Abstract
Supernumerary breast components occur predominantly between the breast and umbilicus.1 Carcinoma of this ectopic, or accessory breast tissue (ABT), is exceedingly rare, accounting for <1% of breast cancer cases.1,2 Historically, ectopic breast carcinoma was considered aggressive with poor outcome. In 1995, Evans et al reported 90 cases spanning from 1929–1993 with a 9.4% survival beyond 4 years.2 More-contemporary studies reveal improvement in both treatment and survival. There is currently no consensus on whether prophylactic excision of an ipsilateral supernumerary nipple at the time of initial breast cancer diagnosis is necessary. The following describes a patient with an ipsilateral tumor uniquely located within her supernumerary nipple 5 years after mastectomy.
Keywords: ipsilateral supernumerary nipple, ectopic tissue, invasive ductal carcinoma, accessory nipple carcinoma, new primary cancer, chest wall lesion
CASE REPORT
The patient is a 44-year-old female with a history of T2N1 triple negative invasive ductal carcinoma (IDC) in the upper outer quadrant of the left breast. She underwent a modified radical mastectomy with immediate reconstruction. Final pathology revealed a 2.8cm IDC with 1/15 positive lymph nodes. She received adjuvant chemotherapy with dose-dense Adriamycin, Cytoxan followed by Taxol.
Five years later, she palpated a mass at the inframammary fold under her supernumerary nipple (Figure 1). Ultrasound revealed an irregular shape (Figure 2), and biopsy revealed a poorly differentiated lobular carcinoma (ER 5%, PR 5%, HER2-). PET scan did not reveal distant metastasis, and MRI revealed a 2.5cm mass involving left rectus abdominus muscle. She received neoadjuvant chemotherapy with Cytoxan and Taxotere, and repeat imaging revealed a favorable radiographic response. She underwent a wide-local excision encompassing the accessory nipple. Final pathology revealed a residual 3.3cm invasive lobular carcinoma, hormone receptor positive (ER: 5%, PR: 5–10%) and HER2- with negative margins.
FIGURE 1.

Clinical presentation.
FIGURE 2.
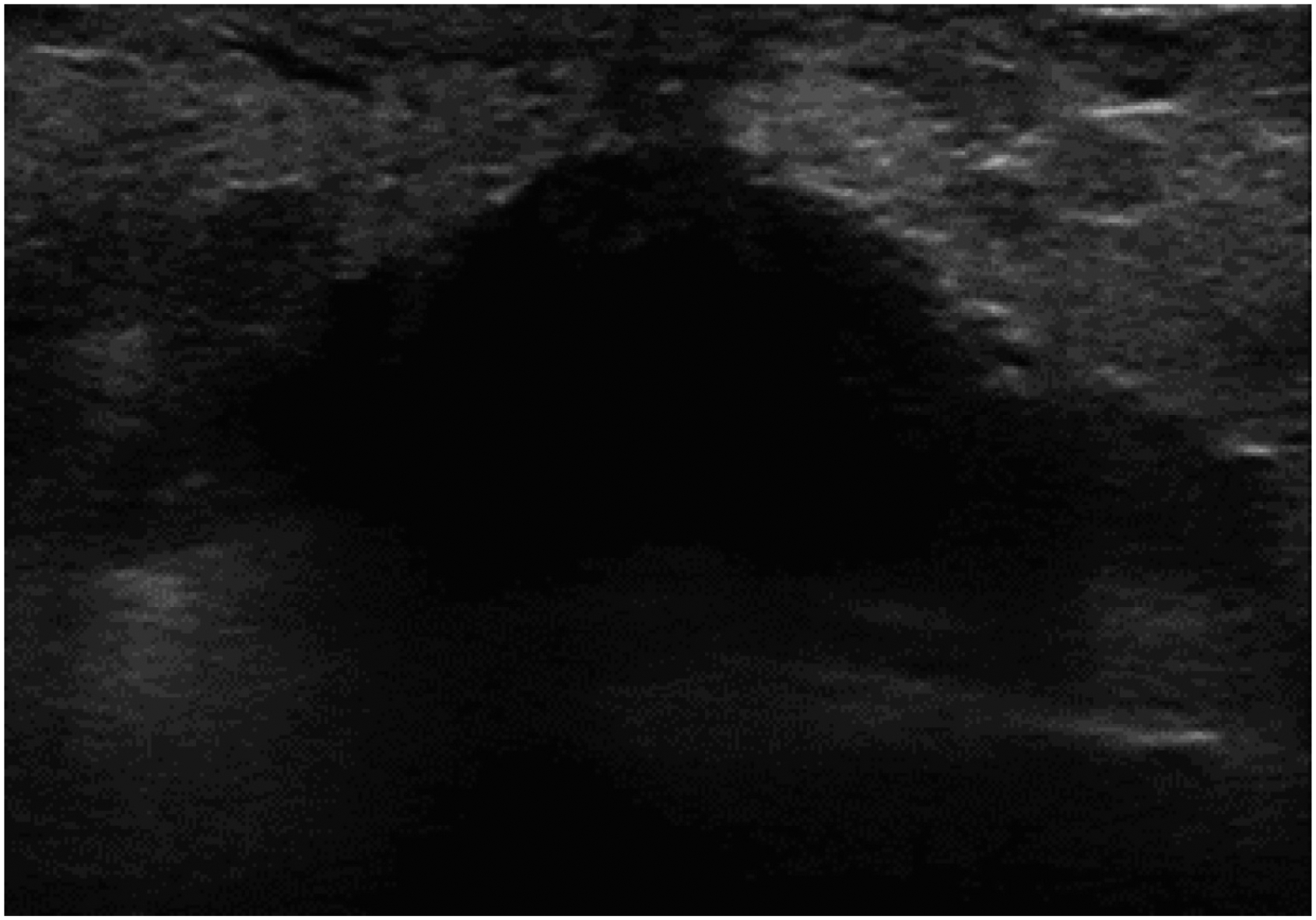
Ultrasound of the breast lesion along the inframammary fold.
Postoperatively, she received Capecitabine followed by endocrine therapy. She completed proton therapy to the chest wall and regional lymph nodes using 2 cobalt Gray-equivalents (CBEs) × 25 plus a tumor bed boost along inframammary fold using 2 CBEs × 8 fractions. Two years later, she underwent revision of her reconstruction using autologous tissue with DIEP flap and is currently NED.
DISCUSSION
The presence of an accessory nipple may necessitate operative intervention in instances of discharge, cyst formation, or tumor.1 Primary carcinoma of ABT is exceedingly rare, and guidance regarding management limited to small observational studies and case reports.2–8 Given the derivation and composition, ABT is subject to the same hormonal influences and physiologic changes as orthotopic breast tissue (OBT) with equivalent susceptibility to malignancy.1 As shown in Table 1, many cases of ABT carcinoma appear to have regional lymph node involvement on final pathology. Due to the insidious nature of its presentation, this finding is likely related to a delay in diagnosis, or possibly greater access to chest-wall lymphatics, rather than a biologically more-aggressive tumor. Current studies demonstrate that cancer arising in ABT does not appear to confer a poorer prognosis by stage compared with cancer arising in OBT,6 but all have limitations given short follow-up and small sample size.
TABLE 1.
Literature review of primary carcinoma in ABT
| Marshall/1994 n (%) | Routiot/1998 n(%) | Nihon-Yanagi/ 2011 n(%) | Zhang/2015 n(%) | Fama/2016 n(%) | |
|---|---|---|---|---|---|
| n | 80 | 21 | 94 | 11 | 4 |
| Average age, years(range) | 50Ŧ(28–90) | 57(39–90) | 57(26–88) | 38 (27–48) | 54(35–73) |
| Median tumor size, cm(range) | 3.6Ŧ(1–15) | 1.8Ŧ(0.8–3.5) | 2.8Ŧ(0.4–1.1) | unknown(0.5–5) | 1.25(0.9–1.2) |
| Histology | |||||
| IDC | 30(38) | 15(71) | 52(75) | 8(73) | 2(50) |
| ILC | 2(3) | 1(5) | 2(3) | 3(27) | 1(25) |
| Other | 7(9) | 4(19) | 14(15) | 0(0) | 1(25) |
| Unknown | 41(51) | 1(5) | 26(28) | 0(0) | 0(0) |
| Operation | |||||
| WLE+/−SLNB+/−ALND | 34(43) | 20(95) | 60(63) | 6(55) | 4(100) |
| Mastectomy+/−ALND | 25(31) | 1(5) | 25(27) | 2(18) | 0(0) |
| None/unknown | 21(26) | 0(0) | 9(10) | 0(0) | 0(0) |
| Lymph node involvement | 20(25)Ŧ | 9(43) | 44(52) | 5(46) | 1(25) |
| Chemotherapy | 2(3) | 4(19)* | 57*(61) | 11(100) | 2(50) |
| Radiation therapy | 14(18)Ŧ | 9(43)* | 13*(14) | 3(27) | 3(75) |
| Endocrine therapy | - | 5(24)* | 40*(43) | 5(46) | 1(25) |
| Recurrences | 16(20)Ŧ | 4(19) | 9(10)Ŧ | 7(64) | 0(0) |
| Deaths | Unknown | 2(9) | 1(1) | 5(45) | 0(0) |
IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; WLE, wide-local excision; ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy
Therapies administered in combination
Incomplete patient information
Treatment of cancer arising in ABT is similar to carcinoma anatomically situated within the breast and includes resection +/− radiation therapy (RT). Data suggest that RT be limited to the tumor cavity and ipsilateral axilla when indicated with regimens for adjuvant systemic therapy identical to orthotopic breast cancer. Carcinoma of ABT occurring concurrently with primary orthotopic invasive breast cancer is extremely uncommon, and the few documented cases appear to have been discovered incidentally.4 Given the long disease-free interval, different histology, and subtype, our patient likely presented with a new primary carcinoma arising in the ABT rather than a recurrent cancer.
Due to its rarity, there is no guidance on whether ipsilateral prophylactic excision of ABT is warranted at the time of the initial operation for orthotopic breast cancer. With accessory nipple preservation, consideration should be made to include the ABT in the breast conservation, or PMRT fields if radiation is recommended. Further study is necessary regarding the management of ABT at the time of treatment of ipsilateral orthotopic breast cancer.
REFERENCES
- 1.Bland KI, Copeland EM III, eds. The Breast: Comprehensive Management of Benign and Malignant Diseases. WB Saunders, Philadelphia, 2018. [Google Scholar]
- 2.Evans DM, Guyton DP. Carcinoma of the axillary breast. J Surg Oncol. 1995;59(3):190–195. [DOI] [PubMed] [Google Scholar]
- 3.Famá F, Cicciú M, Sindoni A, et al. Prevalence of Ectopic Breast Tissue and Tumor: A 20-Year Single Center Experience. Clin Breast Cancer. 2016;16(4):e107–112. [DOI] [PubMed] [Google Scholar]
- 4.Hao JY, Yang CC, Liu FF, et al. Accessory breast cancer occurring concurrently with bilateral primary invasive breast carcinomas: a report of two cases and literature review. Cancer Biol Med. 2012;9(3):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall MB, Moynihan JJ, Frost A, Evans SR. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3(5):295–304. [DOI] [PubMed] [Google Scholar]
- 6.Nihon-Yanagi Y, Ueda T, Kameda N, Okazumi S. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20(1):35–42. [DOI] [PubMed] [Google Scholar]
- 7.Routiot T, Marchal C, Verhaeghe JL, et al. Breast carcinoma located in ectopic breast tissue: a case report and review of the literature. Oncol Rep. 1998;5(2):413–417. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Yu YH, Qu W, Zhang Y, Li J. Diagnosis and treatment of accessory breast cancer in 11 patients. Oncol Lett. 2015;10(3):1783–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]


