ABSTRACT
TMEM41B and VMP1, two endoplasmic reticulum (ER)-resident transmembrane proteins, play important roles in regulating the formation of lipid droplets (LDs), autophagy initiation, and viral infection. However, the biochemical functions of TMEM41B and VMP1 are unclear. A lipids distribution screen suggested TMEM41B and VMP1 are critical to the normal distribution of cholesterol and phosphatidylserine. Biochemical analyses unveiled that TMEM41B and VMP1 have scramblase activity. These findings shed light on the mechanism by which TMEM41B and VMP1 regulate LD formation, lipids distribution, macroautophagy, and viral infection.
KEYWORDS: ER, lipid droplet, lipid transport, macroautophagy, scramblase, TMEM41B, viral infection, VMP1
Lipid droplets (LDs) are conserved dynamic storage organelles that are critical to lipid metabolism, energy homeostasis, signal transduction, membrane trafficking, and innate immunity. The dysregulation of LDs is involved in a variety of diseases, including, but not limited to, neutral lipid storage disease, fatty liver disease, obesity, cholesterol ester storage disease, atherosclerosis, viral infection, neurodegenerative diseases, and so on.
LDs comprise a neutral lipids core wrapped by a phospholipid monolayer decorating with various proteins essential to the function of LDs. Though the molecular mechanism of LD biogenesis remains elusive, LD biogenesis, occurring at the ER, involves several steps that can be divided into: neutral lipid synthesis, nascent LD formation, and LD budding and growth. BSCL2/Seipin, an ER-residing transmembrane protein, is essential for nascent LD formation. BSCL2 has been suggested to regulate the synthesis and distribution of phospholipids in the ER. Moreover, it also has been indicated that BSCL2, together with LDAF1 (lipid droplet assembly factor 1), can form an ~600 kDa oligomeric complex to facilitate LD biogenesis by determining the initiation site in the ER.
Recently, TMEM41B and VMP1, two members of the evolutionarily conserved VTT (VMP1, TMEM41, and Tvp38) domain family, had been shown to participate in autophagy initiation as well as LD formation. Intriguingly, several studies using a genome-scale screen suggested that TMEM41B is a critical host factor for the infection of coronaviruses and flaviviruses, including the notorious SARS-CoV-2. However, the physiological functions of TMEM41B and VMP1 are still elusive. Attractively, giant LDs form in TMEM41B- or VMP1-knockout cells, similar to the phenotype of BSCL2-deficient cells. In addition, both VMP1 and BSCL2 have been suggested to interact with ATP2A/SERCA, a P-type ATPase, and thereby participate in the regulation of cellular calcium signaling.
In light of the clues and existing links between BSCL2, TMEM41B, and VMP1, we focused on the functional and physical relationship among them [1]. Confocal imaging showed that both TMEM41B and VMP1 colocalize with BSCL2 almost perfectly, but co-immunoprecipitation analysis showed that neither TMEM41B nor VMP1 can coprecipitate with BSCL2, indicating that TMEM41B and VMP1 do not interact with BSCL2. BSCL2 is an essential factor for the formation of early LDs. Hence, LD staining was performed to investigate the effect of VMP1 on the formation of LDs. The data showed that the initiation of LD formation is delayed in BSCL2 knockout cells, whereas the early LDs look normal in VMP1-deficient cells, indicating that BSCL2, compared to TMEM41B and VMP1, uses a different mechanism to regulate LD formation.
The proportion and distribution of lipids are critical to the morphology of LDs. Thus, we wondered if TMEM41B and VMP1 affect lipids distribution. A miniscreen detecting lipid distribution was carried out in wild-type and TMEM41B- and VMP1-deficient cells. The sensors for phosphatidic acid (PA), phosphatidylinositol 4,5-bisphosphate, phosphatidylinositol 4-phosphate, and phosphatidylserine (PS) show no difference in WT and TMEM41B- and VMP1-deficient cells. Excitingly, more cholesterol in the plasma membrane (PM) becomes accessible to D4H, a cholesterol sensor, in TMEM41B- and VMP1-deficient cells, compared with WT cells. Moreover, total cellular free cholesterol does not change obviously in TMEM41B- and VMP1-deficient cells, indicating cholesterol might redistribute between the different pools of cholesterol in the PM. Interestingly, overexpressing VMP1 in TMEM41B-deficient cells can rescue the phenotype of cholesterol redistribution and vice versa, suggesting that the functions of TMEM41B and VMP1 are redundant to some extent. Of note, the phenomena aforementioned had been confirmed using different cell lines and cholesterol sensors. Collectively, TMEM41B and VMP1 are important to the physical properties of cellular membrane, of which dysfunction might result in more cholesterol accessible on the inner leaflet of the PM.
Meanwhile, the intensity of the PS sensor on the cytoplasmic side increases dramatically in TMEM41B- and VMP1-deficient cells. Curiously, the lipidomic analysis showed that the total PS in the cell does not change significantly. Digitonin was used to permeabilize the cells, which is too gentle to expose the lumenal side of organelles. Hence, the TMEM41B- and VMP1-deficient cells were treated using Triton X-100, which can permeabilize all cellular membrane and expose both the cytoplasmic leaflet and lumenal leaflet to the PS sensor. Under such conditions, no difference was observed between WT and TMEM41B- and VMP1-deficient cells. Together, TMEM41B and VMP1 may maintain the distribution of PS between cytoplasmic and lumenal leaflets of the ER membrane.
Lipids are transferred between the inner and outer leaflets of the membrane by flippases, floppases, and scramblase. Depending on the hydrolysis of ATP, flippases and floppases transfer lipids to inner leaflet and outer leaflet, respectively. Scramblases can equilibrate lipids across lipid bilayer independent of energy but sometimes requiring Ca2+. Therefore, we examined whether TMEM41B and VMP1 can transfer lipids across a membrane using purified recombinant proteins and a fluorescent-based flippase/floppase/scramblase assay, which can assess whether target proteins can move lipids from the inner leaflet to the outer leaflet of liposomes. The results indicated that TMEM41B and VMP1 can transport lipids between the two leaflets of the lipid bilayer independent of ATP and Ca2+, suggesting TMEM41B and VMP1 might be scramblases. Intriguingly, the scramblase activity of TMEM41B and VMP1 is independent of each other, which is consistent with the observation that overexpressing TMEM41B or VMP1 can rescue the phenotype of knocking out VMP1 or TMEM41B. Additionally, TMEM41B and VMP1 do not show apparent lipids preference in vitro.
Scramblases are essential for the uniform expansion of the ER because the synthesis of phospholipids is finished on the cytoplasmic side. None of the ER scramblases had been identified until ANO10/TMEM16K was identified as an ER-resident lipid scramblase; however, the scramblase activity of ANO10 requires Ca2+. Here, TMEM41B and VMP1 were revealed to have Ca2+-independent scramblase activity (Figure 1). Moreover, the cell biological assays showed that TMEM41B and VMP1 might regulate the PS balance between the two leaflets of the membrane, thereby affecting the LD formation and the accessibility of cholesterol on the PM. Cholesterol is critical to the infection of many viruses. Notably, TMEM41B and several proteins that regulate cholesterol homeostasis were identified as essential factors for viral infection, including SARS-CoV-2. Noteworthily, the Reinisch lab also showed that TMEM41B and VMP1 are scramblases and proposed that TMEM41B and VMP1 facilitate autophagy by reequilibrating the lipids of ER leaflets as the lipids are extracted by ATG2 during autophagosome formation.
Figure 1.
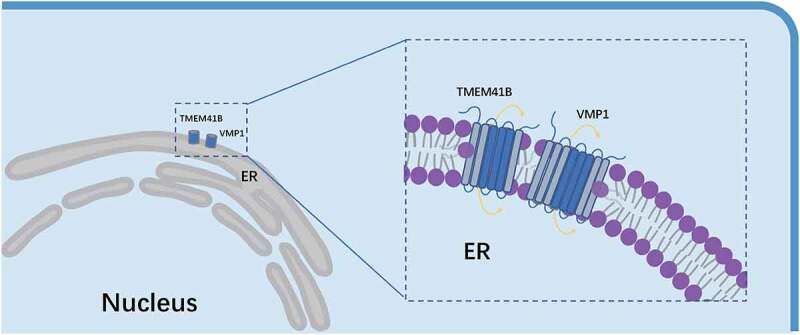
Schematic model of TMEM41B and VMP1 scramblases
ur data, as well as the evidence reported by others, provide a framework for unveiling the physiological function of TMEM41B and VMP1. Nevertheless, the mechanisms of how TMEM41B and VMP1 scramble lipids and affect the physical properties of cellular membrane, and how viruses hijack TMEM41B to facilitate their replication remain to be elucidated.
Funding Statement
This work was supported by National Key R&D Program of China grant 2017YFA0506300 (Q.S.) and NSFC grants 32071214 (Q. S.).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- [1].Li YE, Wang Y, Du X, et al. TMEM41B and VMP1 are scramblases and regulate the distribution of cholesterol and phosphatidylserine. J Cell Biol. 2021;220. DOI: 10.1083/jcb.202103105. [DOI] [PMC free article] [PubMed] [Google Scholar]


