ABSTRACT
Chaperone-mediated autophagy (CMA), as one of the main pathways of lysosomal catabolism, plays essential roles for the maintenance of cellular homeostasis. To date, the absence of any identifiable LAMP2A – the necessary and limiting protein required for CMA – in non-tetrapod lineages, led to the paradigm that this cellular process was restricted to mammals and birds. The recent findings of Lescat et al., demonstrating the existence of a CMA activity in fish, now reshuffle the cards regarding how the entire evolution of CMA function should be considered and appreciated across metazoans. Hence, beyond challenging the current tetrapod-centered accepted view, the work of Lescat et al. tackles the possibility – or the compelling need – of using complementary and powerful genetic models, such as zebrafish or medaka, for studying this fundamental function from an evolutionary perspective.
KEYWORDS: Chaperone-mediated autophagy, CMA, evolution, fish, Lamp2a, medaka, zebrafish
Chaperone-mediated autophagy (CMA) is an intracellular catabolic pathway that mediates the degradation of specific soluble proteins within lysosomes. Because CMA defects are associated to several human pathologies, including neurodegenerative diseases, cancers and immune disorders, research efforts over the past years have been undertaken to study that essential cellular function. Accordingly, recent findings emphasized the fundamental role of CMA for regulating numerous cellular functions including cellular energetics, transcriptional programs, cell death, and cell survival mechanisms or DNA repair.
Briefly, during CMA, cytosolic proteins containing a KFERQ-like motif are first recognized by HSPA8/HSC70 (heat shock protein family A [Hsp70] member 8) and co-chaperones. The substrate-chaperone complex then docks at the lysosomal membrane through specific binding to the cytosolic tail of LAMP2A (lysosomal associated membrane protein 2A). LAMP2A then organizes into a multimeric complex that allows the substrate to translocate across the lysosomal membrane where it is degraded by acid hydrolases. LAMP2A is one of the three variants (namely, LAMP2A, LAMP2B and LAMP2 C) that originate from alternative splicing of the LAMP2 gene. All three splice variants share a common lumenal domain and only differ by their cytosolic and transmembrane regions. CMA activity has been directly correlated to the amount of LAMP2A (but not those of LAMP2B and LAMP2 C) at the lysosomal membrane. As such, LAMP2A has been described as being the limiting component for CMA activity.
Until recently, the absence of any clearly identifiable LAMP2A protein outside of the mammalian and bird species raised concerns about the presence of CMA in other vertebrate lineages. However, using Basic Local Alignment Search Tools (BLASTs) against different expression databases of ray-finned fish species, we were able to identify several expressed sequence tags (ESTs) displaying high homology with mammalian LAMP2A. This suggested that this protein possibly appeared much earlier during evolution than initially thought, and provided the grounds for looking at the existence of a “genuine” CMA function in fish. In a just released paper, Lescat et al. [1] now provide evidence in this direction. The authors first demonstrated that the LAMP2 gene and its structure containing the three alternatively spliced exons (B, A and C) encoding the transmembrane domain and cytoplasmic tail specific to each isoform (LAMP2B, LAMP2A and LAMP2 C, respectively), is not restricted to mammals or birds, but is also present in the genome of different fish species. In contrast, no homologous sequence was found in invertebrate species, suggesting that CMA is indeed restricted to vertebrates. It was then shown that the splice variant lamp2a is expressed from the earliest stages of development as well as in several adult tissues of the medaka fish model species (Oryzias latipes), supporting the idea that fish might exhibit CMA activity – or at least a CMA-like process. In order to firmly establish whether or not CMA exists in fish, a medaka fibroblast cell line was transfected with the photoactivable KFERQ-PA-mCherry construct, which has proven to be a reliable reporter for tracking and measuring CMA activity in mammalian cells. Results clearly showed that, upon long-term starvation, this CMA reporter accumulates in characteristic puncta that colocalize with lysosomes and/or late endosomes, and that specific knockdown of lamp2a results in a significant loss of these puncta, thereby providing functional evidence for the existence of CMA activity in fish. Finally, to address the physiological role of Lamp2a in fish, a medaka knockout for the splice variant lamp2a was generated. These KO fish display severe alterations in carbohydrate and fat metabolism, similar to what has been observed in the liver of mice deficient for CMA. These results further demonstrated that the CMA function is definitively not restricted to mammals and birds.
Overall, these findings open up new and exciting perspectives to approach (or differently appreciate) CMA under a novel angle. For instance, the relative sequence variability observed within the different functional domains of LAMP2A between phylogenetically distant species will certainly be informative for identifying evolutionarily conserved, or species-dependent, key residues necessary for the structure function relationship of this protein (Figure 1).
Figure 1.
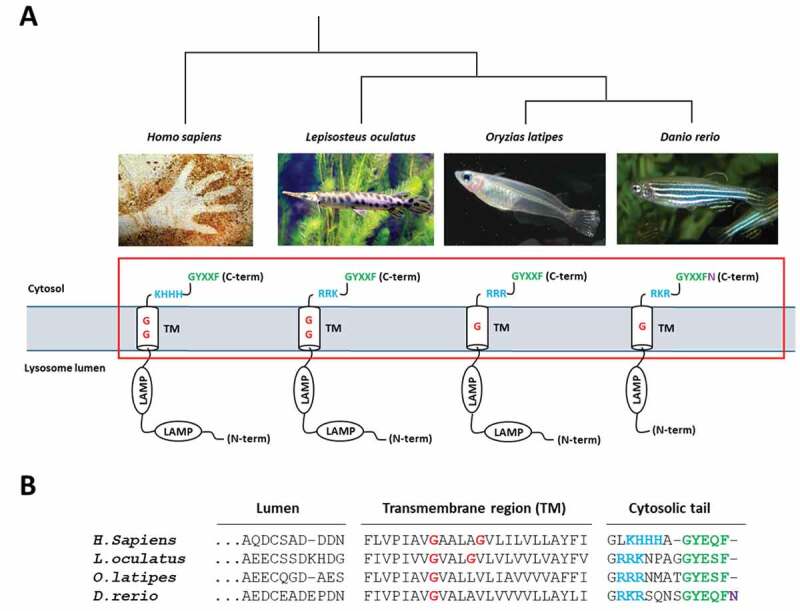
Sequence variability within different functional domains of LAMP2A between phylogenetically distant species. (A) Schematic representation of selected LAMP2As. (B) Sequence alignment of the boxed region. Three positively charged amino acids (in blue) necessary for the binding of substrate proteins are present in fish sequences. However, whereas two glycine (G) residues (in red) located within the transmembrane (TM) region are essential for the multimerization of LAMP2A in rodents, only one G is found in that region in some fish species, including medaka. A GYXXF sequence at the C terminus (in green) is required for targeting LAMP2A to the lysosomal membrane. Although conserved in most teleosts, the divergence of that motif in zebrafish, encoding an additional asparagine residue (N, purple), raises a question about the ability of this species to perform CMA, and certainly deserves special attention.
Beyond these perspectives on the structure-function relationship of LAMP2A, these new findings also emphasize the interest of teleost fish, which diverged from the tetrapod lineage early during vertebrate evolution, as attractive and unique models at the functional interface between invertebrates (assumed to lack any CMA activity, but relying on an endosomal microautophagy [eMI]-like system for targeting KFERQ-like-motif-containing proteins) and mammals (making use of both eMI and CMA functions), for studying the interplay between these two related pathways. In this respect, the evolutionary history and requirement of the main effectors and regulators of CMA and eMI, such as HSPA8/HSC70 (known to control both functions) or the endosomal sorting complexes required for transport/ESCRT machinery (specific to eMI) in teleosts will warrant close consideration.
Hence, much more than challenging the currently tetrapod-centered paradigm, the study of Lescat et al. tackles the urgent need of considering complementary and powerful alternative genetic models, for approaching the entirety of that fundamental catabolic process from an evolutionary perspective.
Funding Statement
This work was supported by the INRAE “Animal Physiology and Livestock Systems” Division, the French National Research Agency (ANR-17-CE20-0033 “Fish-and-Chap”) and the Doctoral School of Exact Sciences and their Applications (ED211) of the UPPA.
Disclosure statement
No potential conflict of interests was reported by the authors.
Reference
- [1].Lescat L, Véron V, Mourot B, et al. Chaperone-mediated autophagy in the light of evolution: insight from fish. Mol Biol Evol. 2020. DOI: 10.1093/molbev/msaa127. [DOI] [PubMed] [Google Scholar]


