Abstract
Polypyrimidine tract-binding protein (PTB) is an abundant vertebrate hnRNP protein. PTB binding sites have been found within introns both upstream and downstream of alternative exons in a number of genes that are negatively controlled by the binding of PTB. We have previously reported that PTB binds to a pyrimidine tract within an RNA processing enhancer located adjacent to an alternative 3′-terminal exon within the gene coding for calcitonin and calcitonin gene-related peptide. The enhancer consists of a pyrimidine tract and CAG directly abutting on a 5′ splice site sequence to form a pseudoexon. Here we show that the binding of PTB to the enhancer pyrimidine tract is functional in that exon inclusion increases when in vivo levels of PTB increase. This is the first example of positive regulation of exon inclusion by PTB. The binding of PTB was antagonistic to the binding of U2AF to the enhancer-located pyrimidine tract. Altering the enhancer pyrimidine tract to a consensus sequence for the binding of U2AF eliminated enhancement of exon inclusion in vivo and exon polyadenylation in vitro. An additional PTB binding site was identified close to the AAUAAA hexanucleotide sequence of the exon 4 poly(A) site. These observations suggest a dual role for PTB in facilitating recognition of exon 4: binding to the enhancer pyrimidine tract to interrupt productive recognition of the enhancer pseudoexon by splicing factors and interacting with the poly(A) site to positively affect polyadenylation.
Alternative polyadenylation frequently occurs and results in multiple mRNA products from a single precursor RNA (reviewed in references 10, 14 and 64). One type of alternative polyadenylation involves the inclusion or exclusion of a 3′-terminal exon embedded within a multiexon transcript (Fig. 1). The two best-studied examples of this type of processing occur in the Drosophila doublesex gene (24, 35–38, 54, 57–59) and the human calcitonin/calcitonin gene-related peptide (CT/CGRP) gene (2, 11, 12, 23, 29–32, 52, 63). Although similar in exon and intron architecture, the two genes are regulated at different steps in processing and by different types of elements. The doublesex alternative processing choice is regulated at the level of splicing via the binding of multiple splicing regulators within the family of serine- and arginine-rich RNA binding proteins to exonic splicing enhancers of simple sequences (24, 35–38, 54, 57–59).
FIG. 1.
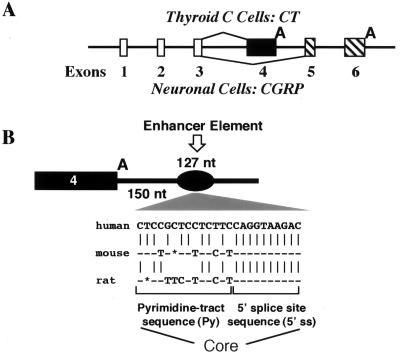
Diagram of CT/CGRP alternative RNA processing pathways and its intron 4 enhancer. (A) Schematic diagram of the CT/CGRP gene and its alternative RNA processing in thyroid and neuronal cells. (B) Diagram showing location of the intron enhancer (black oval) downstream of exon 4 and sequence of the enhancer core including the abutting pyrimidine tract sequence (Py) and 5′ splice site sequence (5′ ss). Sequence differences between the human, mouse, and rat enhancer cores are indicated.
Processing of the CT/CGRP precursor RNA, in contrast, is regulated at the level of polyadenylation through the action of an intron enhancer located downstream of the regulated exon (30–32). This enhancer is a complicated 127-nucleotide (nt) region with multiple sequence elements important for full enhancer activity. The most important enhancer element is a core sequence that resembles a pseudoexon, complete with branch point, pyrimidine tract, CAG, and 5′ splice site. There are, however, no internal sequences within the pseudoexon, so the CAG directly abutts on the +1 nucleotide of the 5′ splice site (Fig. 1).
Previously we have shown that the core pyrimidine tract is important for enhancer activity (30–32). Mutation of the pyrimidine tract by insertion of purines reduced in vivo exon inclusion and in vitro polyadenylation. In addition, mutation decreased binding of the hnRNP protein, polypyrimidine tract-binding protein (PTB), to the pyrimidine tract (31), suggesting a role for PTB in the enhancer function. PTB was originally isolated as a protein binding to 3′ splice site polypyrimidine tracts (6, 15, 16, 18, 47). PTB has been observed to bind to the 3′ polypyrimidine tracts of multiple regulated exons. PTB action has uniformly been associated with inhibition of exon recognition, not enhancement (3, 8, 20, 22, 28, 43–45, 47, 60). Thus, the CT/CGRP system offers an interesting variation in which PTB binds to a pseudoexon regulatory element resembling a 3′ splice site to stimulate recognition of a neighboring exon. It is unclear in other systems if PTB-mediated induction of exon skipping involves enhancement of flanking exons or merely repression of a central alternative exon.
We were interested to see if the pseudoexon model for CT/CGRP enhancer function was a good one. The normal splicing factor that binds the 3′ splice site polypyrimidine tract is U2AF (1, 26, 27, 55, 60, 65, 67–69). Binding of PTB can compete binding of U2AF (3, 55). Therefore, we designed a set of experiments to see if PTB affected CT/CGRP splicing in vivo and if U2AF was also involved. Transfection experiments indicated that increasing PTB levels affected CT/CGRP processing so as to favor exon inclusion, suggesting that PTB binding to CT/CGRP sequences positively affects processing. U2AF, however, did not behave as an enhancer binding protein in cells normally including the alternative exon. When the enhancer pyrimidine tract was altered to a consensus binding site for U2AF65, PTB binding to the enhancer decreased and U2AF65 binding increased. The same mutation severely depressed in vivo exon inclusion and in vitro polyadenylation. Our results suggest, therefore, that PTB does bind to the enhancer pyrimidine tract, thereby excluding U2AF. Thus, the CT/CGRP enhancer pseudoexon behaves very similarly to other alternative exons regulated negatively by PTB in nonneuronal cells (3, 8, 20, 22, 28, 43–45, 48, 61). This similarity suggests that intronic pseudoexons may exist to regulate other systems.
MATERIALS AND METHODS
Plasmids.
The minigene constructs depicted in Fig. 2 consist of CT/CGRP exons 4 to 6 with natural intron sequences fused to a heterologous first exon from adenovirus (11, 30). The minigenes used for Fig. 3, in which the CT/CGRP exon 4 and surrounding intron sequences have been placed into intron 1 of the human metallothionein gene, have also been described previously (29, 30). Construction of in vitro exon 4 polyadenylation substrates, including mutant substrates, was described previously (31). The RNA probes a and b used for Fig. 7 were generated by in vitro transcription of linearized plasmid coding for the in vitro polyadenylation precursor RNA. Probe a contains 187 nt of exon 4 upstream of the hexanucleotide AAUAAA and was transcribed from a SacI-digested plasmid; probe b contains 244 nt of exon 4 and 95 nt of the downstream intron sequence and was transcribed from an NheI-digested plasmid. PCR-directed mutagenesis was used to create new mutant templates, including minigenes, in vitro polyadenylation templates, and isolated transcribable enhancer sequences. New mutant templates were sequenced prior to use.
FIG. 2.
In vivo inclusion of CT/CGRP exon 4 is stimulated by cotransfection of PTB. (A) Diagram of the CT/CGRP minigene and RT-PCR oligonucleotides (arrows). This gene has natural CT/CGRP sequences from intron 3 through exon 6 fused to a heterologous first exon. HeLa cells that have a mixed inclusion-exclusion phenotype were cotransfected with the CT/CGRP minigene and increasing levels of a cDNA coding for PTB to test the ability of the protein to stimulate inclusion. (B) RT-PCR assay of total RNA from transfections with the diagrammed CT/CGRP minigene with a wild-type enhancer (lanes 1 to 7) or an enhancer in which the core pyrimidine tract had been mutated from CUCCGCUCCUCUUC to CUACGCGCAUCGUC (lanes 8 to 11). The cotransfecting plasmids coded for either His-tagged LacZ or PTB. Amplification bands resulting from inclusion (319 nt) or exclusion (280 nt) are indicated. The percent inclusion of exon 4 is indicated below each lane with standard deviations (n = 5). Higher-molecular-weight amplification products result from precursor RNA or activation of cryptic splicing. The latter product has been characterized previously (30) and results from activation of a cryptic 5′ splicing site within exon 4 that changes exon 4 from a terminal exon to an internal exon. (C) Western blots documenting the level of protein being produced from the LacZ or PTB cotransfecting plasmids. Both proteins were detected with tag-specific anti-Xpress antibody. (D) Western blots documenting the level of total nuclear PTB in PTB-transfected cells. Nuclear extracts were prepared from control plasmid or PTB-transfected cells (32). Equal amounts of protein from each nuclear extract preparation were loaded. Protein blots were probed with PTB antibody DH7 (left) or anti-CstF 64-kDa antibody (right) as a control for equal protein load.
FIG. 3.
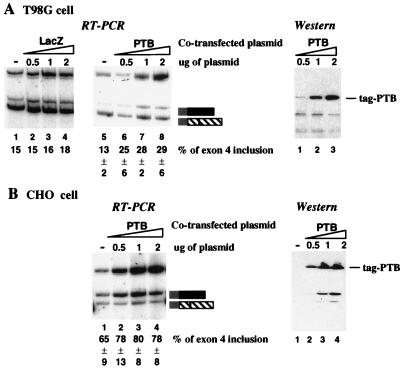
Increasing PTB levels facilitates exon 4 recognition in multiple cell lines, including T86G cells. In vivo transfections similar to those described in the Fig. 2 legend were performed with T98G cells (A) or CHO cells (B). Analysis was done as for Fig. 2; percent product resulting from inclusion or exclusion of exon 4 is shown below each lane with standard deviations (n = 3). The band at the top of each panel resulted from unprocessed precursor RNA. Western blots developed with the tag-specific antibody are shown for each cell line.
FIG. 7.
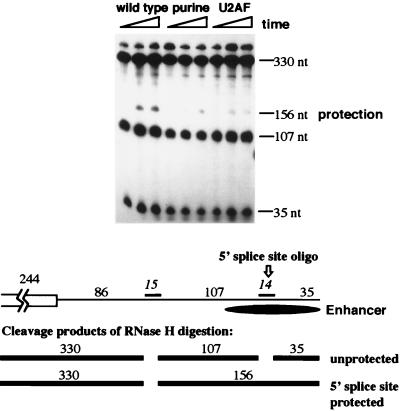
Evidence for interaction between the core pyrimidine tracts and the 5′ splice site sequence by using an RNA protection assay. Binding of factors to the enhancer 5′ splice site sequence was monitored by using an RNase protection assay as diagrammed in the lower part of the figure. Polyadenylation reaction mixtures containing the diagrammed precursor encompassing the last 244 nt of exon 4 and the first 257 nt of intron 4, including the enhancer, were incubated for 10 min. The two antisense DNA oligonucleotides shown in the diagram (an upstream 15-mer hybridizing 86 nucleotides downstream of the polyadenylation cleavage site and a 14-mer complementary to the enhancer 5′ splice site beginning 208 nt downstream of the polyadenylation cleavage site) were added, and incubation was continued for another 10 min in the presence of RNase H. Cleavage products were resolved on a denaturing acrylamide gel. Cleavage and protection products resulting from the binding of factors to the enhancer 5′ splice site sequence are indicated.
The glutathione S-transferase (GST)–PTB construct was a gift from M. Garcia-Blanco (Duke University Medical Center), and the GST-U2AF construct was obtained from M. Green (Massachusetts University Medical Center). The PTB and U2AF expression plasmids were generated by subcloning the PTB or U2AF sequence into the pCDNA3.1His expression vector (Invitrogen), which contains a His tag and an Xpress antigen. LacZ plasmid was from Invitrogen.
Cell transfections and RNA and protein analysis.
Three cell lines, HeLa, T98G, and CHO cells, were cotransfected with CT/CGRP minigenes and either the PTB expression plasmid or a control LacZ plasmid. The basic transfection procedure was previously described (30). Cotransfections each used 2 μg of the CT/CGRP minigene and an increasing amount of PTB or LacZ expression plasmid (0.5, 1, and 2 μg). Cells in each transfection plate were scraped and divided into two parts: one for RNA isolation and one for protein isolation. Procedures for total cell RNA isolation and reverse transcription (RT)-PCR analysis was described previously (30). The RT-PCR analysis used two reverse primers, one for exon 4 and one for exon 5 or metallothionein exon 3. This permitted simultaneous visualization of exon 4 skipping and inclusion products. The RT-PCR protocol was determined to accurately monitor exon 4 inclusion and exclusion products by a set of experiments in which we tested various combinations of the two reverse primers from a number of oligonucleotides complementary to either exon 4 or 5. We also determined that low-cycle (20 to 22 cycles) PCR permitted determination of the relative abundance of individual RNA species with our protocol. Quantification of exon inclusion was determined with a PhosphorImager (Molecular Dynamics). The results shown are representative of at least three transfections for each experiment. Percent inclusion was determined as follows: level of inclusion/(level of inclusion + level of exclusion). Standard deviations were determined on the basis of three to five transfections. Absolute levels of exon 4 inclusion varied from transfection to transfection. However, relative levels of exon 4 inclusion between constructs containing a wild-type or mutant enhancer remained the same (e.g., mutation of the core 5′ splice site sequence decreased exon 4 inclusion to about 15% of the wild-type level). Total cell proteins were prepared in lysis buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 0.1% sodium dodecyl sulfate, and 1% Nonidet P-40. Levels of overexpressed proteins were examined by Western blot analysis with the antitag antibody anti-Xpress (Invitrogen). Levels of total nuclear PTB (both endogenous and exogenous) were examined by Western blot analysis by using the anti-PTB antibody DH7 (21). The nuclear extract was prepared from cells transfected with PTB plasmid as previously described (32).
In vitro assays.
In vitro polyadenylation conditions have been described in detail previously by Lou et al. (31). The procedure for RNase H protection assay was also described (31). Gel shift assays were performed by using recombinant GST-PTB or GST-U2AF prepared from bacteria and in vitro-transcribed RNA substrates. The reactions were carried out in 25 μl containing 50% Roeder D (31), 20 mM creatine phosphate, 2 mM ATP, 2 mg of heparin per ml, 1 mg of bovine serum albumin per ml, 0 to 4 μg of recombinant protein, and 25,000 cpm of 32P-labeled RNA. RNA oligonucleotides were used as competitors in gel shift assays (see Fig. 4B). The reactions were stopped after 10 min of incubation at 30°C by addition of loading buffer containing 50% glycerol and 1% dye, and the complex was separated on a 4% nondenaturing polyacrylamide gel in 1× TG buffer (0.5 M Tris and 0.5 M glycine).
FIG. 4.
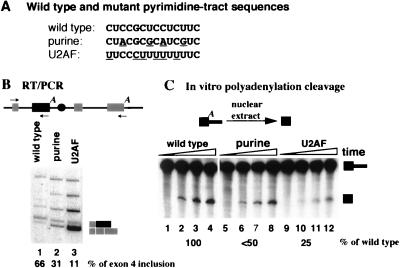
Altering the enhancer pyrimidine tract to a better binding site for U2AF depresses in vivo exon 4 inclusion and in vitro polyadenylation of the exon 4 poly(A) site. (A) Sequence of the wild-type and mutant enhancer pyrimidine tracts. Purine insertions (purine) have been characterized previously (31) and were modeled after mutations used in other studies to inactivate standard 3′ splice sites. Uridine insertions (U2AF) convert the polypyrimidine tract to a site preferred by U2AF (55). (B) In vivo splicing of wild-type and mutant constructs. Total cell RNA from transfected CHO cells was monitored for splicing by using RT-PCR as described for Fig. 2. The minigene for these studies had exon 4 and surrounding intron sequences inserted into intron 1 of the mouse metallothionein gene. Splicing of this construct has been characterized previously (30). The percentage of exon 4 inclusion (versus exclusion) for each construct is shown below the figure. The higher-molecular-weight products result from activation of cryptic splicing which was characterized and described in detail previously (30). (C) In vitro polyadenylation cleavage of wild-type and mutant precursor RNAs. The utilized precursor contained the last half of exon 4 and the beginning of intron 4, including the enhancer sequence. Processing from this precursor has been described previously (31). Reaction mixtures contained 3′-dATP to prevent poly(A) addition. The position of full-length precursor and cleaved but not polyadenylated RNA is indicated. The percent cleavage relative to that of wild-type RNA is shown at the bottom of the figure.
Procedures for UV cross-linking and immunoprecipitation of cross-linked proteins have been previously described (31). The PTB-specific antibodies DH3 and DH7 have been described (21). To examine the U2AF binding by UV cross-linking and immunoprecipitation, an antitag antibody, anti-Xpress, was used to detect the binding of the tagged U2AF protein in nuclear extract isolated from cells transfected with U2AF plasmid. The procedure for preparing this extract was described in detail elsewhere (32).
RESULTS
We have previously demonstrated that four splicing factors—U1 snRNA, ASF/SF2, SRp20, and PTB—bind to the splice site-like sequences within the enhancer (31, 32). Both the sequences and the factors suggested that the enhancer could be modeled after a pseudomicroexon with 0 nt within the exon. To understand the advantages and limitations of such a model, we undertook a study to characterize the functionality of PTB binding to the enhancer pseudoexon and compare it to the normal binding of U2AF to 3′ splice sites.
Raising the in vivo concentration of PTB increases the relative amount of exon 4 inclusion.
In vitro studies identified PTB as one protein that bound to the enhancer pyrimidine tract in an in vitro polyadenylation assay (31). Mutations which significantly decreased PTB binding also inactivated the enhancer, suggesting that PTB might play a role in enhancer activity. Because of the abundance of PTB, it was difficult to determine the role of PTB in enhancer function by using in vitro approaches via PTB inactivation or depletion. We therefore turned to an in vivo approach to establish the role of PTB in enhancer activity. For this purpose we cotransfected various cell lines with a CT/CGRP minigene and a tagged cDNA coding for PTB. The RNA processing phenotype of the minigene was examined by low-cycle RT-PCR. To monitor the relative levels of exon 4 inclusion versus skipping, two 3′ primers complementary to either exon 4 or exon 5 were used in the same reaction. The chosen primers and low-cycle conditions were previously shown to accurately represent relative levels of RNA resulting from exon 4 inclusion or exclusion (see Materials and Methods).
Three cell lines were chosen: HeLa cells, CHO cells, and a glioblastoma cell line, T98G. These three cell lines represent three extremes for the processing of CT/CGRP from the utilized minigene (30, 32). HeLa cells normally direct 40 to 60% inclusion of exon 4. Inclusion rises to 60 to 70% in CHO cells, and falls to 10 to 20% in T98G cells. Therefore, the three cell lines present a spectrum of inherent inclusion efficiencies against which to test the effect of cotransfected PTB.
All three cell lines responded to increasing amounts of PTB in such a way as to raise the percent inclusion levels of exon 4 (Fig. 2 and 3). In HeLa cells inclusion increased from 42 to 76% in CHO cells inclusion increased from 65 to 78%, and in T98G cells inclusion rose from 13 to 29%. Increasing amounts of PTB also lowered overall mRNA levels, so that 2 μg of transfecting PTB plasmid inhibited CT/CGRP mRNA processing and caused an increase in precursor RNA. The increase of precursor RNA was probably the result of the general negative effect of PTB on splicing via its ability to compete for binding of U2AF to 3′ splice sites. Those RNAs that were processed, however, were processed preferentially by a pathway with exon 4 as the terminal exon.
The effect of PTB cotransfection on CT/CGRP RNA processing was indeed the result of increased levels of PTB protein. We detected PTB levels using both tag-specific and PTB-specific antibodies. Western blot analysis using PTB-specific antibodies capable of detecting both endogenous and exogenous PTB (Fig. 2D) demonstrated a significant increase in the level of nuclear PTB in HeLa cells transfected with our PTB cDNA. Because the transfection efficiency in HeLa cells is usually 70 to 90% (32), the data in Fig. 2D represent an underestimate of the actual PTB level in individual transfected cells.
A similar positive effect on exon 4 inclusion of increasing PTB levels was observed when a CT/CGRP minigene construct containing an enhancer pyrimidine tract interrupted by purines was used in cotransfection (Fig. 2B). Several explanations for this observation are possible. First, purine interruption of the pyrimidine tract lowers but does not abolish PTB binding in an in vitro assay (31) (see Fig. 6B). The increased level of PTB protein could compensate for lowered affinity for PTB in the mutant. Second, multiple PTB binding sites surround exon 4 (see below), and PTB could affect exon inclusion by binding to these other sites when the core pyrimidine tract is mutated.
FIG. 6.
Improving the enhancer core to a preferred binding site for U2AF depresses association of PTB with the enhancer. (A) Gel shift analysis of the binding of PTB (top) and U2AF (bottom) to the 127-nt complete enhancer containing the wild-type pyrimidine tract (wild type), purine mutation (purine), or U2AF mutation (U2AF). Mutations are as shown in Fig. 4. Binding utilized purified GST-tagged recombinant proteins under polyadenylation reaction conditions in the presence of 2 mg of heparin per ml. (B) UV cross-linking of PTB and U2AF to the wild-type and mutant enhancer core in nuclear extract. Radiolabeled RNAs containing the indicated wild-type or mutated enhancer sequences were incubated for 10 min in a standard in vitro polyadenylation assay. Reaction mixtures were subjected to UV cross-linking and immunoprecipitation (IP) by using antibodies specific for PTB (top) or U2AF (bottom). The PTB experiment used standard nuclear extract. Because our available anti-U2AF antibodies were inactive for immunoprecipitation, a different technique was used for the U2AF immunoprecipitation. In this experiment, extract was made from cells expressing a tagged version of U2AF by using a technique we have recently developed (32). Immunoprecipitation of cross-linked proteins with this extract utilized tag-specific antibody.
Examination of the splicing patterns of the three cell lines indicated that the effect of increasing PTB concentration was to raise inclusion of exon 4, not to decrease skipping of exon 4 via splicing to exon 5. In both CHO and T98G cells, a reproducible increase in the absolute amount of mRNA resulting from inclusion of exon 4 was observed. Of course, we cannot rule out a concomitant effect on exon 4 skipping arising from the negative effect on splicing of increasing concentrations of PTB. At the concentrations shown in Fig. 2 and 3, however, the splicing of a reporter gene with strong splice sites was not affected by the cotransfecting PTB, suggesting no total poisoning of the processing machinery (data not shown).
PTB exists in at least three variants, PTB, PTB1, and PTB2 proteins, which are derived from the same pre-mRNA through alternative splicing (18). In the cotransfections described here, the PTB form with the lowest molecular weight was used. Identical effects were observed with the other two forms (data not shown). In addition, RT-PCR analysis of endogenous PTB RNA indicated similar ratios of the three forms in HeLa and T98G cells (data not shown). Therefore, each of the three forms has the ability to alter CT/CGRP splicing, and differences in these three forms between cell types are unlikely to be at the heart of alternative CT/CGRP processing.
Altering the enhancer pyrimidine tract to a U2AF binding consensus sequence inhibits in vivo recognition of exon 4 and in vitro polyadenylation cleavage.
The binding of PTB to splicing regulatory sequences is normally considered inhibitory for splicing (3, 8, 20, 22, 28, 43–45, 48, 61). In at least some models, this inhibition is thought to result from competition for binding of U2AF to the polypyrimidine tract of the 3′ splice site. The preceding results suggested that binding of PTB to the enhancer pyrimidine tract activated processing for CT/CGRP exon 4. This “backwards” effect prompted us to ask if the binding of U2AF to the enhancer 3′ pseudo-splice site would be inhibitory for enhancer activity. We therefore assayed the processing phenotypes of precursor RNAs containing a pyrimidine tract in which the natural C-rich tract of CUCCGCUCCUCUUC was altered to a more canonical U2AF binding pyrimidine tract, UUCCCUUUUUUUUC (55). The effect of these changes was compared to that obtained with a mutated pyrimidine tract in which purines were inserted (CUACGCGCAUCGUC). This latter mutation should inhibit the binding of both PTB and U2AF (48, 55).
In vivo, these mutations lowered exon inclusion from 66% to 31% and 11%, respectively, in CHO cells, which normally include exon 4 from the majority of the minigene pre-mRNA (Fig. 4B). In vitro, the two mutations reduced polyadenylation cleavage activity to one-half or one-quarter of that observed with the wild-type sequence (Fig. 4C). Thus, altering the enhancer pyrimidine sequence to a preferred U2AF binding signal caused a direct effect on polyadenylation activity in a precursor RNA that is not undergoing splicing. For both in vivo inclusion and in vitro polyadenylation, the mutation that created a good U2AF binding site was more deleterious than the mutant that would destroy both the PTB and the U2AF binding. These results suggest that the enhancer needs to bind the negative splicing regulator PTB, and not U2AF for maximal in vivo exon 4 recognition and in vitro polyadenylation.
Both PTB and U2AF bind to the enhancer-located pyrimidine tract.
To ascertain if PTB and U2AF bind to the wild-type and mutant enhancer pyrimidine tracts, gel shift and UV cross-linking assays were employed (Fig. 5 and 6). Recombinant GST-tagged PTB and U2AF65 bound to the wild-type pyrimidine sequence, although the binding of PTB was considerably better than that of U2AF. Neither bound well to a pyrimidine tract substrate which had been mutated through the insertion of purines (Fig. 5A). Binding of both proteins could be competed by cold specific pyrimidine oligonucleotides but not by nonspecific oligonucleotides (Fig. 5B and C). Both proteins also bound to the pyrimidine tract mutated to resemble a U2AF preferred binding site, in agreement with published binding preferences (48, 55) for the two proteins (Fig. 6A). In the absence of other proteins, PTB bound strongly to both the wild-type pyrimidine tract and the U2AF binding site. U2AF, however, showed a remarkable preference for the synthetic U-rich RNA, as predicted from its sequence.
FIG. 5.
Both PTB and U2AF can bind the wild type enhancer core pyrimidine tract. Recombinant GST-tagged PTB and U2AF65 were tested for their ability to bind to the CT/CGRP enhancer by using gel shift experiments. The RNA substrates consisted of the isolated 127-nt complete enhancer region, including the core sequence (30) with either a wild-type or purine mutant enhancer sequence (Fig. 4). Binding reactions were carried out under polyadenylation conditions in the presence of 2 mg of heparin per ml. (A) Binding of increasing amounts of GST-PTB or GST-U2AF to wild-type or mutant enhancer sequences; (B) competition of the binding of GST-PTB (1.6 μg of GST-PTB) to the wild-type enhancer with 500 pmol of RNA oligonucleotides containing consensus sequences defined as preferred binding sites for PTB (GCCUGCUGCUCCUCUUCUGUC), U2AF (UUUUCCCUUUUUUUUC), or a nonspecific U3 snRNA sequence; (C) competition of the binding of GST-U2AF (8 μg of protein) to the wild-type enhancer by increasing amounts (20, 100, or 500 pmol) of U2AF-specific or U3 RNA oligonucleotides.
In the presence of extract under standard polyadenylation conditions, greater selectivity of the two proteins was observed. Under these conditions, PTB could be efficiently UV cross-linked only to an RNA containing the enhancer with the wild-type enhancer pyrimidine tract, and U2AF could be cross-linked only to enhancer RNA containing the U-rich pyrimidine tract mutation resembling a U2AF binding site (Fig. 6B). Thus, in the presence of extract factors, considerable preference was observed for both proteins. This difference indicates that the inhibition of in vitro polyadenylation and in vivo inclusion of exon 4 upon altering the enhancer pyrimidine site to a U-rich sequence is accompanied by loss in binding of PTB and acquisition of binding of U2AF. Furthermore, these results suggest that binding of U2AF to the pseudoexon and the 3′ splice site within the enhancer is detrimental to enhancer-mediated exon 4 recognition.
A wild-type enhancer pyrimidine tract is required for maximal binding of factors to the enhancer-located 5′ splice site sequence.
The enhancement of specificity in binding for PTB in complete extract versus with purified protein suggested that PTB interacts with other factors when binding to the enhancer-located pyrimidine tract. One logical binding site for accessory factors is the enhancer-located 5′ splice site sequence. To monitor binding of factors to the 5′ splice site sequence, an RNase protection assay was utilized. A DNA oligonucleotide complementary to the enhancer-located 5′ splice site sequence was added to an in vitro polyadenylation assay in the presence of RNase H. If factors bind to the 5′ splice site sequence, accessibility of the oligonucleotide to the precursor will be limited, cleavage will not occur, and a protection band should be seen. Appearance of maximal protection of the 5′ splice site sequence was dependent upon the wild-type enhancer pyrimidine tract (Fig. 7). Changing the sequence to either the purine or U-rich mutant version lowered protection, indicating that alteration of the PTB binding site lowered recognition of the pseudo-5′ splice site. This observation indicates that recognition of the splice site-like elements within the enhancer is concerted, much like the recognition of natural exons during exon definition (4, 5, 51). Furthermore, it suggests that interactions at the 5′ splice site sequence could alter interactions at the pyrimidine tract and cause the increased binding specificity shown in Fig. 6 for PTB in the context of complete extract.
PTB binds within the CT/CGRP exon 4.
What is the function of the binding of PTB to the enhancer polypyrimidine tract? One answer seems to be that positioning PTB on the enhancer prevents U2AF binding and activation of splice sites within the enhancer to direct cryptic splicing. An alternative hypothesis, however, was that PTB interacted directly with sequences or factors near the exon 4 poly(A) site. This idea seemed particularly attractive because pyrimidine tracts near polyadenylation consensus sequences have been observed to be the binding site for factors that activate polyadenylation (7, 9, 13, 17, 19, 33, 34, 39–41, 50, 53, 56, 62, 66).
Several pyrimidine tracts are present upstream and immediately downstream of the exon 4 AAUAAA sequence. Mutation of the upstream sequences did not affect PTB binding or in vitro polyadenylation activity (data not shown). Between the AAUAAA sequence and the cleavage site, however, is the pyrimidine sequence UUUUUCCCC. As shown in Fig. 8, this sequence bound PTB. Two experimental approaches were used to monitor binding. In the first, gel shift experiments were performed with recombinant PTB and RNA corresponding to regions of exon 4 or intron 4 (Fig. 8A). An RNA containing sequences upstream of the AAUAAA was not bound by recombinant PTB in vitro. In contrast, both an RNA containing the sequences between the hexanucleotide and the cleavage site (Fig. 8A) and an RNA corresponding to the enhancer bound PTB.
FIG. 8.
PTB binds to a second pyrimidine tract adjacent to the polyadenylation consensus AAUAAA element. RNAs a, b, and c as diagrammed were used for gel shift (A) and UV cross-linking (B) studies. RNAs a, b, and c include sequences from −244 to −58, −244 to +91, or +153 to +280 with respect to the exon 4 polyadenylation cleavage site. (A) Binding of recombinant GST-PTB to segments of exon 4 and intron 4. The indicated RNAs were incubated with GST-PTB in the presence of 2 mg of heparin per ml. (B) UV cross-linking and immunoprecipitation (IP) of cross-linked PTB to an exon 4 polyadenylation precursor RNA lacking the enhancer but including wild-type or mutant exon sequences. Two mutants were employed. Mutant 1 had an altered AAUAAA consensus sequence (hex mut); mutant 2 had the sequence adjacent to the AAUAAA altered from UUAUUUUUCCC to UUAUAUGUCACC (Py mut). The precursor RNAs were incubated in nuclear extract under polyadenylation conditions for 10 min and subjected to UV cross-linking and immunoprecipitation with PTB-specific antibodies.
More important, an RNA substrate containing exon 4 and intron 4 sequences immediately downstream of exon 4 but lacking the enhancer could be UV cross-linked to PTB (Fig. 8B). Cross-linking was depressed when the pyrimidine tract adjacent to the AAUAAA element was mutated, indicating that this sequence was important for PTB binding. Mutations in the AAUAAA element also lowered cross-linking, but to a lesser extent. These results indicate the presence of a PTB binding site within exon 4, suggesting that PTB could interact with both the enhancer and with exon 4. The functionality of this binding, however, could not be determined, because mutation of this binding sequence alone did not depress polyadenylation cleavage in the presence or absence of the enhancer (data not shown).
DISCUSSION
We demonstrate here that the CT/CGRP enhancer prefers binding of PTB but not U2AF. In addition, the binding of PTB appears to be functional, because increasing the concentration of PTB in vivo increased the percentage of RNA including the alternative exon. A number of alternatively processed mRNAs contain PTB binding sites (3, 8, 20, 22, 28, 43–45, 48, 61) whose mutation alters inclusion, but conclusive evidence for a PTB protein requirement has not been obtained. The data presented here suggest that PTB levels can be determinative for processing.
One of the types of processing frequently regulated by sequences that bind to PTB is neuronal versus nonneuronal exon inclusion. For processing of both c-src and gamma amino-butyric acid type A receptor (GABAA) γ2, PTB binds to pyrimidine tracts adjacent to the regulated exon necessary for exon exclusion in nonneuronal cells (3, 8). In the case of CT/CGRP, binding of PTB to an intronic pseudoexon is necessary for inclusion of a neighboring exon. Binding also seems to repress recognition of the enhancer-located splice sites by positive splicing factors such as U2AF. Therefore, the CT/CGRP system resembles other PTB-regulated genes in that the recognition of an exon is repressed by PTB. It would be interesting to know if PTB could facilitate the splicing of flanking exons in the context of other PTB-regulated genes without a concomitant negative effect on a central exon. Removal of PTB binding domains within α-tropomyosin can cause skipping of the upstream nonregulated exon (47a), suggesting that PTB may have positive as well as negative effects.
The important question for all neuronal alternative splicing events involving PTB is that of how the effect of PTB is negated in neuronal cells. In the case of CT/CGRP, there are other sequences within the enhancer core that when mutated cause increased exon inclusion in cells that normally skip exon 4 (30). Two of these resemble known splicing signals, a 5′ splice site and a purine enhancer (32a), suggesting that other splicing proteins may bind to the enhancer and regulate PTB binding in neuronal cells. Alternatively, neuronal forms of PTB may exist (3, 8).
We do not yet know how PTB affects exon 4 polyadenylation. Here we show that PTB can bind to a pyrimidine tract within exon 4 located between the AAUAAA and the cleavage site. It is possible that a PTB dimer with multiple RNA-binding domains (46, 49) binds to both the exon and the enhancer and thereby brings other enhancer-binding factors such as U1 snRNPs and SR proteins into proximity with polyadenylation factors (Fig. 9). It is also possible that PTB has a direct effect on exon 4 polyadenylation. It has been recently shown that PTB binds to pyrimidine tracts adjacent to the AAUAAA element in the mouse C2 complement gene (42). These sequences are required for maximal polyadenylation, suggesting that PTB can directly interact with one or more polyadenylation proteins.
FIG. 9.
A model for the role of PTB in the intron enhancer-mediated facilitation of CT exon 4 polyadenylation.
Other models are also possible. The data presented here suggest that binding of U2AF to the enhancer pyrimidine tract is inhibitory for enhancer activity. PTB, therefore, may be required for enhancer activity simply to prevent U2AF binding to the enhancer core. Thus, the pseudoexon within the enhancer core binds a negatively acting splicing factor to its 3′ splice site sequence but positively acting factors, including U1 snRNPs and ASF/SF2, to its 5′ splice site sequence (31). Mutation of either the 3′ or 5′ splice site sequence within the enhancer core prevents the binding of another positively acting splicing factor, SRp20 (32). This observation suggests the existence of interactions between factors that bind the splice site sequences within the core. In agreement with this observation, we have seen that protection of the core 5′ splice site sequence by factors decreases when the pyrimidine tract is mutated. We do not yet understand how the core binding factors interact when binding to the core. SRp20 has been proposed to bind to the 3′ splice site within its own pre-mRNA (25). Thus, both PTB and SRp20 may be binding to the enhancer pyrimidine tract. Given the absence of any direct evidence for an interaction between PTB and any SR protein, including SRp20, it is possible that the association of these two proteins with the pyrimidine tract is sequential rather than simultaneous.
We began this study asking if our model for the enhancer core as a pseudoexon was useful for understanding regulation by this unusual enhancer. Our data suggest that it is so, as long as we present the enhancer core as a pseudoexon binding the negative splicing regulator PTB. In this fashion, the enhancer core resembles many PTB-regulated alternative exons as they are recognized in the tissues in which they are not included (3, 8, 20, 22, 28, 43–45, 48, 61). The abundance of the factors we have observed recognizing this pseudoexon is high, raising the intriguing possibility that pseudoexon elements could be frequent within large vertebrate introns and could be used to promote splicing of flanking exons.
ACKNOWLEDGMENTS
We thank Mariano Garcia-Blanco and Michael Green for providing GST-PTB and GST-U2AF clones, respectively. We acknowledge the helpful advice of members in the Gagel and Berget laboratories.
This work was supported by an ACS grant to S.M.B. and USPHS grants (RO1-DK38146 to R.F.G. and 2P30-CA16672) to the M.D. Anderson Cancer Center. D.M.H. was supported by NIH grant GM43049.
REFERENCES
- 1.Adams M D, Rudner D Z, Rio D C. Biochemistry and regulation of pre-mRNA splicing. Curr Opin Cell Biol. 1996;8:331–339. doi: 10.1016/s0955-0674(96)80006-8. [DOI] [PubMed] [Google Scholar]
- 2.Amara S G, Jonas V, Rosenfeld M G. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- 3.Ashiya M, Grabowski P J. A neuron-specific splicing switch mediated by an array of pre-mRNA repressor sites: evidence of a regulatory role for the polypyrimidine tract binding protein and a brain-specific PTB counterpart. RNA. 1997;3:996–1015. [PMC free article] [PubMed] [Google Scholar]
- 4.Berget S M. Exon recognition in vertebrate splicing. J Biol Chem. 1995;2:2411–2414. doi: 10.1074/jbc.270.6.2411. [DOI] [PubMed] [Google Scholar]
- 5.Black D L. Finding splice sites in a wilderness of RNA. RNA. 1995;1:763–771. [PMC free article] [PubMed] [Google Scholar]
- 6.Bothwell A L, Ballard D W, Philbrick W M, Lindwall G, Maher S E, Bridgett M M, Jamison S F, Garcia-Blanco M A. Murine polypyrimidine tract binding protein. Purification, cloning, and mapping of the RNA binding domain. J Biol Chem. 1991;266:24657–24663. [PubMed] [Google Scholar]
- 7.Brackenridge S, Ashe H L, Giacca M, Proudfoot N J. Transcription and polyadenylation in a short human intergenic region. Nucleic Acids Res. 1997;25:2326–2335. doi: 10.1093/nar/25.12.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan R C, Black D L. The polypyrimidine tract binding protein binds upstream of neural cell-specific c-src exon N1 to repress the splicing of the intron downstream. Mol Cell Biol. 1997;17:4667–4676. doi: 10.1128/mcb.17.8.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou Z-F, Chen F, Wilusz J. Sequence and position requirements for uridylate-rich downstream elements of polyadenylation. Nucleic Acids Res. 1994;22:2525–2531. doi: 10.1093/nar/22.13.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colgan D F, Manley J L. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 1997;11:2755–2766. doi: 10.1101/gad.11.21.2755. [DOI] [PubMed] [Google Scholar]
- 11.Cote G J, Stolow D T, Peleg S, Berget S M, Gagel R F. Identification of exon sequences and an exon binding protein involved in alternative RNA splicing of calcitonin/CGRP. Nucleic Acids Res. 1992;20:2361–2366. doi: 10.1093/nar/20.9.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crenshaw E B, Russo A F, Swanson L W, Rosenfeld M G. Neuron-specific alternative RNA processing in transgenic mice expressing a metallothionein-calcitonin fusion gene. Cell. 1987;49:389–398. doi: 10.1016/0092-8674(87)90291-1. [DOI] [PubMed] [Google Scholar]
- 13.DeZazzo J D, Imperiale M J. Sequences upstream of AAUAAA influence poly(A) site selection in a complex transcription unit. Mol Cell Biol. 1989;9:4951–4961. doi: 10.1128/mcb.9.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwalds-Gilbert G, Veraldi K L, Milcarek C. Alternative poly(A) site selection in complex transcription units: means to an end? Nucleic Acids Res. 1997;25:2547–2561. doi: 10.1093/nar/25.13.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Blanco M, Jamison S, Sharp P A. Identification and purification of a 62,000-dalton protein that binds specifically to the polypyrimidine tract of introns. Genes Dev. 1986;3:1874–1886. doi: 10.1101/gad.3.12a.1874. [DOI] [PubMed] [Google Scholar]
- 16.Ghetti A, Pinol-Roma S, Michael W, Morandi C, Dreyfuss G. HnRNP I, the polypyrimidine tract-binding protein: distinct nuclear localization and association with hnRNAs. Nucleic Acids Res. 1992;20:3671–3678. doi: 10.1093/nar/20.14.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gil A, Proudfoot N J. A sequence downstream of AAUAAA is required for rabbit b globin mRNA 3′ end formation. Nature. 1984;312:473–474. doi: 10.1038/312473a0. [DOI] [PubMed] [Google Scholar]
- 18.Gil A, Sharp P A, Jamison S F, Garcia-Blanco M A. Characterization of cDNAs encoding the polypyrimidine tract-binding protein. Genes Dev. 1991;5:1224–1236. doi: 10.1101/gad.5.7.1224. [DOI] [PubMed] [Google Scholar]
- 19.Gilmartin G M, Fleming E S, Oetjen J, Graveley B R. CPSF recognition of an HIV-1 mRNA 3′-processing enhancer: multiple sequence contacts involved in poly(A) site definition. Genes Dev. 1995;9:72–83. doi: 10.1101/gad.9.1.72. [DOI] [PubMed] [Google Scholar]
- 20.Gooding C, Roberts G C, Smith C W. Role of an inhibitory pyrimidine element and polypyrimidine tract binding protein in repression of a regulated alpha-tropomyosin exon. RNA. 1998;4:85–100. [PMC free article] [PubMed] [Google Scholar]
- 21.Grossman J S, Meyer M I, Wang Y-C, Mulligan G J, Kobayashi R, Helfman D M. The use of antibodies to the polypyrimidine tract binding protein (PTB) to analyze the protein components that assemble on alternatively spliced pre-mRNAs that use distant branch points. RNA. 1998;4:613–625. doi: 10.1017/s1355838298971448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo W, Mulligan G J, Wormsley S, Helfman D M. Alternative splicing of beta-tropomyosin pre-mRNA: cis-acting elements and cellular factors that block the use of a skeletal muscle exon in nonmuscle cells. Genes Dev. 1991;5:2096–2107. doi: 10.1101/gad.5.11.2096. [DOI] [PubMed] [Google Scholar]
- 23.Hedjran F, Yeakley J M, Huh G S, Hynes R O, Rosenfeld M G. Control of alternative pre-mRNA splicing by distributed pentameric repeats. Proc Natl Acad Sci USA. 1997;94:12343–12347. doi: 10.1073/pnas.94.23.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinrichs V, Baker B S. The Drosophila SR protein RBP1 contributes to the regulation of doublesex alternative splicing by recognizing RBP1 RNA target sequences. EMBO J. 1995;14:3987–4000. doi: 10.1002/j.1460-2075.1995.tb00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jumaa H, Nielsen P J. The splicing factor SRp20 modifies splicing of its own mRNA and ASF/SF2 antagonizes this regulation. EMBO J. 1997;16:5077–5085. doi: 10.1093/emboj/16.16.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanaar R, Roche S E, Beall E L, Green M R, Rio D C. The conserved pre-mRNA splicing factor U2AF from Drosophila: requirement for viability. Science. 1993;262:569–572. doi: 10.1126/science.7692602. [DOI] [PubMed] [Google Scholar]
- 27.Krámer A. The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu Rev Biochem. 1996;65:367–409. doi: 10.1146/annurev.bi.65.070196.002055. [DOI] [PubMed] [Google Scholar]
- 28.Lin C H, Patton J G. Regulation of alternative 3′ splice site selection by constitutive splicing factors. RNA. 1995;1:234–245. [PMC free article] [PubMed] [Google Scholar]
- 29.Lou H, Cote G J, Gagel R F. The calcitonin exon and its flanking intronic sequences are sufficient for the regulation of human calcitonin/calcitonin gene-related peptide alternative splicing. Mol Endocrinol. 1994;8:1618–1626. doi: 10.1210/mend.8.12.7535892. [DOI] [PubMed] [Google Scholar]
- 30.Lou H, Yang Y, Cote G J, Berget S M, Gagel R F. An intron splicing enhancer containing a 5′ splice site sequence in the human calcitonin/calcitonin gene-related peptide gene. Mol Cell Biol. 1995;15:7135–7142. doi: 10.1128/mcb.15.12.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lou H, Gagel R F, Berget S M. An intron enhancer recognized by splicing factors activates polyadenylation. Genes Dev. 1996;1:208–219. doi: 10.1101/gad.10.2.208. [DOI] [PubMed] [Google Scholar]
- 32.Lou H, Neugebauer K M, Gagel R F, Berget S M. Regulation of alternative polyadenylation by U1 snRNPs and SRp20. Mol Cell Biol. 1998;18:4977–4985. doi: 10.1128/mcb.18.9.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Lou, H., and S. M. Berget. Unpublished data.
- 33.Lutz C S, Alwine J C. Direct interaction of the U1 snRNP-A protein with the upstream efficiency element of the SV40 late polyadenylation signal. Genes Dev. 1994;8:576–586. doi: 10.1101/gad.8.5.576. [DOI] [PubMed] [Google Scholar]
- 34.Lutz C S, Murthy K G K, Schek N, O’Connor J P, Manley J L, Alwine J C. Interaction between the U1 snRNP-A protein and the 160-kD subunit of cleavage-polyadenylation specificity factor increases polyadenylation efficiency in vitro. Genes Dev. 1996;10:325–337. doi: 10.1101/gad.10.3.325. [DOI] [PubMed] [Google Scholar]
- 35.Lynch K W, Maniatis T. Synergistic interactions between two distinct elements of a regulated splicing enhancer. Genes Dev. 1995;9:284–293. doi: 10.1101/gad.9.3.284. [DOI] [PubMed] [Google Scholar]
- 36.Lynch K W, Maniatis T. Assembly of specific SR protein complexes on distinct regulatory elements of the Drosophila doublesex splicing enhancer. Genes Dev. 1996;10:2089–2101. doi: 10.1101/gad.10.16.2089. [DOI] [PubMed] [Google Scholar]
- 37.Mattox W, Baker B S. Autoregulation of the splicing of transcripts from the transformer-2 gene of Drosophila. Genes Dev. 1991;5:786–796. doi: 10.1101/gad.5.5.786. [DOI] [PubMed] [Google Scholar]
- 38.Mattox W, Ryner L, Baker B S. Autoregulation and multifunctionality among trans-acting factors that regulate alternative pre-mRNA processing. J Biol Chem. 1992;267:19023–19026. [PubMed] [Google Scholar]
- 39.McDevitt M A, Hart R P, Wong W W, Nevins J R. Sequences capable of restoring poly(A) site function define two distinct downstream elements. EMBO J. 1986;5:2907–2913. doi: 10.1002/j.1460-2075.1986.tb04586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLauchlan J, Gaffney J L, Whitton J L, Clements J B. The consensus sequence YGTGTTYY located downstream from the AAUAAA signal is required for efficient formation of mRNA 3′ termini. Nucleic Acids Res. 1985;13:1347–1368. doi: 10.1093/nar/13.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreira A, Wollerton M, Monks J, Proudfoot N J. Upstream sequence elements enhance poly(A) site efficiency of the C2 complement gene and are phylogenetically conserved. EMBO J. 1995;14:3809–3819. doi: 10.1002/j.1460-2075.1995.tb00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreira A, Takagaki Y, Brackenridge S, Wollerton M, Manley J L, Proudfoot N J. The upstream sequence element of the C2 complement poly(A) signal activates mRNA 3′ end formation by two distinct mechanisms. Genes Dev. 1998;12:2522–2534. doi: 10.1101/gad.12.16.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullen M, Smith C, Patton J, Nadal-Ginard B. α-Tropomyosin mutually exclusive exon selection: competition between branchpoint/polypyrimidine tracts determines default exon choice. Genes Dev. 1991;5:642–655. doi: 10.1101/gad.5.4.642. [DOI] [PubMed] [Google Scholar]
- 44.Mulligan G J, Guo W, Wormsley S, Helfman D M. Polypyrimidine tract binding protein interacts with sequences involved in alternative splicing of beta-tropomyosin pre-mRNA. J Biol Chem. 1992;267:25480–25487. [PubMed] [Google Scholar]
- 45.Norton P A. Polypyrimidine tract sequences direct selection of alternative branch sites and influence protein binding. Nucleic Acids Res. 1994;22:3854–3860. doi: 10.1093/nar/22.19.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oh Y L, Hahm B, Kim Y K, Lee H K, Lee J W, Song O, Tsukiyama-Kohara K, Kohara M, Nomoto A, Jang S K. Determination of functional domains in polypyrimidine-tract-binding protein. Biochem J. 1998;331:169–175. doi: 10.1042/bj3310169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patton J G, Mayer S A, Tempst P, Nadal-Ginard B. Characterization and molecular cloning of polypyrimidine tract-binding protein: a component of a complex necessary for pre-mRNA splicing. Genes Dev. 1991;5:1237–1251. doi: 10.1101/gad.5.7.1237. [DOI] [PubMed] [Google Scholar]
- 47a.Patton, J. G. Personal communication.
- 48.Perez I, Lin C H, McAfee J G, Patton J G. Mutation of PTB binding sites causes misregulation of alternative 3′ splice site selection in vivo. RNA. 1997;3:764–778. [PMC free article] [PubMed] [Google Scholar]
- 49.Perez I, McAfee J G, Patton J G. Multiple RRMs contribute to RNA binding specificity and affinity for polypyrimidine tract binding protein. Biochemistry. 1997;36:11881–11890. doi: 10.1021/bi9711745. [DOI] [PubMed] [Google Scholar]
- 50.Prescott J, Falck-Pedersen E. Sequence elements upstream of the 3′ cleavage site confer substrate strength to the adenovirus L1 and L3 polyadenylation sites. Mol Cell Biol. 1994;14:4682–4693. doi: 10.1128/mcb.14.7.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reed R. Initial splice-site recognition and pairing during pre-mRNA splicing. Curr Opin Genet Dev. 1996;6:215–220. doi: 10.1016/s0959-437x(96)80053-0. [DOI] [PubMed] [Google Scholar]
- 52.Rosenfeld M G, Amara S G, Evans R M. Alternative RNA processing: determining neuronal phenotype. Science. 1984;225:1315–1320. doi: 10.1126/science.6089345. [DOI] [PubMed] [Google Scholar]
- 53.Russnak R. Regulation of polyadenylation in hepatitis B viruses: stimulation by the upstream activating signal PS1 is orientation-dependent and additive. Nucleic Acids Res. 1991;19:6449–6456. doi: 10.1093/nar/19.23.6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryner L C, Baker B S. Regulation of doublesex pre-mRNA processing occurs by 3′-splice site activation. Genes Dev. 1991;5:2071–2085. doi: 10.1101/gad.5.11.2071. [DOI] [PubMed] [Google Scholar]
- 55.Singh R, Valcárcel J, Green M R. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science. 1995;268:1173–1176. doi: 10.1126/science.7761834. [DOI] [PubMed] [Google Scholar]
- 56.Sitler A, Gallinaro H, Jacob M. Upstream and downstream cis-acting elements for cleavage at the L4 polyadenylation site of adenovirus-2. Nucleic Acids Res. 1994;22:222–231. doi: 10.1093/nar/22.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian M, Maniatis T. Positive control of pre-mRNA splicing in vitro. Science. 1992;256:237–240. doi: 10.1126/science.1566072. [DOI] [PubMed] [Google Scholar]
- 58.Tian M, Maniatis T. A splicing enhancer complex controls alternative splicing of doublesex pre-mRNA. Cell. 1993;74:105–114. doi: 10.1016/0092-8674(93)90298-5. [DOI] [PubMed] [Google Scholar]
- 59.Tian M, Maniatis T. A splicing enhancer exhibits both constitutive and regulated activities. Genes Dev. 1994;8:1703–1712. doi: 10.1101/gad.8.14.1703. [DOI] [PubMed] [Google Scholar]
- 60.Valcárcel J, Gaur R K, Singh R, Green M R. Interaction of U2AF65 RS region with pre-mRNA branch point and promotion of base pairing with U2 snRNA. Science. 1996;273:1706–1709. doi: 10.1126/science.273.5282.1706. [DOI] [PubMed] [Google Scholar]
- 61.Valcárcel J, Gebauer F. Post-transcriptional regulation: the dawn of PTB. Curr Biol. 1997;7:R705–R708. doi: 10.1016/s0960-9822(06)00361-7. [DOI] [PubMed] [Google Scholar]
- 62.Valsamakis A, Zeichner S, Carswell S, Alwine J C. The human immunodeficiency virus type 1 polyadenylation signal: a 3′ long terminal repeat element upstream of the AAUAAA necessary for efficient polyadenylation. Proc Natl Acad Sci USA. 1991;88:2108–2112. doi: 10.1073/pnas.88.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Oers C C M, Adema G J, Zandberg H, Moen T C, Baas P D. Two different sequence elements within exon 4 are necessary for calcitonin-specific splicing of the human calcitonin/calcitonin gene-related peptide I pre-mRNA. Mol Cell Biol. 1994;14:951–960. doi: 10.1128/mcb.14.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wahle E, Keller W. The biochemistry of polyadenylation. Trends Biochem Sci. 1996;21:247–250. [PubMed] [Google Scholar]
- 65.Wang Z, Hoffmann H M, Grabowski P J. Intrinsic U2AF binding is modulated by exon enhancer signals in parallel with changes in splicing activity. RNA. 1995;1:21–35. [PMC free article] [PubMed] [Google Scholar]
- 66.Wilusz J, Shenk T. A uridylate tract mediates efficient heterogeneous nuclear ribonucleoprotein C protein-RNA cross-linking and functionally substitutes for the downstream element of the polyadenylation signal. Mol Cell Biol. 1990;10:6397–6407. doi: 10.1128/mcb.10.12.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zamore P D, Green M R. Identification, purification and characterization of U2 small nuclear ribonucleoprotein auxiliary factor. Proc Natl Acad Sci USA. 1989;86:9243–9247. doi: 10.1073/pnas.86.23.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zamore P D, Green M R. Biochemical characterization of U2 snRNP auxiliary factor: an essential pre-mRNA splicing factor with a novel intracellular distribution. EMBO J. 1991;10:207–214. doi: 10.1002/j.1460-2075.1991.tb07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zuo P, Maniatis T. The splicing factor U2AF35 mediates critical protein-protein interactions in constitutive and enhancer-dependent splicing. Genes Dev. 1996;10:1356–1368. doi: 10.1101/gad.10.11.1356. [DOI] [PubMed] [Google Scholar]