ABSTRACT
The endoplasmic reticulum (ER) is the largest membrane-bound organelle in eukaryotic cells and plays critical roles in diverse processes in metabolism, signaling and intracellular organization. In response to stress stimuli such as nutrient deprivation, accumulation of misfolded proteins or exposure to chemicals, the ER increases in size through upregulated synthesis of its components to counteract the stress. To restore physiological size, the excess ER components are continuously dismantled and degraded by reticulophagy, a form of autophagy that targets, via adaptor molecules called reticulophagy receptors, specific ER portions to the lysosome for degradation. Previous studies have identified several ER resident proteins as reticulophagy receptors. In a recent study, we identified CALCOCO1 as a soluble reticulophagy receptor for the degradation of tubular ER in response to proteotoxic and starvation-induced stress. On the ER membrane, CALCOCO1 interacts with VAPA and VAPB via a FFAT-like motif and recruits autophagy machinery by binding directly to Atg8-family proteins via LIR and UDS interacting region (UIR) motifs acting co-dependently. Depletion of CALCOCO1 in cultured cells led to an impaired ER degradation during stress.
KEYWORDS: Autophagy receptor, CALCOCO1, ER-phagy, FFAT motif, VAPA
CALCOCO1 is a paralog to TAX1BP1 and CALCOCO2/NDP52, two well-characterized autophagy receptors for the degradation of ubiquitinated cargoes and pathogenic bacteria in eukaryotic cells. The three proteins share substantial similarity and identity and have a similar domain structure composed of an N-terminal SKICH domain, an atypical LIR motif (LVV), middle coiled-coil regions and a C-terminal region that contains one or two zinc finger domains. CALCOCO2 and TAX1BP1 participate in selective autophagy pathways as cargo receptors by binding to Atg8-family proteins on the phagophore via the LIR motif, and to degradable cargo via their ubiquitin or galectin binding activity. Lacking ubiquitin binding activity, however, CALCOCO1 has previously not been associated with autophagy receptor functions. Previous studies have suggested that CALCOCO1 functions in transcriptional co-activation, glucose metabolism and calcium signaling. In a recent study, we characterized new biochemical, molecular and interaction features of CALCOCO1 and in the process uncovered its function as a selective reticulophagy receptor [1]. Further, the study revealed CALCOCO1 colocalization with the ER and Golgi, and elucidated its coil-coil domain-mediated homodimerization property. Hence, CALCOCO1 is a component of ER- and Golgi-associated protein complexes.
While it retains the Atg8-family proteins binding activity of its paralogs, CALCOCO1 binds to potential cargo through direct interaction with ER resident proteins VAPA and VAPB via a novel FFAT-like motif on its C terminus. The mode of interaction with Atg8-family proteins, however, differs from that of CALCOCO2 because in addition to the atypical LIR motif, we demonstrated that CALCOCO1 also interacts through a C-terminal proximal region that binds to the UIM-docking site (UDS) of Atg8-family proteins. The UDS interacts with ubiquitin-interacting motif (UIM)-like sequences found in some autophagy receptors and it is located on the opposite side of the Atg8-family proteins relative to the LIR-docking sites (LDS). The CALCOCO1 UDS-binding region, however, does not have homology to UIM-like sequences and therefore we call it a UDS-interacting region (UIR). Both the LIR and UIR of CALCOCO1 are required for strong interaction with Atg8-family proteins and for its efficient degradation by autophagy. TAX1BP1 also has a C-terminal UIR that binds to the UDS site of GABARAP but with no sequence similarity with the UIR of CALCOCO1.
The FFAT motif targets proteins to the cytoplasmic face of the ER by binding to the VAP protein family. Our data revealed that CALCOCO1 is targeted to the ER by interacting with VAPA/B via a FFAT-like motif to mediate reticulophagy during proteotoxic and starvation-induced stress. In response to ER stress, the unfolded protein response (UPR) increases the size of peripheral ER through upregulated generation of sheets and tubules. Tubular ER is characterized by the presence of key proteins including VAPs, TEX264 and RTN3. The latter two are known reticulophagy receptors. To restore homeostasis, the cell engages reticulophagy to degrade the excess portions of the ER. Our studies revealed that VAPA and VAPB recruit CALCOCO1 during proteotoxic and starvation-induced stress to mediate degradation of the excess tubular ER. Depletion of VAP proteins in cells led to an accumulation of CALCOCO1 and RTN3, indicating inefficient degradation of CALCOCO1 and tubular ER. Conversely, knockout of the gene encoding CALCOCO1 causes an accumulation of tubular ER proteins, including VAPs, during starvation and proteotoxic stress, implying an impaired turnover. The turnover is, however, rescued by ectopic expression of CALCOCO1 in the knockout cells. Depletion of VAPs in the rescued cells, however, blocks the CALCOCO1-mediated turnover of tubular ER proteins, indicating that CALCOCO1-VAP interaction is required for CALCOCO1-mediated reticulophagy.
We suggest a model where CALCOCO1, bound to VAPs via its FFAT-like motif, recruits the core autophagy machinery via the Atg8-family proteins, to specific ER subdomains to initiate autophagosome biogenesis and capture of the degradable cargo (Figure 1). This is consistent with the notion that receptors act upstream of the autophagy machinery and supported by our observation that early phagophore markers WIPI2 and ATG13 are colocalized with CALCOCO1 puncta. Because VAP proteins are upregulated during stress conditions, we envisage that the targeted regions of the ER are VAP-rich tubular extrusions generated by remodeling of the ER. We postulate that the oligomeric nature of CALCOCO1 and VAPs enhances tight ER membrane targeting and recruitment of autophagy machinery proteins, consequently, leading to ER morphological changes and fragmentation. We suggest to classify CALCOCO1 as a soluble reticulophagy receptor because it is peripherally recruited to the ER, akin to SQSTM1/p62 acting with ER-anchored TRIM13 to mediate reticulophagy, and therefore distinguishing it from other ER-resident reticulophagy receptors.
Figure 1.
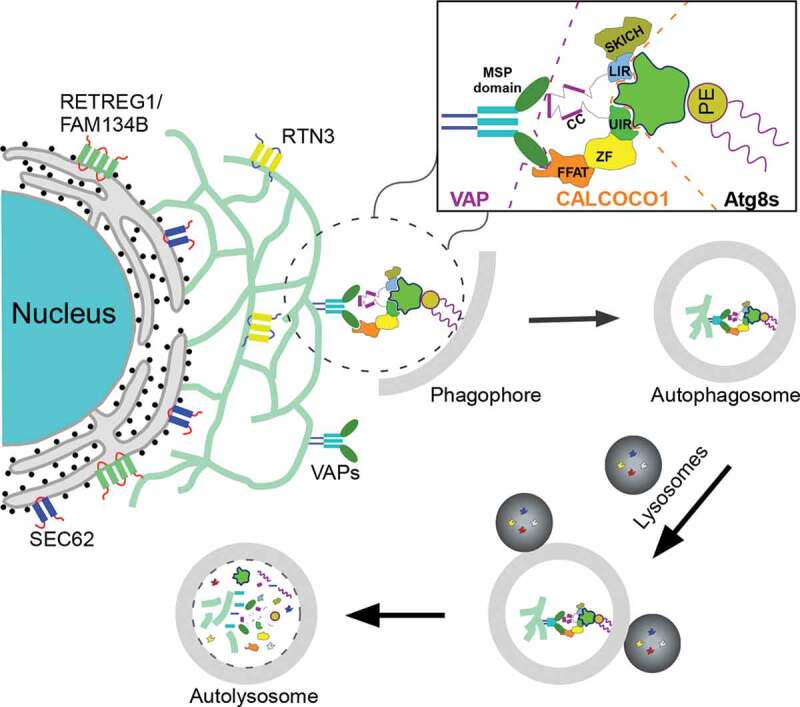
Role of CALCOCO1 during reticulophagy. During proteotoxic or nutrient stress, CALCOCO1 mediates the degradation of tubular ER through interaction with VAP proteins via its C-terminal FFAT-like motif. VAP-bound CALCOCO1 then recruits the autophagy machinery to the ER sites via LIR-LDS- and UIR-UDS-mediated interactions with Atg8-family proteins to initiate autophagosome biogenesis and capture of the degradable cargo. The cargo is subsequently sequestered into phagophores and then degraded when the mature autophagosomes fuse with lysosomes. CC, coiled coil; MSP, major sperm protein; PE, phosphatidylethanolamine; ZF, zinc finger.
The FFAT-VAP interactions have been associated with cytoskeletal organization, membrane trafficking, calcium signaling, ER‐associated degradation/ERAD, membrane contact sites and autophagosome biogenesis. An important question for future investigation is whether, apart from reticulophagy, CALCOCO1 is involved in any of these VAP-associated cellular functions. While the study revealed that a FFAT-mediated interaction of CALCOCO1 with VAPs targets tubular ER for degradation by autophagy, it is unclear how the process is regulated, considering there are many FFAT‐containing proteins conceivably competing for VAP interaction.
In conclusion, our study identified a new reticulophagy mechanism during stress conditions involving CALCOCO1 as a soluble autophagy receptor. CALCOCO1 interacts with ER resident VAPA and VAPB proteins via a novel FFAT-like motif, and with Atg8-family proteins via LIR and UIR motifs acting co-dependently.
Funding Statement
This work was funded by the TOPPFORSK program of The Research Council of Norway (grant number 249884) and the Norwegian Cancer Society (grant number 190214) to TJ.
Disclosure statement
The authors declare that they have no conflict of interest.
Reference
- [1].Nthiga TM, Kumar Shrestha B, Sjøttem E, et al. CALCOCO 1 acts with VAMP ‐associated proteins to mediate ER ‐phagy. Embo J. 2020;39:e103649. [DOI] [PMC free article] [PubMed] [Google Scholar]


