.8% and 3% PCL/PEG-NB fiber grafts, as well as PCL fiber graft. (B) Illustration of strain sweep results, showing G′ and G′′ obtained from the rheometer measurements of 1.8 and 3% PCL/PEG-NB grafts. (C) Storage modulus (G′) of grafts composed of 1.8% or 3% PCL/PEG-NB coaxial fibers. “**”: p < 0.01 compared to 1.8% PCL/PEG-NB.
Because an adequate BP must be achieved for clinical uses of grafts, we determined from tensile measurements the average BPs of vascular grafts: 2232 mmHg (297.5 kPa) for 1.8% PCL/PEG-NB, 6834 mmHg (911.1 kPa) for 3% PCL/PEG-NB, and 21045 mmHg (2805.8 kPa) for 3% PCL control. All these values are greater than the BP values reported for saphenous vein grafts (in the range of 1600–2100 mmHg).7,18 Additionally, because the normal range of human blood pressure is only 90–140 mmHg,19 our developed vascular grafts with coaxial fibers possess more than adequate physical strength, providing a safe range sufficient for grafts to operate within physiological pressures.20
Suture retention is another critical requirement for graft mechanics and surgical performances. Figure S4 shows the suture strength of the three microfiber grafts. Results showed that PCL fiber grafts have the highest suture strength with an average peak load of 1308 kPa, followed by 3% PCL/PEG-NB with 306.3 kPa. The 1.8% PCL/PEG-NB graft was shown to have the lowest value (249 kPa). These results agree with tensile data, showing higher stiffness and fracture strength of 3% coaxial fiber grafts compared to 1.8% coaxial fiber grafts. Therefore, the former with improved strength was preferred.
Because coaxial fibers in the grafts were fully hydrated, their viscoelastic performances were investigated with strain sweep under a rheometer (Figure 2B). All crosslinked grafts displayed elastic behaviors, showing higher storage moduli (G′) when compared to loss moduli (G″). The effect of PCL concentration was assessed using G′ measurements in the linear region (Figure 2C). In agreement with tensile data and fiber structure (i.e., thickness, porosity), a lower PCL concentration (1.8%) resulted in ~32% reduction in G′ values. However, there was one limitation in such measurement: the discontinuity of the coaxial fiber grafts, similar to hydrogels, may not be accurately measured with the rheometry test, which assumes a continuous material such as a composite. This might explain the large discrepancy in the storage modulus and elastic modulus values.
To further determine local matrix stiffness, a parameter that modulates vascular cell activity and stem cell fate,13,21 hydrated tubular vascular grafts consisting of 1.8 or 3% PCL/PEG-NB coaxial fibers, and the 3% PCL fiber graft were cut open for AFM indentation measures (Figure S5). The compressive elastic moduli obtained from AFM indentation measures were in agreement with the shear storage moduli from rheometry measures, with PCL grafts having the highest elastic modulus, followed by 3% PCL/PEG-NB. Grafts consisting of 1.8% PCL/PEG-NB displayed the lowest elastic modulus.
3.3. In Vitro Stem Cell Adherence, Proliferation, and Differentiation on Coaxial Fiber Vascular Grafts.
The bioactivity of coaxial fiber vascular grafts was provided by the peptides covalently bonded to the 4-arm thiol–ene network after the hydrogel polymerization. In order to determine an appropriate RGD concentration for cell attachment, the RGD concentrations were varied (5 vs 25 mM). Fluorescent images of cytoskeletal F-actin (Figure 3A) illustrate the significant increases in the cell numbers on day 4 with respect to day 1, indicating cell proliferation on both coaxial fiber grafts. Results also showed that 25 mM RGD conjugated to the 3% PCL/thiol-PEG-NB fibers outperformed 5 mM in terms of cell adhesion and proliferation (Figure 3B), exhibiting more cells on the material on both days. Therefore, the RGD concentration of 25 mM was selected for the cell differentiation study and in vivo experiments.
Figure 3.
Cell compatibility of the coaxial fiber grafts. (A) In vitro evaluation of hMSC attachment (day 1) and proliferation (day 4) on the coaxial fiber grafts, assessing different RGD concentrations. Fluorescent images display F-actin stain (in green) in hMSCs cultured for 24 and 96 h on the grafts consisting of PCL/PEG-NB. Results demonstrate preferential cell attachment and proliferation on the grafts treated with 25 mM RGD. Images were taken at 10×. Scale bar = 100 μm. (B) Cell density on coaxial grafts treated with different RGD concentrations, measured at day 1 and day 4. “**”: p < 0.01 compared to 5 mM, “****”: p < 0.0001 compared to 5 mM, “##”: p < 0.01 compared to day 1. (C) Cell density on PCL/PEG-NB grafts functionalized with RGD or RGD + TGF-β1, as well as PCL fiber graft, measured at day 4. Fluorescent images display DAPI stain (in blue) in hMSCs cultured for 4 days on these grafts. (D) CCK assay of the cell cultures on the coaxial fiber grafts demonstrating higher viability of cells cultured on the coaxial fiber grafts compared to PCL graft. The absorbances were taken at 450 nm 96 h after cell seeding. “*” p < 0.05 and “**” p < 0.01, compared to PCL graft.
The potent effects of TGF-β1 on directing hMSC differentiation into mature vascular smooth muscle cells have been demonstrated by our studies and others.13,21-23 Herein, TGF-β1 was covalently tethered to the coaxial fiber grafts in order to promote vascular smooth muscle regeneration. The presence of TGF-β1 in the coaxial scaffolds was attested with the ELISA assay result (Figure S6). Cell assays were then performed on PCL/PEG-NB grafts functionalized with RGD or RGD/TGF-β1, in comparison with PCL fiber grafts. Cell adhesion and proliferation results showed differences in the cell number on day 4 among these grafts (Figure 3C), with the highest cell density in the PCL/PEG-NB + RGD graft and the lowest in the PCL graft. Similarly, the CCK assay of cell cultures demonstrated a similar trend for the three fibrous grafts on day 4 (Figure 3D), with significantly higher cell viability and proliferation in functionalized coaxial fiber grafts (PCL/PEG-NB + RGD or PCL/PEG-NB + RGD/TGF-β1) compared to PCL fiber graft.
To assess the cell differentiation into smooth muscle cells, two important smooth muscle markers, α-SMA and SM-MHC, were investigated after 6 days of cell culture by immunostaining and confocal imaging (Figures 4A and S7A). To validate the protein expression results, relevant gene expression using qPCR was obtained (Figure 4B). Expression of α-SMA, SM-MHC, elastin, and collagen I mRNA was normalized to human GAPDH (Figure 4B) or HPRT (Figure S7B). Figure 4B quantitatively demonstrated significantly higher SM-MHC intensity in PCL/PEG-NB + RGD/TGF-β1 compared to PCL/PEG-NB + RGD and PCL grafts. Additionally, both coaxial fiber grafts functionalized with peptides showed much stronger α-SMA signals than PCL grafts. This indicated the capability of the developed coaxial grafts to promote not only cell attachment and proliferation but also cell differentiation into vascular lineage. Furthermore, these results suggested that the addition of TGF-β1 to the grafts further improved cell differentiation into smooth muscle cells.
Figure 4.
Cell differentiation in the coaxial fiber vascular grafts. (A) Confocal microscopy images showing α-SMA and MHC stains in hMSCs cultured for 6 days on scaffolds made of PCL fibers, PCL/PEG-NB + RGD, and PCL/PEG-NB + RGD/TGF-β1. DAPI stain (blue) showing cell nuclei is shown as well. Images were taken at 10×. Scale bar = 50 μm. (B) Gene expression on fiber scaffolds by qPCR showing α-SMA (a), SM-MHC (b), elastin (c), and collagen I (d). GAPDH was used as the reference house-keeping gene. (C) Average intensity calculated as the total mean grey value divided by the total number of cells from confocal images of the scaffolds. “*” p < 0.05, “**” p < 0.01, “***” p < 0.001, compared to PCL. “###” p < 0.001 compared to PCL/PEG-NB + RGD.
The results on α-SMA and SM-MHC expressions agreed with the immunofluorescent analysis results (Figure 4C). Interestingly, collagen I and elastin genes were only expressed in the coaxial scaffolds. Overall, our results showed that TGF-β1 induces MSC differentiation into vascular smooth muscle phenotype, which is in agreement with previous work.13,22
3.4. In Vivo Arterial Grafting Showed Expeditious Cell and ECM Regeneration in Coaxial Fiber Grafts.
To assess our newly developed 3% PCL/PEG-NB grafts conjugated with bioactive RGD and RGD/TGF-β1, the abdominal aortic interposition graft model in adult rats was used. Arterial regeneration was evaluated on grafts with or without preseeding of MSCs on the graft lumen, using histology, immunohistochemistry, immunofluorescence, and SHG-TPEF analyses.
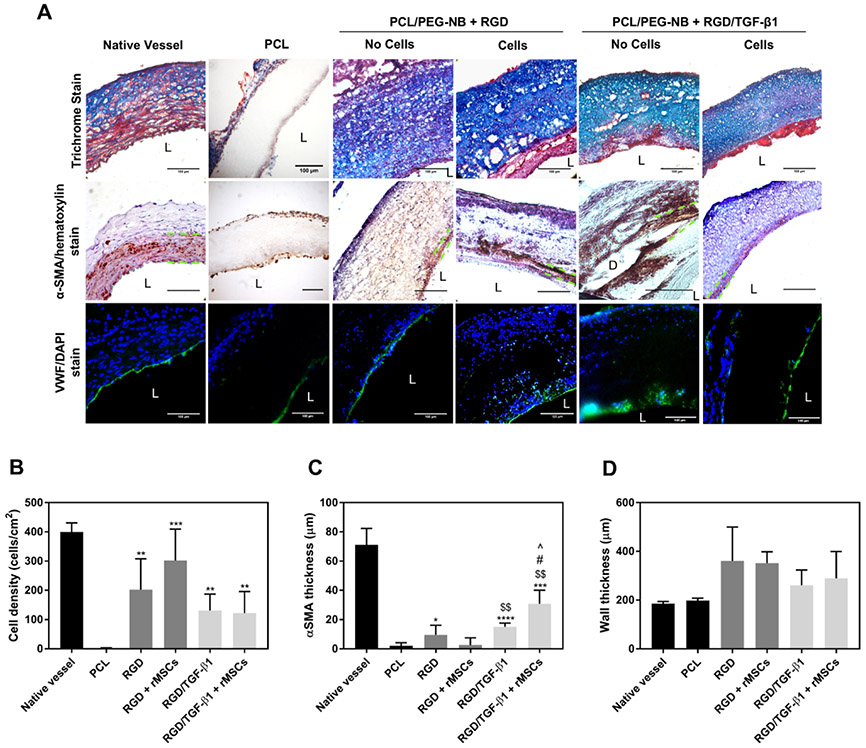
To identify the presence of cells and ECM proteins, the explanted grafts were stained with Masson’s trichrome. Figure 5A shows a significantly larger amount of deposited collagen in all coaxial fiber grafts (with or without MSCs), compared to the PCL graft. Additionally, images with hematoxylin and DAPI stains showed a much larger number of cells in the coaxial grafts, compared to the PCL graft. Although no statically significant differences were found among all coaxial fiber grafts, coaxial grafts functionalized with only RGD, with or without preseeded MSCs, seemed to show a trend of increased cell infiltration into the grafts (Figure 5B). Endothelial and smooth muscle cell layers were found in all explanted coaxial fiber grafts (Figure 5A), while there were sparse populations of endothelial cells (vWF+) and smooth muscle cells (α-SMA+) in the PCL graft. The smooth muscle layer appeared thicker in the coaxial grafts functionalized with RGD/TGF-β1 (Figure 5C), likely due to TGF-β1-induced differentiation. The thickest smooth muscle cell layer was found in the RGD/TGF-β1-functionalized graft preseeded with MSCs. The α-SMA-positive (α-SMA+) cells were detected not only in the area close to the graft lumen but also within the graft-surrounding neotissues away from the lumen, which might result from cell migration. This was in agreement with other studies that reported the presence of vascular smooth muscle cells in synthetic vascular grafts 4 weeks after implantation.24,25 Individual cells stained with hematoxylin and α-SMA stain are more clearly visualized in Figure S8. To investigate the inflammation around grafts, immunofluorescent staining was performed with CD68 for macrophage detection together with DAPI counterstain (Figure S9). Results showed an overall very low level of inflammation in all grafts, with slightly more macrophages detected in the PCL graft compared to the coaxial grafts, suggesting that PEG-NB sheath material served as less reactive coating around PCL, causing minimal inflammation in the coaxial fiber grafts, and therefore better material integration. Figure 5D shows the measured thickness of the explanted graft wall. Coaxial fiber grafts showed thicker wall compared to the PCL graft, which had a wall thickness close to the initial wall thickness or native vessel wall thickness. The thicker wall of coaxial fiber grafts might be due to the 4-armed PEG thiol–ene hydrogel sheath, which swelled with large amounts of water. The recruited cells and matrix production could also play a role in the increased wall thickness.
Figure 5.
Biological performances of vascular grafts. (A) Histological analyses, displaying cell infiltration and ECM production in the explanted grafts, as well as smooth muscle and endothelial layers. Masson’s trichrome stain displays collagen and mucus (blue), cell nuclei (black), and cell cytoplasm, keratin, muscle, and intercellular fiber (red). α-SMA/hematoxylin stain shows smooth muscle cells in brown, and cell nuclei in purple. The space between green dotted lines denotes α-SMA layer in implanted vascular grafts. For native artery, it denotes the smooth muscle layer or tunica media. vWF/DAPI stain shows cell nuclei in blue, and endothelial cell in green. “L” denotes the lumen. “D” denotes graft delamination. Scale bar = 100 μm. (B) The cell penetration, (C) smooth muscle cell layer thickness, and (D) graft wall thickness measured on the explanted grafts. Significance comparisons include: “*” compared to PCL, “$” compared to RGD + rMSCs, “#” compared to RGD/TGF-β1, and “^” compared to RGD.
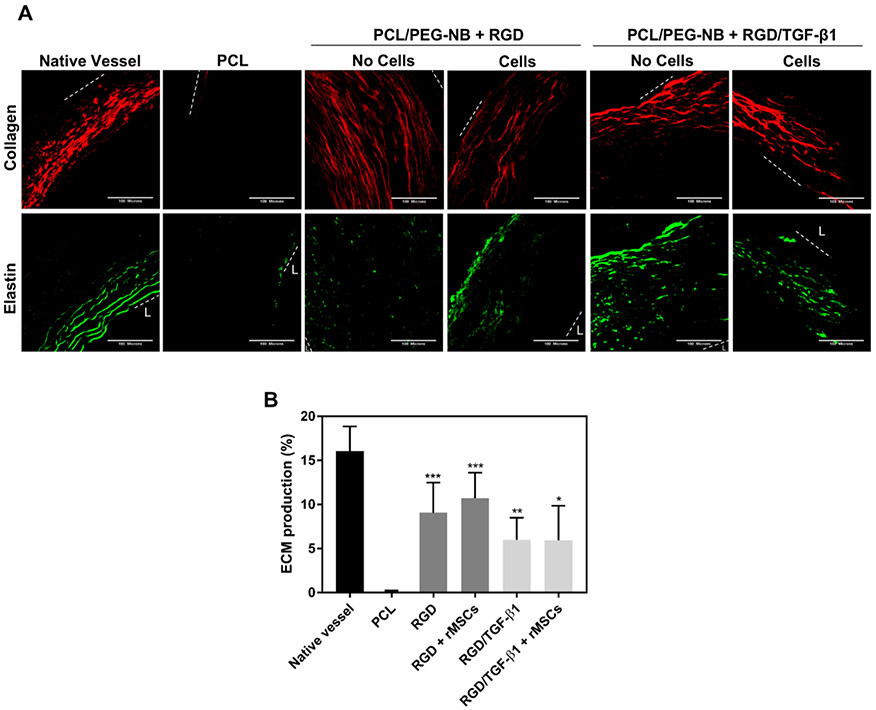
Dual multiphoton imaging modality (SHG-TPEF) was used to further detect the matrix composition produced in the explanted vascular grafts. Graft samples from the anastomosis site were evaluated. Figure 6A shows the newly synthesized fibrillar collagen (mainly collagen types I and III) and elastin. All coaxial fiber grafts showed significantly increased depositions of collagen and elastin compared to the PCL graft because of the ECM production of infiltrated cells. Though no statistical differences existed among all coaxial fiber grafts, the coaxial grafts treated with only RGD, with or without preseeded MSCs, tended to show a larger amount of ECM deposition (Figure 6B), which was in line with our results showing more cells present in those groups. This might be due to the TGF-β1 effect on smooth muscle differentiation reducing cell proliferation and synthesis—smooth muscle cells possess two basic phenotypes, synthetic or highly proliferative phenotype and contractile or highly differentiated phenotype. In addition to the graft samples from the anastomosis site of the grafts, samples from the middle of the grafts were also evaluated (Figure S10). The ECM production existed in these samples as well, although to a lesser extent. This was probably due to a large amount of trans-anastomotic cell infiltration at the anastomosis sites, with cells that slowly migrated to the middle of the graft, together with possible adhesion of a relatively small number of blood–borne cells. In accordance with the results found with samples at the anastomosis sites, more ECM production appeared in the grafts functionalized with only RGD, with or without preseeded MSCs, although no statistically significant differences existed among all coaxial samples. However, 1 week implantation might be short for the evaluation of ECM production in the middle portion of the grafts. Overall, the newly produced ECM in coaxial fiber grafts looked different from the natural ECM, in both quality and quantity. In terms of the ECM quality, the elastin fiber content, structure and arrangement were much lower, fragmented and unaligned in all coaxial fiber grafts, while the collagen fibers were more sparsely distributed, less dense or localized compared to collagen fibers in the native vessel, where thick collagen fibrils were located only in the adventitial layer.
Figure 6.
ECM production measured in the anastomosis site of the explanted vascular grafts. (A) Multiphoton imaging of the grafts. Images display the produced collagen in red (from SHG imaging) and elastin in green (from TPE imaging). The area between white dotted lines shows the implanted vascular grafts. For native artery, it shows the area between tunica intima and adventitia. “L” represents the lumen. Scale bar = 100 μm. (B) The ECM production measured on the explanted grafts. “*” Compared to PCL.
4. DISCUSSION
This study has developed an innovative platform that creates vascular grafts with not only mimetic arterial mechanics, including high strength and elasticity, but also physiologically relevant 3D arterial environments, such as hydrated surfaces and biochemical cues for regulated cell behaviors in vivo. This study has achieved distinctive features. First, coaxial electrospinning was used to fabricate nanostructured hybrid fibers that mimic both structural and mechanical properties of the vascular ECM. Structurally, the porous microfiber hydrogel provided soft, hydrated, bioactive environments for the cell infiltration and ECM production. Mechanically, the step-growth polymerization between PEG-NB in the fiber sheath and PEG dithiol resulted in fully crosslinked hydrogels with structural stability, high elasticity and strain-to-failure. Overall, tensile test results showed that the mechanical properties of coaxial PCL/PEG-NB fibers were comparable to those of native vessels such as veins or arteries of a similar size (1–2 mm), in terms of the modulus (~200–800 kPa), strength (~150–1400 kPa), and maximum elongation (~45–175%).26-30 Also, the coaxial fiber grafts presented a more linear behavior compared to hyper-elastic materials, likely because of the interfibrous crosslinks originated from polymerization. These crosslinks could efficiently hamper extensive rearrangement of the coaxial fibers before they were ruptured. Also, the nearly linear stress–strain behaviors of our PCL/PEG-NB grafts are different from rat aortas, which display an overall non-linear stress–strain behavior, with two distinct regions of relatively linear behavior—modulus in the range of 0.05–0.2 MPa in the lower modulus region linear up to about 50% strain, and modulus in the range of 1–2.5 MPa in the upper modulus region.31 Our PCL/PEG-NB grafts, with a modulus of ~1 MPa for 1.8% PCL/PEG-NB grafts and ~2.3 MPa for 3% PCL/PEG-NB, seem close to the rat aorta modulus in the upper modulus region (under non-physiological strains).
Compared to PCL grafts, PCL/PEG-NB grafts mimic native ECM by showing: (1) hydrogel properties with high water absorption, withholding water or blood within the grafts and offering hydrated microenvironments for molecular diffusion and cell migration; (2) much higher pore size, even in the wet fibers (~7–9 times), which facilitate cell infiltration; and (3) crosslinked fiber network, maintaining high structural stability and avoiding delamination under repeated loading of blood flow stresses. Because of these unprecedented features of polymer grafts, we have shown expeditious and abundant vascular cell infiltration and a large amount of vascular ECM production, the two main criteria of arterial regeneration, within only 1 week of implantation. The arterial regeneration was significantly improved with respect to those reported in previous studies,6,24,25,32 even though adult rats used in this study might be less regenerative than widely used juvenile rats. These previous studies reported arterial regeneration on electrospun synthetic grafts implanted in either the abdominal aorta or the carotid artery of rats, as early as two weeks after implantation. The endothelialization of the luminal surface and the presence of α-SMA+ cells and matrices in the grafts occurred after at least 2–4 weeks of implantation, with no α-SMA+ cells detected after 1 week of implantation.6 Therefore, our results demonstrated faster arterial tissue regeneration in the implanted PCL/PEG-NB vascular grafts compared to other synthetic grafts, with endothelialization, presence of α-SMA+ cells, deep cell infiltration into the graft material, and ECM production within 1 week after implantation.
The long-term patency of readily available small-diameter vascular grafts requires both strength and elasticity of graft materials. Results from this study showed that increasing the PCL concentration (3 vs 1.8%) in the fiber core enhanced the graft strength, yielding stronger graft material more adequate for vascular graft applications. Also, the suture retention values were close to other synthetic PCL-based vascular grafts’ reported values,11 although they were lower than other native vessels such as saphenous veins11,30,33 and coronary arteries,30 as well as PTFE grafts.11 When hydrated, the grafts made of 3% PCL/PEG-NB fibers showed more superior mechanical performances than grafts made of 1.8% PCL/PEG-NB fibers, which might derive from their structural features characterized by a lower porosity and a larger fiber diameter with thicker core and sheath. Herein, the PCL core played a crucial role in reinforcing the hydrogel, which otherwise would collapse under the normal blood pressure. During initial in vivo evaluations, the 1.8% PCL/PEG-NB graft, due to its high porosity, turned out to be too permeable for proper graft implantation. However, further increasing the PCL concentration beyond 3% tended to increase stiffness and reduce porosity, resulting in a material mechanically stiff and structurally uninviting for cell infiltration and regeneration. Therefore, 3% PCL/PEG-NB graft provided an excellent balance for vascular grafting, being strong, elastic, soft, porous and thus optimal for graft implantation. Additionally, the PEG-NB polymerization process made the scaffold mechanically stable because of interfibrous crosslinks as well as intrafibrillar interactions predominantly in the sheath of the coaxial fibers.
Another unique feature of using 4-armed, thiol–ene photoclickable chemistry is that the cell-recognizable, bioactive peptides can be covalently tethered with varied densities after hydrogel crosslinking. Different from other vascular graft materials, the local stiffness of 3D fiber hydrogels rendered well-defined, physiological arterial stiffness to drive the smooth muscle differentiation of MSC in vitro and in vivo. Interestingly, when combined with cell differentiation growth factor such as TGF-β1, we found synergistic effects of local stiffness and differentiating factor on MSC differentiation into vascular smooth muscle cell in vitro and in vivo, which was consistent with our previous studies13,21,22,34 and others’.23 While both types of coaxial fiber grafts (RGD and RGD/TGF-β1) stimulated cell proliferation and infiltration as well as ECM production in vitro and in vivo, the coaxial fiber grafts with tethered RGD/TGF-β1 further enhanced the number of differentiated (contractile) smooth muscle cells. Therefore, the novel platform could offer arterial ECM-like structure, tunable composition and stiffness for the remodeling process and functional activity on the graft wall. It may also be used as stent coating, together with recent innovations in metal stents.35-37
Though our in vivo graft outcomes were in good agreement with in vitro results, highlighting the great performances of functionalized coaxial fiber grafts, our studies also led to an intriguing finding about allogenic MSC function in vivo. No statistically significant differences between the functionalized coaxial grafts with and without preseeded MSCs suggest that highly functional, soft grafts, capable of stimulating regeneration and minimizing inflammation, might have overridden the potential roles of MSCs in anti-inflammation and proregeneration. Nevertheless, the puzzling roles of MSCs in vivo as well as the optimization of MSC-seeded functional grafts demand further investigations, such as using autologous MSCs or immunodeficient rats.38 Additionally, several limitations of the present study are acknowledged: (1) the implantation time is only 1 week. Further studies into future clinical utility of our grafts will be extended to several months. (2) To better understand the cell activities during the course of in situ regeneration, a larger number of animals in each study group will be desired for long-term implantation, as well as a different animal model because of the rapid re-endothelialization of synthetic grafts in rodents.24 (3) The fate and function of preseeded MSCs remain elusive. Based on previous studies,13,39,40 we would expect expeditious loss of preseeded allogenic cells from the grafts upon implantation and prompt penetration of host cells in the grafts. Despite of this loss, allogenic cells have been shown to significantly influence graft performances. (4) As fast degradation is considered important to in situ regeneration,41 materials with similar physical and mechanical properties but faster degradation rates, such as PLGA and PEG-NB conjugated with MMP-sensitive peptides, may reduce overall wall thickness and improve regeneration outcomes in the long-term run. The mechanical strength of the graft in vivo depends on the biological and mechanical deterioration or degradation of the graft and the tissue regeneration within the graft. An ideal graft would maintain a delicate balance between the tissue regeneration and the graft deterioration or degradation over time in vivo. Because expeditious, significant tissue regeneration was found around our coaxial fiber grafts, future efforts will be on designing strategies to stabilize graft mechanical properties over time by balancing the graft deterioration or degradation with the tissue regeneration. In particular, appropriate cell types should be induced to produce ECM that closely matches the natural ECM, both qualitatively and quantitatively.
5. CONCLUSIONS
As far as we know, this study is the first to report the employment of tunable ECM-like composition and stiffness in strong, compliant vascular grafts for guided cell remodeling on the graft wall. We report high cell infiltration and ECM production in both acellular and cellular small-caliber vascular grafts for 1 week implantation, indicating high regenerative potential. This versatile fibrous hydrogel system, with excellent regenerative and mechanical properties, would meet the needs of replacing a broad range of tissue regenerations in numerous clinical settings, including a clear application as vascular graft.
Supplementary Material
ACKNOWLEDGMENTS
Transmission electron microscopy was done with the technical assistance of staff at the EM Services Core Facility in the Department of MCD Biology, the University of Colorado—Boulder.
Funding
The work was financially supported by National Heart, Lung, and Blood Institute R01HL119371 (W.T.).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsabm.0c01114.
MSC seeded coaxial graft lumen prior to implantation; blood flow through and morphology of coaxial grafts; SEM image of PCL fibers; suture strength of the coaxial grafts; elastic modulus of the coaxial grafts; confirmation of tethered TGF-β1 on coaxial fiber scaffolds; hMSC differentiation in the vascular graft fibers; α-SMA/hematoxylin stain showing smooth muscle cells in the explanted coaxial grafts; evaluation of macrophage presence on the explanted vascular grafts; and ECM production measured in the middle of the explanted vascular grafts (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Criqui MH; Aboyans V Epidemiology of Peripheral Artery Disease. Circ. Res 2015, 116, 1509–1526. [DOI] [PubMed] [Google Scholar]
- (2).Cameron RB Bioengineered Vascular Grafts: So Close and yet so Far. J. Thorac. Cardiovasc. Surg 2018, 156, 1823–1824. [DOI] [PubMed] [Google Scholar]
- (3).Carrabba M; Madeddu P Current Strategies for the Manufacture of Small Size Tissue Engineering Vascular Grafts. Front. Bioeng. Biotechnol 2018, 6, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Dimitrievska S; Wang J; Lin T; Weyers A; Bai H; Qin L; Li G; Cai C; Kypson A; Kristofik N; Gard A; Sundaram S; Yamamoto K; Wu W; Zhao L; Kural MH; Yuan Y; Madri J; Kyriakides TR; Linhardt RJ; Niklason LE Glycocalyx-Like Hydrogel Coatings for Small Diameter Vascular Grafts. Adv. Funct. Mater 2020, 30, 1908963. [Google Scholar]
- (5).Luo J; Qin L; Zhao L; Gui L; Ellis MW; Huang Y; Kural MH; Clark JA; Ono S; Wang J; Yuan Y; Zhang S-M; Cong X; Li G; Riaz M; Lopez C; Hotta A; Campbell S; Tellides G; Dardik A; Niklason LE; Qyang Y Tissue-Engineered Vascular Grafts with Advanced Mechanical Strength from Human IPSCs. Cell Stem Cell 2020, 26, 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Zhu M; Wu Y; Li W; Dong X; Chang H; Wang K; Wu P; Zhang J; Fan G; Wang L; Liu J; Wang H; Kong D Biodegradable and Elastomeric Vascular Grafts Enable Vascular Remodeling. Biomaterials 2018, 183, 306–318. [DOI] [PubMed] [Google Scholar]
- (7).Pashneh-Tala S; MacNeil S; Claeyssens F The Tissue-Engineered Vascular Graft - Past, Present, and Future. Tissue Eng., Part B 2016, 22, 68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Song H-HG; Rumma RT; Ozaki CK; Edelman ER; Chen CS Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 2018, 22, 340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Kurobe H; Maxfield MW; Breuer CK; Shinoka T Concise Review: Tissue-Engineered Vascular Grafts for Cardiac Surgery: Past, Present, and Future. Stem Cells Transl. Med 2012, 1, 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Singha K; Singha M Cardio Vascular Grafts: Existing Problems and Proposed Solutions. Int. J. Biol. Eng 2012, 2, 1–8. [Google Scholar]
- (11).Madhavan K; Elliott WH; Bonani W; Monnet E; Tan W Mechanical and Biocompatible Characterizations of a Readily Available Multilayer Vascular Graft. J. Biomed. Mater. Res., Part B 2013, 101, 506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Iglesias-Echevarria M; Durante L; Johnson R; Rafuse M; Ding Y; Bonani W; Maniglio D; Tan W Coaxial PCL/PEG-Thiol-Ene Microfiber with Tunable Physico-Chemical Properties for Regenerative Scaffolds. Biomater. Sci 2019, 7, 3640–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ding Y; Johnson R; Sharma S; Ding X; Bryant SJ; Tan W Tethering Transforming Growth Factor B1 to Soft Hydrogels Guides Vascular Smooth Muscle Commitment from Human Mesenchymal Stem Cells. Acta Biomater. 2020, 105, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hamrang A; Howell BA; Zaikov GE; Haghi AK Nanomaterials: Dos and don’ts of Production Process in Laboratory. In Foundations of High Performance Polymers: Properties, Performance, and Applications; Hamrang A, Howell BA, Eds.; Apple Academic Press: Oakville, 2013; Chapter 9, pp 202–209. [Google Scholar]
- (15).Golecki HM; Yuan H; Glavin C; Potter B; Badrossamay MR; Goss JA; Phillips MD; Parker KK Effect of Solvent Evaporation on Fiber Morphology in Rotary Jet Spinning. Langmuir 2014, 30, 13369–13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Liu C; Sun J; Shao M; Yang B A Comparison of Centrifugally-Spun and Electrospun Regenerated Silk Fibroin Nanofiber Structures and Properties. RSC Adv. 2015, 5, 98553–98558. [Google Scholar]
- (17).Li Y; Ceylan M; Shrestha B; Wang H; Lu QR; Asmatulu R; Yao L Nanofibers Support Oligodendrocyte Precursor Cell Growth and Function as a Neuron-Free Model for Myelination Study. Biomacromolecules 2014, 15, 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Konig G; McAllister TN; Dusserre N; Garrido SA; Iyican C; Marini A; Fiorillo A; Avila H; Wystrychowski W; Zagalski K; Maruszewski M; Jones AL; Cierpka L; de la Fuente LM; L’Heureux N Mechanical Properties of Completely Autologous Human Tissue Engineered Blood Vessels Compared to Human Saphenous Vein and Mammary Artery. Biomaterials 2009, 30, 1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wang S; Zhang Y; Yin G; Wang H; Dong Z Electrospun Polylactide/Silk Fibroin-Gelatin Composite Tubular Scaffolds for Small-Diameter Tissue Engineering Blood Vessels. J. Appl. Polym. Sci 2009, 113, 2675–2682. [Google Scholar]
- (20).Drilling S; Gaumer J; Lannutti J Fabrication of Burst Pressure Competent Vascular Grafts via Electrospinning: Effects of Microstructure. J. Biomed. Mater. Res., Part A 2009, 88, 923–934. [DOI] [PubMed] [Google Scholar]
- (21).Ding Y; Xu X; Sharma S; Floren M; Stenmark K; Bryant SJ; Neu CP; Tan W Biomimetic Soft Fibrous Hydrogels for Contractile and Pharmacologically Responsive Smooth Muscle. Acta Biomater. 2018, 74, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Floren M; Bonani W; Dharmarajan A; Motta A; Migliaresi C; Tan W Human Mesenchymal Stem Cells Cultured on Silk Hydrogels with Variable Stiffness and Growth Factor Differentiate into Mature Smooth Muscle Cell Phenotype. Acta Biomater. 2016, 31, 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Park KM; Gerecht S Harnessing Developmental Processes for Vascular Engineering and Regeneration. Development 2014, 141, 2760–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Qiu X; Lee BL-P; Ning X; Murthy N; Dong N; Li S End-Point Immobilization of Heparin on Plasma-Treated Surface of Electrospun Polycarbonate-Urethane Vascular Graft. Acta Biomater. 2017, 51, 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Henry JJD; Yu J; Wang A; Lee R; Fang J; Li S Engineering the Mechanical and Biological Properties of Nanofibrous Vascular Grafts for in Situ Vascular Tissue Engineering. Biofabrication 2017, 9, 035007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Claes E; Atienza JM; Guinea GV; Rojo FJ; Bernal JM; Revuelta JM; Elices M Mechanical Properties of Human Coronary Arteries. 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, August31–Sept 4, 2010; IEEE, 2010; p 3792. [DOI] [PubMed] [Google Scholar]
- (27).Khamdaeng T; Luo J; Vappou J; Terdtoon P; Konofagou EE Arterial Stiffness Identification of the Human Carotid Artery Using the Stress-Strain Relationship in Vivo. Ultrasonics 2012, 52, 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Holzapfel GA; Sommer G; Gasser CT; Regitnig P Determination of Layer-Specific Mechanical Properties of Human Coronary Arteries with Nonatherosclerotic Intimal Thickening and Related Constitutive Modeling. Am. J. Physiol.: Heart Circ. Physiol 2005, 289, H2048–H2058. [DOI] [PubMed] [Google Scholar]
- (29).Sommer G; Regitnig P; Költringer L; Holzapfel GA Biaxial Mechanical Properties of Intact and Layer-Dissected Human Carotid Arteries at Physiological and Supraphysiological Loadings. Am. J. Physiol.: Heart Circ. Physiol 2010, 298, H898–H912. [DOI] [PubMed] [Google Scholar]
- (30).Vatankhah E; Prabhakaran MP; Semnani D; Razavi S; Morshed M; Ramakrishna S Electrospun Tecophilic/Gelatin Nanofibers with Potential for Small Diameter Blood Vessel Tissue Engineering. Biopolymers 2014, 101, 1165–1180. [DOI] [PubMed] [Google Scholar]
- (31).Sokolis DP; Lampropoulos KM; Dimitriou CA; Balafas E; Boudoulas H; Karayannacos PE Time-Course of Mechanical Changes of the Rat Aorta Following Chronic Beta-Blocker Treatment. Hell. J. Cardiol 2010, 51, 19–26. [PubMed] [Google Scholar]
- (32).Tang D; Chen S; Hou D; Gao J; Jiang L; Shi J; Liang Q; Kong D; Wang S Regulation of Macrophage Polarization and Promotion of Endothelialization by NO Generating and PEG-YIGSR Modified Vascular Graft. Mater. Sci. Eng., C 2018, 84, 1–11. [DOI] [PubMed] [Google Scholar]
- (33).Nagiah N; Johnson R; Anderson R; Elliott W; Tan W Highly Compliant Vascular Grafts with Gelatin-Sheathed Coaxially Structured Nanofibers. Langmuir 2015, 31, 12993–13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Wingate K; Floren M; Tan Y; Tseng PON; Tan W Synergism of Matrix Stiffness and Vascular Endothelial Growth Factor on Mesenchymal Stem Cells for Vascular Endothelial Regeneration. Tissue Eng., Part A 2014, 20, 2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Su Y; Cockerill I; Wang Y; Qin Y-X; Chang L; Zheng Y; Zhu D Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Nuhn H; Blanco CE; Desai TA Nanoengineered Stent Surface to Reduce In-Stent Restenosis in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 19677–19686. [DOI] [PubMed] [Google Scholar]
- (37).Yang Z; Zhao X; Hao R; Tu Q; Tian X; Xiao Y; Xiong K; Wang M; Feng Y; Huang N; Pan G Bioclickable and Mussel Adhesive Peptide Mimics for Engineering Vascular Stent Surfaces. Proc. Natl. Acad. Sci. U.S.A 2020, 117, 16127–16137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Fukunishi T; Best CA; Ong CS; Groehl T; Reinhardt J; Yi T; Miyachi H; Zhang H; Shinoka T; Breuer CK; Johnson J; Hibino N Role of Bone Marrow Mononuclear Cell Seeding for Nanofiber Vascular Grafts. Tissue Eng., Part A 2018, 24, 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Elliott MB; Ginn B; Fukunishi T; Bedja D; Suresh A; Chen T; Inoue T; Dietz HC; Santhanam L; Mao H-Q; Hibino N; Gerecht S Regenerative and Durable Small-Diameter Graft as an Arterial Conduit. Proc. Natl. Acad. Sci. U.S.A 2019, 116, 12710–12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Hibino N; Duncan DR; Nalbandian A; Yi T; Qyang Y; Shinoka T; Breuer CK Evaluation of the Use of an Induced Puripotent Stem Cell Sheet for the Construction of Tissue-Engineered Vascular Grafts. J. Thorac. Cardiovasc. Surg 2012, 143, 696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Wissing TB; Bonito V; Bouten CVC; Smits AIPM Biomaterial-Driven in Situ Cardiovascular Tissue Engineering—a Multi-Disciplinary Perspective. npj Regener. Med 2017, 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.